- 1College of Electronics and Information Engineering, University of Sichuan, Chengdu, China
- 2Department of Gynecology and Obstetrics, West China Second Hospital, University of Sichuan, Chengdu, China
- 3Key Laboratory of Obstetric and Gynecologic and Pediatric Diseases and Birth Defects of Ministry of Education, West China Second Hospital, University of Sichuan, Chengdu, China
Observational studies have reported an association between gastroesophageal reflux disease (GERD) and endometriosis. We conducted a two-sample and bidirectional Mendelian randomization analysis to determine whether those associations are causal. Two-sample and bidirectional MR analyses were performed using summary statistics from the European Individual Genome-Wide Association Study (GWAS). The inverse variance weighting (IVW) method is used as the main analysis method to evaluate causality. Sensitivity analyses were performed to assess heterogeneity, horizontal versatility, and stability. The results showed no significant causal association between GERD in women with endometriosis in the UK Bank database [ratio (OR) ≈ 0, 95% adjusted interval (CI) 1.0007∼1.0044, P = 0.006] and Finn databases [ratio (OR) = 1.29, 95% adjusted interval (CI) 0.99∼1.67, P = 0.06]. However, when studying the Finn database only for endometriosis, which is confined to the uterus, a significant increase in GERD was limited to the risk of endometriosis in the uterus [ratio (OR) = 1.47, 95% adjusted interval (CI) 1.00∼2.17, P = 0.05]. Sensitivity analysis showed that the results were robust and did not detect multi efficacy or heterogeneity. Meanwhile, reverse MR analysis showed that endometriosis did not increase the risk of GERD. This MR study supports a causal relationship between GERD and an increased risk of endometriosis confined to the uterus. Therefore, patients with gastric esophageal reflux should be treated with gynecological examination to avoid and prevent the development of endometriosis.
1 Introduction
Endometriosis is a common benign disease in gynecology, affecting approximately 10% (190 million) of women and girls of childbearing age worldwide (1). It is a chronic disease that is affected by estrogen regulation and is associated with dysmenorrhea, sexual intercourse, bowel pain and/or urination pain, chronic pelvic pain, bloating, nausea, and fatigue, and some patients also suffer from depression, anxiety and infertility (2). Patients bear a severe burden of life and psychology, with enormous social and economic burdens (3–6). In addition, endometriosis sufferers often experience symptoms of intestinal or bladder irritation due to chronic pain comorbidities, which overlap with other diseases, leading to significant delays in the diagnosis of endometriosis after the onset of symptoms (7). Therefore, it is important to explore the factors associated with endometriosis to guide the early diagnosis and treatment of the disease.
Gastroesophageal reflux disease (GERD) refers to the reflux of gastroduodenal contents into the esophagus causing acid reflux, heartburn and other symptoms. Reflux can cause tissue damage to the mouth, throat, and bronchial tract and other tissue damage near the esophagus. Esophageal manifestations include asthma, chronic cough, idiopathic pulmonary fibrosis, hoarseness, chronic sore throat and tooth erosion (8). These nonspecific symptoms can cause overlap or confusion with other diseases (8). A clinical report in the United States showed that after long-term gastric esophageal reflux treatment, patients with GERD had a history of endometriosis and endometriosis resection and showed continued progression of symptoms of dysphagia, vomiting and reflux, and weight loss, with unknown causes and complications (9). The American Gastroenterological Society study also suggests that intestinal endometriosis can present with acute abdominal pain and small intestinal obstruction on CT. Therefore, when women of childbearing age have acute abdominal pain, the possibility of endometriosis involving the gastrointestinal tract should be considered (10, 11). Over the past five years, the American Gastrointestinal Association has also reported a possible association between a history of GERD and a history of hysterectomy in women (12). In addition, in recent new drug reports, domestic and foreign research institutes and companies have reported the invention of novel prevention and treatment drugs for both endometriosis and gastrointestinal diseases, which have synergistic effects (13–15). Although the underlying mechanisms of these phenomena are unclear, some evidence may support the potential of endometriosis to cause GERD, which in turn can lead to elevated levels of inflammation, leading to the development of endometriosis.
Although clinical observations and some current evidence suggest a possible association between GERD and endometriosis, it has not been possible to establish a causal link between them. Mendelian randomization (MR) is an innovative approach to optimizing observational epidemiology and can be used to investigate the causal effects of altered exposure on health outcomes (16).
The method introduces instrumental variables that affect exposure only, independent of potential confounding factors associated with outcomes and exposure outcomes, and will use single nucleotide polymorphisms (SNPs), which are highly correlated with exposure and randomized genetic variation, as instrumental variables to assess the causal relationship between the variable exposure and the outcome (17). SNPs have characteristics that precede disease occurrence and are unaffected by the outcome and the correlation between many confusing exposures and outcomes; thus, MR studies can reduce the risk of potential bias from confounding factors and reverse causation and effectively evaluate the causal relationship between exposure and outcome (18). To date, only one study of the correlation between GERD and endometriosis using MR has been retrieved (19). However, the study did not address the correlation between endometriosis and GERD at different sites. Therefore, this study explores the causal relationship between GERD and endometriosis through MR and further explores the correlation between endometriosis at different sites. It seeks a new research direction for exploring the pathogenesis of endometriosis at different sites and provides a theoretical basis for endometriosis screening and early accurate diagnosis of endometriosis in patients with GERD.
