- 1Department of Gastroenterology, The Anqing 116 Hospital, China RongTong Medical Healthcare Group Co. Ltd., Anqing, Anhui, China
- 2Department of Gastroenterology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Background and aim: Gastroesophageal reflux disease (GERD) patients often report sleep disturbance (SD); however, the relationship between GERD and SD is unknown. This study investigated whether SD affects symptoms, acid reflux, and autonomic function in GERD patients.
Methods: A total of 257 subjects (126 patients with SD and 99 patients without SD) participated in this survey from January 2020 to August 2022. Participants were required to complete questionnaires including the GERD impact scale (GIS), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD). Esophageal mucosal injury, acid exposure, peristaltic function, and autonomic function were assessed by upper endoscopy, high-resolution esophageal manometry (HRAM), 24-h multichannel intraluminal impedance with pH (24 h-MII-pH), and electrocardiography (ECG).
Results: Gastroesophageal reflux disease patients with SD experienced a higher frequency of prolonged reflux (p < 0.001), longest reflux event (p < 0.001), acid exposure time (p < 0.001) during the recumbent period, and a higher incidence of erosive esophagitis (EE) (59.5 vs. 45.5%, p = 0.036) than those without SD. Pearson’s correlation analysis showed that SD was positively correlated with GIS (r = 0.725, p < 0.001), HAMA (r = 0.680, p < 0.001), and HAMD (r = 0.323, p < 0.001) scores, and negatively correlated with parasympathetic or vagal nerve activity (r = −0.770, p < 0.001).
Conclusion: Gastroesophageal reflux disease patients with SD experience more severe reflux symptoms and nocturnal acid reflux, which may be related to autonomic dysfunction.
1 Introduction
Gastroesophageal reflux disease (GERD) is a chronic disease caused by the reflux of gastroduodenal contents due to various causes, leading to uncomfortable symptoms and complications (1). 13% of the world’s population is estimated to experience GERD symptoms at least once per month (2). Proton-pump inhibitors (PPIs) remain the mainstay of treatment for GERD, but 40% of patients reportedly experience no significant relief of reflux symptoms after 8 weeks of full dosage and full course of acid suppressant (3). Interestingly, Schey et al. showed that sleep deficiency (SD) could induce esophageal hypersensitivity in GERD patients and may be one of the reasons why reflux symptoms are difficult to control in some patients (4).
Sleep disturbance (SD) usually refers to a subjective experience whereby the patient is dissatisfied with the duration and/or quality of sleep and affects daytime social functioning (5). Indeed, good sleep hygiene is an important component of overall health and quality of life. People that get less than 5–6 h of sleep per night are classified as sleep deprived. Over the past century, the average night sleep has decreased by 2 h (6, 7). SD can lead to many health-related diseases such as obesity, diabetes, cardiovascular disease, functional gastrointestinal disorders (FGIDs), reduced immune response, impaired cognition, anxiety and depression, poor quality of life, and increased all-cause mortality (8). SD is also associated with disorders of gut-brain interaction (DGBI), such as irritable bowel syndrome (IBS) and functional dyspepsia (FD), and often results in various gastrointestinal symptoms, including abdominal pain, bloating, and belching (5, 7). Current evidence suggests increased risk of multiple gastrointestinal complaints and reduced quality of life are associated with SD (9). A recent study emphasized the importance of properly coordinating the sleep/wake cycle with the body’s circadian cycle for achieving healthy sleep. Therefore sleep, wake, and meal times should match the circadian cycle as closely as possible, to obtain optimal sleep and gut-related health (7).
Proton-pump inhibitors continue to be the preferred pharmacological treatment for gastroesophageal reflux disease (GERD). However, numerous studies have highlighted potential adverse events associated with their use, thereby casting doubt on the safety of prolonged administration and heightening concerns regarding the overprescription of these medications. Additionally, recent evidence has surfaced concerning the viability of surgical and endoscopic alternatives for managing this condition. Meanwhile, mineral waters, especially those based on bicarbonate-magnesium, were also validated for the management of GERD (10, 11).
It has been established that GERD is a kind of FGID often associated with SD. However, most studies have demonstrated the association between gastrointestinal symptoms and SD, while the relationship between SD and esophageal motility in GERD has been largely understudied. Therefore, this study aimed to investigate the effect of SD on esophageal motility and autonomic function in GERD (10).
2 Materials and methods
2.1 Subjects
Patients diagnosed with GERD based on diagnostic endoscopy and reflux monitoring at the Department of Gastroenterology, the First Affiliated Hospital of the University of Science and Technology of China (USTC) from January 2020 to August 2022 were enrolled in the study (1). The diagnosis of GERD was defined based on Lyon consensus 2.0 (12). In detail, the diagnostic criteria of included patients were: (1) LA grade B, C, and D esophagitis, biopsy proven Barrett’s esophagus and peptic stricture; (2) AET > 6.0%, >80 reflux episodes, and MNBI value <1,500 ohms; (3) borderline evidence with supportive adjunctive metrics according to the Lyon Consensus. The exclusion criteria consisted of: (1) patients with presence of other pathologies leading to altered sleep, such as heart, lung, liver, kidney diseases, or other serious comorbidities, (2) patients with any previously serious psychiatric comorbidity, such as HAMD>20; (4) patients who used psychotropic drugs or medication influencing sleep; (5) patients who had taken PPI 1 week prior to inclusion.
The study was approved by the Ethics Committee of the First Affiliated Hospital of USTC (Registration No: 2022-RE-296) and was performed in accordance with the Declaration of Helsinki. All participants gave written informed consent for enrollment in this study.
2.2 Study protocol
The patients included in this study were divided into two groups based on their Pittsburgh Sleep Quality Index (PSQI) scores: GERD patients with SD (PSQI scores ≥ 5) and GERD patients without SD (PSQI scores < 5). All participants were required to complete questionnaires, including PSQI, GERD impact scale (GIS), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD). Moreover, all participants underwent High-resolution esophageal manometry (HRAM), 24-h multichannel intraluminal impedance with pH (24 h-MII-pH), upper endoscopy, and electrocardiogram (ECG).
2.3 Sample size
The sample size was calculated, referencing a previous clinical study (13). As a case–control study, in the control group, the sleep disturbance (defined as PSQI) prevalence was 65.61% (332/506), while the adjusted OR value was 3.5. To achieve a power of 80%, a sample size of 62 patients in case and control group was necessary, considering a 20% dropout rate and an alpha level of 5%. Therefore, the sample size of this study is sufficient to demonstrate statistical differences.
2.4 Measurements
2.4.1 Questionnaires
2.4.1.1 Pittsburgh sleep quality index
The Pittsburgh Sleep Quality Index score is a standardized rating scale that captures the global sleep status in different populations, including the general population and individuals with psychiatric disorders. It identifies sleep disorders by assessing seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, sleep medication use, and daytime dysfunction. Each category is scored on a scale of 0–3, and these factors are added together to give a global score (maximum global score of 21). Higher scores indicate poorer sleep, with a global score above 5 considered “SD” (14–16).
2.4.1.2 GERD impact scale
The GERD impact scale is a practical and easily administered instrument targeted for use in the primary care setting. The questionnaire includes nine items covering five symptom domains (chest pain, heartburn, acid reflux, epigastric pain, and hoarseness) and four questions on the quality of life (QOL). Their frequency of symptoms and poor quality of life in the past 1 month were assessed separately using a modified five-point Likert scale (0, never; 1, 1–2 times per month; 2, 1–2 times per week; 3, 3–4 times per week; 4, daily). The GIS scores range from 0 to a maximum of 36 (17).
