
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 11 September 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1442283
 Sarah Dehne1
Sarah Dehne1 Lina Kirschner1
Lina Kirschner1 Rosa Klotz2
Rosa Klotz2 Samuel Kilian3
Samuel Kilian3 Christoph W. Michalski2
Christoph W. Michalski2 Thilo Hackert2†
Thilo Hackert2† Markus W. Büchler2
Markus W. Büchler2 Markus A. Weigand1
Markus A. Weigand1 Jan Larmann1*
Jan Larmann1*Background: Intraoperative end-tidal carbon dioxide concentrations (EtCO2) values are associated with recurrence-free survival after colorectal cancer surgery. However, it is unknown if similar effects can be observed after other surgical procedures. There is now evidence available for target EtCO2 and its relation to surgical outcomes following pancreatic cancer surgery.
Methods: In this single-center, retrospective cohort study, we analyzed 652 patients undergoing elective resection of pancreatic cancer at Heidelberg University Hospital between 2009 and 2016. The entire patient cohort was sorted in ascending order based on mean intraoperative EtCO2 values and then divided into two groups: the high-EtCO2 group and the low-EtCO2 group. The pre-specified primary endpoint was the assessment of recurrence-free survival up to the last known follow-up. Cardiovascular events, surgical site infections, sepsis, and reoperations during the hospital stay, as well as overall survival were pre-specified secondary outcomes.
Results: Mean EtCO2 was 33.8 mmHg ±1.1 in the low-EtCO2 group vs. 36.8 mmHg ±1.9 in the high-EtCO2 group. Median follow-up was 2.6 (Q1:1.4; Q3:4.4) years. Recurrence-free survival did not differ among the high and low-EtCO2 groups [HR = 1.043 (95% CI: 0.875–1.243), log rank test: p = 0.909]. Factors affecting the primary endpoint were studied via Cox analysis, which indicated no correlation between mean EtCO2 levels and recurrence-free survival [Coefficient −0.004, HR = 0.996 (95% CI:0.95–1.04); p = 0.871]. We did not identify any differences in the secondary endpoints, either.
Conclusions: During elective pancreatic cancer surgery, anesthesiologists should set EtCO2 targets for reasons other than oncological outcome until conclusive evidence from prospective, multicenter randomized controlled trials is available.
During surgery, the measurement of end-tidal carbon dioxide concentration (EtCO2) serves as a non-invasive method to estimate arterial carbon dioxide pressure (PaCO2) (1), reflecting the amount of carbon dioxide (CO2) dissolved in arterial blood. CO2 can impact cellular mechanism such as cell movement, apoptosis, and cell growth (2–5). In vitro, elevated CO2 levels may enhance the invasive capabilities of various cancer cells (6–9). In colon cancer cells, exposure to CO2 exhibits in heightened proliferation, adhesion disorder and elevated levels of growth factor expression (7, 10). Effects of CO2 exposure on tumor biology have also been demonstrated in pancreatic ductal adenocarcinoma cells. Exposure to hypercapnia can result in increased cell colony formation, elevated cell division process, and higher radio- and chemotherapy resistance (11, 12).
During surgical procedures, EtCO2 is influenced by mechanical ventilation and typically ranges from 30 to 45 mmHg (13–15). Currently, there are no recommendations for precise intraoperative target EtCO2 levels derived from outcome studies (2). Intraoperative EtCO2 levels are influenced by several factors, such as the presence of existing pulmonary conditions, the kind of surgery, and the anesthesiologist's preferences (2).
In our retrospective cohort study involving 528 patients undergoing colorectal cancer surgery, lower EtCO2 values were associated with enhanced oncological outcome (16). Since in vitro experiments suggest that CO2 may potentially affect the tumor biology of pancreatic cancer cells and because the impact of different intraoperative EtCO2 values on oncological outcome in patients undergoing pancreatic cancer surgery is unknown, we performed this retrospective cohort study to examine the association between intraoperative EtCO2 levels and recurrence-free survival.
A retrospective cohort study was conducted involving patients who received general anesthesia for elective pancreatic cancer surgery at the Department of General, Visceral, and Transplant Surgery, Heidelberg University Hospital, Heidelberg, Germany.
Our study protocol (S-723/2021) received approval by the local Ethics Committee of the Medical Faculty of Ruprecht-Karls-University, Heidelberg, Germany, on 29 March, 2022. The principles described in the Declaration of Helsinki and the STROBE guidelines for observational studies have been followed in preparing this report (17).