2 Materials and methods
2.1 GWAS summary-level data of GERD and endometriosis
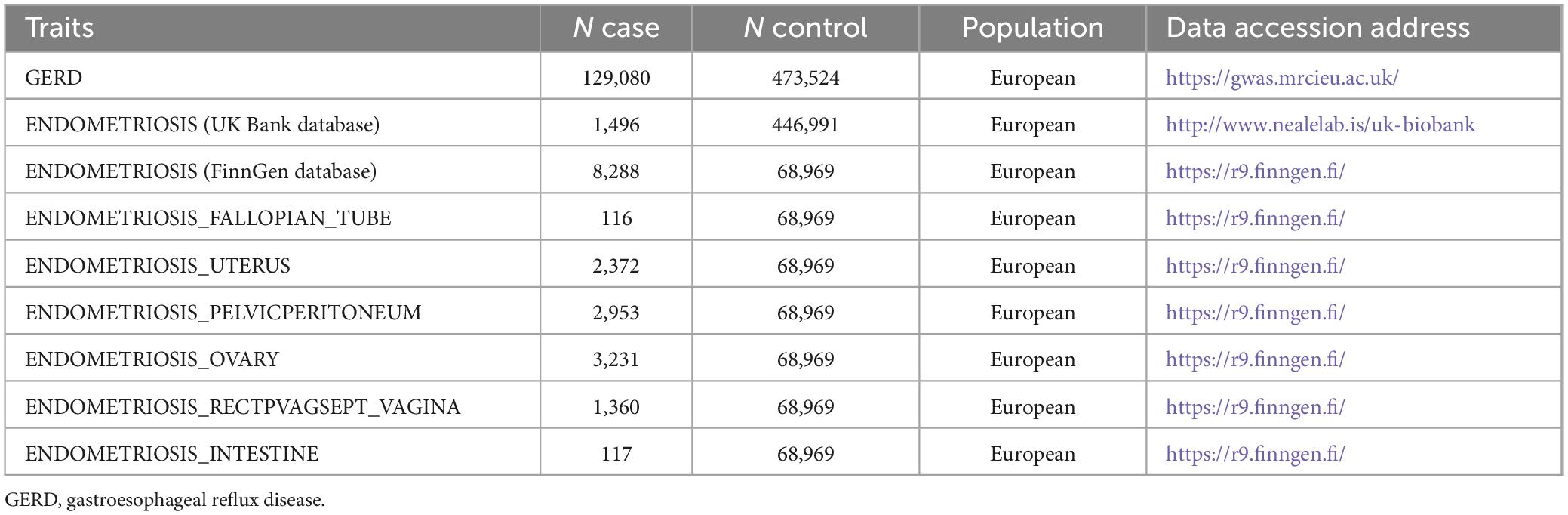
The overall flow chart of the bidirectional MR study is shown in Figure 1. A genome-wide association study (GWAS) meta-analysis was used to study European GERD data, which included 78,707 patients with GERD in Europe and 288,734 healthy controls (20). These data are available in the GWAS Catalog project database (21). In addition, the aggregate GWAS statistics for endometriosis are from the FinnGen database (8,288 cases of endometriosis and 9,972 cases of healthy controls) and the UK Bank database (1,496 cases of endometriosis) (22). Among them, the FinnGen database includes subsets of endometriosis occurring in fallopian tubes (116 cases of endometriosis and 146 cases of healthy controls), the uterus (2,372 cases of endometriosis and 1,600 cases of healthy controls), the pelvic peritoneum (2,953 cases of endometriosis and 3,940 cases of healthy controls), the ovaries (3,231 cases of endometriosis and 3,865 cases of healthy controls), the rectal vaginal and vaginal compartments (1,360 cases of endometriosis and 1,570 cases of healthy controls) and the intestines (117 cases of endometriosis and 375 cases of healthy controls). Table 1 provides details of the GWAS summary level data of exposure and outcome analyzed in this MR study. All data analyzed in this study were obtained from publicly available databases in which ethical approval was obtained for each cohort, and informed consent was obtained from all participants prior to participation. Figure 2 shows endometriosis at different sites. The specific analysis process (Example: GERD as an exposure/endometriosis as an outcome example) is shown in Figure 3.

Figure 1. The overall flow chart of the bidirectional MR study. SNPs, single nucleotide polymorphisms. There are three assumptions of Mendelian randomization design. The first assumption is that the genetic variants used as instrumental variables should be robustly associated with the exposure; the second assumption is that the used genetic variants should not be associated with any confounders; and the third assumption is that the selected genetic variants should affect the risk of the outcome merely through the risk factor, not via alternative pathways.
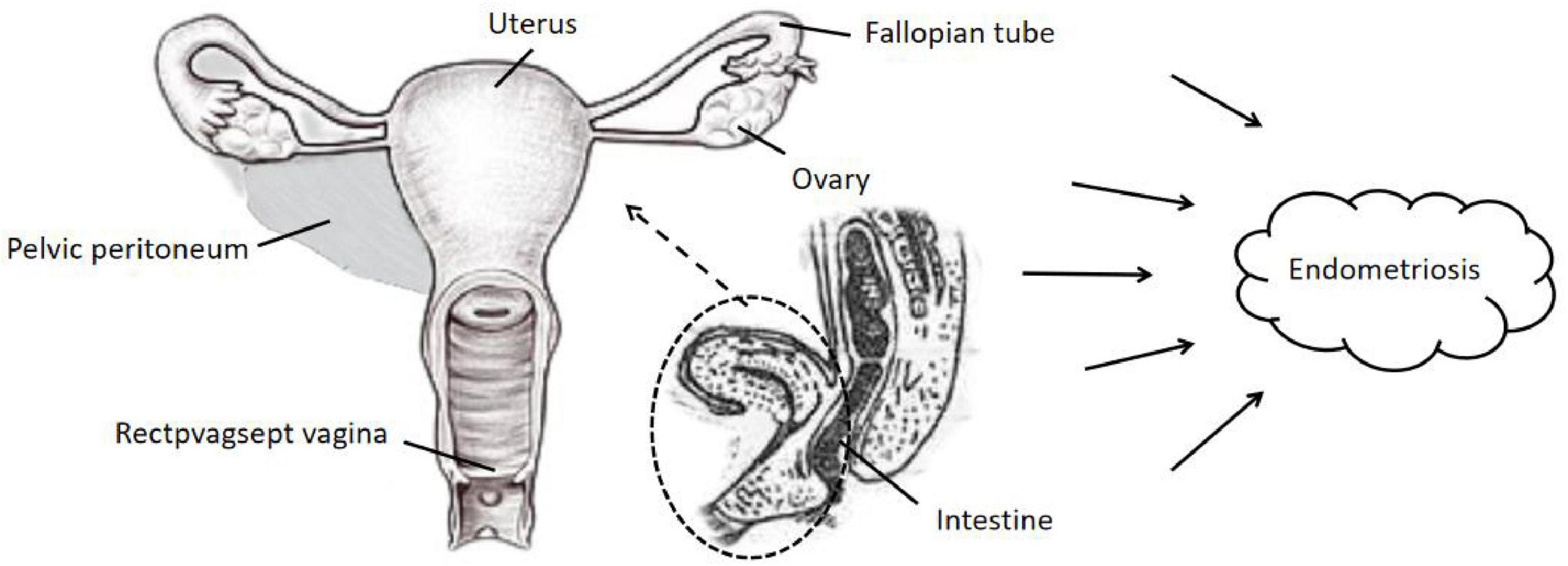
Figure 2. Classification of endometriosis occurring at different sites. The main possible locations where endometriosis occurs in the tissues surrounding the body of the uterus in women: pelvic peritoneum, rectpvagsept vagina, fallopian tubes, uterus, ovaries, intestine, etc.