2.4.1.3 HAMA and HAMD scale
The Hamilton anxiety scale and Hamilton depression scale have been widely used to assess the mental state of patients (18, 19). The Hamilton anxiety scale contains 14 items; each assessed separately using a five-point Likert scale ranging from grade 0 to grade 4. A global HAMA score of >6 indicates “anxiety.” HAMD contains 17 items; each scored on a five-point Likert scale. A global HAMA score of >7 indicates “depression” (20, 21).
2.4.2 Upper endoscopy
Upper endoscopy is the most widely used examination to assess the esophageal mucosa, especially the distal portion of the esophagus, during standard endoscopy to determine the presence of mucosal injury. The Los Angeles (LA) classification was used to assess the severity of erosive esophagitis (EE) as follows: Grade A (one or more esophageal mucosal breaks with a maximum diameter < 5 mm), Grade B (one or more esophageal mucosal breaks with a maximum diameter > 5 mm but no fused lesions), Grade C (esophageal mucosal breaks with fusion but <75% of the circumference of the esophagus), and Grade D (esophageal mucosal breaks with fusion to at least 75% of the circumference of the esophagus) (1, 22).
2.4.3 High-resolution esophageal manometry
A water-perfused esophageal manometric catheter with 24 pressure sensors spaced at 1 cm intervals (MedKinetic, Ningbo, China) was used to assess the motility of the esophagus. All patients were taken off acid suppressants and prokinetic drugs for at least 1 week before HREM, and subjects were required to fast for at least 8 h before the test. During the examination, the HREM catheter was inserted through the patient’s unobstructed nasal cavity, and the patient was placed in a prone position after successful insertion. After recording the resting state pressure, the patient swallowed 5 mL of water 10 times every 30 s. The catheter was removed after manometry was completed (23).
2.4.4 Multichannel intraluminal impedance with pH testing
A combined 24-h multichannel intraluminal impedance with a pH (pH-MII) testing system (Jinshan, Chongqing, China) and a 6MII-1 pH electrode catheter (JSIpS-1) were used to perform 24-h pH-impedance monitoring of the esophagus. After the position of the LES was determined by HREM, the monitoring catheter was inserted through the nasal cavity on one side of the patient and placed, ensuring that the pH electrode was located 5 cm above the upper edge of the LES. After fixing the catheter and the monitoring system was turned on, the patient was instructed to record his diet, postural changes and the onset of GERD symptoms during the monitoring period (24).