We evaluated the association between intraoperative EtCO2 levels and recurrence-free survival following pancreatic cancer surgery. Patients who underwent pancreatic cancer surgery between 2009 and 2016 were analyzed. Only patients ≥18 years of age with a minimum of 180 days of follow-up data were eligible for inclusion. Patients with distant metastases at the time of surgery were not included in this study. Furthermore, the histopathological examination following elective pancreatic tumor surgery had to show either an R1 result (microscopic residual tumor) or an R0 result (no residual tumor) for inclusion. Exclusion criteria included identification of peritoneal carcinomatosis during surgery or cases where histological analysis could not verify the presence of cancer tissue, such as those following neoadjuvant chemotherapy or radiotherapy.
Data were accessed from patients' medical records and the prospectively-maintained electronic databases of the Department of Surgery at Heidelberg University Hospital. Data incorporated demographic information, American Society of Anesthesiologists physical status classification (ASA), body mass index (BMI), pre-existing conditions, neoadjuvant and adjuvant therapy, duration of surgery, intraoperative opioid usage and transfusions, performance of intraoperative radiation therapy (IORT), duration of hospital and intensive care unit stay, and outcome parameters. Resection margin status, tumor grade, and TNM (tumor, node, metastasis) classification were obtained from pathology findings. The mean EtCO2 was determined using EtCO2 values documented in the anesthesia records every quarter hour from intubation to extubation throughout the entire surgery. The EtCO2 was measured in real-time using infrared spectroscopy in the “main stream” method. The documented EtCO2 values correspond to the plateau values of the EtCO2 curves displayed by the ventilator.
The primary outcome measure was recurrence-free survival in the period from index surgery until the last known follow-up, with a median follow-up duration of 2.6 years (Q1:1.4; Q3:4.4). Recurrence-free survival was defined as the time from index surgery to the first documented event of local cancer recurrence, newly diagnosed metastases, or death. During the follow-up examinations, abdominal ultrasounds, computer tomographies, physical examinations and blood samples were carried out at regular intervals or when new suspicious symptoms occurred. If there were no documented instances of cancer recurrence, new metastases, or death, we recorded the date of the last follow-up or doctor-patient contact with negative findings. Surgical site infections (SSI), sepsis, reoperation due to surgical complications, and cardiovascular events (transitory ischemic attack, and cerebral- or myocardial infarction) during the hospital stay and overall survival were secondary outcomes.
The entire patient cohort was sorted in ascending order based on mean intraoperative EtCO2 values and then divided into two groups: the high-EtCO2 group and the low-EtCO2 group, each consisting of 326 patients. Descriptive analyses involved calculating the mean, standard deviation (SD), median, and first and third quartiles for continuous variables, and absolute and relative proportions for categorical variables. The chi-square test was employed to compare the distribution of categorical variables among the different groups, whereas differences in continuous variables were evaluated using the Mann-Whitney U test. Survival analysis for the predefined primary endpoint was performed using the Kaplan-Meier method (18), and group comparisons were made using the log-rank test (19). Bar charts were created for each year to compare the rates of a composite endpoint, including local cancer recurrence, newly diagnosed metastases, and death between the low and high EtCO2 groups throughout the respective follow-up periods. To compare major differences in mean EtCO2 values, the entire patient cohort was sorted by ascending mean intraoperative EtCO2 values and then stratified into five groups for further survival analysis using the Kaplan-Meier method. Furthermore, a subgroup analysis was conducted to account for the duration of the respective EtCO2 values. Therefore, patients were categorized into three subgroups according to their duration of surgery. The high and low EtCO2 groups within these subgroups were subsequently re-evaluated for recurrence-free survival using Kaplan-Meier method. Thereafter, the primary outcome was analyzed by the Cox proportional hazard model (20), in which the effect of mean EtCO2 on recurrence-free survival was estimated after adjusting for the following covariates: age, gender, BMI, diabetes mellitus, smoking, UICC (tumor classification according to the Union for International Cancer Control) stage, epidural anesthesia, intraoperative sufentanil consumption and transfusions, resection margin status, tumor grade, and neoadjuvant, intraoperative, and adjuvant therapies. Using the Wald-Test, p-values for regression coefficients were obtained. Hazard ratios (HRs) were calculated using Cox analysis and presented with their respective 95% confidence intervals (CIs). Significance was defined as a two-sided p-value < 0.05. Survival rates at 1–5 years were assessed using the Kaplan-Meier technique, and statistical significance was evaluated employing the log-rank test. Prism 9.0.0 (GraphPad Prism Software, Inc., San Diego, CA) and IBM SPSS Statistics 28.0 (SPSS, Chicago, IL) and were used for statistical analysis and graphical representation.