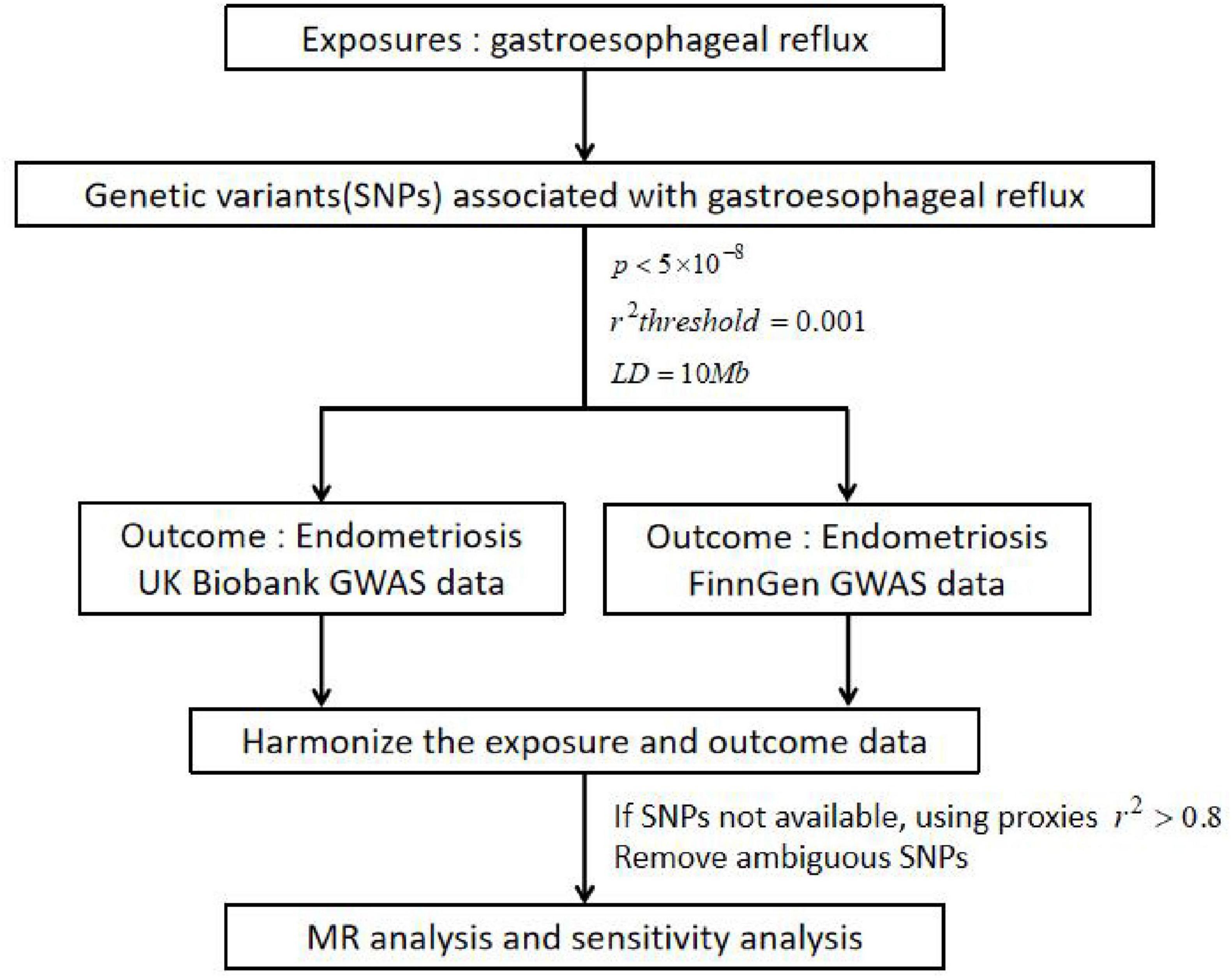
Figure 3. Specific analysis process. Example: GERD as a exposure/endometriosis as an outcome example; GERD, gastroesophageal reflux disease; SNPs, single nucleotide polymorphisms; p, statistical p-value; r2, correlation index for evaluating the effect of the fitted regression; LD, linkage disequilibrium; GWAS, genome-wide association study. GERD was selected as the exposure and endometriosis was selected as the outcome. The screened instrumental variable (SNPs) was associated with exposure, fulfilling the following three conditions: p < 5×10−8, r2threshold = 0.001 and LD=10Mb. In addition, the aggregate GWAS statistics for endometriosis are from FinnGen database (8,288 cases of endometriosis and 9,972 cases of health control) and UK Bank database (1,496 cases of endometriosis). If SNPs not available, using proxies r2 > 0.8, remove ambiguous SNPs and harmonize the exposure and outcome data. Finally, MR analysis and sensitivity analysis were performed.
2.2 Selection of instrumental variables
Mendelian randomization is a method of studying the causal relationship between exposure and outcome using genetic variation as an instrumental variable in medical research observations (23). Instrumental variable (IV) selection satisfies correlation with exposure, and IVs should be independent of any confusion associated with the exposure result, which means that there are no causal pathways from IVs to results, except through exposure (24, 25).
The selection of gene variants involves controlling genome-wide significance thresholds (p < 5×10−8) and screening SNPs as IVs for MR analysis (16). Consideration of chained unbalanced SNPs had an impact on the resulting effect values by removing SNPs with r2 < 0.001 to the most significant SNP in the 10,000kb range of chromosomes to satisfy near-perfect chained equilibrium between the two SNPs and to ensure the independence of each instrumental variable. Additionally, palindromic SNPs, outcome-associated SNPs (p < 0.05), and SNPs not present in the resultant GWAS pooled data were removed. The extent of weak instrumental bias was assessed according to the f-statistic formula, and IVs with F > 10 were retained to avoid bias caused by weak IVs (26).
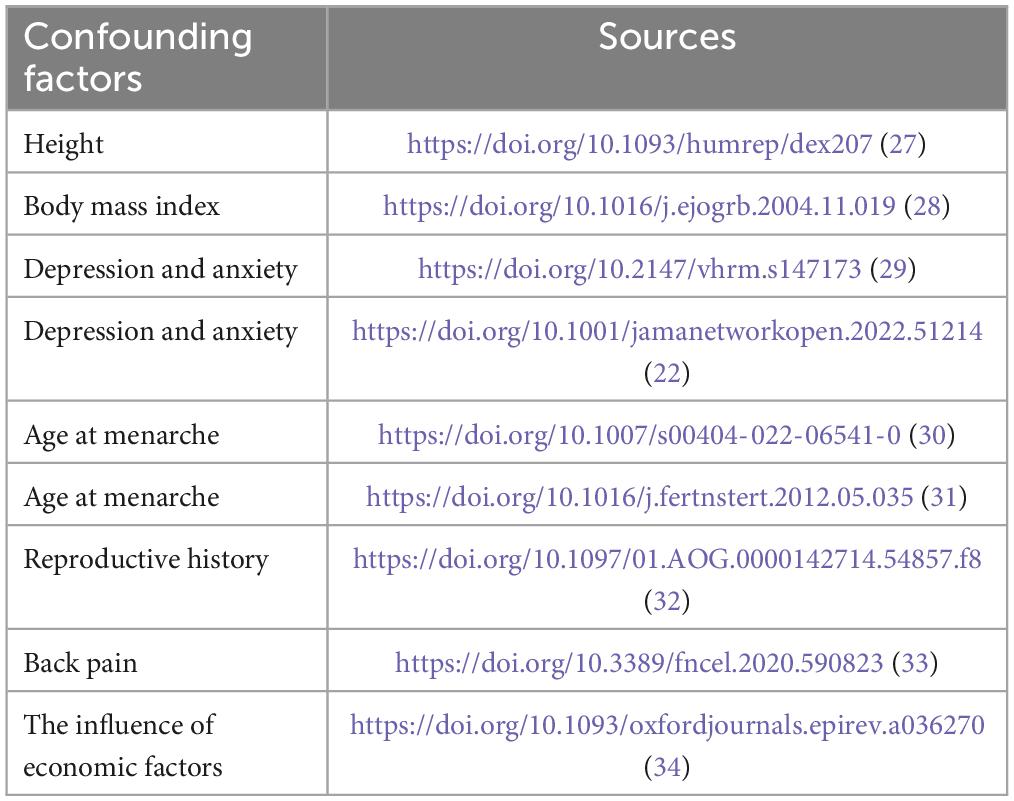
Body mass index, height, depression and anxiety, menarche, reproductive history, back pain, and the influence of economic factors may be potential confounders affecting GERD and endometriosis (22, 27–34). To increase the credibility of the findings, SNPs associated with these confounders (p < 5×10−8) were retrieved from the IEU Open GWAS program database and excluded, and the number of these confounding accessions is shown in Table 2.
