- Department of Medical Oncology, Universitair Ziekenhuis Brussel (UZ Brussel) and Vrije Universiteit Brussel (VUB), Brussels, Belgium
Clonal MAPK-pathway activating mutations in the MAP2K1 (MEK1) gene are present in approximately 9% of cutaneous melanomas. These mutations are divided into three classes: RAF-dependent, RAF-regulated, RAF-independent. Cell lines with class-2 or RAF-regulated MAP2K1-mutations are most responsive to MEK-inhibitors. We present a patient with a class-2 MAP2K1-mutant stage IV-M1d melanoma who experienced extra- and intracranial progressive disease following treatment with immune-checkpoint inhibitors. The patient was treated with the MEK-inhibitor trametinib (2 mg OD) to which a low-dose of dabrafenib (50 mg BID) was added to mitigate skin-toxicity. Following documentation of a partial response (PR), she developed one new, and increase in volume of two pre-existing brain metastases that were treated with stereotactic radiosurgery (SRS) while continuing trametinib and dabrafenib. Thereafter, a deep partial radiologic and metabolic response both extra-and intra-cranially was achieved and is ongoing 88 weeks after initiating trametinib. She experienced no grade > 2 adverse events. Focal post-radiation necrosis at site of an irradiated brain metastasis developed 9 months after SRS and is successfully being treated with low-dose bevacizumab. This is the first published case of a durable intracranial disease control with the MEK-inhibitor trametinib of a stage IV-M1d class-2 MAP2K1-mutant melanoma. This illustrates the utility of NGS profiles that include class-1/2 MAP2K1-mutations in patients with melanoma and other malignancies to provide valuable information on a potentially active individualized treatment option. A prospective clinical trial that further evaluates the efficacy of MEK-inhibitor therapies in MAP2K1-mutated tumors is justified.
1 Introduction
Activating mutations of the mitogen-activated protein kinase (MAPK)-pathway drive proliferation, invasion and metastasis of melanoma (1, 2). Mutations in BRAF (mostly V600E/K), NRAS (mostly Q61R/K/L) and NF1 occur in approximately 50, 30, and 25% of cutaneous melanomas, respectively, and are mutually exclusive (3). Patients with triple wild-type melanoma may carry other oncogenic driver mutations (e.g., KIT, MAP2K1), which mostly cause direct or indirect activation of the MAPK-pathway (4).
Regardless of mutational status, improved overall survival (OS) can be achieved in advanced melanoma with immune checkpoint blockade (ICB) (5, 6). Additionally, targeted therapy with BRAF-/MEK-inhibitors increases survival in BRAFV600mutant melanoma (7–10). To date, no targeted therapy improves OS in BRAFV600wild-type melanoma. Preclinical data indicate that MEK-inhibition can be effective in triple wild-type melanoma (11). Arm B of the phase II clinical trial TraMel-WT evaluated trametinib (competitive MEK1/2-inhibitor) combined with low-dose dabrafenib (to mitigate MEK-inhibitor-induced skin-toxicity) in 24 patients with pretreated BRAFV600/NRASQ61wild-type melanoma (NCT04059224). The overall response rate was 29%, median progression-free survival (mPFS) and mOS were 13.3 and 54.3 weeks, respectively (12).
MAP2K1, also known as MAPK/ERK-kinase-1 (MEK1), is a serine/threonine/tyrosine kinase that is activated by upstream RAF kinases. Clonal mutations in MAP2K1 are present in approximately 9% of cutaneous melanomas and activate the MAPK-pathway (4).
In this report, we present in more detail the case of a patient with a triple wild-type, class-2 MAP2K1-mutant AJCC stage IV-M1d melanoma, treated in the TraMel-WT trial, who had a deep and durable response to MEK-inhibitor treatment with excellent treatment tolerance (12). The patient provided written informed consent for publication.
