- 1Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China
- 2Postgraduate College, China Medical University, Shenyang, China
- 3Postgraduate College, Shenyang Pharmaceutical University, Shenyang, China
- 4Postgraduate College, Dalian Medical University, Dalian, China
Background: Primary liver cancer (PLC) is one of the most common cancers worldwide. ABO blood groups and rhesus (Rh) factor are inherited characteristics. Their association with the presence of PLC remains unclear in cirrhotic patients. Hence, the purpose of this cross-sectional study was to evaluate whether blood groups were risk factors for the presence of PLC in cirrhosis.
Methods: Patients with liver cirrhosis who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command from 1 January 2010 to 30 June 2014 were retrospectively screened. Logistic regression analyses were performed to explore the association of ABO blood groups and Rh factor with PLC in cirrhotic patients. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were calculated after adjusting for gender, age, family history of liver cirrhosis, HBV-DNA positivity, and etiology of cirrhosis. Subgroup analyses were performed according to the etiology of liver cirrhosis.
Results: Overall, 1,158 cirrhotic patients without PLC and 240 cirrhotic patients with PLC were included in the study. After adjusting for confounding factors, non-O (aOR = 0.763; 95%CI = 0.449–1.298, p = 0.319), A (aOR = 0.643; 95%CI = 0.332–1.246, p = 0.191), B (aOR = 0.835; 95%CI = 0.453–1.540, p = 0.564), AB (aOR = 0.888; 95%CI = 0.363–2.170, p = 0.795), and Rh (+) (aOR = 0.239; 95%CI = 0.036–1.571, p = 0.136) blood groups were not independently associated with PLC in cirrhotic patients. In the subgroup analysis of HBV-related cirrhotic patients, the proportion of A blood group was significantly lower in cirrhotic patients with PLC than in those without PLC (24.17% vs. 33.99%, p < 0.001); however, in HCV- and alcohol-related cirrhotic patients, the proportions of ABO blood groups and Rh factor were not significantly different between the two groups.
Conclusion: ABO blood groups and Rh factor may not be associated with the presence of PLC in cirrhotic patients.
1 Introduction
Primary liver cancer (PLC) is the sixth most common cancer and the third leading cause of cancer-related death worldwide in 2020, with approximately 906,000 new cases and 830,000 deaths (1). PLC primarily occurs as a consequence of chronic liver diseases, including hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and alcoholic or non-alcoholic fatty liver diseases (2). Smoking, obesity, diabetes, iron overload, and aflatoxin B1 exposures are also identified as major risk factors associated with PLC (3). Except for environmental and lifestyle-related factors, epigenetics also plays an important role in the pathogenesis of PLC (4). The ABO blood groups and rhesus (Rh) factor are inherited characteristics, and the expression of ABO and Rh antigens varies among individuals. Nowadays, several malignancies, including pancreatic (5), gastric (6), skin (7), and ovarian (8) cancers, are assumed to be associated with ABO blood groups and Rh factor. In recent years, there is a growing body of evidence on the association of ABO blood groups and Rh factor with PLC (9–12), but their relationship remains controversial. Li et al. found that A blood group was associated with a higher risk of HCV-related hepatocellular carcinoma (HCC) (9). Similarly, Li et al. found that after adjusting for age, sex, type 2 diabetes, cirrhosis, hepatitis B e antigen, and HBV-DNA, A blood group was associated with a higher risk of HBV-related HCC (10). However, Lu et al. found that ABO blood groups were not associated with HBV-related HCC (11). Huang et al. found that AB blood group was associated with a higher risk of HCC (12).
To date, the association of ABO blood groups and Rh factor with the risk of developing PLC in cirrhotic patients is still unclear. To the best of our knowledge, only one study by Iavarone et al. which retrospectively included 215 cirrhotic patients without HCC and 194 cirrhotic patients with HCC, found that non-O blood group was significantly associated with a higher risk of HCC. However, this association was weak probably due to a limited sample size (13). In this setting, we carried out a cross-sectional study to evaluate the association of ABO blood groups and Rh factor with the presence of PLC in cirrhotic patients.
2 Methods
2.1 Study design
We retrospectively reviewed the medical records of patients with liver cirrhosis who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command from 1 January 2010 to 30 June 2014. This study was carried out following the rules of the 1975 Declaration of Helsinki and approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number [Y (2024) 008]. Patients’ written informed consents were waived by the Medical Ethical Committee of the General Hospital of Northern Theater Command due to the retrospective nature of this study.
The exclusion criteria were as follows: (i) repeated admissions of the same patient; (ii) age < 18 years; (iii) patients who did not have sufficient data about ABO blood groups; (iv) patients who had a history or evidence of other non-hepatic malignancy; and (v) patients who did not have imaging-based evidence for a definite diagnosis of liver cirrhosis during their hospitalizations.
2.2 Diagnosis and definitions
Liver cirrhosis was diagnosed based on clinical manifestations, laboratory tests, imaging, liver stiffness measurement, and histopathological examinations, if necessary. PLC was primarily diagnosed by findings from contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) scans with or without a serum alpha-fetoprotein (AFP) level of greater than 400 ng/mL, or histology, if necessary (2, 10).
According to the presence of A and B antigens on the red blood cells (RBC), the ABO blood group includes (i) A blood group characterized as anti-A (+) and anti-B (−); (ii) B blood group as anti-A (−) and anti-B (+); (iii) O blood group as anti-A (−) and anti-B (−); and (iv) AB blood group as anti-A (+) and anti-B (+). According to the presence or absence of D antigen on the RBC, the Rh factor includes (i) Rh (+) blood group characterized as anti-D (+) and (ii) Rh (−) blood group as anti-D (−).
2.3 Data collection
Demographic, clinical, and laboratory data at admissions were collected, including age, gender, smoking, drinking, etiology of cirrhosis (i.e., HBV infection, HCV infection, and alcohol abuse), hypertension, diabetes, family history of liver cirrhosis, ABO blood groups, Rh factor, red blood cells (RBC), hemoglobin (Hb), white blood cell (WBC), platelet count (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), gamma-glutamyltransferase (GGT), serum creatinine (Scr), blood urea nitrogen (BUN), serum sodium (Na), international normalized ratio (INR), activated partial thromboplastin time (APTT), and AFP. Child–Pugh score and class and Model for End-Stage Liver Disease (MELD) score were also calculated.