After applying the inclusion and exclusion criteria, the final analysis comprised 652 patients with pancreatic adenocarcinoma (Figure 1).
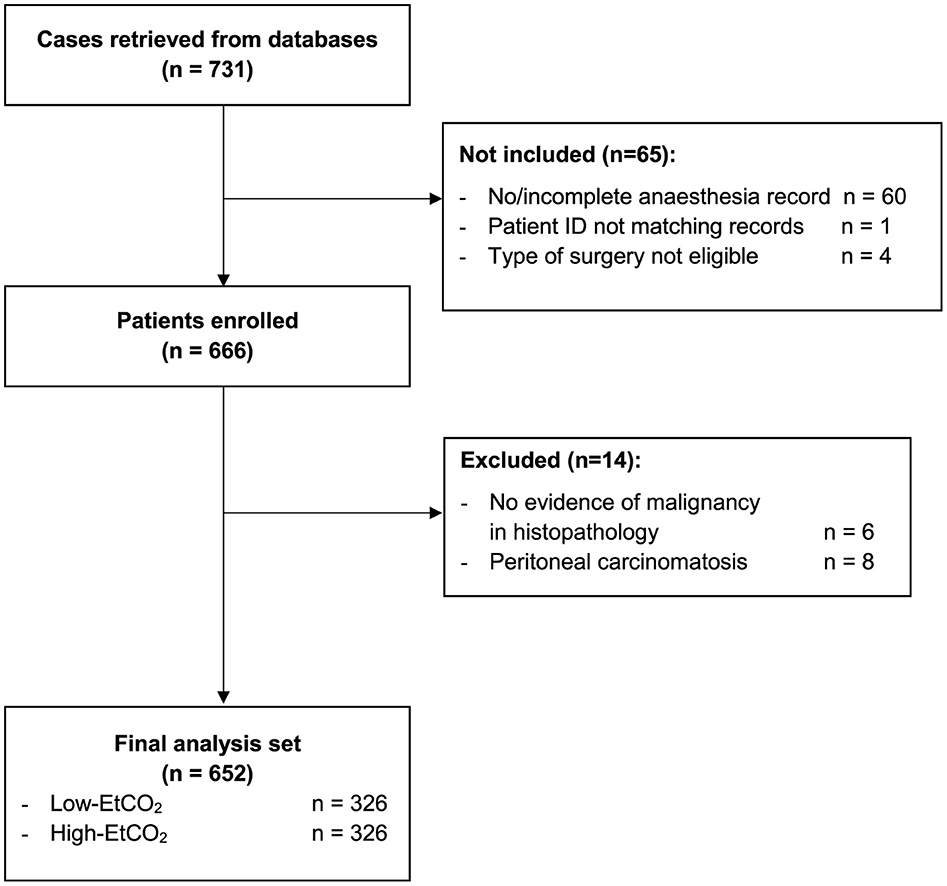
Figure 1. Participant flow chart. EtCO2, end-tidal carbon dioxide concentration; ID, identity document.
Table 1 and Supplementary Table 1 contain the primary clinical and demographic baseline characteristics. At the time of surgery, the mean age was 63 ± 10 years. The average BMI was 25.2 kg m−2 and 48.3% of patients were female.
There were no disparities between high and low EtCO2 levels in baseline characteristics or transfusion requirement. Furthermore, we noted no disparities concerning UICC stage, neoadjuvant therapy, resection margin and tumor grading status, or adjuvant therapy between groups. IORT was less prevalent in the high-EtCO2-group [8 (2.5%) vs. 21 (6.4%), high- vs. low-EtCO2, p = 0.014].
General anesthesia was performed as balanced anesthesia. In two participants (0.31%), fentanyl was administered as the intraoperative opioid, requiring the calculation of an equivalent dose to sufentanil (21). Epidural anesthesia was less prevalent in the high-EtCO2-group [230 (70.8%) vs. 258 (79.1%); high- vs. low-EtCO2, p = 0.014]. Intraoperative consumption of sufentanil was lower in the low-EtCO2-group (71.6 ± 34.7 vs. 76.7 ± 33.9; high vs. low-EtCO2, p = 0.014).