2 Case description
A 40-year-old Caucasian female was diagnosed with a pT4bN1aM0 BRAFV600wild-type cutaneous nodular melanoma on the left shoulder. During adjuvant pembrolizumab treatment, a solitary subcutaneous metastasis was resected and irradiated. One year after diagnosis, [18F]-fluorodeoxyglucose-positron emission tomography computed tomography ([18F]FDG-PET/CT) revealed supra- and infradiaphragmatic lymph node and bone metastases. Treatment with ipilimumab/nivolumab was initiated. She developed immune-related colitis after two cycles and progressed with seven new brain metastases (AJCC stage IV-1Md) (Figure 1A). Comprehensive genomic profiling through next generation sequencing (NGS) revealed a clonal class-2 RAF-regulated MAP2K1 mutation (Q58_E62del, allele frequency 37%, in-frame-deletion; the complete NGS results can be found in Table 1). The patient initiated trametinib 2 mg once and dabrafenib 50 mg twice daily in the TraMel-WT trial in September 2022. Low-dose dabrafenib was associated upfront to mitigate skin-toxicity (12, 13). She did not receive any local intracranial therapy at this time for the brain metastases. After 5 weeks, brain MRI revealed no new lesions and baseline lesions were considered stable according to RANO-BM criteria (14). Response assessment in week 7 with [18F]FDG-PET/CT showed complete metabolic response of extracranial lesions (Figure 1B). After 14 weeks of treatment, brain MRI indicated complete regression of five brain metastases. However, there was one new lesion (6 mm longest diameter) and an increased diameter of two preexisting metastases (Figure 1C). At the patient’s request, close surveillance rather than immediate radiotherapy was applied while continuing trametinib and dabrafenib. Following confirmed progression in these three lesions nearly 2 months later, they were treated with stereotactic radiosurgery (1 × 20 Gy) (Figure 1D). A follow-up MRI (week 32) showed decrease in all three lesions and no new lesions. After 66 weeks of treatment, the patient remained in complete metabolic remission extracranially (Figure 1E). The week-59 brain MRI confirmed a continuing decrease in diameter of one of the irradiated lesions, and complete regression of the others (Figure 1E).
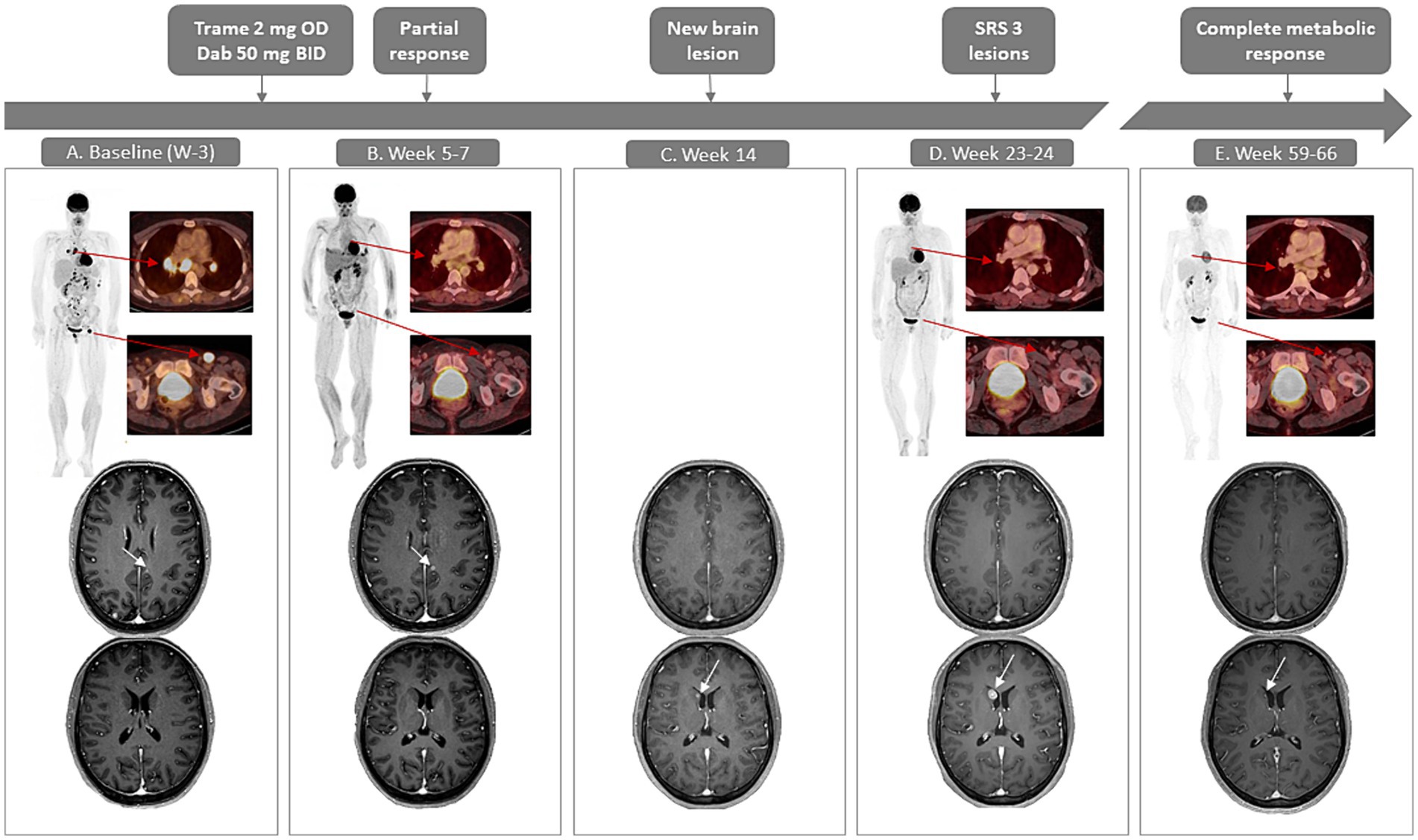
