- School of Health Professions, Shenandoah University, Winchester, VA, United States
Long COVID is a condition that develops in a subset of patients after COVID-19 infection comprising of symptoms of varying severity encompassing multiple organ systems. Currently, long COVID is without consensus on a formal definition, identifiable biomarkers, and validated treatment. Long COVID is expected to be a long-term chronic condition for a subset of patients and is associated with suffering and incapacity. There is an urgent need for clear management guidelines for the primary care provider, who is essential in bridging the gap with more specialized care to improve quality of life and functionality in their patients living with long COVID. The purpose of this mini review is to provide primary care providers with the latest highlights from existing literature regarding the most common long COVID symptoms and current management recommendations. This review also highlights the underutilized interventions of stellate ganglion blocks and low-dose naltrexone, both with well-established safety profiles demonstrated to improve quality of life and functionality for patients suffering with some symptoms of long COVID, and encourages prompt referral to interventional pain management.
1 Introduction
The recent Coronavirus disease 2019 (COVID-19) pandemic elicits many challenges in healthcare. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 infection (1, 2). SARS-CoV-2 utilizes angiotensin-converting-enzyme 2 (ACE2) to bind with spike protein (S-protein) to enter the cell (2). ACE2 is found in several body tissues such as the heart, lungs, kidneys, and gastrointestinal system (3). Most people infected by COVID-19 make a full recovery however, there are varying estimates from 0 to 93% of those infected developing a long-term condition comprised of often severe symptoms affecting multiple organ systems known as long COVID (1, 2, 4, 5). These estimate are highly varied based on several factors such as the definition of long COVID, settings such as hospital-based versus outpatient, reporting methods such as medical records versus self-report, and vaccination status (5, 6). In a subset of patients, long COVID is expected to be a chronic illness with high impacts to healthcare utilization, workforce and employment. The long COVID burden is estimated to be $2.6 trillion per year in the USA (7). The pandemic united the world in a global effort for rapid development and delivery of COVID vaccinations but the lack of sufficient data and recommendations for managing long COVID complications remain.
There are no current validated biomarkers or validated treatments for long COVID making management difficult for providers and frustrating for patients already living with long COVID. Due to the lack of sufficient data many recommendations for managing long COVID are based on expert opinion thus there is urgent need for more research. Patients living with long COVID experience varying severity of debility. Many current strategies include self-management and rehabilitation. Proposed treatments include: apheresis (8), nirmatrelvir/ritonavir (Paxlovid) (9), antihistamines such as loratadine (10), fexofenadine, and famotidine (11), anticoagulants such as apixaban (10) and antiplatelet agents such as aspirin and clopidogrel (12). Thrombolytics such as nattokinase, serrapeptase, lumbrokinase, and bromelain have also been proposed as potential treatments for long COVID (13).
Missed opportunities for intervention are imputable to the paucity of randomized controlled trials. Long COVID patients are more likely to utilize services from their primary care providers to seek further care for their new chronic disease. The aim of this mini review is to provide primary care providers the latest highlights from existing literature regarding current recommendations and feature two safe and underutilized interventions that may be helpful in improving functionality and quality of life for their patients already suffering with some symptoms of long COVID with an emphasis on prompt referral to interventional pain management.
2 Background
COVID-19 was declared a pandemic on March 11, 2020 and as of March 2024, the World Health Organization reports over 775 million cases of COVID-19 worldwide; 103 million cases are in the United States alone (14). The prevalence of long COVID may be underestimated due to the breadth and variability of symptoms (15). There is consistent evidence that women are more likely to report symptoms of long COVID and have a diagnosis compared to men (6, 16–18). An analysis of repeat cross-sectional data collected by US Census Bureau from June 2022 to June 2023 determined the highest prevalence of long COVID and prevalence of associated significant activity limitation was found in adults 35–44 years of age (19). This group encompasses the largest portion of the US workforce as the median age of the labor force is 41.8 (20).
Long COVID pathophysiology remains unclear. Current hypotheses underlying pathophysiology include: Post-viral immune dysregulation triggering multi-organ inflammation, reactivation of latent pathogens, autoimmunity, and formation of microclots (21, 22).
Long COVID is without specific biomarkers for detection and without validated effective treatments (4, 18, 23). Over 200 symptoms encompassing multiple organ systems have been reported and severity varies from mild and reversible to moderate or severe and persistent (18). Long COVID is expected to be a long-term chronic condition for a subset of patients that may last months to years and is associated with suffering and incapacity highlighting an urgent need for clearer management guidelines and interventions.
3 Long COVID definitions
Long COVID has also been referred to as post COVID conditions (PCC), post-acute sequelae of SARS-CoV-2 infection (PASC), and long-haul COVID (7, 24, 25). In 2021, long COVID condition was assigned an International Classification of Diseases, Tenth Revision (ICD-10) code, U90.9 (26).