Surgery was always performed as open surgery in this observed study cohort.
After sorting the entire patient cohort in ascending order based on their mean intraoperative EtCO2 values, they were divided into two groups: the high- and low-EtCO2 groups, the determined cut-off value was 35.3 mmHg. The mean EtCO2 in the high-EtCO2 group was 36.8 mmHg ±1.9, compared to 33.8 mmHg ± 1.1 in the low-EtCO2 group.
After dividing the cohort into five groups, sorted according to the mean intraoperative EtCO2 value, mean EtCO2 values were 32.72 ± 0.97 mmHg, 34.26 ± 0.27 mmHg, 35.19 ± 0.26 mmHg, 36.09 ± 0.28 mmHg and 38.31 ± 2.45 mmHg, in ascending order.
During the observation period, cancer recurrence was diagnosed in 493 patients (75.6%). Of these patients, 154 (31.2 %) had a local cancer recurrence, 298 (60.4 %) had distant metastases, and 32 patients (6.5 %) experienced both. Information regarding the nature of recurrence was not accessible for nine patients (1.8%). Throughout the observation period, 386 patients (59.2%) died. 373 patients (57.2%) succumbed to their cancer disease. Two patients (0.3%) passed away following a cardiovascular event. The reason for death was not available for 11 patients (1.7%). There was no difference in recurrence-free survival between the high and low-EtCO2 groups [HR = 1.043 (95% CI: 0.875–1.243), log rank test: p = 0.909] (Figure 2), nor did it differ among the groups when the patient cohort was divided into five groups [HR 1.011 (95% CI 0.950-1.075), log rank test: p = 0.917] (Figure 3). Bar charts revealed no differences in the occurrence of the composite endpoint, including local cancer recurrence, newly diagnosed metastases, and death between the high and low EtCO2 groups across the respective time periods (Supplementary Figure 1). Likewise, the subgroup analysis for different durations of surgery did not reveal differences between the respective high and low EtCO2 groups (Supplementary Figure 2).
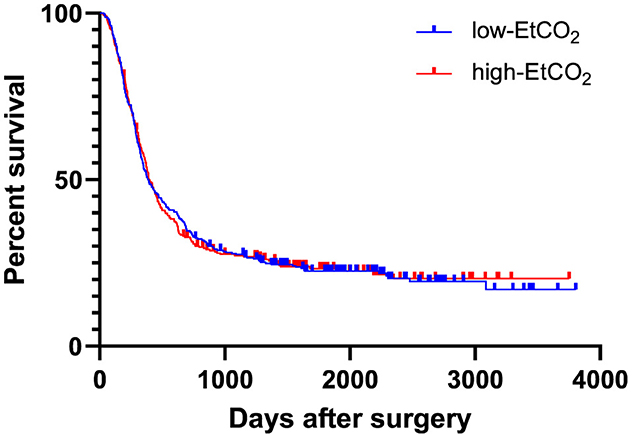
Figure 2. EtCO2 and recurrence-free survival. Patients were divided into low-EtCO2 group and high-EtCO2 groups. There was no difference in recurrence-free survival between the high and low-EtCO2 groups [HR 1.043 (95% CI: 0.875–1.243), log rank test: p = 0.909]. CI, confidence interval; EtCO2, end-tidal carbon dioxide concentration.
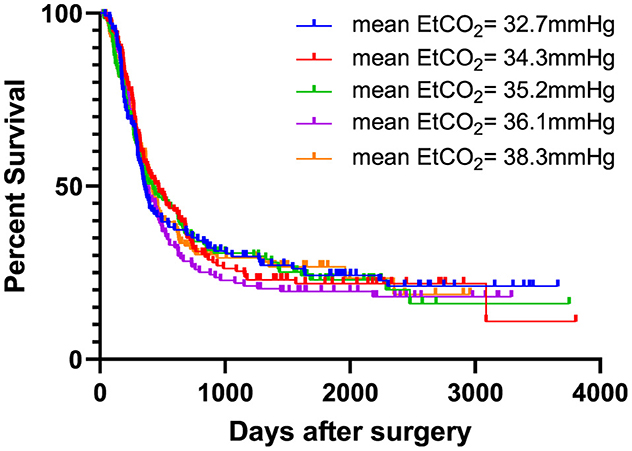
Figure 3. EtCO2 and recurrence-free survival stratified in five groups. Patients were categorized into five groups based on increasing mean EtCO2 values. There was no difference in recurrence-free survival between the five groups [HR 1.011 (95% CI 0.950–1.075), log rank test: p = 0.917]. EtCO2, end-tidal carbon dioxide concentration.