Figure 1. Case presentation with radiological evaluation with [18F]FDG-PET/CT (maximum intensity projections and fused axial images) and contrast-enhanced T1-weighted MRI of the brain. (A) Baseline, week minus 3 shows hypermetabolic supra-and infradiaphragmatic lesions on PET/CT. In the brain, a lesion can be noted parafalcine in the left (L) occipital lobe (4 mm; upper image) and no lesion is noted in the right (R) nucleus caudatus (lower image). (B) PET/CT in week 7 shows a significant decrease in metabolic activity as well as a decrease in size of the lesions (unconfirmed partial response). Brain MRI at week 5 shows a slight increase of the L occipital parafalcine lesion (7 mm, upper image), however there are no new lesions and considering a lead time bias of 3 weeks the treatment is continued. (C) On brain MRI in week 14, the parafalcine L occipital lesion has disappeared (upper image), a new lesion in the nucleus caudatus has emerged (lower image). (D) PET/CT in week 24 shows a metabolic remission extracranially. MRI shows an increased size of the lesion on the nucleus caudatus (lower image); SRS is performed for this lesion and 2 other progressive lesions (not shown). (E) PET-CT in week 66 shows a sustained complete metabolic remission, MRI in week 59 shows a shrinkage of the lesion in the nucleus caudatus (lower image), no other intracranial lesions are detectable on MRI. BID, twice a day; dab, dabrafenib; OD, once daily; SRS, stereotactic radiosurgery; trame, trametinib.
Treatment was well tolerated. She intermittently reported low-grade nausea, pruritus, epigastric pain and fatigue, but did not experience skin-toxicity. No dose reductions of trametinib or dabrafenib were required.
While extra- and intracranial responses persisted, the week-59 brain MRI revealed signs of focal post-radiation necrosis of the brain (fRNB) approximately 8 months after SRS for the lesion in the paramedian frontal right region (Figure 2A). A brain MRI, repeated 6 weeks later, showed increase in contrast-enhancement and size of the region (Figure 2B), which was associated with headaches. In order to differentiate between fRNB and tumor progression, an [18F]FDG-PET/CT of the brain was performed and demonstrated focal hypometabolism at the region of the gadolinium-contrast-enhancement, supporting the diagnosis of fRNB. A low-dose bevacizumab treatment regimen (previously established as effective treatment for fRNB) was initiated to treat the perilesional edema (15) (Figure 2C). After two doses of bevacizumab (8 weeks) the headache subsided and MRI showed an important decrease in contrast-enhancement and edema (Figure 2D).