2.3 Statistical methods
The MR study relied on three core instrumental variable assumptions (correlation with exposure, independence from confounders, and exclusion of restrictions unrelated to outcome) to test the causal effect of exposure on outcome (16). Inverse variance weighted (IVW) analysis was used to estimate the causal effect of exposure and outcome using the Wald ratio estimator based on the principles of meta-analysis (35). To demonstrate the stability and directionality of the results, in addition to the IVW method, two other MR methods [MR-Egger method and weighted median method] were used to assess causality. The MR-Egger method estimates the causal effect of genes on traits by fitting a linear regression model that relates the effect of genetic variation on traits to the effect of genetic variation on gene expression. It also provides unbiased estimates, detecting and correcting for propensity and reverse causation bias in causal effect estimates (36). The weighted median method weights the causal effects of different genetic variants on a trait and then takes the weighted median as the final causal effect estimate. This method is robust and can reduce bias due to deviations in the estimates of certain genetic variants. However, the criterion for using the weighted median method is that at least 50% of the SNPs must satisfy the prerequisite of valid IVs (37). A significance threshold of p < 0.05 was set, and the causal association results were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs).
2.4 Reverse MR analysis
Reverse MR analysis was performed to assess whether endometriosis affects GERD, and screening instrumental variables, Mendelian randomization analysis, and sensitivity analysis were performed sequentially. Instrumental variables were selected as described in Section “2.2 Selection of instrumental variables,” and statistical methods were selected as described in Section “2.3 Statistical methods.”
3 Results
3.1 Results of MR analysis using IVs based on genome-wide significance screening
MR results were based on instrumental variables screened at the genome-wide significance threshold (p < 5×10−8), and a total of 44 SNPs associated with confounding factors (body mass index, height, depression and anxiety, menarche, reproductive history, back pain, and the influence of economic factors) were excluded. The causal effect of GERD on endometriosis and on endometriosis occurring in different locations was assessed based on 33 instrumental variables after removing the palindromic SNPs, outcome-associated SNPs (p < 0.05), and SNPs that were not present in the outcome GWAS pooled data. Detailed information on the confounding SNPs associated with the results is provided in Supplementary Table 1, and detailed information on the instrumental variables for MR and the results of the analyses are provided in Supplementary Table 2.
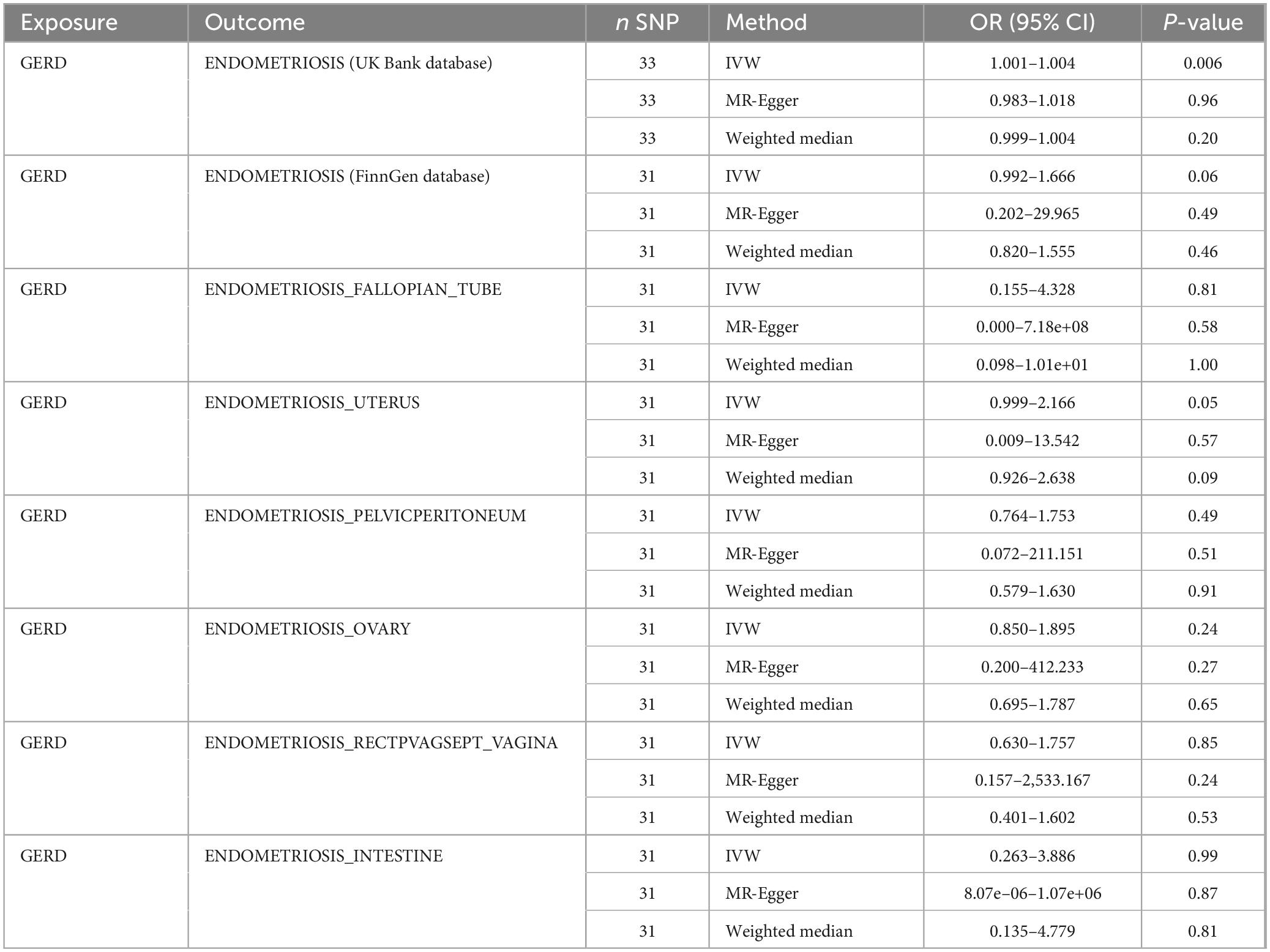
In all endometriosis databases, the f-statistics of all IVs were greater than 10, ranging from 29.75∼45.55, which excluded the interference of weak instrumental variables on the results. In addition, the results of MR analysis for IVs screened based on genome-wide significance thresholds are shown in Table 3. The MR results indicated that there was no significant causal relationship between GERD and the occurrence of endometriosis (UK Bank: OR ≈ 0, 95% CI 1.0007–1.0044, P = 0.006; FinnGen: OR = 1.29, 95% CI 0.99–1.67, P = 0.06). In addition, MR results, occurring in the subdatabases of fallopian tubes, pelvic peritoneum, ovaries, rectovaginal septum with vagina and intestines, yielded the same conclusions as described above, and the results of MR analysis are shown in Table 3.