Factors affecting the primary endpoint were studied via Cox analysis, which indicated no correlation between mean EtCO2 levels and recurrence–free survival [Coefficient −0.004, HR = 0.996 (95% CI: 0.95–1.04); p = 0.871] (Table 2). Variables that were associated with the primary endpoint included: BMI [Coefficient: −0.026, HR = 0.974 (95% CI: 0.950–0.999), p = 0.044], UICC stage IIb–III [Coefficient 0.402, HR = 0.669 (95% CI:0.523–0.855), p = 0.001], no grading due to adjuvant therapy [Coefficient: 0.421, HR = 0.656 (95% CI: 0.535–0.805), p < 0.001] and grading 3–4 [Coefficient 0.931; HR = 0.394 (95% CI: 0.201–0.774), p = 0.007].
1-and 5-year survival rates did not differ between the high- and low-EtCO2 groups (1-year survival: 87.9% vs. 86.4%, high- vs. low-EtCO2, p = 0.855; 5-year survival: 31.1% vs. 25.2 %, high- vs. low-EtCO2, p = 0.855) (Table 3).
There were also no differences between the groups in terms of cardiovascular events, sepsis rate, surgical site infection rate or the rate of reoperations (Table 4).
In this retrospective cohort study, we did not identify a correlation between intraoperative EtCO2 and recurrence-free survival in patients undergoing pancreatic cancer surgery. This finding differs from our previous observation in other tumor entities (16), and it is in contrast our expectations that were based on in vitro studies (11, 12). BMI, UICC stage IIb-III, no grade due to adjuvant therapy, and grade G3-4 were independently associated with recurrence-free survival. In our secondary endpoint analysis, overall survival, incidence of cardiovascular events, sepsis, SSI, and need for reoperation did not differ between the high- and low-EtCO2 groups.
The correlation between EtCO2 levels and oncological outcome in pancreatic cancer patients has not yet been investigated. CO2 exerts diverse effects on tumor biology (2, 8–10). Effects of CO2 on mitochondrial metabolism (22), the cellular microenvironment (23), the expression of Vascular Endothelial Growth Factor (7), E-cadherin (7), and various matrix metalloproteinases (24, 25), which are known to influence cancer cell invasion and metastasis (26, 27), have been reported. Hypercapnic conditions in cervical cancer cells stimulate tumor cell proliferation. At the same time, hypercapnia led to a reduction in invasion, migration and adhesion (8, 9). However, potential effects of hypocapnic conditions remain uncertain. Exposing colon cancer cells to CO2 exhibits in heightened proliferation, adhesion disorder and elevated levels of growth factor expression (7, 10). The effect of CO2 on pancreatic cancer cells has rarely been studied. Nevler et al. exposed two pancreatic ductal adenocarcinoma cell lines to normocapnic (5% CO2) and hypercapnic (10% CO2) conditions (11). Hypercapnia led to increased tumor proliferation, radio resistance, and chemoresistance (11). In the first retrospective cohort study of 528 colorectal cancer patients investigating the association between intraoperative EtCO2 and oncological outcome, we demonstrated that lower EtCO2 levels were independently associated with enhanced recurrence-free survival (HR = 1.138, 95% CI:1.02-1.28, p = 0.027) at a median follow-up of 3.8 with an interquartile range of 2.5 to 5.1 years (16). The hazard of cancer recurrence decreased by 12.1% with each 1 mmHg reduce in mean EtCO2 (16). Surprisingly, in this study of patients who underwent pancreatic cancer surgery, EtCO2 level was not associated with recurrence-free survival. In addition to the various CO2-related effects on tumor biology in different tumor types (2, 8–10, 28), the biological effects of hypercapnia on tumor development are also discussed as a function of time and CO2 concentration (23). The EtCO2 values only differed by approximately 10% (3 mmHG) between the high and low EtCO2 groups. However, when patients were stratified into 5 groups, not even the extreme cases differed regarding recurrence-free survival.
Our study also found no differences in 1- and 5-year survival rates between the high and low EtCO2 groups. In contrast, in patients undergoing colorectal cancer surgery, both 1- and 5-year survival rates were higher in patients with lower EtCO2 values (16). The type of cancer itself significantly influences the average survival time of a patient (29–31). Individuals with pancreatic cancer typically experience a shorter average survival time compared to those with colorectal cancer (29–31). Factors such as tumor grading, UICC stage, or resection margin status appear to have a stronger influence on recurrence-free survival than EtCO2 in pancreatic cancer patients.