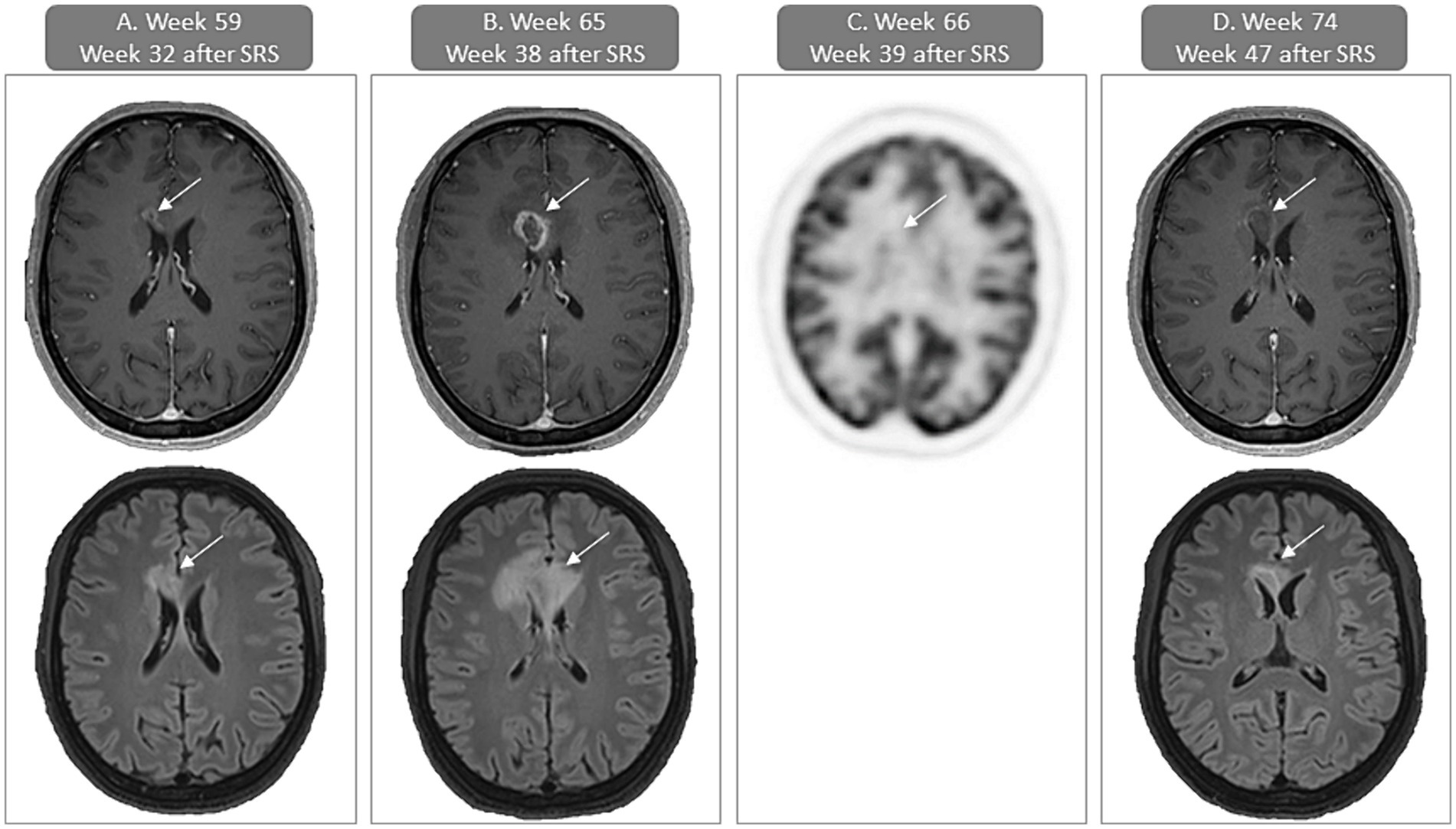
Figure 2. Case presentation of focal post-radiation necrosis of the brain (fRNB) with gadolinium contrast-enhanced T1-weighted and FLAIR MRI of the brain and [18F]FDG-PET/CT (FDG-uptake images). (A) First sign of fRNB (week 59 or 32 weeks after SRS) with contrast-enhancement in the paramedian frontal right region on the T1-weighted axial image (upper) and surrounding edema on the FLAIR axial image (lower). (B) In week 65 or 38 weeks after SRS there is an increase of contrast-enhancement in the paramedian frontal right region on the T1-weighted axial image (upper) and surrounding edema on the FLAIR axial image (lower). (C) PET-CT performed 1 week later (week 66 or 39 weeks after SRS) shows no increased uptake of [18F]FDG, supporting the diagnosis of fRNB. (D) In week 74 or 8 weeks after bevacizumab initiation there is a decrease of contrast-enhancement in the paramedian frontal right region on the T1-weighted axial image (upper) and surrounding edema on the FLAIR axial image (lower). SRS, stereotactic radiosurgery.
At the moment of writing, after 88 weeks of treatment, the patient continues to have a radiological response and remains on treatment (trametinib with low-dose dabrafenib and bevacizumab), while maintaining an active lifestyle.
3 Discussion
No approved therapies have shown to improve OS in patients with BRAFV600wild-type melanoma who progress on ICB. In melanoma with non-NRASQ61/BRAFV600, MAPK-pathway activating mutations, MEK-inhibitor therapy has shown anti-tumor activity (12, 16, 17). In vitro trametinib has activity in triple wild-type melanoma cell lines (18). In MAP2K1-mutant melanoma, there is one case report of a partial response to trametinib in a patient with stage IV-M1c melanoma, but resistance developed 3 months later and dose reduction was needed because of trametinib-induced skin-toxicity (Table 2) (19). To the best of our knowledge, this is the first case report of stage IV-M1d MAP2K1-mutant melanoma with a durable extracranial complete response and intracranial disease control with trametinib and low-dose dabrafenib. Our patient initially had unconfirmed PR, thereafter oligo-progression in the brain, which was managed locally with SRS while continuing trametinib and dabrafenib. Eventually, a deep and durable disease control, both extra- and intracranially, was achieved.