2.4 Statistical analyses
All statistical analyses were performed using SPSS version 26.0 statistical software (IBM Corp, Armonk, New York, USA). Continuous variables were expressed as mean ± standard deviation and median (range) and compared using the independent sample t-tests for normal distribution or the Mann–Whitney U-tests for non-normal distribution. Categorical variables were expressed as frequency (percentage), and their difference between the groups was evaluated using Chi-squared or Fisher’s exact tests. Logistic regression analyses were used to explore whether ABO blood groups and Rh factor were significantly associated with PLC in cirrhotic patients. Crude odds ratios (cORs) with 95% confidence intervals (CIs) were calculated in univariate analyses. Adjusted odds ratios (aORs) with 95%CIs were calculated after adjusting for gender, age, family history of liver cirrhosis, HBV-DNA, and etiology of cirrhosis. Finally, subgroup analyses were performed to explore the association of ABO blood groups and Rh factor with PLC in patients with different etiologies of liver cirrhosis. Interactions between etiologies of liver cirrhosis and ABO blood groups or Rh factor were tested in subgroup analyses, if appropriate. A two-tailed p < 0.05 was considered statistically significant.
3 Results
3.1 Description of overall patients
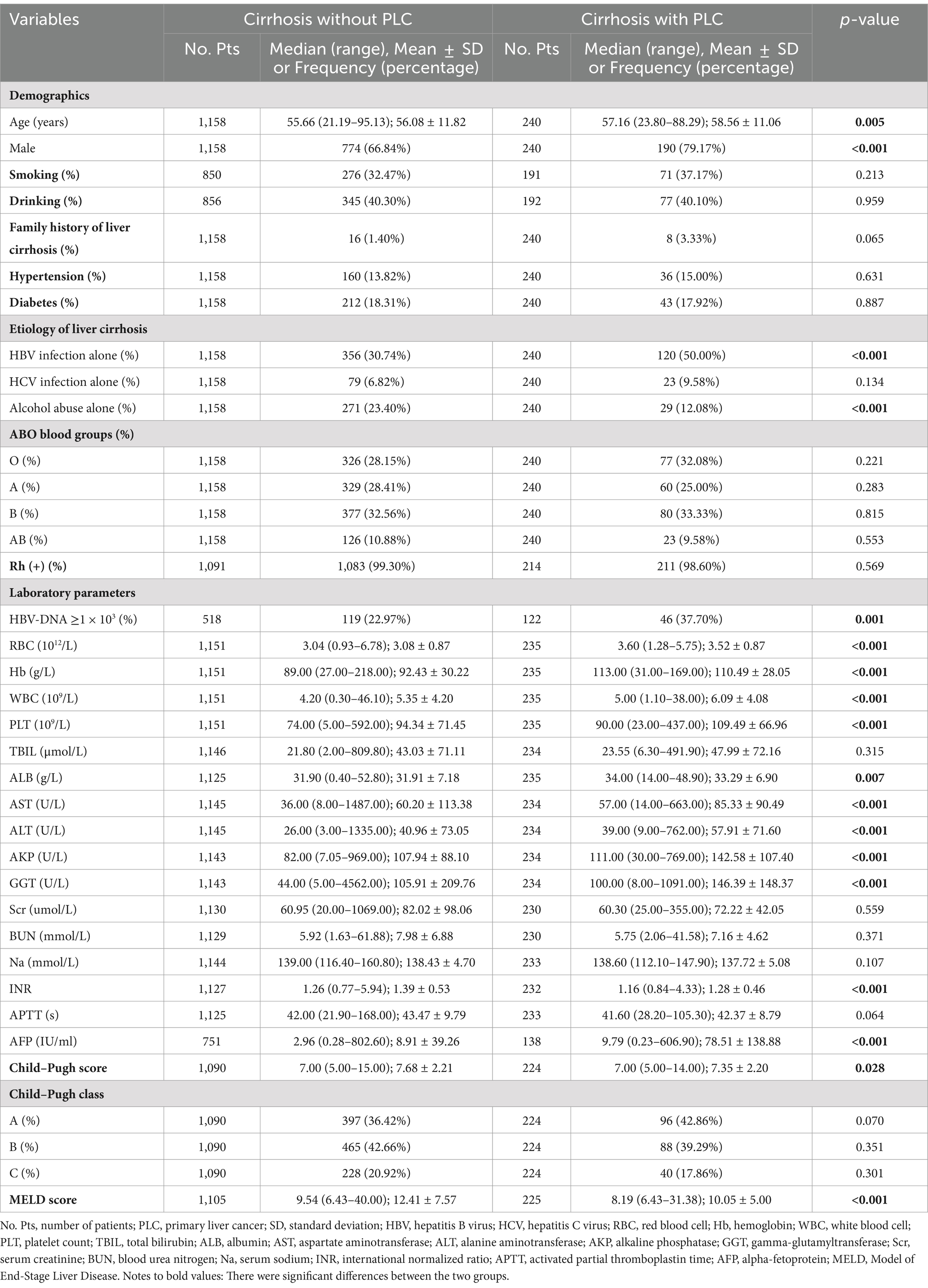
A total of 1,158 cirrhotic patients without PLC and 240 cirrhotic patients with PLC were included in the study (Figure 1). The mean age of cirrhotic patients without and with PLC was 56.08 and 58.56 years, respectively, and the proportion of male subjects without and with PLC was 66.84% (774/1158) and 79.17% (190/240), respectively.
3.2 Overall comparison between cirrhotic patients without and with PLC
The proportions of male subjects (66.84% vs. 79.17%, p < 0.001) and HBV-DNA ≥ 1 × 103 (22.97% vs. 37.70%, p = 0.001), age (56.08 ± 11.82 years vs. 58.56 ± 11.06 years, p = 0.005), and AFP level (8.91 ± 39.26 vs. 78.51 ± 138.88, p < 0.001) were significantly different between cirrhotic patients without and with PLC. Cirrhotic patients with PLC had a significantly higher prevalence of HBV infection alone (50.00% vs. 30.74%, p < 0.001) but a lower prevalence of alcohol abuse alone (12.08% vs. 23.40%, p < 0.001) than those without. The proportions of A (28.41% vs. 25.00%, p = 0.283), B (32.56% vs. 33.33%, p = 0.815), O (28.15% vs. 32.08%, p = 0.221), AB (10.88% vs. 9.58%, p = 0.553), and Rh (+) (99.30% vs. 98.60%, p = 0.569) blood groups were not significantly different between cirrhotic patients without and with PLC (Table 1).