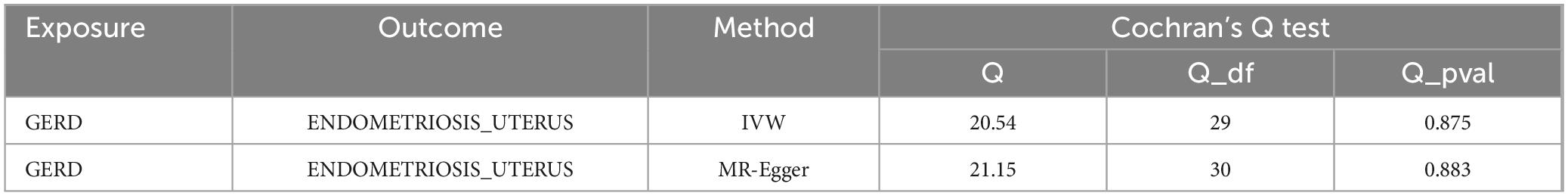
However, in the subdatabase of endometriosis confined to the uterine corpus, MR results demonstrated a causal relationship between GERD and the development of endometriosis. Specifically, MR results in IVW indicated that GERD significantly increased the risk of endometriosis (OR = 1.47, 95% CI 1.00–2.17, P = 0.05) (Table 3). In addition, two other MR methods yielded similar causal estimates, including MR-Egger and weighted median (Table 3 and Figure 4). Sensitivity analyses were conducted to assess the robustness of the MR results. The MR Steiger test indicated that the inferred causal direction between exposure (GERD) and outcome (endometriosis) was in the “right direction” (p < 0.05). The Cochran’s Q test indicated that there was no heterogeneity between IVs (p > 0.05) (Table 4). The results of the MR- Egger intercept test and the MRPRESSO global test indicated that the MR analyses were not potentially affected by any level of pleiotropy (p > 0.05) (Table 5). Leave-one-out sensitivity analyses confirmed the robustness of the MR results, as there were no prior SNPs that severely affected the results upon exclusion (Supplementary Figure 1).
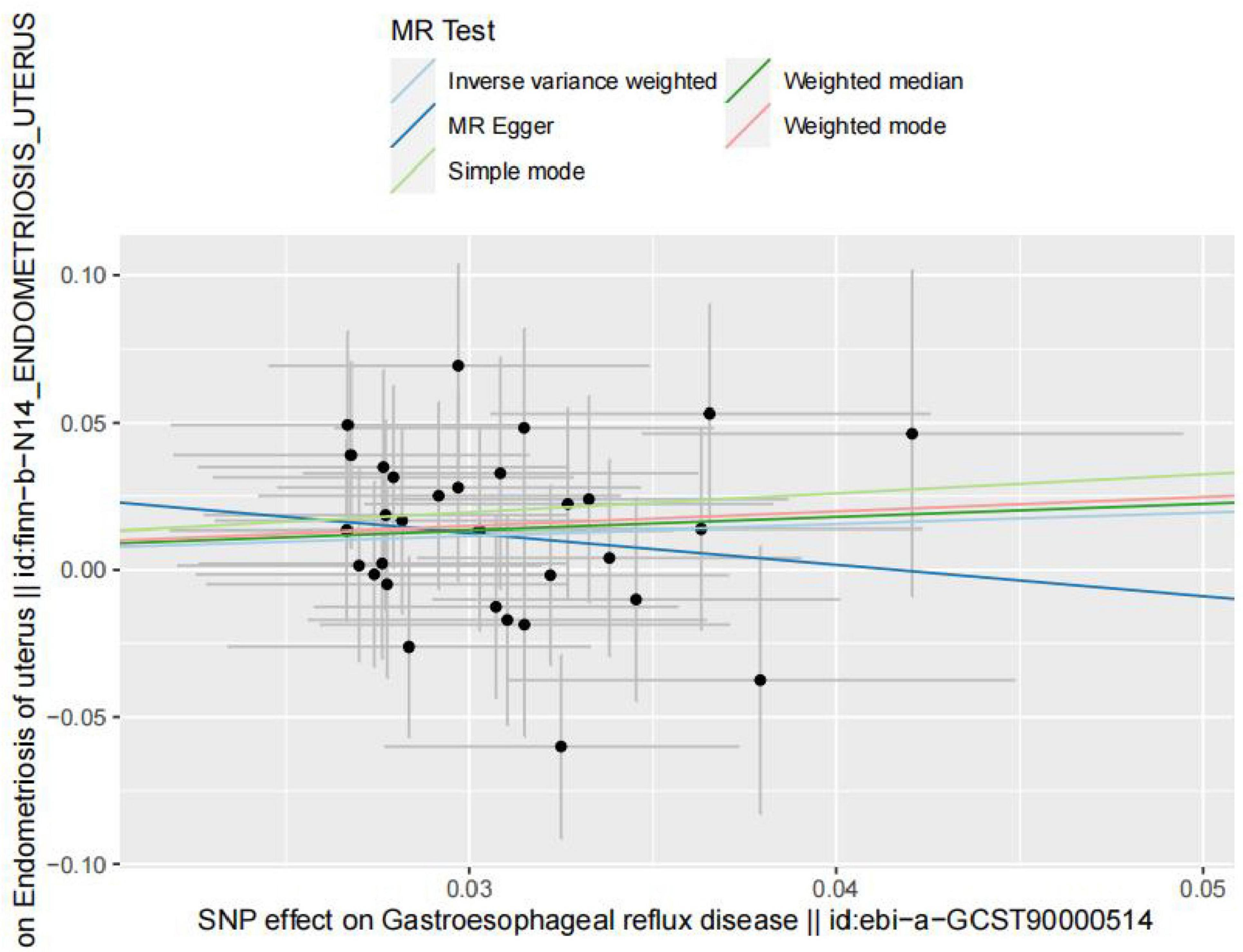
Figure 4. Scatterplot of genetic correlations between exposure (endometriosis occurring at the uterus) and outcome (gastroesophageal reflux disease) based on IVs screened at genome-wide significance thresholds.