There is not an established definition for long COVID, though sometimes it includes symptom duration or clusters of symptoms and may not always be straightforward (27, 28). Raveendran (28) proposes clinical and essential criteria to facilitate categorizing long COVID into four categories: confirmed, probable, possible, or doubtful. The National Institute for Health and Care Excellence (NICE) defines long COVID as “signs and symptoms that continue or develop after acute COVID-19” and “includes both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more)” (29). The National Institutes of Health (NIH) Researching COVID to Enhance Recovery (RECOVER) Initiative program defines long COVID as “ongoing, relapsing, or new symptoms, or other health effects occurring after the acute phase of SARS-CoV-2 infection (i.e., present four or more weeks after acute infection)” (22).
A Delphi process conducted in partnership between the World Health Organization (WHO) Clinical Case Definition Working Group on Post-COVID-19 Condition and an international panel of patients, providers, researchers and WHO staff, provides the following definition for long COVID: A condition occurring in individuals with SARS-CoV-2 infection history at least 3 months post-acute infection with persistent symptoms of at least 2 months duration in which an alternative diagnosis cannot be obtained (30). The US Centers for Disease Control and Prevention (CDC), defines long COVID as new onset or persistent symptoms of at least 4 weeks’ duration from acute infection (31). Commonalities in the definition of long COVID is comprised of signs and symptoms occurring 3 months after acute COVID infection and persistent for at least 2 months. Differences in the timelines defining long COVID have significant effects on comparing research among these studies. Several studies note that long COVID symptoms change over time from predominantly respiratory towards neuropsychiatric symptoms.
4 Preliminary workup
Diagnosing long COVID is difficult with over 200 associated/related symptoms. Long COVID is not exclusively associated with severe acute COVID infections. Many studies focus on hospitalized COVID patients thus there is an underrepresentation of non-hospitalized COVID patients (32). The most commonly reported long COVID symptoms include: fatigue, dyspnea, cognitive impairment, myalgias and arthralgias, headache, cough, chest pain, smell and taste alterations (17, 26, 33, 34). No specific biomarkers exist to detect long COVID (4, 15, 18) although proposed biomarkers include: C-reactive protein (35), interferon gamma (36), interleukin-6 (35, 37), and tumor necrosis factor alpha (38). Antibodies do not facilitate the retrospective diagnosis of infection in patients with long COVID. Antibodies are only found in about 80% of patients that seroconvert and these levels decrease over time and are undetectable at 3 months (26). Most studies measured spike antibodies, which are no longer relevant post-vaccination. Anti-nucleocapsid protein antibodies also diminish over 6 months in 50% of patients hospitalized with COVID-19 (39).
Assessment of patients with high suspicion for long COVID should include a comprehensive evaluation with collection of a thorough history. Physical examination should include baseline vital signs and bloodwork such as complete blood count, electrolytes, creatinine, random blood glucose, hemoglobin A1c, thyroid panel, and an electrocardiogram (EKG) (40). Tools such as the World Health Organization (WHO) Post-COVID-19 Functional Status (PCFS) Scale and the Montreal Cognitive Assessment (MOCA) test may be helpful to assess for resolution of acute COVID symptoms, recurrence of symptoms, and/or development of new symptoms within the first year following acute COVID infection (41). After thorough work-up to exclude other alternative diagnoses and referrals to appropriate specialists when indicated, management comprises of symptom control and interventions for treatable characteristics.
5 Management
Current and recommended management strategies differ widely across the literature. Strategies consist primarily of supportive care or based on system involvement. Current care recommendations include multidisciplinary rehabilitation, self-management and self-pacing (42–44). Self-management approaches include appropriate rest, practicing good sleep hygiene, energy pacing methods, diet control, and distraction (41, 42, 45). Pacing strategies are aimed at coping with inconsistent and decreased energy levels by adapting or adjusting efforts to different activities (43). This may include energy conservation which integrates activity prioritization, task delegation, assistive device utilization, and alternating between activity and rest to complete daily activities, and activity pacing which incorporates activity goals and gradual increase of activity levels (43). Higher pacing adherence is associated with higher rates of recovery and improvement (43).