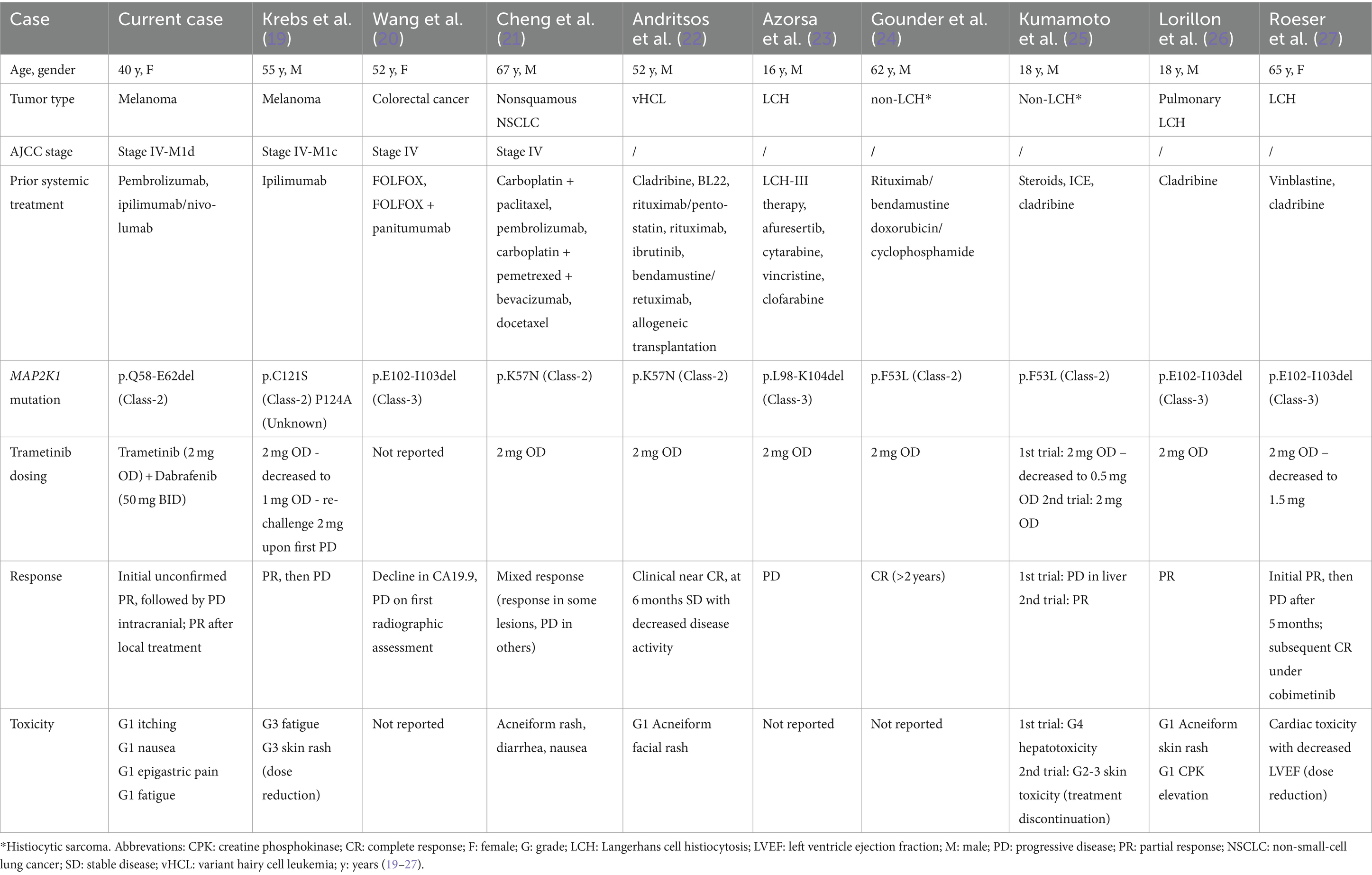
Table 2. Overview of case reports describing patients with an MAP2K1-mutation treated with trametinib.
The prognosis and natural evolution of AJCC stage IV-M1d, BRAF-wild-type melanoma with active brain metastasis progressing on ICB is very poor. In this patient, the brain metastases were successfully controlled: the majority disappeared with trametinib and no new lesions emerged following SRS, maintaining disease control for more than 20 months at moment of writing.