Collectively, ABO blood groups and Rh factor were not associated with PLC in overall patients.
3.3 Logistic regression analyses regarding the association of ABO blood groups and Rh factor with PLC in liver cirrhosis
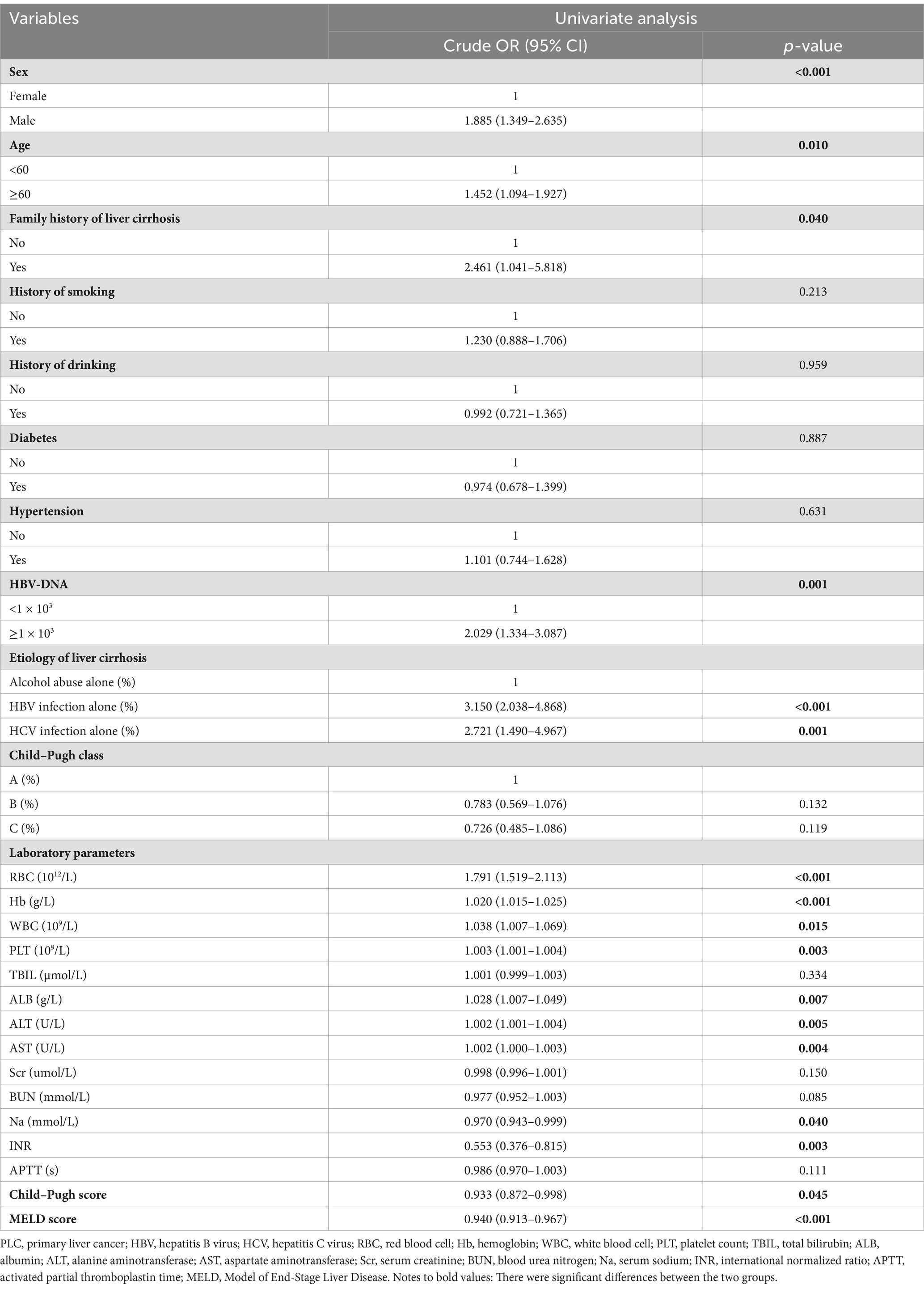
Univariate logistic regression analyses showed that male subjects (cOR = 1.885, 95%CI = 1.349–2.635, p < 0.001), age ≥ 60 years (cOR = 1.452, 95%CI = 1.094–1.927, p = 0.010), family history of liver cirrhosis (cOR = 2.461, 95%CI = 1.041–5.818, p = 0.040), HBV-DNA ≥ 1 × 103 (cOR = 2.029, 95%CI = 1.334–3.087, p = 0.001), HBV infection alone (cOR = 3.150, 95%CI = 2.038–4.868, p < 0.001), and HCV infection alone (cOR = 2.721, 95%CI = 1.490–4.967, p = 0.001) were significantly associated with PLC in cirrhotic patients (Table 2).
Compared with O blood group, non-O (cOR = 0.829, 95%CI = 0.615–1.119, p = 0.221), A (cOR = 0.772, 95%CI = 0.533–1.119, p = 0.171), B (cOR = 0.898, 95%CI = 0.635–1.270, p = 0.544), and AB (cOR = 0.773, 95%CI = 0.465–1.286, p = 0.321) blood groups were not significantly associated with PLC in cirrhotic patients. Multivariate logistic regression analyses also showed that non-O (aOR = 0.763, 95%CI = 0.449–1.298, p = 0.319), A (aOR = 0.643, 95%CI = 0.332–1.246, p = 0.191), B (aOR = 0.835, 95%CI = 0.453–1.540, p = 0.564), and AB (aOR = 0.888, 95%CI = 0.363–2.170, p = 0.795) blood groups were not independently associated with PLC in cirrhotic patients (Table 3).