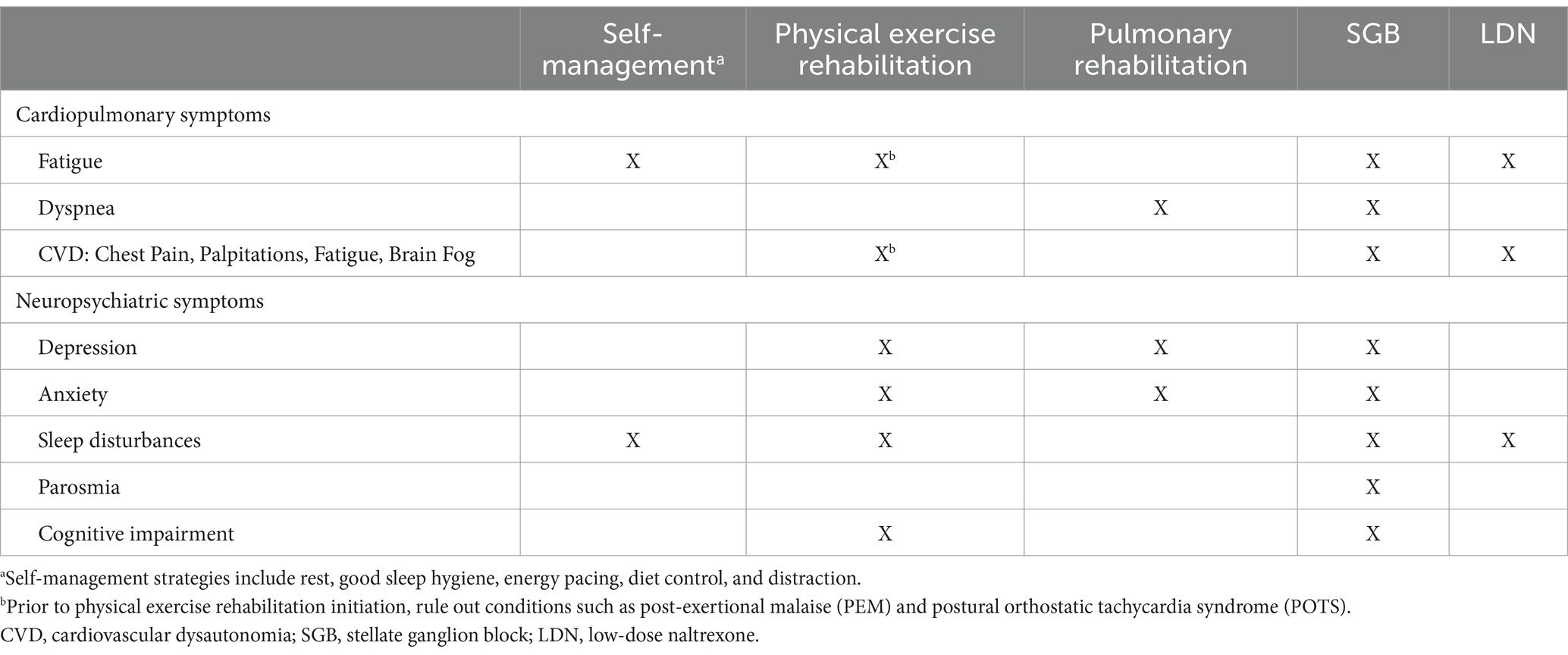
Described hereafter are management recommendations for the most common long COVID presentations that cause the most morbidity and disability. Management recommendations by symptom is presented in Table 1.
5.1 Cardiopulmonary symptoms
Fatigue and dyspnea are two of the most common complaints in patients with long COVID (41, 42, 46–51). Fatigue screening may be achieved by utilizing validated tools such as the Fatigue Assessment Scale (FAS), Visual Analogue Fatigue Scale (VAFS), or Fatigue Severity Scale (FSS) (41, 43). Objective testing for fatigue may be performed using tests such as the 6-min walk test (6MWT) and the Timed Up and Go (TUG) Test (41). Dyspnea is associated with poor sleep, mood, life quality and strength particularly at 12 months post-COVID (52). Dyspnea and depressive symptoms at 3 months post-COVID are predictors of severity of dyspnea 12 months post-COVID and may be screened with standardized dyspnea and mood questionnaires (52). Assessment of dyspnea may include pulmonary function tests (PFTs), transthoracic echocardiogram (TTE), and the 6-min walk test (6MWT) (41). Patients with pre-existing cardiac history should have regular monitoring of troponin and inflammatory panels, electrocardiogram, chest x-ray, echocardiogram, holter-EKG and spirometry (18).
Cardiovascular dysautonomia symptoms include chest pain, palpitations, fatigue, and brain fog due to deficient function of the autonomic nervous system (ANS) (52). The most prevalent type is postural orthostatic tachycardia syndrome (POTS) (52, 53). POTS may be screened and assessed through utilization of orthostatic vital signs and tilt table testing (41, 53). Symptom management for patients with confirmed POTS may include compression stockings, hydration, and behavioral modification (41, 53). Pharmacological management may also be employed based on target symptom. For example, to reduce tachycardia and improve exercise or orthostatic intolerance, beta-blockers may be utilized (53).