MAP2K1 mutations can be classified into three groups. Class-1, RAF-dependent MAP2K1-mutations are dependent on upstream RAF-activation to induce high levels of activated phosphorylated ERK (pERK), likewise the pathway is self-limited by feedback inhibition of RAS or RAF. Class-2, RAF-regulated MAP2K1-mutations are partially dependent on RAF-activation but have varying amounts of RAF-independent activity. Finally, class-3, RAF-independent mutations induce high levels of pERK without upstream RAF-activation and are not susceptible to feedback inhibition (11). Class-1/2 MAP2K1-mutations are usually associated with upstream mutations in RAS, RAF, or NF1, while class-3 MAP2K1-mutations do not coexist with other mutations. MAP2K1-mutations have been successfully targeted in vitro with MEK-inhibitors that preferably bind to the inactive, unphosphorylated form of the MEK1-enzyme. Both class-1 and -2 MAP2K1-mutations are (partially) RAF-dependent, suggesting a significant inactive fraction of the mutant MEK1-enzymes and thus sensitivity to trametinib. Class-3 mutated proteins on the other hand are resistant to allosteric MEK-inhibitors due to their permanently active conformation (11). The MAP2K1-mutation (Q58-E62del) found in our patient, results in an in-frame deletion and is classified as class-2 RAF-regulated MAP2K1-mutation (28). In accordance with these preclinical results, the melanoma lesions in our case responded to trametinib.
A recent retrospective review of the AACR genie, a clinico-genomic database showed that co-occurring MAPK-pathway mutations (e.g., NRAS, NF1) are significantly more likely with class-1 MAP2K1-mutations (82.3%) compared to class-2 (30.9%) and class-3 (10.6%) in any tumor type. This highlights that additional activation of the MAPK-pathway is needed to induce malignant cell growth in class-1 RAF-dependent MAP2K1-mutant tumors, that this is to a lesser extent necessary in class-2 MAP2K1-mutant and not necessary in class-3 MAP2K1-mutant tumors. Class-2/3 MAP2K1-mutations can therefore act as a driver mutation. Additionally, patients receiving MAPK-inhibitors, with class-2 MAP2K1-mutations derived the most benefit, translating to a longer PFS (4.0 months) and duration of response (23.8 months) (29).
Several other cases of successful MEK-inhibitor use in MAP2K1-mutant malignancies have been documented, primarily non-Langerhans cell histiocytosis and hairy cell leukemia (Table 2) (30). Responses were observed in cases with class-2 and -3 MAP2K1-mutations, however in the class-3 cases, responses were mostly short-lived. These data show the importance of precision oncology and systematic genomic analysis through NGS in both triple wild-type melanoma and other malignancies in which no classical driver mutations have been identified to expand possible treatment options.
Another point of interest is that in five of the seven cases reporting adverse events, rash or acneiform dermatitis were reported (Table 2). In one case this led to treatment discontinuation, in another trametinib dosing was reduced (19, 25). In our patient trametinib-induced skin-toxicity was successfully prevented by adding low-dose BRAF-inhibitor, as previously reported by our group, seemingly without compromising MEK-inhibitor activity (12, 13). Consequently, the patient has an excellent and durable tolerance of full dose trametinib.
Of note, the patient developed fRNB 8 months after SRS, a late side effect of SRS with increasing frequency as more effective therapy for brain metastases becomes available. [18F]FDG-PET/CT helps distinguish fRNB from tumor progression, with fRNB showing decreased [18F]FDG uptake (hypometabolism) and tumor progression showing increased uptake (hypermetabolism) (15, 31). Our group recently reported a case series of successful fRNB treatment using low-dose bevacizumab (loading dose of 400 mg, followed by 100 mg q4w) (15). This regimen was effective and well tolerated alongside trametinib and dabrafenib in our patient.
4 Conclusion
We report the first case of durable intra- and extracranial response to trametinib, following local control with SRS of intracranial oligo-progression, in a patient with stage IV-M1d class-2 MAP2K1-mutant melanoma. Association of low-dose BRAF-inhibitor prevented MEK-inhibitor-induced skin-toxicity. Precision oncology using NGS data to screen for MAP2K1-mutations offers valuable treatment insights. A cross-tumor prospective trial is needed to evaluate the efficacy of MEK-inhibitors in MAP2K1-mutated tumors.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the dataset generated for this case report contains confidential patient information but can be made available upon request to interested researchers. Requests to access these datasets should be directed to ID, aXJpcy5kaXJ2ZW5AdXpicnVzc2VsLmJl.