2.4.5 Autonomic functions
The Heart Rate Variability (HRV) was documented by electrocardiogram (ECG) recording (ct-082, Hangzhou Baihui Electrocardiograms, China) and imported into HRV analysis software (Cardiotrak Holtersystem version: 1.2.0.0, Hangzhou Baihui Electrocardiograms, China). HRV frequency domain indicators include a low frequency (LF) band (LF power in the range of 0.04–0.15 Hz) and a high frequency (HF) band (HF power in the range of 0.15–0.40 Hz). Given that LF is an indicator of sympathetic activity, and HF is an indicator of parasympathetic or vagal activity, the LF/HF ratio can be used to assess sympathetic/parasympathetic balance (25).
2.5 Statistical methods
All statistical analyses were performed using SPSS version 22.0 software. Continuous variables were expressed as mean ± standard deviation (SD) and using independent samples t-test analysis. Categorical variables were expressed as rates and compared using the χ2 test. Correlations were assessed using Pearson’s correlation test. A p value <0.05 was statistically significant.
3 Results
3.1 Patient characteristics
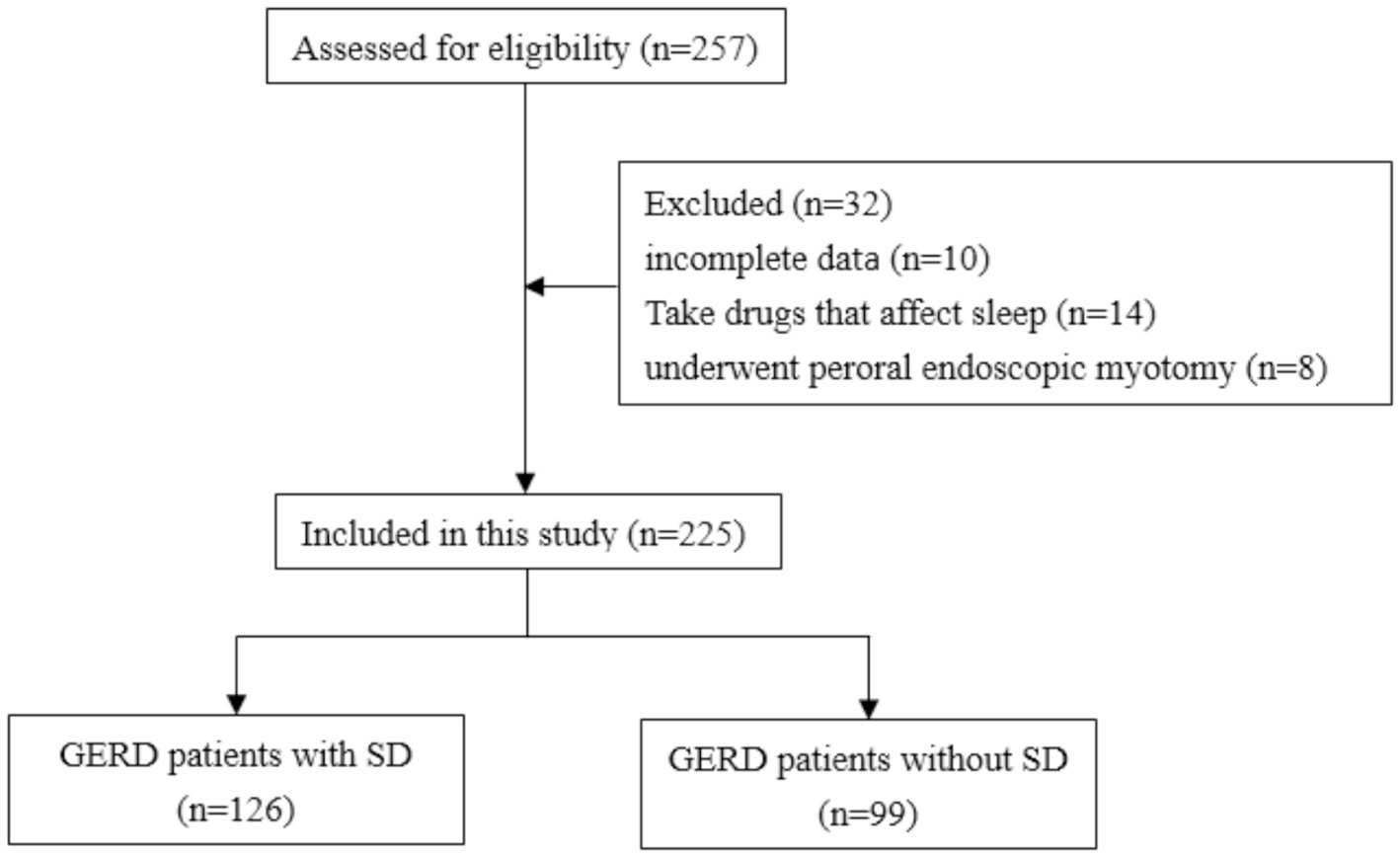
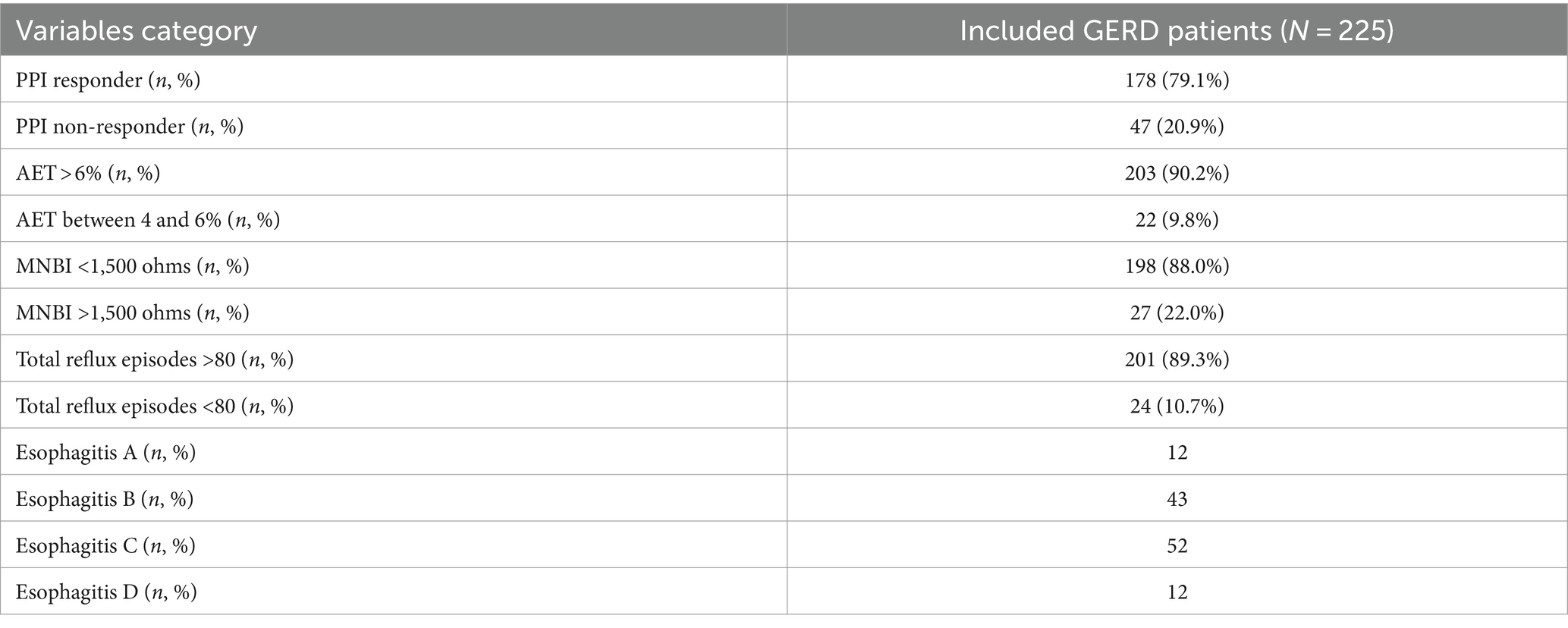
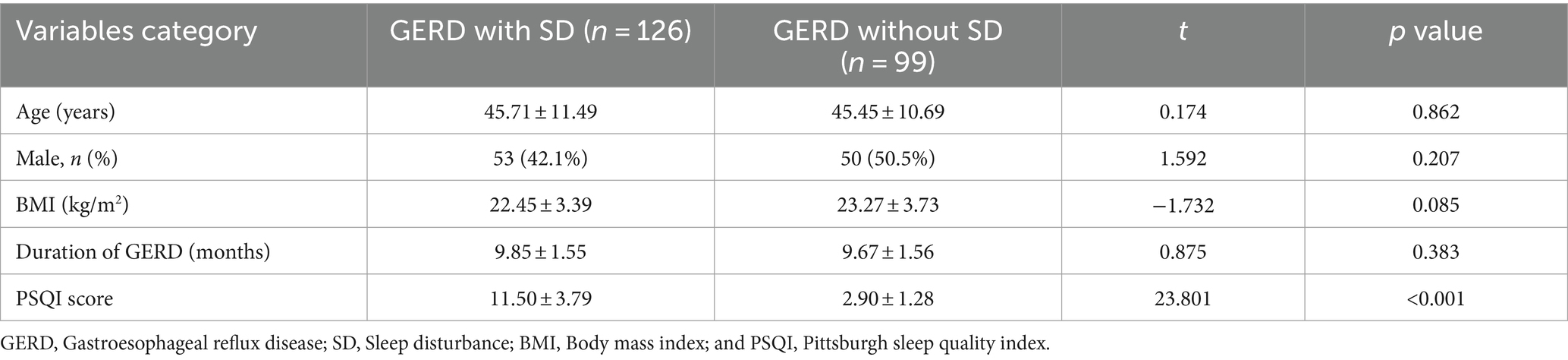
257 GERD patients initially participated in this study. 32 patients were excluded due to incomplete data (n = 10), use of drugs that affected sleep (n = 14), or history of peroral endoscopic myotomy (n = 8). Finally, 225 GERD patients were enrolled in this study (Figure 1) with a mean age of 45.60 ± 11.12 years (range 22–83 years) and separated into patients with (n = 126) or without (n = 99) SD. The detailed characteristics of included patients were shown in Table 1. A total of 12 patients with esophagitis A had pathologic pH-impedance (AET > 6%, reflux episodes >80, and MNBI <1,500 ohms) as evidence with supportive adjunctive metrics according to the Lyon Consensus. A were the sex ratio, mean age and body mass index did not differ significantly between both patient groups (Table 2).