CO2 has been linked to multiple effects on the cardiovascular system. Hypocapnia can reduce the cerebral blood flow, impact the airway resistance, provoke cardiac arrythmias and trigger vasoconstrictions (15, 32, 33). Dony et al. demonstrated that hypocapnia, was linked to elevated 30-day postoperative mortality and extended hospitalization duration in patients undergoing non-cardiac surgery (32). In contrast, negative effects on the cardiovascular system due to hypercapnia have also been described. Increased blood CO2 levels can elevate the heart's oxygen consumption, potentially resulting in tachycardia and hypertension (34, 35). In this patient cohort, as well as in patients undergoing colorectal cancer surgery (16), intraoperative EtCO2 did not affect the incidence of cardiovascular events or the length of hospital stay.
Moreover, some authors suggest that hypercapnia has anti-inflammatory properties and improves tissue perfusion and oxygenation, potentially lowering the rate of SSI (34, 36–38). In our study, no association was observed between EtCO2 levels and SSI or the occurrence of sepsis. However, the incidence of sepsis and wound infections was low in this observed patient cohort.
Our study has several limitations that warrant consideration. The presence of digital anesthesia records constrained the duration of observation period, thereby limiting the sample size. Due to the study design, the representativeness, validity, and reliability of the results are limited. Moreover, the study design lacks the ability to exercise complete control over unmeasured biases.
The selection process for EtCO2 values lacked standardization, and the entire cohort was split into two equal groups based on the calculated mean EtCO2 values. The mean for the low EtCO2 group was in the mildly hypocapnic range, whereas the mean for the high EtCO2 group was in the normocapnic range. Despite comparing the intraoperative EtCO2 values by calculating individual mean values, we did not consider the individual dosages during surgery. The duration of EtCO2 levels was considered only in a subgroup analysis with different operation durations and not continuously. Additionally, inspiratory CO2 levels could not be considered. However, it is reasonable to assume that higher inspiratory CO2 levels would also result in elevated EtCO2 levels.
Because PaO2 values are determined irregularly in contrast to the continuous non-invasive measurement of EtCO2, we refrained from exploring the correlation between PaO2 and the measured EtCO2 values.
In conclusion, we demonstrated, that intraoperative EtCO2 during pancreatic cancer surgery is not associated with increased or decreased recurrence-free survival. EtCO2 was not associated with the secondary endpoints, namely overall survival, cardiovascular events, SSI, incidence of sepsis, or reoperations. During elective pancreatic cancer surgery, anesthesiologists should set EtCO2 targets for reasons other than oncological outcome until conclusive evidence from prospective, multicenter randomized controlled trials is available.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Local Ethics Committee of the Medical Faculty of Ruprecht-Karls-University, Heidelberg, Germany (S-723/2021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SD: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. LK: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing. RK: Data curation, Resources, Writing – review & editing. SK: Formal analysis, Methodology, Writing – review & editing. CM: Data curation, Resources, Writing – review & editing. TH: Data curation, Resources, Writing – review & editing. MB: Data curation, Resources, Writing – review & editing. MW: Data curation, Resources, Writing – review & editing. JL: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this study was supported by institutional resources from the Departments of General, Visceral, and Transplant Surgery, and Anesthesiology at Heidelberg University Hospital, Heidelberg, Germany.
We extend our gratitude to Ulf Hinz, Department of General, Visceral and Transplant Surgery, Heidelberg University Hospital, Heidelberg, Germany, for his support and cooperating throughout the data collection process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1442283/full#supplementary-material
ASA, American Society of Anesthesiologists physical status classification; BMI, body mass index; CI, confidence interval; CO2, carbon dioxide; COPD, chronic obstructive pulmonary disease; CRC, colorectal cancer; EtCO2, end-tidal carbon dioxide concentration; FFP, fresh frozen plasma; HR, hazard ratios; IORT, intraoperative radiation therapy; PaCO2, arterial carbon dioxide pressure; PC, pancreatic surgery; PLT, Platelet concentrates; R0, no residual tumor; R1, microscopic residual tumor; RBC, Red blood cells; SD, standard deviation; SSI, surgical site infections; TNM, tumor, node, metastasis; TU, Transfusion units; UICC, tumor classification according to the Union for International Cancer Control.