Ethics statement
The studies involving humans were approved by Commissie Medische Ethiek Universitair Ziekenhuis Brussel O.G. 016; protocol code 2018/429; date of approval 19 December 2018. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ID: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. EC: Formal analysis, Writing – original draft. GA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Writing – review & editing. MV: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. BN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
Funding
The authors declare that financial support was received for the research of this article. Research funding from Novartis was received for the trial in which the patient was included (NCT04059224). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shain, AH, Yeh, I, Kovalyshyn, I, Sriharan, A, Talevich, E, Gagnon, A, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. (2015) 373:1926–36. doi: 10.1056/NEJMoa1502583
2. Pearson, G, Robinson, F, Beers Gibson, T, Xu, BE, Karandikar, M, Berman, K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. (2001) 22:153–83. doi: 10.1210/edrv.22.2.0428
3. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. (2015) 161:1681–96. doi: 10.1016/j.cell.2015.05.044
4. Newell, F, Johansson, PA, Wilmott, JS, Nones, K, Lakis, V, Pritchard, AL, et al. Comparative genomics provides etiologic and biological insight into melanoma subtypes. Cancer Discov. (2022) 12:2856–79. doi: 10.1158/2159-8290.CD-22-0603
5. Wolchok, JD, Chiarion-Sileni, V, Gonzalez, R, Rutkowski, P, Grob, JJ, Cowey, CL, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med. (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
6. Hodi, FS, O'Day, SJ, McDermott, DF, Weber, RW, Sosman, JA, Haanen, JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
7. Hauschild, A, Grob, JJ, Demidov, LV, Jouary, T, Gutzmer, R, Millward, M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. (2012) 380:358–65. doi: 10.1016/S0140-6736(12)60868-X
8. Chapman, PB, Robert, C, Larkin, J, Haanen, JB, Ribas, A, Hogg, D, et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol. (2017) 28:2581–7. doi: 10.1093/annonc/mdx339
9. Robert, C, Karaszewska, B, Schachter, J, Rutkowski, P, Mackiewicz, A, Stroiakovski, D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. (2015) 372:30–9. doi: 10.1056/NEJMoa1412690
10. Dummer, R, Flaherty, K, Robert, C, Arance, AM, Groot, JW, Garbe, C, et al. Five-year overall survival (OS) in COLUMBUS: a randomized phase 3 trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients (pts) with BRAF V600-mutant melanoma. J Clin Oncol. (2021) 39:9507. doi: 10.1200/JCO.2021.39.15_suppl.9507
11. Gao, Y, Chang, MT, McKay, D, Na, N, Zhou, B, Yaeger, R, et al. Allele-specific mechanisms of activation of MEK1 mutants determine their PropertiesAllele-specific activation mechanisms of MEK1 mutants. Cancer Discov. (2018) 8:648–61. doi: 10.1158/2159-8290.CD-17-1452
12. Awada, G, Dirven, I, Schwarze, JK, Tijtgat, J, Fasolino, G, Kockx, M, et al. Phase II clinical trial of Trametinib and low-dose Dabrafenib in advanced, previously treated BRAF V600/NRAS Q61 wild-type melanoma (TraMel-WT). JCO Precis Oncol. (2024) 8:e2300493. doi: 10.1200/PO.23.00493
13. Awada, G, Schwarze, JK, Tijtgat, J, Fasolino, G, Everaert, H, and Neyns, B. A phase 2 clinical trial of trametinib and low-dose dabrafenib in patients with advanced pretreated NRASQ61R/K/L mutant melanoma (TraMel-WT). Cancers. (2021) 13:2010. doi: 10.3390/cancers13092010
14. Lin, NU, Lee, EQ, Aoyama, H, Barani, IJ, Barboriak, DP, Baumert, BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. (2015) 16:e270–8. doi: 10.1016/S1470-2045(15)70057-4
15. Tijtgat, J, Calliauw, E, Dirven, I, Vounckx, M, Kamel, R, Vanbinst, AM, et al. Low-dose bevacizumab for the treatment of focal radiation necrosis of the brain (fRNB): a single-center case series. Cancers. (2023) 15:2560. doi: 10.3390/cancers15092560
16. Menzer, C, Menzies, AM, Carlino, MS, Reijers, I, Groen, EJ, Eigentler, T, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol. (2019) 37:3142–51. doi: 10.1200/JCO.19.00489