3.2 Comparison of the GERD impact scale, anxiety, and depression score between both groups
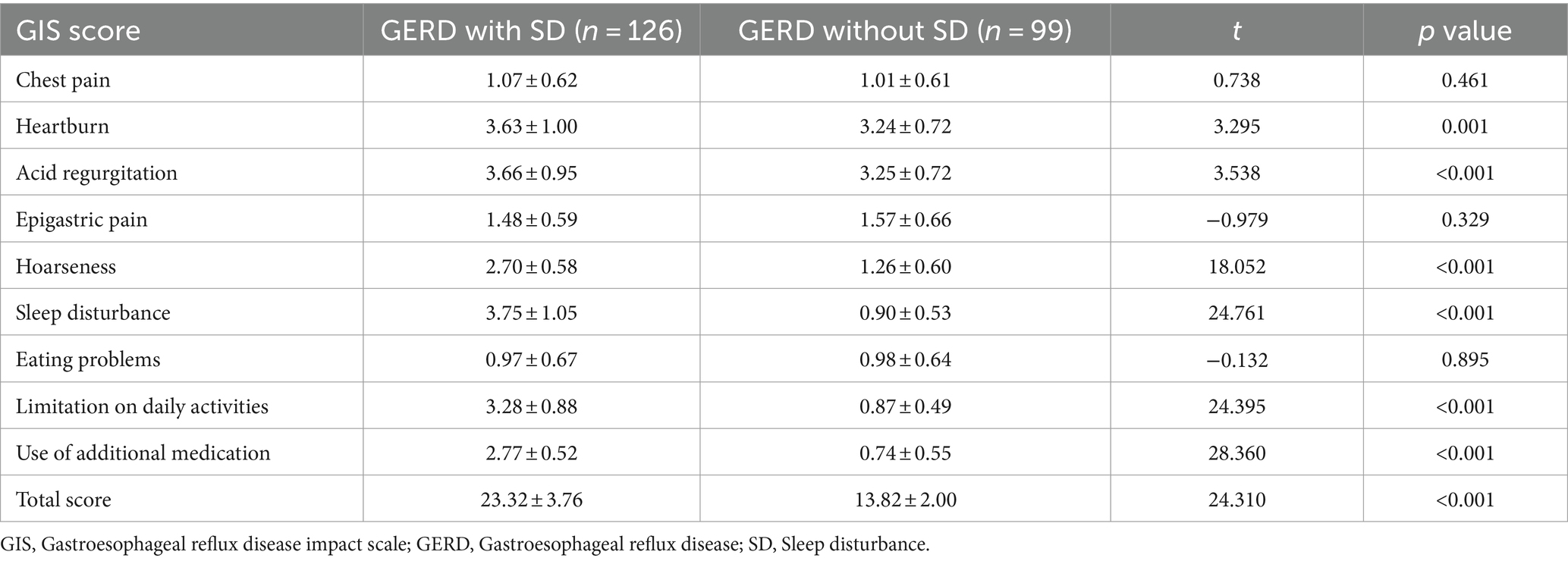
Comparison of both groups showed that the frequency of heartburn (3.63 ± 1.00 vs. 3.24 ± 0.72, p = 0.001), acid regurgitation (3.66 ± 0.95 vs. 3.25 ± 0.72, p < 0.001), and hoarseness (2.70 ± 0.58 vs. 1.26 ± 0.60, p < 0.001) was significantly higher in GERD patients with SD, while the frequency of chest pain (p = 0.461) and epigastric pain (p = 0.329) was comparable. The frequency of sleep disturbance (3.75 ± 1.05 vs. 0.90 ± 0.53, p < 0.001), limitation on daily activities (3.28 ± 0.88 vs. 0.87 ± 0.49, p < 0.001), and use of additional medication (2.77 ± 0.52 vs. 0.74 ± 0.55, p < 0.001) were higher in GERD patients with SD. There was no statistical difference regarding eating problems (p = 0.895) among these two groups. The total GIS score (23.32 ± 3.76 vs. 13.82 ± 2.00, p < 0.001) was significantly higher in GERD patients with SD (Table 3). Meanwhile, GERD patients with SD had higher HAMA (9.54 ± 3.13 vs. 5.56 ± 2.69, p < 0.001) and HAMD (8.20 ± 3.20 vs. 5.97 ± 2.67, p < 0.001) scores compared with GERD patients without SD (Table 4).
3.3 Comparison of the severity of erosive esophagitis between the two groups
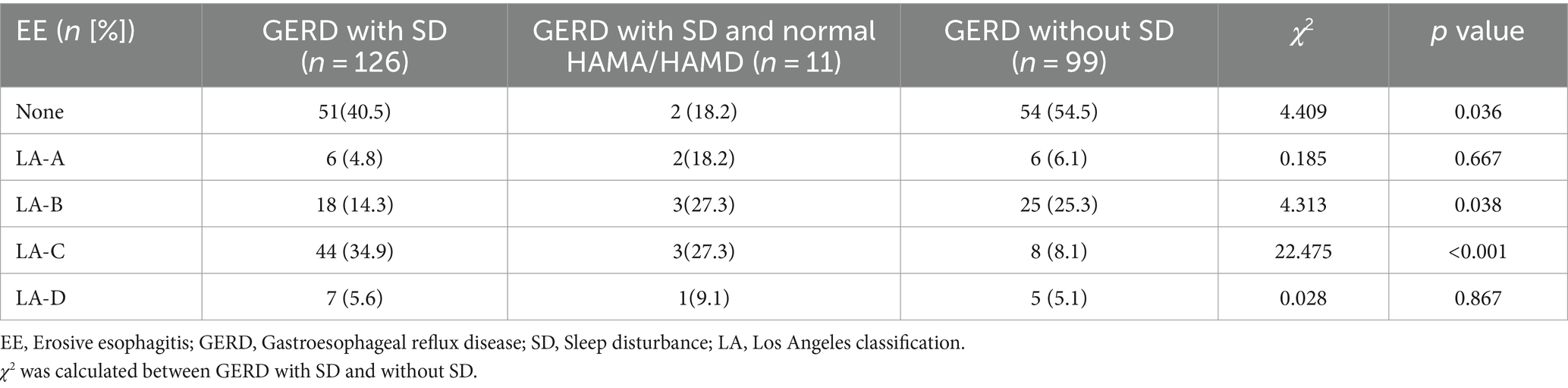
Erosive esophagitis was found more in GERD patients with SD than those without SD (59.5 vs. 45.5%, p = 0.036). According to the LA classification, the proportion of grade A (p = 0.667) and grade D (p = 0.867) was comparable between the two groups. The proportion of grade B GERD patients with SD was 14.3%, lower than GERD patients without SD (p = 0.038), while the proportion of grade C was 34.9%, higher than GERD patients without SD (p < 0.001) (Table 5).
3.4 Comparison of the high-resolution esophageal manometry between the two groups
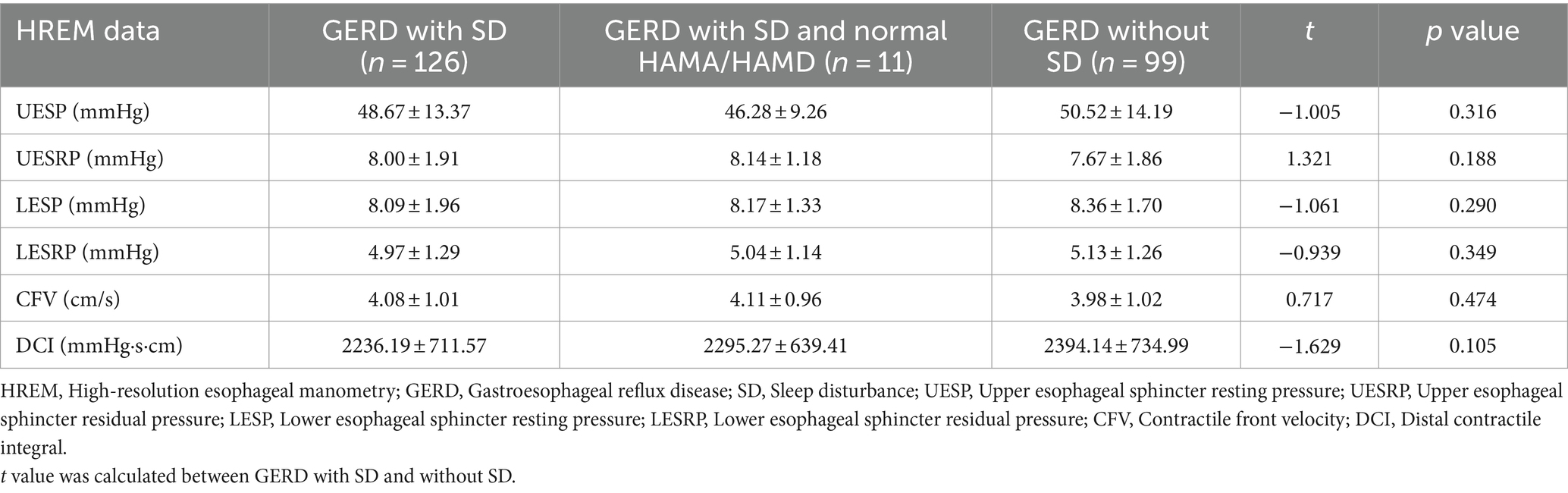
The Lower Esophageal Sphincter resting Pressure (LESP) (p = 0.290) and Lower Esophageal Sphincter Residual Pressure (LESRP) (p = 0.349) were lower in GERD patients with SD than without SD; however, there was no significant difference between the two groups. Meanwhile, the Upper Esophageal Sphincter resting Pressure (UESP) (p = 0.316), Upper Esophageal Sphincter Residual Pressure (UESRP) (p = 0.188), Contractile Front Velocity (CFV) (p = 0.474) and Distal Contractile Integral (DCI) (p = 0.105) were not significantly different between the two groups (Table 6).