1. Razi E, Moosavi GA, Omidi K, Khakpour Saebi A, Razi A. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res. (2012) 1:58–62. doi: 10.5812/atr.6444
2. Gutt CN, Kim ZG, Hollander D, Bruttel T, Lorenz M. CO2 environment influences the growth of cultured human cancer cells dependent on insufflation pressure. Surg Endosc. (2001) 15:314–8. doi: 10.1007/s004640000321
3. Guais A, Brand G, Jacquot L, Karrer M, Dukan S, Grévillot G, et al. Toxicity of carbon dioxide: a review. Chem Res Toxicol. (2011) 24:2061–70. doi: 10.1021/tx200220r
4. Strowitzki MJ, Nelson R, Garcia MP, Tuffs C, Bleul MB, Fitzsimons S, et al. Carbon dioxide sensing by immune cells occurs through carbonic anhydrase 2-dependent changes in intracellular pH. J Immunol. (2022) 208:2363–75. doi: 10.4049/jimmunol.2100665
5. Cummins EP, Strowitzki MJ, Taylor CT. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol Rev. (2020) 100:463–88. doi: 10.1152/physrev.00003.2019
6. Obata S, Goi T, Nakazawa T, Kimura Y, Katayama K, Yamaguchi A. Changes in CO2 concentration increase the invasive ability of colon cancer cells. Anticancer Res. (2013) 33:1881–5.
7. Cai KL, Wang GB, Xiong LJ. Effects of carbon dioxide and nitrogen on adhesive growth and expressions of E-cadherin and VEGF of human colon cancer cell CCL-228. World J Gastroenterol. (2003) 9:1594–7. doi: 10.3748/wjg.v9.i7.1594
8. Lin F, Pan L, Li L, Li D, Mo L. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit. (2014) 20:2497–503. doi: 10.12659/MSM.891179
9. Lv H, Zhou T, Rong F. Proteomic analysis of the influence of CO(2) pneumoperitoneum in cervical cancer cells. J Cancer Res Ther. (2021) 17:1253–60. doi: 10.4103/jcrt.jcrt_638_21
10. Kim ZG, Mehl C, Lorenz M, Gutt CN. Impact of laparoscopic CO2-insufflation on tumor-associated molecules in cultured colorectal cancer cells. Surg Endosc. (2002) 16:1182–6. doi: 10.1007/s00464-001-9194-3
11. Nevler A, Brown SZ, Nauheim D, Portocarrero C, Rodeck U, Bassig J, et al. Effect of hypercapnia, an element of obstructive respiratory disorder, on pancreatic cancer chemoresistance and progression. J Am Coll Surg. (2020) 230:659–67. doi: 10.1016/j.jamcollsurg.2019.12.033
12. Nevler A, Khalilieh S, Lavu H, Bowne W, Yeo CJ. Hypercapnic tissue gene expression and survival in early-stage pancreatic ductal adenocarcinoma. J Am College Surg. (2023) 236:4. doi: 10.1097/XCS.0000000000000552
13. Akkermans A, van Waes JAR, Thompson A, Shanks A, Peelen LM, Aziz MF, et al. An observational study of end-tidal carbon dioxide trends in general anesthesia. Can J Anaesth. (2019) 66:149–60. doi: 10.1007/s12630-018-1249-1
14. Wax DB, Lin HM, Hossain S, Porter SB. Intraoperative carbon dioxide management and outcomes. Eur J Anaesthesiol. (2010) 27:819–23. doi: 10.1097/EJA.0b013e32833cca07
15. Way M, Hill GE. Intraoperative end-tidal carbon dioxide concentrations: what is the target? Anesthesiol Res Pract. (2011) 2011:271539. doi: 10.1155/2011/271539
16. Dehne S, Kirschner L, Strowitzki MJ, Kilian S, Kummer LC, Schneider MA, et al. Low intraoperative end-tidal carbon dioxide levels are associated with improved recurrence-free survival after elective colorectal cancer surgery. J Clin Anesth. (2024) 96:111495. doi: 10.1016/j.jclinane.2024.111495
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
18. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (1958) 53:457–81. doi: 10.1080/01621459.1958.10501452
19. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Repor. (1966) 50:163–70.