17. Nebhan, CA, Johnson, DB, Sullivan, RJ, Amaria, RN, Flaherty, KT, Sosman, JA, et al. Efficacy and safety of trametinib in non-V600 BRAF mutant melanoma: a phase II study. Oncologist. (2021) 26:731–e1498. doi: 10.1002/onco.13795
18. Ranzani, M, Alifrangis, C, Perna, D, Dutton-Regester, K, Pritchard, A, Wong, K, et al. BRAF/NRAS wild-type melanoma, NF1 status and sensitivity to trametinib. Pigment Cell Melanoma Res. (2015) 28:117–9. doi: 10.1111/pcmr.12316
19. Krebs, FS, Moura, B, Missiaglia, E, Aedo-Lopez, V, Michielin, O, Tsantoulis, P, et al. Response and resistance to Trametinib in MAP2K1-mutant triple-negative melanoma. Int J Mol Sci. (2023) 24:4520. doi: 10.3390/ijms24054520
20. Wang, C, Sandhu, J, and Fakih, M. A case of class 3 MEK1 mutated metastatic colorectal cancer with a non-durable tumor marker response to MEK and ERK inhibitors. J Gastrointest Oncol. (2019) 10:1140–3. doi: 10.21037/jgo.2019.08.02
21. Cheng, ML, Lee, JK, Kumar, R, Klein, H, Raskina, K, Schrock, AB, et al. Response to MEK inhibitor therapy in MAP2K1 (MEK1) K57N non-small-cell lung Cancer and genomic landscape of MAP2K1 mutations in non-small-cell lung Cancer. JCO Precis Oncol. (2022) 6:e2200382. doi: 10.1200/PO.22.00382
22. Andritsos, LA, Grieselhuber, NR, Anghelina, M, Rogers, KA, Roychowdhury, S, Reeser, JW, et al. Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma. (2018) 59:1008–11. doi: 10.1080/10428194.2017.1365853
23. Azorsa, DO, Lee, DW, Wai, DH, Bista, R, Patel, AR, Aleem, E, et al. Clinical resistance associated with a novel MAP2K1 mutation in a patient with Langerhans cell histiocytosis. Pediatr Blood Cancer. (2018) 65:e27237. doi: 10.1002/pbc.27237
24. Gounder, MM, Solit, DB, and Tap, WD. Trametinib in histiocytic sarcoma with an activating MAP2K1 (MEK1) mutation. N Engl J Med. (2018) 378:1945–7. doi: 10.1056/NEJMc1511490
25. Kumamoto, T, Aoki, Y, Sonoda, T, Yamanishi, M, Arakawa, A, Sugiyama, M, et al. A case of recurrent histiocytic sarcoma with MAP2K1 pathogenic variant treated with the MEK inhibitor trametinib. Int J Hematol. (2019) 109:228–32. doi: 10.1007/s12185-018-2553-9
26. Lorillon, G, Jouenne, F, Baroudjian, B, de Margerie-Mellon, C, Vercellino, L, Meignin, V, et al. Response to trametinib of a pulmonary Langerhans cell histiocytosis harboring a MAP2K1 deletion. Am J Respir Crit Care Med. (2018) 198:675–8. doi: 10.1164/rccm.201802-0275LE
27. Roeser, A, Jouenne, F, Vercellino, L, Calvani, J, Goldwirt, L, Lorillon, G, et al. Dramatic response after switching MEK inhibitors in a patient with refractory mixed Histiocytosis. J. Hematol. (2022) 11:185–9. doi: 10.14740/jh1030
28. Mizuno, S, Ikegami, M, Koyama, T, Sunami, K, Ogata, D, Kage, H, et al. High-throughput functional evaluation of MAP2K1 variants in cancer. Mol Cancer Ther. (2023) 22:227–39. doi: 10.1158/1535-7163.MCT-22-0302
29. Dankner, M, Wang, Y, Fazelzad, R, Johnson, B, Nebhan, CA, Dagogo-Jack, I, et al. Clinical activity of mitogen-activated protein kinase-targeted therapies in patients with non-V600 BRAF-mutant tumors. JCO Precis Oncol. (2022) 6:e2200107. doi: 10.1200/PO.22.00107
30. Aaroe, A, Kurzrock, R, Goyal, G, Goodman, AM, Patel, H, Ruan, G, et al. Successful treatment of non-Langerhans cell histiocytosis with the MEK inhibitor trametinib: a multicenter analysis. Blood Adv. (2023) 7:3984–92. doi: 10.1182/bloodadvances.2022009013
Keywords: MAP2K1-mutation, MEK1-mutation, stage IV melanoma, trametinib, MEK-inhibitor, focal post-radiation necrosis, brain metastasis, case report
Citation: Dirven I, Calliauw E, Awada G, Vounckx M, Kessels JI and Neyns B (2024) Successful treatment of MAP2K1 mutant stage IV-M1d melanoma with trametinib plus low-dose dabrafenib: a case report. Front. Med. 11:1436774. doi: 10.3389/fmed.2024.1436774
Edited by:
Erika Bandini, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Kevinn Eddy, Eurofins Scientific SE, United StatesElizabeth Gaughan, University of Virginia, United States
Copyright © 2024 Dirven, Calliauw, Awada, Vounckx, Kessels and Neyns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iris Dirven, aXJpcy5kaXJ2ZW5AdXpicnVzc2VsLmJl
 Iris Dirven
Iris Dirven Evan Calliauw
Evan Calliauw Gil Awada
Gil Awada Bart Neyns
Bart Neyns