3.5 Comparison of the 24 h-MII-pH testing between the two groups
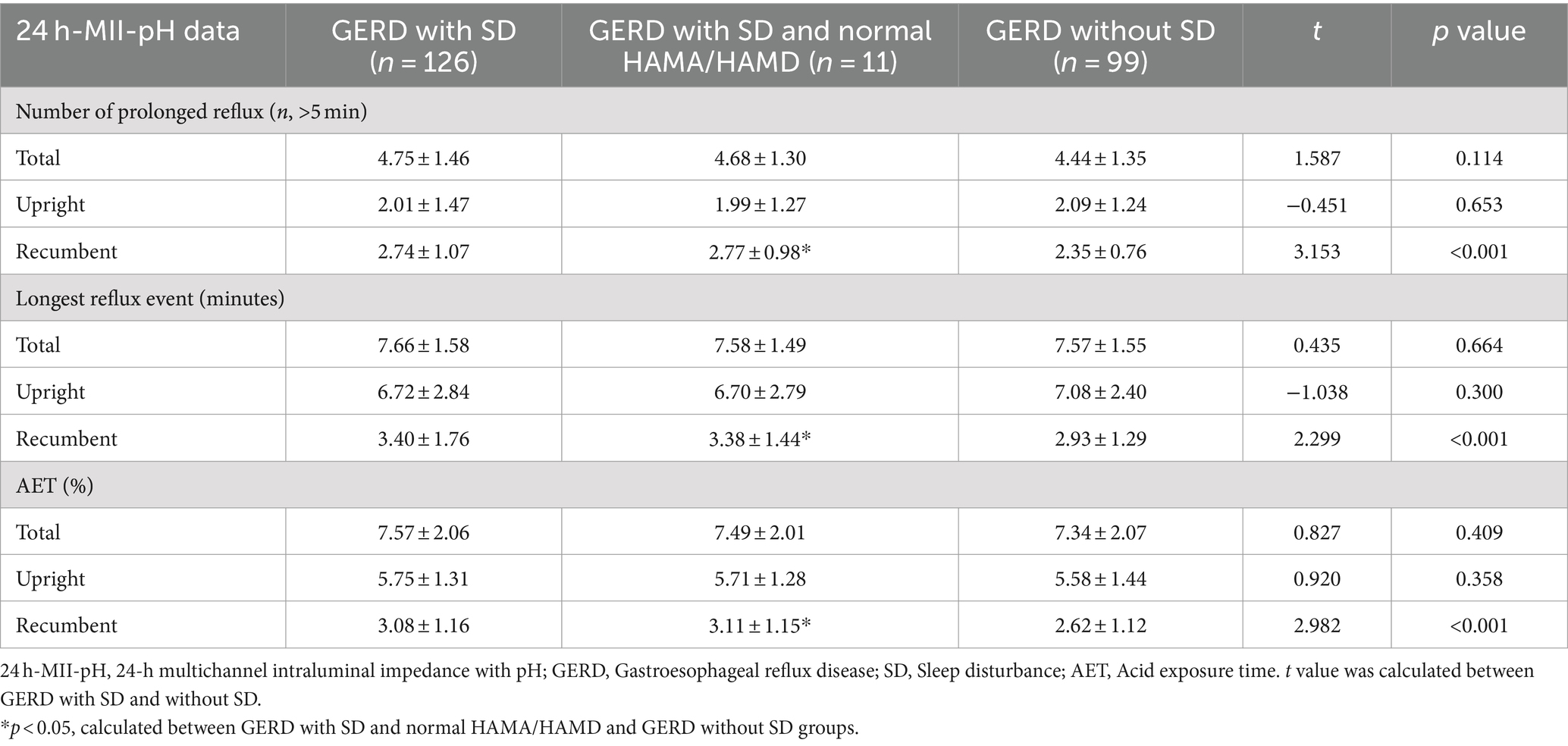
The frequency of prolonged reflux during the recumbent period was significantly greater in GERD patients with SD than without SD (2.74 ± 1.07 vs. 2.35 ± 0.76, p < 0.001), while no significant differences were found for the overall and upright periods in both groups (p = 0.114 and p = 0.653, respectively). Although there was no difference in longest reflux time during the overall and upright periods in both groups (p = 0.664 and p = 0.300, respectively), GERD patients with SD experienced a significant increase in the longest reflux time compared with those without SD during the recumbent period (3.40 ± 1.76 vs. 2.93 ± 1.29, p < 0.001). Moreover, AET was significantly greater in GERD patients with SD than without SD in the recumbent period (3.08 ± 1.16 vs. 2.62 ± 1.12, p < 0.001) (Table 7).
3.6 Comparison of the autonomic functions between the two groups
Gastroesophageal reflux disease patients with SD had a higher LF/HF ratio than in patients without SD (1.19 ± 0.24 vs. 0.89 ± 0.12, p < 0.001), and had a lower HF/LF ratio than in patients without SD (0.88 ± 0.19 vs. 1.15 ± 0.16, p < 0.001) (Table 8).
3.7 Correlations between Pittsburgh sleep quality index and GERD impact scale, anxiety score, depression score, and autonomic functions
Pittsburgh Sleep Quality Index was positively correlated with the GIS score (r = 0.725, p < 0.001), HAMA score (r = 0.680, p < 0.001), HAMD score (r = 0.323, p < 0.001), and LF/HF (r = 0.793, p < 0.001) in GERD patients. However, PSQI was negatively correlated with HF/LF (r = −0.770, p < 0.001) in GERD patients (Figures 2A–E).