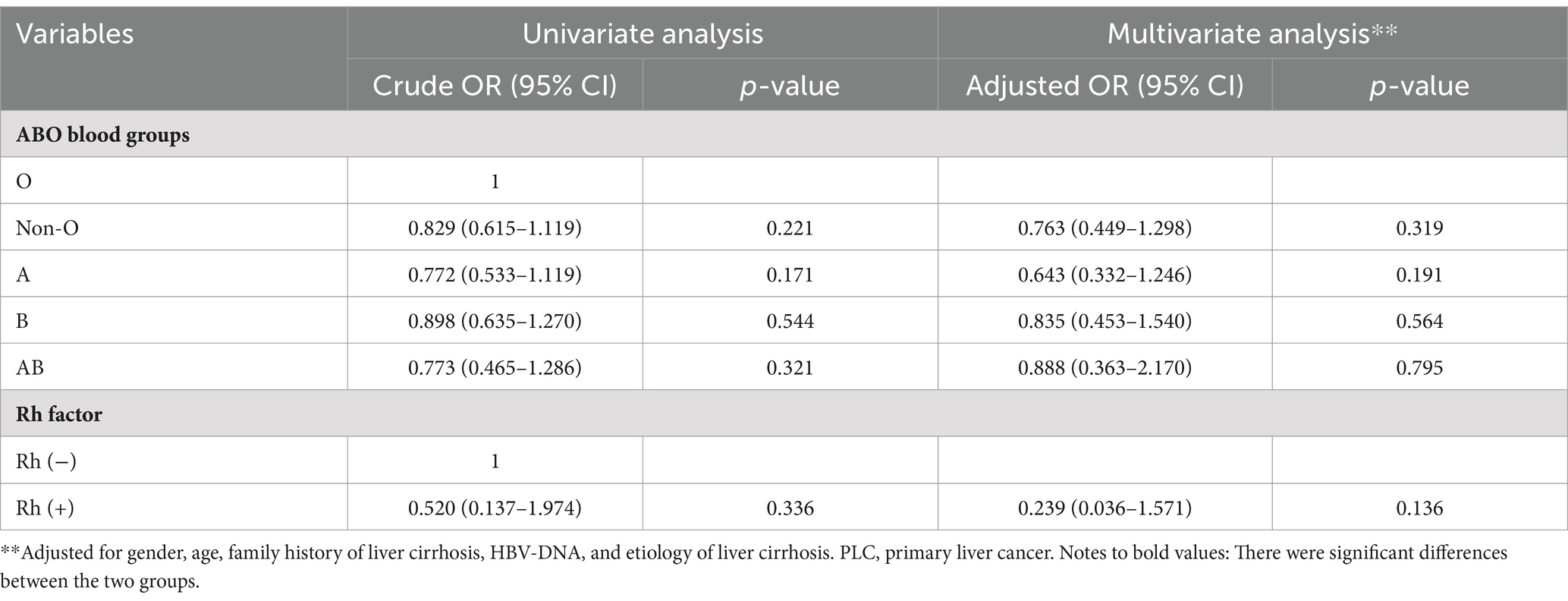
Table 3. Univariate and multivariate logistic regression analyses of ABO blood groups and Rh factor in cirrhotic patients with PLC.
Compared with Rh (−) blood group, Rh (+) blood group (cOR = 0.520, 95%CI = 0.137–1.974, p = 0.336) was not significantly associated with PLC in cirrhotic patients. Multivariate logistic regression analysis showed that Rh factor (aOR = 0.239, 95%CI = 0.036–1.571, p = 0.136) was not independently associated with PLC in cirrhotic patients (Table 3).
Collectively, ABO blood groups and Rh factor were not significantly associated with PLC in cirrhotic patients.
3.4 Subgroup comparison between cirrhotic patients without and with PLC
3.4.1 HBV infection alone
In the subgroup of patients with HBV infection alone, the proportion of A blood group was significantly higher in cirrhotic patients without PLC than those with PLC (33.99% vs. 24.17%, p < 0.001), but the proportions of B, O, AB, and Rh (+) blood groups were not significantly different between cirrhotic patients without and with PLC (Table 4).
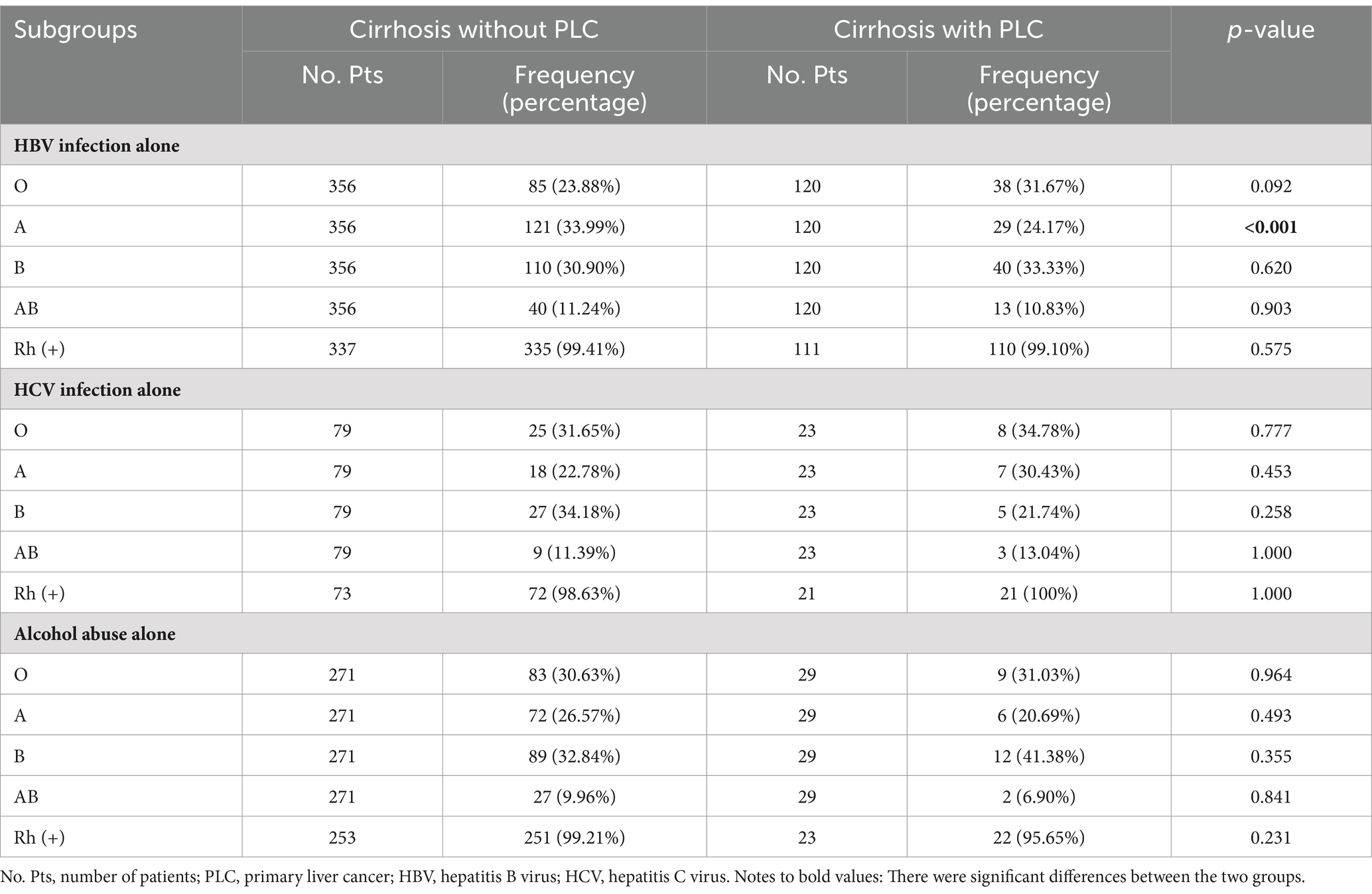
Table 4. Comparison of the proportions of ABO blood groups and Rh factor in subgroup analyses between cirrhotic patients without and with PLC.