Physical exercise rehabilitation has also been shown to provide improvement of fatigue but it is essential to rule out conditions such as post-exertional malaise (PEM) and POTS as exercise may exacerbate symptoms and be harmful (41, 48, 51). A randomized controlled trial (RCT) examined functional versus aerobic exercise telerehabilitation programs in combination with breathing techniques to improve long COVID symptoms and demonstrated both improved quality of life and stress symptoms (54). However, functional exercise exhibited more significant results improving fatigue and functional performance (54). Physical exercise rehabilitation should be performed in a clinical setting with direct supervision to ensure patient safety (51, 55).
Pulmonary rehabilitation has positive effects on dyspnea, physical function, quality of life, anxiety and depression, however not as significant on fatigue (56, 57). Face-to-face rehabilitation and telerehabilitation both demonstrate improved outcomes with face-to-face delivery faring slightly better in terms of improved quality of life (57).
5.2 Neuropsychiatric symptoms
The most common neuropsychiatric symptoms in long COVID include depression, anxiety, sleep disturbances, parosmia, and cognitive impairment (58, 59). “Brain fog” is a term used by patients to describe their cognitive impairment experience and may include any of the following: concentration difficulty, feelings of confusion, cognitive slowing, mental fuzziness, forgetfulness, word finding, mental fatigue (45, 59).
Neuropsychiatric screening may include formal psychological assessment, testing for autonomic dysfunction, and cognitive impairment screening with the MOCA test (26, 41). Sleep quality and mental health should also be assessed (41). Autonomic dysfunction may be screened with the Composite Autonomic Symptom Scale-31 (COMPASS 31) and diagnosis may be facilitated through evaluation of beat-to-beat blood pressure and heart rate variability (HRV) (26). Dysautonomia symptoms that are also components of long COVID include changes to smell and taste, headaches, and hypoxia (26).
Utilization of psychological aides such as cognitive behavioral therapy and antidepressants for mental health conditions associated with long COVID may provide benefit (26).
Parosmia is a sense of smell distortion and negatively impacts quality of life as a significant number of patients report associated weight loss, reductions in enjoyment of food, and depression (60). For persistent parosmia, management includes olfactory training, nasal corticosteroid sprays, and/ or vitamin A drops (41, 60).
Exercise based rehabilitation is also recommended to manage long COVID mental health and sleep-related problems once other conditions such as POTS and PEM have been ruled out (41, 48, 56).
5.3 Underutilized modalities
Long COVID symptoms contribute to social and economic hardship for individuals and their families highlighting the urgent need for interventions to provide relief (61). The following highlights two underutilized interventions with well-established safety profiles that may improve functionality and quality of life in patients suffering with long COVID.
5.3.1 Stellate ganglion block
Overactivity in the sympathetic nervous system coupled with underactivity in the vagus nerve may contribute to the persistent inflammation found in long COVID (62). This persistent inflammation unsettles the sympathetic and parasympathetic nervous systems’ balance and likely contributes to the characteristic symptoms of long COVID (62). A safe and underutilized intervention to target the autonomic nervous system to potentially relieve long COVID symptoms is the stellate ganglion block (SGB). SGBs have been a treatment modality for nearly a century, particularly for several sympathetically mediated conditions such as complex regional pain syndrome (CRPS), postherpetic neuralgia, and refractory cardiac arrhythmias (32, 63). SGBs are considered an emergent modality in conditions such as anosmia, chronic fatigue syndrome, and anxiety and depression in PTSD (64).
SGBs are performed with imaging guidance while patients are in the supine position, head turned opposite from the procedure side, and with the head of the bed slightly elevated to reduce risk of adverse events such as pneumothorax or injury of adjacent structures (62, 64). This positioning also facilitates decompression of the subclavian vessels and reduces the distance of the stellate ganglion from the needle entry point (62).
Complications that may occur after SGB delivery include systemic or local adverse events. Systemic adverse events include the most common complaints of hoarseness and lightheadedness, followed by cough, dyspnea, migraine headaches, or ptosis (65). Local adverse events include hematoma formation, dural puncture, and local infection (two cases with patients on either concomitant oral or system steroids potentially increasing infection risk during the peri-procedure period) (65). While most complications are transient, these potential adverse events must be weighed against the patient’s personal symptom burden prior to SGB recommendation.
Parosmia is associated with poor quality of life and significant weight loss (60). In a study comparing interventions for parosmia, while SGBs had the lowest utilization in comparison to oral steroids and smell training, it had the highest reported percentage improvement among participants with maintained benefit (60). The classic protocol as designed by Hummel et al. for smell training, or olfactory training, involves patients sniffing four odors for at least 10 s, twice daily, for at least 3 months (66, 67).