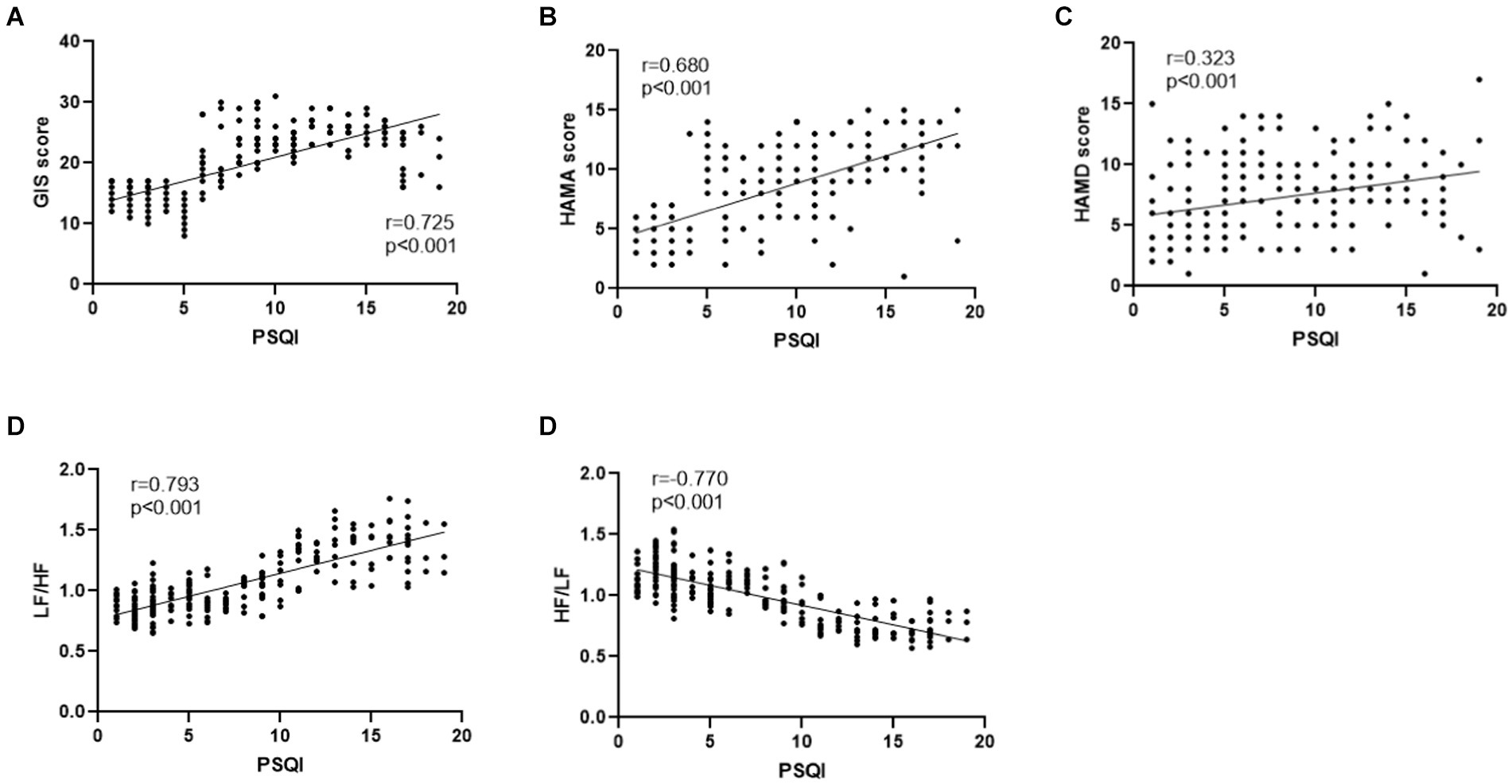
Figure 2. (A) PSQI was positively correlated with GIS in GERD patients (r = 0.725, p < 0.001). (B) PSQI was positively correlated with HAMA score in GERD patients (r = 0.680, p < 0.001). (C) PSQI was positively correlated with HAMD score in GERD patients (r = 0.323, p < 0.001). (D) PSQI was positively correlated with LF/HF in GERD patients (r = 0.793, p < 0.001). (E) PSQI was negatively correlated with HF/LF in GERD patients (r = −0.770, p < 0.001).
4 Discussion
This study revealed the effects of SD on reflux symptoms in GERD patients and explored the underlying autonomic mechanisms through spectral analysis of heart rate variability. Our data showed that GERD patients with SD experience a higher incidence of esophageal symptoms (especially heartburn, acid regurgitation and hoarseness) and nocturnal acid reflux than those without SD, with decreased quality of life and increased anxiety and depression. Notably, we found that GERD patients with SD had a higher incidence of erosive esophagitis with more severe damage to the esophageal mucosa. Meanwhile, SD increased sympathetic activity while suppressing parasympathetic or vagal activity. Pearson correlation analysis showed that SD was positively correlated with GIS scores, HAMA scores, HAMD scores, and sympathetic activity and negatively correlated with parasympathetic or vagal activity. Notably, subgroup analysis on patients with increased PSQI but with normal HAMA and HAMD was also conducted. We found that GERD patients with SD and normal HAMA/ HAMD also had a higher incidence of erosive esophagitis with more severe damage to the esophageal mucosa. Meanwhile, GERD patients with SD and normal HAMA/HAMD also had increased sympathetic activity while decreased parasympathetic or vagal activity, which bring valuable information on the influence of sleep on GERD.
The sample size can mainly provide medium-to-large effect sizes, and we found that the sample size of this study is sufficient to demonstrate statistical differences although the statistical power is not very high. The association between SD and GERD has long been reported in the literature (26). A large cross-sectional study documented that heartburn and regurgitation were almost twice as likely in patients with SD as in those without SD (27). In an Australian community-based study where 1,612 men completed a GERD symptom questionnaire, it was found that GERD symptom severity was significantly positively associated with self-reported poor quality of sleep (28). This association was further supported by a recent study investigating patients with various gastrointestinal disorders in a tertiary gastrointestinal clinic (29). Meanwhile, PSQI is widely acknowledged as a major tool for assessing sleep quality. We found that PSQI scores in GERD patients with SD were positively correlated with the severity of GERD symptoms. SD is widely thought to be a transdiagnostic symptom for many mental disorders, being most closely related to depression and anxiety (30, 31). SD was likewise found to influence anxiety and depressive states and positively correlate with those states in patients in our study.
Dickman et al. used questionnaires to evaluate SD in GERD patients and found that erosive reflux disease subjects woke up with reflux symptoms more frequently than those with non-erosive reflux disease (32). A Japanese study found that the rate of SD in patients with GERD was about one-third and that patients with EE had significantly shorter sleep times (33), given that nocturnal acid reflux might interfere with normal sleep by inducing conscious awakening during sleep. Recumbent acid reflux is associated with GERD complications such as increased EE (34). Our study found that EE was more frequent and mucosal damage was more severe in GERD patients with SD, consistent with the literature (32). Previous studies substantiated that GERD patients with SD experience reduced esophageal mechanosensitivity in generating distension-induced secondary peristalsis and a reduced successful peristaltic response (35). However, we did not find a significant correlation between SD, esophageal sphincter pressure, and esophageal motility in GERD patients. Interestingly, a Swedish study found that patients with persistent nocturnal GERD symptoms experienced a significantly higher prevalence of daytime sleepiness than those without nocturnal GERD symptoms. Moreover, benzodiazepines could affect the basal lower esophageal sphincter pressure and transient lower esophageal sphincter relaxation rate (36).
It has been established that GERD can affect the sleep–wake cycle and lead to SD. Conversely, SD has a strong impact on GERD. Therefore, sleep and GERD are bidirectionally associated (37). A previous study reported that feedback loops in the gut-brain axis could influence the regulation of circadian rhythms via mechanisms, including immune activation and intestinal endocrine signaling (38). 24 h-MII-pH showed an association between impaired sleep quality and nocturnal acid reflux events and longer reflux duration, consistent with the findings of Dickman et al. (32). This phenomenon may be attributed to decreased esophageal peristalsis and prolonged esophageal acid clearance at night. Accordingly, GERD patients have nocturnal reflux symptoms resulting in an increased risk of SD (39). A study reported that SD increased esophageal acid exposure in GERD patients, resulting in abnormal pH tests in nearly half of healthy individuals (40). Recent studies have found that the left lateral position significantly reduced the duration of nocturnal esophageal acid exposure time and accelerated esophageal acid clearance compared to the supine and right lateral positions (41). Meanwhile, the position of the head during sleep may also cause heartburn and affect sleep quality. One study investigated the effect of head position on reflux symptoms and sleep quality by gradually elevating the head over 7 days, and the results indicated that elevating the head reduced acid exposure and acid clearance time of the esophagus in the recumbent period (42). Meanwhile, our previous study (43) found that SD is associated with worse constipation related symptoms, and impaired autonomic function in functional constipation patients.