Compared with O blood group, A blood group (cOR = 0.536, 95%CI = 0.307–0.936, p = 0.028), rather than non-O, B, and AB blood groups, was significantly associated with a lower risk of PLC in cirrhotic patients with HBV infection alone (Figure 2). However, multivariate logistic regression analyses showed that A blood group was not independently associated with PLC in cirrhotic patients with HBV infection alone (Figure 3).
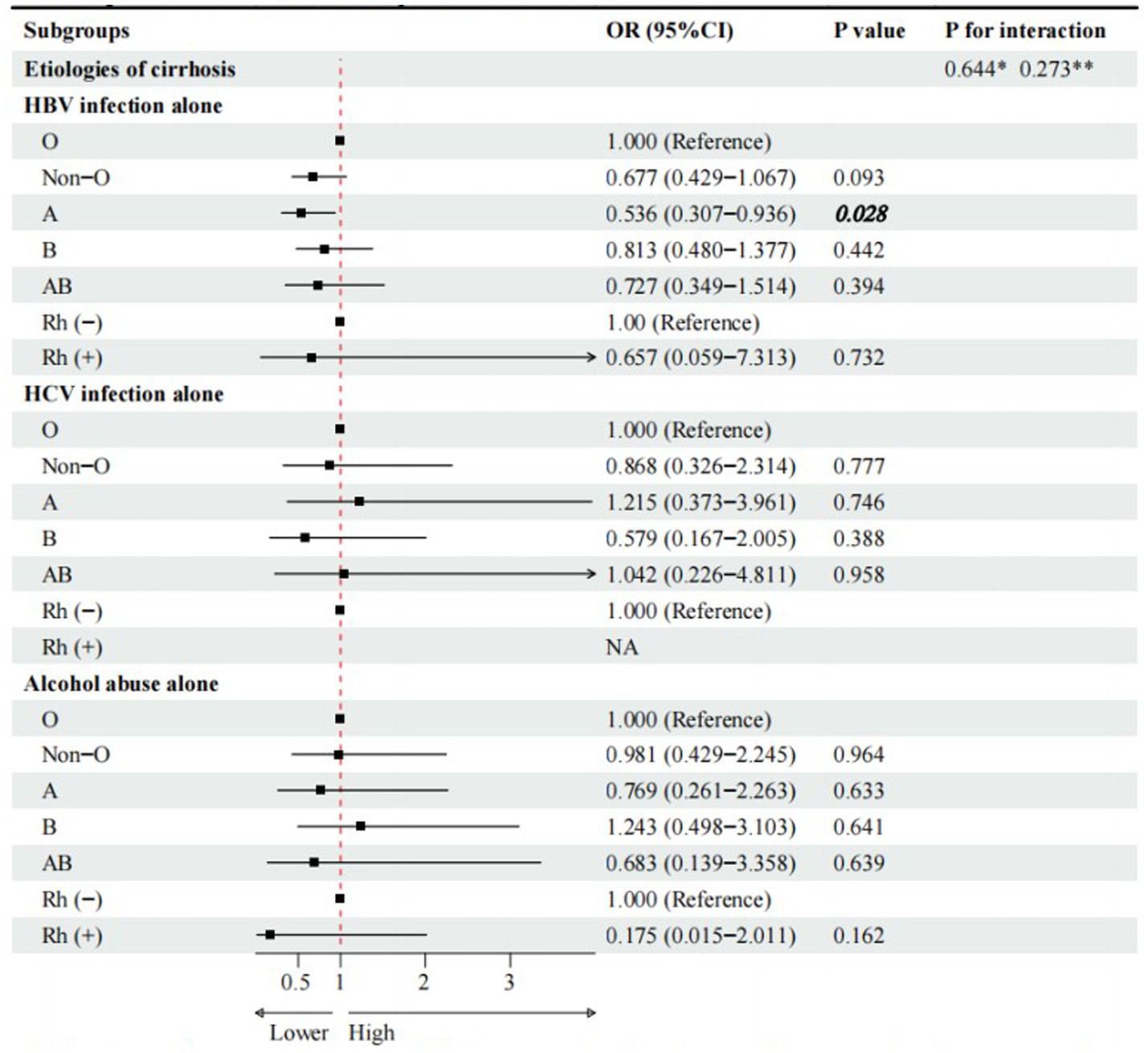
Figure 2. Univariate logistic regression analyses for subgroup analyses of cirrhotic patients with PLC. * Interaction among different etiologies of liver cirrhosis with ABO blood groups. **Interaction among different etiologies of liver cirrhosis with Rh factor. Notes to bold values: There were significant differences between the two groups.