A retrospective cohort study of 41 participants evaluated the use of SGBs for long COVID symptoms and initial symptoms included: fatigue (85%), brain fog (80%), post-exertional malaise (66%), mood changes (51%), taste or smell changes (44%), shortness of breath (41%), sleep problems (34%), tachycardia or palpitations (22%) (62). Reduction of at least one symptom was reported in 86% and relief of all presenting initial symptoms was reported in 61% of participants (62). Most patients reported symptom improvement within 15-min of SGB delivery while other symptoms that could not be evaluated immediately such as fatigue or brain fog, improvement was reported over 1–2 weeks from delivery (62). At 9-to-12-month follow-up, only 2 patients reported return of symptoms (62). Long term follow-up studies of long COVID patients after SGB are recommended to evaluate duration of benefit. Pearson et al. (62) urge for prompt consideration of SGBs to treat long COVID symptoms as diminished response has been observed in other chronic post-viral diseases such as Lyme disease or myalgic encephalopathy and is theorized to be attributed to time-critical neuroadaptive changes.
For patients suffering with long COVID, consider timely referral to interventional pain management for SGB consideration.
5.3.2 Low-dose naltrexone
Another underutilized intervention with an established safety profile for a variety of conditions is the pharmacological agent naltrexone. Naltrexone is a non-selective opioid antagonist currently approved by the US Food and Drug Administration (FDA) for the treatment of alcohol and opioid dependence and is prescribed at 50–150 mg daily (68, 69). At doses below 5 mg, it is considered low-dose naltrexone (LDN), exhibits anti-inflammatory and analgesic properties, and has been used off-label to reduce severity of symptoms in conditions such as fibromyalgia, multiple sclerosis, complex regional pain syndrome, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and Crohn’s disease (69–71). LDN is widely available with a prescription, also at a low cost, and is associated with minimal side effects (69). LDN has been associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality and pattern, brain fog, post-exertional malaise, headache, and demonstrated reduction of symptoms and improvement of functionality (70). In patients living with long COVID presenting with neuropsychiatric symptoms, fatigue, or exertional intolerance, consider utilization or adjunct therapy with LDN.
5.4 Prevention
There is general agreement across the literature stating the most effective way to prevent long COVID is to prevent COVID-19 infection with appeals for strong vaccination efforts (15, 18, 72). Vaccination may decrease prevalence of long COVID among US adults by almost 20.9% with at least two-dose vaccination associated with lower risk of persistent fatigue and pulmonary complaints (72, 73). Vaccination reduces the risk of long COVID (40, 41, 46, 74, 75). Several studies demonstrate protective effects of vaccination against long COVID (46, 76–79). Additionally, vaccination alleviates the severity of long COVID symptoms (26, 41, 80–82).
6 Conclusion
Long COVID is an emerging condition without formal consensus on its definition, diagnostic tools, or validated treatments. In a subset of patients, long COVID is likely a chronic disease with long-term disability which will contribute to rising healthcare utilization, and decreased workforce and productivity. There is an urgent need for more knowledge regarding long COVID identification, treatments, and outcomes to direct management guidelines particularly for primary care providers who serve as the gap to more specialized care for patients living with long COVID. Multidisciplinary rehabilitation is the most recommended course of care for patients with long COVID (2, 18). However, it is not recommended for patients with irreversible lung damage (70), ME/CFS (32), or POTS (18).
ME/CFS and long COVID have many overlapping symptoms and features making it difficult to distinguish one from another. Most notable differences include: changes to smell and taste, rash and loss of hair more likely in long COVID compared to ME/CFS (83). Higher inflammatory response reflected by stronger cytokine levels has been demonstrated in ME/CFS compared to long COVID however larger scale studies are needed to confirm (84). Employ caution with physical exercise rehabilitation recommendations as exercise may exacerbate symptoms and be detrimental for conditions such as PEM and POTS (41, 48, 51). In ME/CFS patients, NICE guidelines have recommendations for pacing methods for these patients as to not overexert themselves or aggravate their symptoms (32). Long COVID symptoms can be debilitating to its sufferers and impact not only patients but their families. In addition to multidisciplinary rehabilitation, consider early referral to interventional pain management for consideration of SGB or initiation of LDN to alleviate long COVID symptoms. Vaccination not only reduces the risk of and is protective against long COVID, but also alleviates the severity of long COVID symptoms (26, 40, 41, 46, 74–77, 80–82).
Primary care providers are at the forefront of care for their patients living with long COVID. With this up-to-date information, they will be able to identify the most common symptoms and presentation, validate patients’ experiences, provide care recommendations to improve quality of life and functionality, and coordinate future long-term supportive care.