Table 5. Results of MR-Egger intercept test and MR-PRESSO global test for horizontal multivariate validity.
3.2 Reverse MR results
Reverse MR analysis of the UK Bank database and endometriosis occurring at the uterus with no valid IVs after removal of the palindromic SNPs, outcome-associated SNPs (p < 0.05), SNPs not present in the resultant GWAS pooled data, and SNPs associated with confounders. The FinnGen database assessed the causal effect of endometriosis on GERD based on five IVs. Detailed information on the IVs for reverse MR analysis is shown in Supplementary Tables 3, 4. None of the MR methods showed a causal relationship between endometriosis and GERD (p > 0.05) (Table 6). The Cochran’s Q test showed that reverse MR analysis was affected by heterogeneity (p < 0.05) (Table 7). In addition, the MR-Egger intercept test and MR-PRESSO global test showed that the reverse MR analysis was not affected by horizontal pleiotropy (p > 0.05) (Table 8). Finally, leave-one-out sensitivity analysis confirmed the robustness of the reverse MR results (Supplementary Figure 2).

Table 8. Horizontal multivariate results of MR-Egger intercept test and MR-PRESSO global test in MR reverse analysis.
4 Discussion
In this study, bidirectional MR analysis was performed using a variety of MR methods, and the results showed that from the entire endometriosis dataset, no significant causality with GERD was found in either forward or reverse MR analysis (even though a significant causality was shown in the UK bank database, the OR was approximately equal to 1, suggesting that the occurrence of GERD did not significantly increase the risk of developing endometriosis). These associations were robust in sensitivity analyses, with no detectable heterogeneity or pleiotropy. The above results were largely consistent in MR analysis using IVs screened based on genome-wide significance thresholds from databases in different countries, adding more confidence to the results. Our findings are consistent with the results of previous reports on this type of disease by Adewuyi et al. (19). Surprisingly, however, when we analyzed the data using the Finn database, which provides subdatasets of endometriosis occurring in different locations, and when analyzing each subdataset individually, we found that genetically predicted GERD significantly increased the risk of endometriosis occurring in the uterine corpus, while at the same time, the reverse MR analysis revealed that confinement to the body of the uterus of the endometriosis did not appear to be causally related to GERD.
Previously reported observational studies have hinted at a possible relationship between GERD and endometriosis. Seaman et al. (38) found that endometriosis may coexist with the manifestation of gastrointestinal symptoms compared to healthy controls. Smorgick et al. (39) noted that the associations were closer relative to adolescents and young women, particularly in the adult female subgroup. Similarly, a cross-sectional cohort study involving Danish women found an association between gastrointestinal symptoms in patients with endometriosis, with cause and effect unknown (40). El Moaein and Carpentier (9) performed a clinical report showing the complex impact of a previous history of endometriosis on gastroesophageal reflux disease. In addition, the severity of gastroesophageal reflux can contribute to the development of endometriosis. For example, Mysior et al. (10) and Dasari et al. (11) reported that endometriosis involving the gastrointestinal tract should be considered when identifying acute abdominal pain in women of childbearing age. Although these observational studies do not explain causality, they provide sufficient evidence for an association between GERD and endometriosis.
Endometriosis confined to the uterine corpus, also known as adenomyosis, is a diffuse or confined lesion formed by the invasion of endometrial glands and mesenchyme into the myometrium, and its pathogenesis and pathophysiology have not yet been clarified, although it has been reported to have some genetic homology with endometriosis in other sites. The association of the study population with other diseases is shown in Table 9, from which it can be seen that the overlap between patients with adenomyosis and those with intestinal endometriosis was 2.82%, and with other gastroesophageal and gastrointestinal diseases was less than 2%. There was about 7% overlap between these study participants and those identified as being in stages 1,2 of endometriosis American Society for Reproductive Medicine (ASRM) and stages 3,4 of endometriosis ASRM. Notably, the patients with adenomyosis studied had an overlap of more than 20% with benign uterine fibroids with endometriosis and endometriosis. Meanwhile, Table 10 provides the drug use in the study population, which shows that the overlap between the adenomyosis patients and the concomitant drug group was less than 2% in all cases, with the exception of the “Triptan medication for migraine,” which also had an approximate overlap of 2%. Therefore, the conclusion of our study that gastroesophageal reflux may somewhat increase the risk of developing adenomyosis was less affected by the medications used by the study subjects, and it can be ruled out that these patients were not at pathogenic risk due to the use of medications. In the present MR study, GERD can lead to an increased risk of adenomyosis without a significant causal relationship with other sites of endometriosis, which explores the possible different pathogenesis of adenomyosis and other endometriosis from another new angle and provides a new direction for further pathologic studies.
MR research is an innovative approach to inferring causality. Compared to traditional observational studies, MR studies eliminate confounding variables and reverse causation. Compared to randomized controlled trials, MR studies are more effective, and there are no ethical restrictions on their implementation. The MR results showed that GERD significantly increased the risk of endometriosis confined to the uterus (IVW: OR = 1.47, 95% CI 1.00–2.17, P = 0.05) using IVs based on genome-wide significance threshold screening. In addition, the extrapolation of the weighted median approach was consistent with the results of IVW (37). Subsequently, various sensitivity tests further demonstrated the validity of the results.
Meanwhile, the reason for analyzing the slight difference with the results of Adewuyi et al. (19) is most likely related to the exclusion of confounders in the instrumental variables, and the literature on MR analysis of GERD and endometriosis was cited in the latest review of the causal relationship between different exposures and endometriosis using Mendelian randomization, which referred to the association between GERD and depression, and GERD may play a role as a mediating variable in depression and anxiety affecting the occurrence of endometriosis, implying that previous studies did not exclude the interference of depression and anxiety as a confounding factor on the research results, and can also indicate that this paper based on the results of the latest research progress to exclude the new confounding factors may get different analytical conclusions of research and practical significance, and can provide a new reference value (41). On the basis of previous studies and conclusions, we continue to dig deeper into endometriosis occurring in different locations and find that GERD may increase the risk of adenomyosis, which can also prove the results of previous studies to a certain extent.