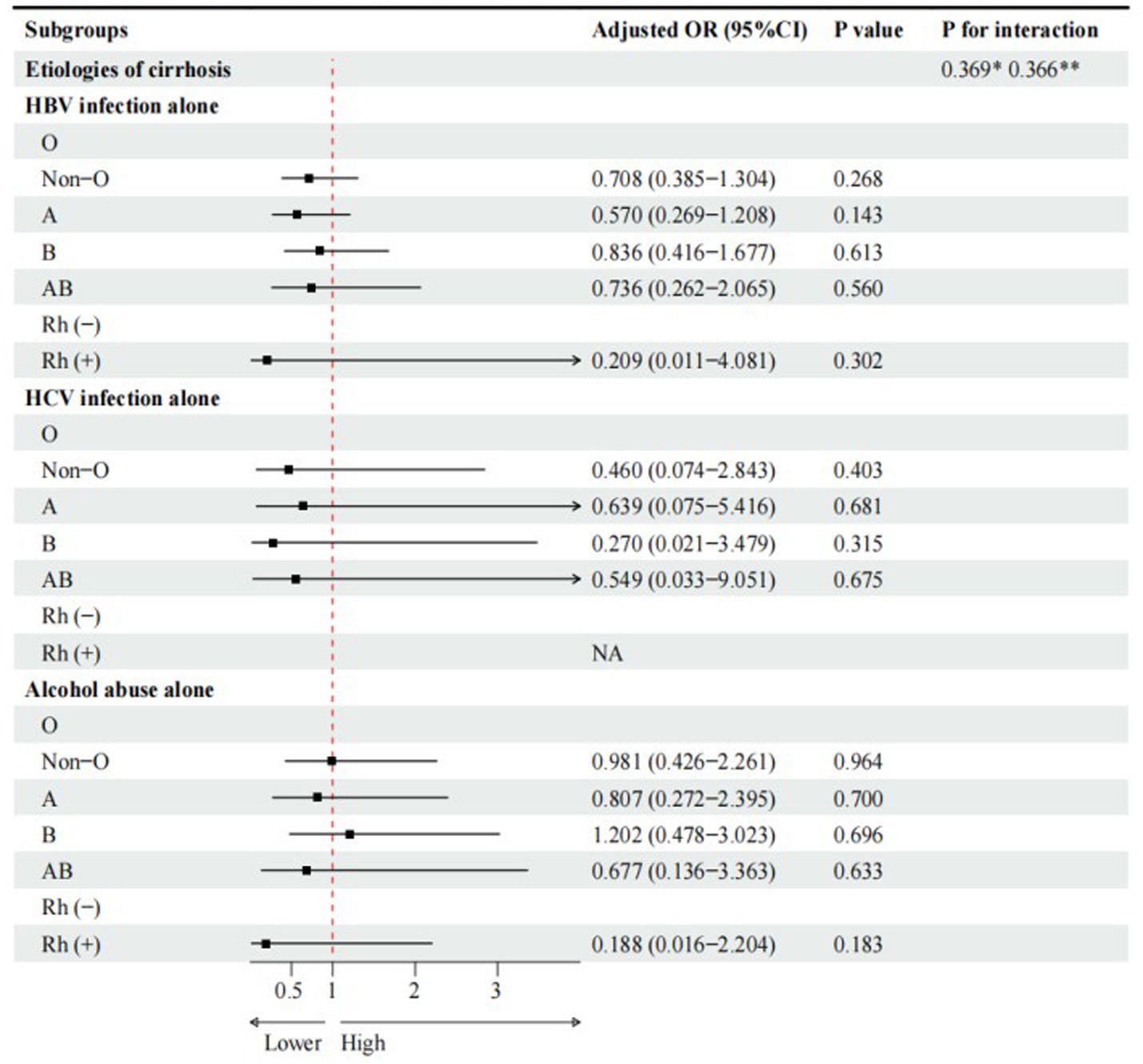
Figure 3. Multivariate logistic regression analyses for subgroup analyses of cirrhotic patients with PLC. Adjusted for gender, age, family history of liver cirrhosis, HBV-DNA. * Interaction among different etiologies of liver cirrhosis with ABO blood groups. **Interaction among different etiologies of liver cirrhosis with Rh factor.
Compared with Rh (−) blood group, Rh (+) blood group (cOR = 0.657, 95%CI = 0.059–7.313, p = 0.732) was not significantly associated with PLC in cirrhotic patients with HBV infection alone (Figure 2). Multivariate logistic regression analysis showed that Rh factor (aOR = 0.209, 95%CI = 0.011–4.081, p = 0.302) was not independently associated with PLC in cirrhotic patients with HBV infection alone (Figure 3).
3.4.2 HCV infection alone
In the subgroup of patients with HCV infection alone, the proportions of A, B, O, AB, and Rh (+) blood groups were not significantly different between cirrhotic patients without and with PLC (Table 4).
Compared with O blood group, non-O, A, B, and AB blood groups were not significantly associated with PLC in cirrhotic patients with HCV infection alone (Figure 2). Multivariate logistic regression analyses showed that non-O, A, B, and AB blood groups were not independently associated with PLC in cirrhotic patients with HCV infection alone (Figure 3).
3.4.3 Alcohol abuse alone
In the subgroup of patients with alcohol abuse alone, the proportions of A, B, O, AB, and Rh (+) blood groups were not significantly different between cirrhotic patients without and with PLC (Table 4).
Compared with O blood group, non-O, A, B, and AB blood groups were not significantly associated with PLC in cirrhotic patients with alcohol abuse alone (Figure 2). Multivariate logistic regression analyses showed that non-O, A, B, and AB blood groups were not independently associated with PLC in cirrhotic patients with alcohol abuse alone (Figure 3).
Compared with Rh (−) blood group, Rh (+) blood group (OR = 0.175, 95%CI = 0.015–2.011, p = 0.162) was not significantly associated with PLC in cirrhotic patients with alcohol abuse alone (Figure 2). Multivariate logistic regression analysis also showed that Rh factor (aOR = 0.188, 95%CI = 0.016–2.204, p = 0.183) was not independently associated with PLC in cirrhotic patients with alcohol abuse alone (Figure 3).