Author contributions
TD: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. KB: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
A special thank you to KB (Shenandoah University, Winchester) for her unwavering support, invaluable guidance, and constructive feedback during the development of this article. All opinions, omissions, and errors remain my own.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo, YR, Cao, QD, Hong, ZS, Tan, YY, Chen, SD, Jin, HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. (2020) 7:11. doi: 10.1186/s40779-020-00240-0
2. Yan, Z, Yang, M, and Lai, CL. Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. (2021) 9:966. doi: 10.3390/biomedicines9080966
3. Li, R, and Qin, C. Expression pattern and function of SARS-CoV-2 receptor ACE2. Biosaf Health. (2021) 3:312–8. doi: 10.1016/j.bsheal.2021.08.003
4. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
5. Woodrow, M, Carey, C, Ziauddeen, N, Thomas, R, Akrami, A, Lutje, V, et al. Systematic review of the prevalence of long COVID. Open forum. Infect Dis. (2023) 10:ofad233. doi: 10.1093/ofid/ofad233
6. Krishna, B, Wills, M, and Sithole, N. Long COVID: what is known and what gaps need to be addressed. Br Med Bull. (2023) 147:6–19. doi: 10.1093/bmb/ldad016
7. Cutler, DM. The costs of long COVID. JAMA Health Forum. (2022) 3:e221809. doi: 10.1001/jamahealthforum.2022.1809
8. Achleitner, M, Steenblock, C, Dänhardt, J, Jarzebska, N, Kardashi, R, Kanczkowski, W, et al. Clinical improvement of long-COVID is associated with reduction in autoantibodies, lipids, and inflammation following therapeutic apheresis. Mol Psychiatry. (2023) 28:2872–7. doi: 10.1038/s41380-023-02084-1
9. McCarthy, MW. Paxlovid as a potential treatment for long COVID. Expert Opin Pharmacother. (2023) 24:1839–43. doi: 10.1080/14656566.2023.2262387
10. Chee, YJ, Fan, BE, Young, BE, Dalan, R, and Lye, DC. Clinical trials on the pharmacological treatment of long COVID: a systematic review. J Med Virol. (2023) 95:e28289. doi: 10.1002/jmv.28289
11. Salvucci, F, Codella, R, Coppola, A, Zacchei, I, Grassi, G, Anti, ML, et al. Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation. Front Cardiovasc Med. (2023) 10:1202696. doi: 10.3389/fcvm.2023.1202696
12. Samarelli, F, Graziano, G, Gambacorta, N, Graps, E, Leonetti, F, Nicolotti, O, et al. Small molecules for the treatment of long-COVID-related vascular damage and abnormal blood clotting: a patent-based appraisal. Viruses. (2024) 16:450. doi: 10.3390/v16030450
13. Kell, DB, and Pretorius, E. The potential role of ischaemia–reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. (2022) 479:1653–708. doi: 10.1042/BCJ20220154
14. World Health Organization. COVID-19 cases. WHO COVID-19 Dashboard. (2024). Available at: https://data.who.int/dashboards/covid19/cases (Accessed Apr 13, 2024).
15. Iqbal, P, Ata, F, Chaudhry, H, Muthanna, B, Waqas Younas, H, Munamm, SA, et al. Post-COVID-19-associated multiorgan complications or “long COVID” with literature review and management strategy discussion: a meta-analysis. Health Sci Rep. (2023) 6:e1211. doi: 10.1002/hsr2.1211
16. Fernández-de-las-Peñas, C, Palacios-Ceña, D, Gómez-Mayordomo, V, Florencio, LL, Cuadrado, ML, Plaza-Manzano, G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. (2021) 92:55–70. doi: 10.1016/j.ejim.2021.06.009
17. Aiyegbusi, OL, Hughes, SE, Turner, G, Rivera, SC, McMullan, C, Chandan, JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. (2021) 114:428–42. doi: 10.1177/01410768211032850
18. Gyöngyösi, M, Alcaide, P, Asselbergs, FW, Brundel, BJJM, Camici, GG, Martins, PDC, et al. Long COVID and the cardiovascular system—elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint scientific statement of the ESC working groups on cellular biology of the heart and myocardial and pericardial diseases. Cardiovasc Res. (2023) 119:336–56. doi: 10.1093/cvr/cvac115
19. Ford, ND, Slaughter, D, Edwards, D, Dalton, A, Perrine, C, Vahratian, A, et al. Long COVID and significant activity limitation among adults, by age — United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:866–70. doi: 10.15585/mmwr.mm7232a3
20. Median age of the labor force, by sex, race, and ethnicity: U.S. Bureau of Labor Statistics. Available at: https://www.bls.gov/emp/tables/median-age-labor-force.htm (Accessed Mar 30, 2024).