20. Cox DR. Regression models and life-tables. J Royal Stat Soc Series B (Methodological). (1972) 34:187–220. doi: 10.1111/j.2517-6161.1972.tb00899.x
21. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. (2016) 25:733–7. doi: 10.1002/pds.3945
22. Kikuchi R, Tsuji T, Iwai Y, Nakamura H, Aoshiba K. High CO2 tumor microenvironment confers chemoresistance in lung cancer cells. Eur Respirat J. (2017) 50:OA4865. doi: 10.1183/1393003.congress-2017.OA4865
23. Zhang S, Yang Y, Liu S, Dong R, Qian Z. Influence of the hypercapnic tumor microenvironment on the viability of hela cells screened by a CO(2)-gradient-generating device. ACS Omega. (2021) 6:26773–81. doi: 10.1021/acsomega.1c04422
24. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. (2011) 278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x
25. Rydlova M, Holubec L Jr, Ludvikova M Jr, Kalfert D, Franekova J, Povysil C, et al. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res. (2008) 28:1389–97.
26. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. (2004) 23:101–17. doi: 10.1023/A:1025867130437
27. Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. (2003) 22:145–52. doi: 10.1023/A:1023039230052
28. Kikuchi R, Iwai Y, Tsuji T, Watanabe Y, Koyama N, Yamaguchi K, et al. Hypercapnic tumor microenvironment confers chemoresistance to lung cancer cells by reprogramming mitochondrial metabolism in vitro. Free Radic Biol Med. (2019) 134:200–14. doi: 10.1016/j.freeradbiomed.2019.01.014
29. Klaiber U, Schnaidt ES, Hinz U, Gaida MM, Heger U, Hank T, et al. Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann Surg. (2021) 273:154–62. doi: 10.1097/SLA.0000000000003270
30. Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new r-status counts. Ann Surg. (2017) 265:565–73. doi: 10.1097/SLA.0000000000001731
31. Ulrich CM, Gigic B, Böhm J, Ose J, Viskochil R, Schneider M, et al. The ColoCare Study: a paradigm of transdisciplinary science in colorectal cancer outcomes. Cancer Epidemiol Biomark Prev. (2019) 28:591–601. doi: 10.1158/1055-9965.EPI-18-0773
32. Dony P, Dramaix M, Boogaerts JG. Hypocapnia measured by end-tidal carbon dioxide tension during anesthesia is associated with increased 30-day mortality rate. J Clin Anesth. (2017) 36:123–6. doi: 10.1016/j.jclinane.2016.10.028
34. Saghaei M, Matin G, Golparvar M. Effects of intra-operative end-tidal carbon dioxide levels on the rates of post-operative complications in adults undergoing general anesthesia for percutaneous nephrolithotomy: a clinical trial. Adv Biomed Res. (2014) 3:84. doi: 10.4103/2277-9175.127997
35. Mas A, Saura P, Joseph D, Blanch L, Baigorri F, Artigas A, et al. Effect of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. (2000) 28:360–5. doi: 10.1097/00003246-200002000-00012
36. Fleischmann E, Herbst F, Kugener A, Kabon B, Niedermayr M, Sessler DI, et al. Mild hypercapnia increases subcutaneous and colonic oxygen tension in patients given 80% inspired oxygen during abdominal surgery. Anesthesiology. (2006) 104:944–9. doi: 10.1097/00000542-200605000-00009
37. Akça O, Kurz A, Fleischmann E, Buggy D, Herbst F, Stocchi L, et al. Hypercapnia and surgical site infection: a randomized trial. Br J Anaesth. (2013) 111:759–67. doi: 10.1093/bja/aet233
Keywords: carbon dioxide, pancreatic cancer surgery, oncological outcome, recurrence-free survival, perioperative complications
Citation: Dehne S, Kirschner L, Klotz R, Kilian S, Michalski CW, Hackert T, Büchler MW, Weigand MA and Larmann J (2024) Intraoperative end-tidal carbon dioxide levels are not associated with recurrence-free survival after elective pancreatic cancer surgery: a retrospective cohort study. Front. Med. 11:1442283. doi: 10.3389/fmed.2024.1442283
Received: 01 June 2024; Accepted: 13 August 2024;
Published: 11 September 2024.
Edited by:
Ingrid Tennant, University of the West Indies, JamaicaReviewed by:
Hanna Sternby, Lund University, SwedenCopyright © 2024 Dehne, Kirschner, Klotz, Kilian, Michalski, Hackert, Büchler, Weigand and Larmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Larmann, amFuLmxhcm1hbm5AbWVkLnVuaS1oZWlkZWxiZXJnLmRl
†Present address: Thilo Hackert, Department of General, Visceral, and Thoracic Surgery, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.