Motor regulation of the gastrointestinal tract is influenced by the enteric nervous system located within the intestinal wall and combined with extrinsic autonomic innervation. The vagus nerve has a major influence on esophageal peristalsis and LES function. Moreover, sympathetic excitation can reportedly stimulate the esophageal sphincter tone and reduce motility (44). HRV provides a non-invasive method of assessing autonomic function (25). A previous study found that esophageal acid exposure is usually associated with decreased autonomic tone (45). Meanwhile, Wang et al. (46) found that poor sleep resulted in higher LF and a lower HF/LF ratio, reflecting increased sympathetic and decreased parasympathetic activity. Notably, in a previous study, we documented that the imbalance between sympathetic and parasympathetic nerves plays an important role in refractory GERD (23). The current study substantiated that SD affects the balance between parasympathetic and sympathetic, thus significantly decreasing parasympathetic activity.
5 Limitations
Our study had some limitations that need to be addressed. First, although patients with comorbid psychiatric disorders were excluded, it is well-established that there is a definite relationship between sleep problems and mental status, which may affect our results to a certain extent. Besides, we defined SD based on the results of the PSQI questionnaire but did not assess objective sleep parameters, such as polysomnography, which may also affect our results. Finally, participants in this study underwent 24 h-MII-pH, which proved to be less sensitive and led to physical discomfort compared to wireless pH monitoring (47).
6 Conclusion
In summary, this study investigated the potential relationship between SD and GERD symptoms. We found that GERD patients with SD had a higher incidence of erosive esophagitis with more severe damage to the esophageal mucosa. Meanwhile, GERD patients with SD also had increased sympathetic activity while decreased parasympathetic or vagal activity, which indicated that autonomic function may be a potential pathophysiological mechanism.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of USTC. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing. LX: Writing – original draft, Writing – review & editing. WQ: Writing – review & editing. JD: Writing – review & editing. BW: Writing – review & editing. JT: Writing – review & editing. XF: Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank all participants in this research.
Conflict of interest
YH, LX, WQ, JD, and XF were employed by China RongTong Medical Healthcare Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katz, PO, Dunbar, KB, Schnoll-Sussman, FH, Greer, KB, Yadlapati, R, and Spechler, SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. (2022) 117:27–56. doi: 10.14309/ajg.0000000000001538
2. Eusebi, LH, Ratnakumaran, R, Yuan, Y, Solaymani-Dodaran, M, Bazzoli, F, and Ford, AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. (2018) 67:430–40. doi: 10.1136/gutjnl-2016-313589
3. Niu, XP, Yu, BP, Wang, YD, Han, Z, Liu, SF, He, CY, et al. Risk factors for proton pump inhibitor refractoriness in Chinese patients with non-erosive reflux disease. World J Gastroenterol. (2013) 19:3124–9. doi: 10.3748/wjg.v19.i20.3124
4. Schey, R, Dickman, R, Parthasarathy, S, Quan, SF, Wendel, C, Merchant, J, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology. (2007) 133:1787–95. doi: 10.1053/j.gastro.2007.09.039
5. Hyun, MK, Baek, Y, and Lee, S. Association between digestive symptoms and sleep disturbance: a cross-sectional community-based study. BMC Gastroenterol. (2019) 19:19. doi: 10.1186/s12876-019-0945-9
6. Martin, SE, Wraith, PK, Deary, IJ, and Douglas, NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. (1997) 155:1596–601. doi: 10.1164/ajrccm.155.5.9154863
7. Orr, WC, Fass, R, Sundaram, SS, and Scheimann, AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. (2020) 5:616–24. doi: 10.1016/S2468-1253(19)30412-1
8. St-Onge, MP, Grandner, MA, Brown, D, Conroy, MB, Jean-Louis, G, Coons, M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health a scientific statement from the American Heart Association. Circulation. (2016) 134:E367–86. doi: 10.1161/CIR.0000000000000444
9. Jiang, Y, Tang, YR, Xie, C, Yu, T, Xiong, WJ, and Lin, L. Influence of sleep disorders on somatic symptoms, mental health, and quality of life in patients with chronic constipation. Medicine. (2017) 96:e6093. doi: 10.1097/MD.0000000000006093
10. Gravina, AG, Pellegrino, R, Romeo, M, Ventriglia, L, Scognamiglio, F, Tuccillo, C, et al. The use of bicarbonate-sulphate-calcium-magnesium and sodium-low drinkable water improves functional gastrointestinal symptoms in patients with non-alcoholic fatty liver disease: a prospective study. Clin Nutr Espen. (2023) 57:281–7. doi: 10.1016/j.clnesp.2023.07.008
11. Ag, G, Romeo, M, Pellegrino, R, Tuccillo, C, Federico, A, and Loguercio, C. Just drink a glass of water? Effects of bicarbonate-sulfate-calcium-magnesium water on the gut-liver Axis. Front Pharmacol. (2022) 13:869446. doi: 10.3389/fphar.2022.869446
12. Gyawali, CP, Yadlapati, R, Fass, R, Katzka, D, Pandolfino, J, Savarino, F, et al. Updates to the modern diagnosis of Gerd: Lyon consensus 2.0. Gut. (2024) 73:361–71. doi: 10.1136/gutjnl-2023-330616
13. Ju, G, Iy, Y, Lee, S, and Kim, N. Relationships between sleep disturbances and gastroesophageal reflux disease in Asian sleep clinic referrals. J Psychosom Res. (2013) 75:551–5. doi: 10.1016/j.jpsychores.2013.10.004
14. Lei, WY, Chang, WC, Wong, MW, Hung, JS, Wen, SH, Yi, CH, et al. Sleep disturbance and its association with gastrointestinal symptoms/diseases and psychological comorbidity. Digestion. (2019) 99:205–12. doi: 10.1159/000490941
15. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index - a new instrument for psychiatric practice and research [J]. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
16. Huang, Y, and Zhu, ML. Increased global Psqi score is associated with depressive symptoms in an adult population from the United States. Nat Sci Sleep. (2020) 12:487–95. doi: 10.2147/NSS.S256625
17. Jo, SY, Kim, N, Lim, JH, Shin, CM, Park, YS, Lee, DH, et al. Comparison of gastroesophageal reflux disease symptoms and proton pump inhibitor response using gastroesophageal reflux disease impact scale questionnaire. J Neurogastroenterol Motility. (2013) 19:61–9. doi: 10.5056/jnm.2013.19.1.61
18. University of Mostar Clinical Hospital, Department of Gastroenterology and Hepatology, Mostar, Bosnia and HerzegovinaBabic, E, Bevanda, M, Karin, M, Volaric, M, Bogut, A, et al. Anxiety, depression and personality types in patients with inflammatory bowel disease: comparisons with peptic ulcer and the general population. Psychiatr Danub. (2021) 33:48–56. doi: 10.24869/psyd.2021.48
19. Liu, J, Lv, C, Wu, D, Wang, Y, Sun, C, Cheng, C, et al. Subjective taste and smell changes in conjunction with anxiety and depression are associated with symptoms in patients with functional constipation and irritable bowel syndrome. Gastroenterol Res Pract. (2021) 2021:1–9. doi: 10.1155/2021/5491188