3.4.4 Interaction in subgroup analyses
There was no significant interaction between the etiologies of liver cirrhosis and ABO blood groups or Rh factor (Figures 2, 3).
Collectively, ABO blood groups and Rh factor were not independently associated with PLC in patients with different etiologies of liver cirrhosis.
4 Discussion
Our study did not demonstrate any significant association of ABO blood groups and Rh factor with the risk of PLC in cirrhotic patients. Some possible explanations for this finding are as follows: First, the development of PLC is largely influenced by acquired factors but less affected by inherited factors. The majority of our patients had hepatitis B or C virus infection (41.34%) and alcohol abuse (21.46%) as the underlying etiologies of liver diseases, which are also the leading causes of PLC. By comparison, ABO blood groups and Rh factor are inherited characteristics of human populations (14). Second, the development of PLC in cirrhotic patients is largely determined by the regulation of microRNAs, which are a series of small, single-stranded RNAs with approximately 22 nucleotides (15–19) and do not encode the proteins but repress the expression of their target mRNAs on transcriptional and translational levels (20). In cirrhotic patients, multiple signaling pathways and gene expressions can be affected by upgrading the microRNA-21 level and downgrading the microRNA-122, microRNA-29, microRNA-223, and microRNA-193 levels, thereby decreasing cell apoptosis and increasing tumor cell proliferation, invasion, and migration, eventually promoting the development of PLC (21). By comparison, blood groups are differentiated by the presence of A and B antigens on the RBC, which are glycoproteins or glycolipids distributed on the RBC membrane (21) and the products of the gene on chromosome 9q34 (22). Notably, the microRNA level cannot be affected when encoding A and B antigens. Third, the impact of ABO blood groups on PLC may be diluted or even masked by more dominant risk factors, such as hepatitis. Indeed, our previous meta-analysis found that the proportion of O blood group in patients with HCC was significantly lower than that in healthy subjects, but the proportions of ABO blood groups were not significantly different between patients with HCC and hepatitis (23). A case–control study by Shim et al. found that A blood group was associated with a higher risk of PLC in subjects without hepatitis, but ABO blood groups were not significantly associated with PLC in subjects with hepatitis (24). Thus, it can be inferred that ABO blood groups may be associated with PLC in healthy people, but not in patients with hepatitis. Notably, our patients mostly had viral hepatitis, so we did not find any association of ABO blood groups with PLC.
Another finding of our study was that A blood group was a protective factor for PLC in cirrhotic patients with HBV infection alone in univariate logistic regression analyses. However, it should be acknowledged that after adjusting for gender, age, family history of liver cirrhosis, and HBV-DNA, A blood group was not independently associated with PLC in cirrhotic patients with HBV infection alone, which is almost consistent with previous study (11). Therefore, the protective effect of A blood group on PLC, as shown in the univariate analyses, may be caused by confounding factors. Further studies are needed to investigate the association between ABO blood groups and PLC in different settings.
Our study has several advantages as follows. First, to the best of our knowledge, this should be the first study to explore the association of ABO blood groups and Rh factor with the presence of PLC in cirrhotic patients from Liaoning province, China. Second, the selection of eligible patients in our study was more rigorous and reasonable. All subjects included in our study had a diagnosis of cirrhosis. As known, PLC often develops in the setting of cirrhosis. Thus, our data should be more comparable. Third, imaging-based evidence for a definite diagnosis of liver cirrhosis can be obtained in all eligible patients. Thus, our findings should be more accurate and convincing. Fourth, we had a relatively large sample size. Fifth, we adjusted for confounding factors and performed subgroup analyses according to the etiology of liver cirrhosis.
The limitations of our study should not be ignored. First, this was a retrospective cross-sectional study at a single center. Thus, the cause–effect association of ABO blood groups and Rh factor with the development of PLC in cirrhosis could not be clarified. In addition, selection and information bias were inevitable, which may affect the external validity of our study and lead to inaccurate results. Second, most eligible patients included in our study lived in Liaoning province, China. Hence, the findings might be inappropriate to the population from different regions. Third, we did not subdivide the genotypes of the ABO blood groups. In detail, A blood group is composed of AA genotype and AO genotype. B blood group is composed of BB genotype and BO genotype. Therefore, we could not confirm any association of specific ABO genotypes with the risk of PLC in cirrhotic patients.
In conclusion, ABO blood groups and Rh factor may not be associated with the presence of PLC in cirrhotic patients. More large-scale and high-quality prospective studies, multicenter trials, or investigations into the biological mechanisms linking ABO blood groups and Rh factor with PLC are needed to explore the association of ABO blood groups and Rh factor with the risk of PLC among healthy subjects and patients with chronic liver diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors. The datasets presented in this article are not readily available because our data is not open to the public. Requests to access the datasets should be directed to Xingshun Qi, eGluZ3NodW5xaUAxMjYuY29t.
Ethics statement
The studies involving humans were approved by the Medical Ethical Committee of General Hospital of Northern Theater Command. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because patients’ written informed consents were waived by the Medical Ethical Committee of General Hospital of Northern Theater Command due to the retrospective nature of this study.
Author contributions
LD: Writing – original draft, Software, Methodology, Formal analysis, Data curation, Investigation. YY: Writing – original draft, Methodology, Investigation. HL: Software, Data curation, Writing – original draft. DS: Writing – original draft, Data curation. DW: Writing – review & editing, Investigation, Data curation. DZ: Writing – review & editing, Investigation, Data curation. XQ: Writing – review & editing, Supervision, Project administration, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present study was partially supported by the National Key R&D Program of China (2023YFC2507500), Outstanding Youth Foundation of Liaoning Province (2022-YQ-07), and Science and Technology Plan Project of Liaoning Province (2022JH2/101500032).