Several hypotheses could explain the increased risk of adenomyosis caused by GERD. First, previous studies imply that other syndromes of the intestinal tract are closely associated with endometriosis conditions (15), both showing a tendency to increase the overall level of chronic inflammation (39, 42, 43). In this context, the activation of mast cells and their degranulation, followed by the release of lymphokines, tumor necrosis factor-alpha, and the presence of proinflammatory cytokines in mesenchymal tissues, promotes the persistence of a chronic inflammatory situation (42, 44–47). Considering the pathophysiologic mechanisms shared by GERD and endometriosis, the possible diagnosis of both pathologies needs to be investigated in the presence of severe pelvic pain. Second, depression and anxiety may mediate GERD-induced endometriosis (22, 29). Since GERD episodes may lead to elevated levels of central nervous system inflammation, which may trigger depression and anxiety, patients with GERD often suffer from more severe anxiety-depression (48, 49). At the same time, anxiety depression produces estrogen disorders, which exacerbate depression anxiety and make endometriosis, which is regulated by estrogen, possible (2). Therefore, it is necessary to maintain psychological support for patients with GERD to maintain estrogen stability and reduce the risk of subsequent endometriosis.
The current study has some strengths. First, this is an MR investigation assessing the causal relationship between GERD and endometriosis, which obtained not exactly the same conclusions as previous studies and did not find a significant causal relationship between GERD and endometriosis in both the positive and negative directions. Second, the MR analyses in this paper were performed using separate pooled-level data from large-scale GWAS in different countries, which improves the confidence of inferences due to the large sample sizes and different populations, and many MR methods and sensitivity analyses were used to improve the confidence of the results. Third, thanks to the Finn database, which breaks down endometriosis occurring in different locations, the present study enriches and completes the findings of previous studies and is highly likely to imply a causal association between GERD and specific sites of endometriosis occurrence. However, this study has some limitations. First, the original GWAS pooled data analyzed in this study were from a European population; therefore, the findings may not be applicable to other ethnicities. Second, due to the limitations of the GWAS pooled data, it was not possible to stratify the analysis for general factors such as age and gender. Third, it is difficult to ensure that the results are completely independent of horizontal polymorphism effects. Therefore, we performed a series of sensitivity analyses to demonstrate the reliability of the results.
5 Conclusion
Evidence is provided that genetically predicted GERD increases the risk of adenomyosis. Therefore, symptomatic treatment of patients with GERD should be complemented by gynecological examination to avoid and prevent the development of endometriosis.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the datasets analyzed during the current study are available in the GWAS database, https://www.ebi.ac.uk/gwas.
Ethics statement
The studies involving humans were approved by the Medical Ethics Review Board of West China Second Hospital of Sichuan University, and informed consent was obtained from the subjects (Date: March 8, 2019). The datasets analyzed in this study were publicly available summary statistics, and informed consent was obtained from all individuals participating in the study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZS: Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. ZL: Supervision, Validation, Methodology, Formal analysis, Data curation, Conceptualization, Writing – review & editing. KW: Validation, Supervision, Methodology, Funding acquisition, Writing – review & editing. FY: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing, Validation.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (grant number 2022YFC2704103).
Acknowledgments
We thank the Natural Science Foundation of Sichuan Province (No. 2022NSFSC0790).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1440157/full#supplementary-material
Supplementary Figure 1 | Funnel plots of the causal effect between GERD and endometriosis confined to the uterine corpus.
Supplementary Figure 2 | Funnel plots of the causal effect between endometriosis confined to the uterine corpus and GERD.
References
1. Laganà A, Garzon S, Götte M, Viganò P, Franchi M, Ghezzi F, et al. The pathogenesis of endometriosis: Molecular and cell biology insights. Int J Mol Sci. (2019) 20:5615. doi: 10.3390/ijms20225615
2. Koninckx P, Ussia A, Adamyan L. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil Steril. (2019) 111:327–40. doi: 10.1016/j.fertnstert.2018.10.013
3. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. (2019) 15:666–82. doi: 10.1038/s41574-019-0245-z
4. Hämmerli S, Kohl Schwartz A, Geraedts K, Imesch P, Rauchfuss M, Wölfler M, et al. Does endometriosis affect sexual activity and satisfaction of the man partner? A comparison of partners from women diagnosed with endometriosis and controls. J Sex Med. (2018) 15:853–65. doi: 10.1016/j.jsxm.2018.03.087
5. Soliman A, Coyne K, Gries K, Castelli-Haley J, Snabes M, Surrey E. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm. (2017) 23:745–54. doi: 10.18553/jmcp.2017.23.7.745
6. Soliman A, Yang H, Du E. The direct and indirect costs associated with endometriosis: A systematic literature review. Hum Reprod. (2016) 31:712–22. doi: 10.1093/humrep/dev335
7. Saunders P, Horne A. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. (2021) 184:2807–24. doi: 10.1016/j.cell.2021.04.041
8. Katz P, Dunbar K, Schnoll-Sussman F. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. (2021) 117:27–56. doi: 10.14309/ajg.0000000000001538
9. El Moaein T, Carpentier SG. resulting in superior mesenteric artery syndrome: An unfortunate outcome. Am J Gastroenterol. (2018) 113:S1399–401. doi: 10.14309/00000434-201810001-02522
10. Mysior J, Sreenivasan P, Mangla R, Schwender B. Intestinal endometriosis as an unusual cause of small bowel obstruction and abdominal pain. Am J Gastroenterol. (2011) 106:S236. doi: 10.14309/00000434-201110002-00619
11. Dasari V, Grossman E, Dubrovskaya V. Ileocecal valve endometriosis as a cause of acute intestinal obstruction. Am J Gastroenterol. (2012) 107:S383. doi: 10.14309/00000434-201210001-00936
12. Salas Noain J, Gudipally S, Zheng S, Amaral R. 1525 Atypical presentation of colon cancer: Colon-Vaginal fistula. Am J Gastroenterol. (2019) 114:S844–844. doi: 10.14309/01.ajg.0000595628.12916.9a
13. Griffiths MJ, Horne AW, Gibson DA, Roberts N, Saunders PTK. Endometriosis: recent advances that could accelerate diagnosis and improve care. Trends Mol Med. (2024) 30:875–89. doi: 10.1016/j.molmed.2024.06.008
14. Yang F, Wu Y, Hockey R, Doust J, Mishra GD, Montgomery GW, et al. Evidence of shared genetic factors in the etiology of gastrointestinal disorders and endometriosis and clinical implications for disease management. Cell Rep Med. (2023) 4:101250. doi: 10.1016/j.xcrm.2023.101250
15. Viganò D, Zara F, Usai P. Irritable bowel syndrome and endometriosis: New insights for old diseases. Digest Liver Dis. (2018) 50:213–9. doi: 10.1016/j.dld.2017.12.017
16. Skrivankova V, Richmond R, Woolf B, Davies N, Swanson S, VanderWeele T, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
17. Richmond R, Davey Smith G. Mendelian randomization: Concepts and scope. Cold Spring Harb Perspect Med. (2021) 12:a040501. doi: 10.1101/cshperspect.a040501
18. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
19. Adewuyi EO, Mehta D, Sapkota Y, Auta A, Yoshihara K, Nyegaard M, et al. Genetic analysis of endometriosis and depression identifies shared loci and implicates causal links with gastric mucosa abnormality. Hum Genet. (2020) 140:529–52. doi: 10.1007/s00439-020-02223-6
20. Ong J-S, An J, Han X, Law MH, Nandakumar P, Schumacher J, et al. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for Barrett’s oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. (2021) 71:1053–61. doi: 10.1136/gutjnl-2020-323906
21. GWAS Catalog. The NHGRI-EBI catalog of human genome-wide association studies. (2023). Available online at: https://www.ebi.ac.uk/gwas (accessed November 17, 2023).