21. Castanares-Zapatero, D, Chalon, P, Kohn, L, Dauvrin, M, Detollenaere, J, Maertens De Noordhout, C, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. (2022) 54:1473–87. doi: 10.1080/07853890.2022.2076901
22. Sherif, ZA, Gomez, CR, Connors, TJ, Henrich, TJ, Reeves, WB, Mechanistic Pathway Task, RECOVER, et al. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife. (2023) 12:e86002. doi: 10.7554/eLife.86002
23. Bonilla, H, Peluso, MJ, Rodgers, K, Aberg, JA, Patterson, TF, Tamburro, R, et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front Immunol. (2023) 14:1129459. doi: 10.3389/fimmu.2023.1129459
24. Fernández-de-las-Peñas, C, Palacios-Ceña, D, Gómez-Mayordomo, V, Cuadrado, ML, and Florencio, LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. (2021) 18:2621. doi: 10.3390/ijerph18052621
25. Mehandru, S, and Merad, M. Pathological sequelae of long-haul COVID. Nat Immunol. (2022) 23:194–202. doi: 10.1038/s41590-021-01104-y
26. DePace, NL, and Colombo, J. Long-COVID syndrome and the cardiovascular system: a review of neurocardiologic effects on multiple systems. Curr Cardiol Rep. (2022) 24:1711–26. doi: 10.1007/s11886-022-01786-2
27. O’Mahoney, LL, Routen, A, Gillies, C, Ekezie, W, Welford, A, Zhang, A, et al. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. (2023) 55:101762. doi: 10.1016/j.eclinm.2022.101762
28. Raveendran, AV. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr Clin Res Rev. (2021) 15:145–6. doi: 10.1016/j.dsx.2020.12.025
29. National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE; (2020). Available at: https://www.nice.org.uk/guidance/ng188 (Accessed Mar 30, 2024).
30. Soriano, JB, Murthy, S, Marshall, JC, Relan, P, and Diaz, JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. doi: 10.1016/S1473-3099(21)00703-9
31. Lippi, G, Henry, BM, Favresse, J, and Plebani, M. Addressing standardized definitions of post-COVID and long-COVID. Clin Chem Lab Med. (2023) 61:1361–2. doi: 10.1515/cclm-2023-0390
32. Chandan, JS, Brown, KR, Simms-Williams, N, Bashir, NZ, Camaradou, J, Heining, D, et al. Non-pharmacological therapies for post-viral syndromes, including long Covid: a systematic review. Int J Environ Res Public Health. (2023) 20:3477. doi: 10.3390/ijerph20043477
33. Brennan, A, Broughan, J, McCombe, G, Brennan, J, Collins, C, Fawsitt, R, et al. Enhancing the management of long COVID in general practice: a scoping review. BJGP Open. (2022) 6:BJGPO.2021.0178. doi: 10.3399/BJGPO.2021.0178
34. Yelin, D, Moschopoulos, CD, Margalit, I, Gkrania-Klotsas, E, Landi, F, Stahl, JP, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. (2022) 28:955–72. doi: 10.1016/j.cmi.2022.02.018
35. Liu, F, Li, L, Xu, M, Wu, J, Luo, D, Zhu, Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. (2020) 127:104370. doi: 10.1016/j.jcv.2020.104370
36. Krishna, BA, Lim, EY, Metaxaki, M, Jackson, S, Mactavous, L, BioResource, NIHR, et al. Spontaneous, persistent, T cell–dependent IFN-γ release in patients who progress to long Covid. Sci Adv. (2024) 10:eadi9379. doi: 10.1126/sciadv.adi9379
37. Zhang, C, Wu, Z, Li, J-W, Zhao, H, and Wang, G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
38. Queiroz, MAF, Neves, PFMD, Lima, SS, Lopes, JDC, Torres, MKDS, Vallinoto, IMVC, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. (2022) 12:922422. doi: 10.3389/fcimb.2022.922422
39. Krishna, BA, Lim, EY, Mactavous, L, Lyons, PA, Doffinger, R, Bradley, JR, et al. Evidence of previous SARS-CoV-2 infection in seronegative patients with long COVID. EBioMedicine. (2022) 81:104129. doi: 10.1016/j.ebiom.2022.104129
40. Quinn, KL, Lam, GY, Walsh, JF, Bhéreur, A, Brown, AD, Chow, CW, et al. Cardiovascular considerations in the management of people with suspected long Covid. Can J Cardiol. (2023) 39:741–53. doi: 10.1016/j.cjca.2023.04.003
41. Perumal, R, Shunmugam, L, and Naidoo, K. Long COVID: an approach to clinical assessment and management in primary care. South Afr Fam Pract. (2023) 65:e1–e10. doi: 10.4102/safp.v65i1.5751