Acknowledgments
This study has been partially presented as an oral abstract in the section “Hepatocellular Carcinoma – Clinical” of the Asian Pacific Association for the Study of the Liver (APASL) 2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PLC, primary liver cancer; Rh, rhesus; cORs, crude odds ratios; aORs, adjusted odds ratios; 95%CIs, 95% confidence intervals; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; RBC, red blood cell; Hb, hemoglobin; WBC, white blood cell; PLT, platelet count; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; GGT, gamma-glutamyltransferase; Scr, serum creatinine; BUN, blood urea nitrogen; Na, serum sodium; INR, international normalized ratio; APTT, activated partial thromboplastin time; AFP, alpha-fetoprotein; MELD, Model for End-Stage Liver Disease
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Vogel, A, Meyer, T, Sapisochin, G, Salem, R, and Saborowski, A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
3. Center, MM, and Jemal, A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. (2011) 20:2362–8. doi: 10.1158/1055-9965.EPI-11-0643
4. Ghafouri-Fard, S, Honarmand Tamizkar, K, Hussen, BM, and Taheri, M. MicroRNA signature in liver cancer. Pathol Res Pract. (2021) 219:153369. doi: 10.1016/j.prp.2021.153369
5. Engin, H, Bilir, C, Ustun, H, and Gokmen, A. ABO blood group and risk of pancreatic Cancer in a Turkish population in Western Blacksea region. Asian Pac J Cancer Prev. (2012) 13:131–3. doi: 10.7314/apjcp.2012.13.1.131
6. Song, HR, Shin, MH, Kim, HN, Piao, JM, Choi, JS, Hwang, JE, et al. Sex-specific differences in the association between ABO genotype and gastric cancer risk in a Korean population. Gastric Cancer. (2013) 16:254–60. doi: 10.1007/s10120-012-0176-z
7. Xie, J, Qureshi, AA, Li, Y, and Han, J. ABO blood group and incidence of skin cancer. PLoS One. (2010) 5:e11972. doi: 10.1371/journal.pone.0011972
8. Gates, MA, Wolpin, BM, Cramer, DW, Hankinson, SE, and Tworoger, SS. ABO blood group and incidence of epithelial ovarian cancer. Int J Cancer. (2010) 128:482–6. doi: 10.1002/ijc.25339
9. Li, X, Xu, H, Ding, Z, Jin, Q, and Gao, P. Association between ABO blood group and HCV-related hepatocellular carcinoma risk in China. Medicine (Baltimore). (2016) 95:e5587. doi: 10.1097/MD.0000000000005587
10. Li, Q, Yu, CH, Yu, JH, Liu, L, Xie, SS, Li, WW, et al. ABO blood group and the risk of hepatocellular carcinoma: a case-control study in patients with chronic hepatitis B. PLoS One. (2012) 7:e29928. doi: 10.1371/journal.pone.0029928
11. Lu, LL, Zhang, YH, Yao, MH, Lu, JH, Chen, YS, Xu, J, et al. ABO blood groups and liver cancer: prospective results from an HBsAg cohort study. BMJ Open. (2021) 11:e044039. doi: 10.1136/bmjopen-2020-044039
12. Huang, JY, Wang, R, Gao, YT, and Yuan, JM. ABO blood type and the risk of cancer - findings from the Shanghai cohort study. PLoS One. (2017) 12:e0184295. doi: 10.1371/journal.pone.0184295
13. Iavarone, M, Della Corte, C, Pelucchi, C, Marconi, M, Trotti, R, Triolo, M, et al. Risk of hepatocellular carcinoma in relation to ABO blood type. Dig Liver Dis. (2016) 48:94–6. doi: 10.1016/j.dld.2015.10.011
14. Bodmer, W. Genetic characterization of human populations: from ABO to a genetic map of the British people. Genetics. (2015) 199:267–79. doi: 10.1534/genetics.114.173062
15. Krol, J, Loedige, I, and Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. (2010) 11:597–610. doi: 10.1038/nrg2843
16. Borchert, GM, Lanier, W, and Davidson, BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. (2006) 13:1097–101. doi: 10.1038/nsmb1167
17. Macfarlane, LA, and Murphy, PR. MicroRNA: biogenesis, function and role in Cancer. Curr Genomics. (2010) 11:537–61. doi: 10.2174/138920210793175895
18. Lee, RC, Feinbaum, RL, and Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. (1993) 75:843–54. doi: 10.1016/0092-8674(93)90529-y
19. Landgraf, P, Rusu, M, Sheridan, R, Sewer, A, Iovino, N, Aravin, A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. (2007) 129:1401–14. doi: 10.1016/j.cell.2007.04.040
20. Mohr, R, Özdirik, B, Lambrecht, J, Demir, M, Eschrich, J, Geisler, L, et al. From liver cirrhosis to Cancer: the role of Micro-RNAs in Hepatocarcinogenesis. Int J Mol Sci. (2021) 22:1492. doi: 10.3390/ijms22031492
21. Deng, J, Jia, M, Cheng, X, Yan, Z, Fan, D, and Tian, X. ABO blood group and ovarian reserve: a meta-analysis and systematic review. Oncotarget. (2017) 8:25628–36. doi: 10.18632/oncotarget.15759
22. Reid, ME, and Mohandas, N. Red blood cell blood group antigens: structure and function. Semin Hematol. (2004) 41:93–117. doi: 10.1053/j.seminhematol.2004.01.001
23. Liu, F, Li, C, Zhu, J, Ren, L, and Qi, X. ABO blood type and risk of hepatocellular carcinoma: a meta-analysis. Expert Rev Gastroenterol Hepatol. (2018) 12:927–33. doi: 10.1080/17474124.2018.1500174
Keywords: ABO blood groups, rhesus factor, primary liver cancer, liver cirrhosis, risk factor
Citation: Dong L, Yin Y, Lu H, Sun D, Wang D, Zou D and Qi X (2025) No association of ABO blood groups and Rh factor with primary liver cancer in cirrhotic patients: a single-center cross-sectional study. Front. Med. 11:1432137. doi: 10.3389/fmed.2024.1432137
Edited by:
Cataldo Doria, Capital Health, United StatesReviewed by:
Yong Lv, Air Force Medical University, ChinaLiang Shan, Anhui Medical University, China
Copyright © 2025 Dong, Yin, Lu, Sun, Wang, Zou and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deli Zou, em91ZGVsaTIwMjJAMTYzLmNvbQ==; Xingshun Qi, eGluZ3NodW5xaUAxMjYuY29t
†These authors have contributed equally to this work
 Liyan Dong1,2†
Liyan Dong1,2† Xingshun Qi
Xingshun Qi