22. Koller D, Pathak G, Wendt F. Epidemiologic and genetic associations of endometriosis with depression, anxiety, and eating disorders. JAMA Netw Open. (2023) 6:e2251214. doi: 10.1001/jamanetworkopen.2022.51214
23. Burgess S, Small D, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. (2015) 26:2333–55. doi: 10.1177/0962280215597579
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. (2000) 29:722–9. doi: 10.1093/ije/29.4.722
25. Martens E, Pestman W, de Boer A. Instrumental variables. Epidemiology. (2006) 17:260–7. doi: 10.1097/01.ede.0000215160.88317.cb
26. Pierce B, Ahsan H, Vanderweele T. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2010) 40:740–52. doi: 10.1093/ije/dyq151
27. Farland L, Missmer S, Bijon A, Gusto G, Gelot A, Clavel-Chapelon F, et al. Associations among body size across the life course, adult height and endometriosis. Hum Reprod. (2017) 32:1732–42. doi: 10.1093/humrep/dex207
28. Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. (2005) 121:94–8. doi: 10.1016/j.ejogrb.2004.11.019
29. Bussotti M, Sommaruga M. Anxiety and depression in patients with pulmonary hypertension: Impact and management challenges. Vasc Health Risk Manag. (2018) 14:349–60. doi: 10.2147/vhrm.s147173
30. Lu M, Niu J, Liu B. The risk of endometriosis by early menarche is recently increased: A meta-analysis of literature published from 2000 to 2020. Arch Gynecol Obstet. (2022) 307:59–69. doi: 10.1007/s00404-022-06541-0
31. Nnoaham K, Webster P, Kumbang J, Kennedy S, Zondervan K. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril. (2012) 98:702–712.e6. doi: 10.1016/j.fertnstert.2012.05.035
32. Missmer S, Hankinson S, Spiegelman D, Barbieri R, Malspeis S, Willett W, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. (2004) 104:965–74. doi: 10.1097/01.aog.0000142714.54857.f8
33. Maddern J, Grundy L, Castro J, Brierley S. Pain in Endometriosis. Front Cell Neurosci. (2020) 14:590823. doi: 10.3389/fncel.2020.590823
34. Houston D. Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiol Rev. (1984) 6:167–91. doi: 10.1093/oxfordjournals.epirev.a036270
35. Pagoni P, Dimou N, Murphy N, Stergiakouli E. Using Mendelian randomisation to assess causality in observational studies. Evid Based Ment Health. (2019) 22:67–71. doi: 10.1136/ebmental-2019-300085
36. Burgess S, Thompson S. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
37. Bowden J, Davey Smith G, Haycock P, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
38. Seaman H, Ballard K, Wright J, De Vries C. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: Findings from a national case–control study–Part 2. BJOG. (2008) 115:1392–6. doi: 10.1111/j.1471-0528.2008.01879.x
39. Smorgick N, Marsh C, As-Sanie S, Smith Y, Quint E. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J Pediatr Adolesc Gynecol. (2013) 26:171–5. doi: 10.1016/j.jpag.2012.12.006
40. Schomacker M, Hansen K, Ramlau-Hansen C, Forman A. Is endometriosis associated with irritable bowel syndrome? A cross-sectional study. Eur J Obstet Gynecol Reprod Biol. (2018) 231:65–9. doi: 10.1016/j.ejogrb.2018.10.023
41. McGrath I, Montgomery G, Mortlock S. Insights from Mendelian randomization and genetic correlation analyses into the relationship between endometriosis and its comorbidities. Hum Reprod Update. (2023) 29:655–74. doi: 10.1093/humupd/dmad009
42. Polak G, Barczyński B, Bednarek W. Increased levels of proteins of the acute inflammatory phase in the peritoneal fluid of women with advanced stages of endometriosis. Polish Gynaecol. (2015) 86:414–8. doi: 10.17772/gp/2416
43. Sinagra E, Pompei G, Tomasello G, Cappello F, Morreale G, Amvrosiadis G, et al. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol. (2016) 22:2242–55. doi: 10.3748/wjg.v22.i7.2242
44. Héron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. (2013) 264:1–7. doi: 10.1016/j.jneuroim.2013.09.018
45. Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. (2012) 1822:85–92. doi: 10.1016/j.bbadis.2011.06.004
46. Walker M. Are conditions of immune dysregulation risk factors for functional gastrointestinal disorders? Findings from a random population based study. Berlin: Morressier (2016).
47. Kempuraj D, Papadopoulou N, Stanford E, Christodoulou S, Madhappan B, Sant G, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. (2004) 52:267–75. doi: 10.1111/j.1600-0897.2004.00224.x
48. You Z, Perng C, Hu L. Risk of psychiatric disorders following gastroesophageal reflux disease: A nationwide population-based cohort study. Eur J Intern Med. (2015) 26:534–9. doi: 10.1016/j.ejim.2015.05.005
Keywords: bidirectional Mendelian randomization (MR) analysis, gastroesophageal reflux disease, risk of developing endometriosis, endometriosis confined to the uterus, endometriosis
Citation: Shi Z, Li Z, Wang K and Yang F (2024) The causal role of gastroesophageal reflux disease in endometriosis: a bidirectional Mendelian randomization study. Front. Med. 11:1440157. doi: 10.3389/fmed.2024.1440157
Received: 29 May 2024; Accepted: 09 October 2024;
Published: 30 October 2024.
Edited by:
Lan Zheng, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Lidong Zhu, University of Electronic Science and Technology of China, ChinaMi Yang, Chengdu Fourth People’s Hospital, China
Copyright © 2024 Shi, Li, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Yang, c2hhcnJ5NDhAMTYzLmNvbQ==
 Zunlin Shi
Zunlin Shi Zhi Li
Zhi Li Kana Wang
Kana Wang Fan Yang
Fan Yang