20. Hamilton, M. The assessment of anxiety-states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
21. Hamilton, M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
22. Lundell, LR, Dent, J, Bennett, JR, Blum, AL, Armstrong, D, Galmiche, JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. (1999) 45:172–80. doi: 10.1136/gut.45.2.172
23. Yu, Y, Wei, R, Liu, Z, Xu, J, Xu, C, and Jdz, C. Ameliorating effects of transcutaneous electrical Acustimulation combined with deep breathing training on refractory gastroesophageal reflux disease mediated via the autonomic pathway. Neuromodulation. (2019) 22:751–7. doi: 10.1111/ner.13021
24. Liu, J, Wang, W, Wang, Y, Wu, D, Sun, C, Lv, C, et al. Subjective changes of taste and smell in conjunction with anxiety and depression are associated with symptoms in Globus patients without evidence of pathologic acid reflux. J Clin Gastroenterol. (2022) 56:505–11. doi: 10.1097/MCG.0000000000001603
25. Crooks, E, Hansen, DA, Satterfield, BC, Layton, ME, and van Dongen, HPA. Cardiac autonomic activity during sleep deprivation with and without caffeine administration. Physiol Behav. (2019) 210:112643. doi: 10.1016/j.physbeh.2019.112643
26. Jansson, C, Nordenstedt, H, Wallander, MA, Johansson, S, Johansson, R, Hveem, K, et al. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol. (2009) 7:960–5. doi: 10.1016/j.cgh.2009.03.007
27. Cremonini, F, Camilleri, M, Zinsmeister, AR, Herrick, LM, Beebe, T, and Talley, NJ. Sleep disturbances are linked to both upper and lower gastrointestinal symptoms in the general population. Neurogastroenterol Motil. (2009) 21:128–35. doi: 10.1111/j.1365-2982.2008.01181.x
28. Niu, XP, Yu, BP, Wang, YD, Han, Z, Liu, SF, He, CY, et al. The association between gastroesophageal reflux disease with sleep quality, depression, and anxiety in a cohort study of Australian men. J Gastroenterol Hepatol. (2017) 32:1170–7. doi: 10.1111/jgh.13650
29. Ballou, S, Alhassan, E, Hon, E, Lembo, C, Rangan, V, Singh, P, et al. Sleep disturbances are commonly reported among patients presenting to a gastroenterology clinic. Dig Dis Sci. (2018) 63:2983–91. doi: 10.1007/s10620-018-5237-7
30. Nguyen, VV, Zainal, NH, and Newman, MG. Why sleep is key: poor sleep quality is a mechanism for the bidirectional relationship between major depressive disorder and generalized anxiety disorder across 18 years. J Anxiety Disord. (2022) 90:102601. doi: 10.1016/j.janxdis.2022.102601
31. Riemann, D, Krone, LB, Wulff, K, and Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology. (2020) 45:74–89. doi: 10.1038/s41386-019-0411-y
32. Dickman, R, Green, C, Fass, SS, Quan, SF, Dekel, R, Risner-Adler, S, et al. Relationships between sleep quality and pH monitoring findings in persons with gastroesophageal reflux disease. J Clin Sleep Med. (2007) 3:505–13. doi: 10.5664/jcsm.26915
33. Okuyama, M, Takaishi, O, Nakahara, K, Iwakura, N, Hasegawa, T, Oyama, M, et al. Associations among gastroesophageal reflux disease, psychological stress, and sleep disturbances in Japanese adults. Scand J Gastroenterol. (2017) 52:44–9. doi: 10.1080/00365521.2016.1224383
34. Hung, JS, Lei, WY, Yi, CH, Liu, TT, and Chen, CL. Association between nocturnal acid reflux and sleep disturbance in patients with gastroesophageal reflux disease. Am J Med Sci. (2016) 352:141–5. doi: 10.1016/j.amjms.2016.05.017
35. Yi, CH, Lei, WY, Hung, JS, Liu, TT, Orr, WC, and Chen, CL. Relevance of sleep disturbance to the integrity and characteristics of secondary peristalsis in patients with gastroesophageal reflux disease. Scand J Gastroenterol. (2017) 52:136–42. doi: 10.1080/00365521.2016.1235225
36. Hägg, SA, Emilsson, OI, Franklin, K, Janson, C, and Lindberg, E. Nocturnal gastroesophageal reflux increases the risk of daytime sleepiness in women. Sleep Med. (2019) 53:94–100. doi: 10.1016/j.sleep.2018.08.036
37. Iwakura, N, Fujiwara, Y, Shiba, M, Ochi, M, Fukuda, T, Tanigawa, T, et al. Characteristics of sleep disturbances in patients with gastroesophageal reflux disease. Intern Med. (2016) 55:1511–7. doi: 10.2169/internalmedicine.55.5454
38. Morais, LH, Schreiber, HL, and Mazmanian, SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
39. Fujiwara, Y, Arakawa, T, and Fass, R. Gastroesophageal reflux disease and sleep disturbances. J Gastroenterol. (2012) 47:760–9. doi: 10.1007/s00535-012-0601-4
40. Yamasaki, T, Quan, SF, and Fass, R. The effect of sleep deficiency on esophageal acid exposure of healthy controls and patients with gastroesophageal reflux disease. Neurogastroenterol Motil. (2019) 31:e13705. doi: 10.1111/nmo.13705
41. Hägg, SA, Emilsson, ÖI, Franklin, K, Janson, C, and Lindberg, E. Associations between sleep position and nocturnal gastroesophageal reflux: a study using concurrent monitoring of sleep position and esophageal pH and impedance. Am J Gastroenterol. (2022) 117:346–51. doi: 10.14309/ajg.0000000000001588
42. Khan, BA, Sodhi, JS, Zargar, SA, Javid, G, Yattoo, GN, Shah, A, et al. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol. (2012) 27:1078–82. doi: 10.1111/j.1440-1746.2011.06968.x
43. Liu, J, Wang, W, Tian, J, Lv, C, Fu, Y, Fass, R, et al. Sleep deficiency is associated with exacerbation of symptoms and impairment of anorectal and autonomic functions in patients with functional constipation. Front Neurosci. (2022) 16:912442. doi: 10.3389/fnins.2022.912442
44. Ramadi, KB, Srinivasan, SS, and Traverso, G. Electroceuticals in the gastrointestinal tract. Trends Pharmacol Sci. (2020) 41:960–76. doi: 10.1016/j.tips.2020.09.014
45. Milovanovic, B, Filipovic, B, Mutavdzin, S, Zdravkovic, M, Gligorijevic, T, Paunovic, J, et al. Cardiac autonomic dysfunction in patients with gastroesophageal reflux disease. World J Gastroenterol. (2015) 21:6982–9. doi: 10.3748/wjg.v21.i22.6982
46. Hägg, SA, Emilsson, ÖI, Franklin, K, Janson, C, and Lindberg, E. Poor sleep quality associated with high risk of ventricular tachycardia after acute myocardial infarction. Nat Sci Sleep. (2019) 11:281–9. doi: 10.2147/NSS.S222359
Keywords: gastroesophageal reflux disease, sleep disturbance, autonomic dysfunction, anxiety, depression
Citation: Huang Y, Liu J, Xu L, Qi W, Dai J, Wang B, Tian J, Fu X and Yu Y (2024) Exacerbation of symptoms, nocturnal acid reflux, and impaired autonomic function are associated with sleep disturbance in gastroesophageal reflux disease patients. Front. Med. 11:1438698. doi: 10.3389/fmed.2024.1438698
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Georgiana Gîlcă-Blanariu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaRaffaele Pellegrino, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Huang, Xu, Qi, Dai, Liu, Wang, Tian, Fu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, ZmVpeGlsakAxNjMuY29t; Xin Fu, MTUxNTYyNjkwMjFAMTYzLmNvbQ==; Yue Yu, eXV5dWVtZEAxNjMuY29t
†These authors have contributed equally to this work
 Yizhou Huang
Yizhou Huang Jie Liu
Jie Liu Linsheng Xu1†
Linsheng Xu1† Yue Yu
Yue Yu