42. Leggat, FJ, Heaton-Shrestha, C, Fish, J, Siriwardena, AN, Domeney, A, Rowe, C, et al. An exploration of the experiences and self-generated strategies used when navigating everyday life with long Covid. BMC Public Health. (2024) 24:789. doi: 10.1186/s12889-024-18267-6
43. Ghali, A, Lacombe, V, Ravaiau, C, Delattre, E, Ghali, M, Urbanski, G, et al. The relevance of pacing strategies in managing symptoms of post-COVID-19 syndrome. J Transl Med. (2023) 21:375. doi: 10.1186/s12967-023-04229-w
44. O’Kelly, B, Vidal, L, McHugh, T, Woo, J, Avramovic, G, and Lambert, JS. Safety and efficacy of low dose naltrexone in a long Covid cohort; an interventional pre-post study. Brain Behav Immun Health. (2022) 24:100485. doi: 10.1016/j.bbih.2022.100485
45. Gross, M, Lansang, NM, Gopaul, U, Ogawa, EF, Heyn, PC, Santos, FH, et al. What do I need to know about long-Covid-related fatigue, brain fog, and mental health changes? Arch Phys Med Rehabil. (2023) 104:996–1002. doi: 10.1016/j.apmr.2022.11.021
46. Català, M, Mercadé-Besora, N, Kolde, R, Trinh, NTH, Roel, E, Burn, E, et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir Med. (2024) 12:225–36. doi: 10.1016/S2213-2600(23)00414-9
47. Cha, C, and Baek, G. Symptoms and management of long COVID: a scoping review. J Clin Nurs. (2024) 33:11–28. doi: 10.1111/jocn.16150
48. Chuang, H-J, Lin, C-W, Hsiao, M-Y, Wang, T-G, and Liang, H-W. Long COVID and rehabilitation. J Formos Med Assoc. (2024) 123:S61–9. doi: 10.1016/j.jfma.2023.03.022
49. Laguarta-Val, S, Varillas-Delgado, D, Lizcano-Álvarez, Á, Molero-Sánchez, A, Melian-Ortiz, A, Cano-de-la-Cuerda, R, et al. Effects of aerobic exercise therapy through Nordic walking program in lactate concentrations, fatigue and quality-of-life in patients with long-COVID syndrome: a non-randomized parallel controlled trial. J Clin Med. (2024) 13:1035. doi: 10.3390/jcm13041035
50. Lewthwaite, H, Byrne, A, Brew, B, and Gibson, PG. Treatable traits for long COVID. Respirology. (2023) 28:1005–22. doi: 10.1111/resp.14596
51. Zheng, C, Chen, XK, Sit, CHP, Liang, X, Li, MH, Ma, ACH, et al. Effect of physical exercise–based rehabilitation on long Covid: a systematic review and meta-analysis. Med Sci Sports Exerc. (2024) 56:143–54. doi: 10.1249/MSS.0000000000003280
52. Grewal, JS, Carlsten, C, Johnston, JC, Shah, AS, Wong, AW, and Ryerson, CJ. Post-COVID dyspnea: prevalence, predictors, and outcomes in a longitudinal, prospective cohort. BMC Pulm Med. (2023) 23:84. doi: 10.1186/s12890-023-02376-w
53. Mallick, D, Goyal, L, Chourasia, P, Zapata, MR, Yashi, K, and Surani, S. Covid-19 induced postural orthostatic tachycardia syndrome (POTS): a review. Cureus. (2023) 15:e36955. doi: 10.7759/cureus.36955
54. Espinoza-Bravo, C, Arnal-Gómez, A, Martínez-Arnau, FM, Núñez-Cortés, R, Hernández-Guillén, D, Flor-Rufino, C, et al. Effectiveness of functional or aerobic exercise combined with breathing techniques in telerehabilitation for patients with long Covid: a randomized controlled trial. Phys Ther. (2023) 103:pzad118. doi: 10.1093/ptj/pzad118
55. Binetti, J, Real, M, Renzulli, M, Bertran, L, Riesco, D, Perpiñan, C, et al. Clinical and biomarker profile responses to rehabilitation treatment in patients with long COVID characterized by chronic fatigue. Viruses. (2023) 15:1452. doi: 10.3390/v15071452
56. Pollini, E, Lazzarini, SG, Cordani, C, Del Furia, MJ, Kiekens, C, Negrini, S, et al. Effectiveness of rehabilitation interventions on adults with Covid-19 and post–Covid-19 condition. A systematic review with meta-analysis. Arch Phys Med Rehabil. (2024) 105:138–49. doi: 10.1016/j.apmr.2023.08.023
57. Martínez-Pozas, O, Meléndez-Oliva, E, Rolando, LM, Rico, JAQ, Corbellini, C, and Sánchez Romero, EA. The pulmonary rehabilitation effect on long Covid-19 syndrome: a systematic review and meta-analysis. Physiother Res Int. (2024) 29:e2077. doi: 10.1002/pri.2077
58. Efstathiou, V, Stefanou, M-I, Demetriou, M, Siafakas, N, Makris, M, Tsivgoulis, G, et al. Long COVID and neuropsychiatric manifestations (review). Exp Ther Med. (2022) 23:363. doi: 10.3892/etm.2022.11290
59. Nouraeinejad, A. Brain fog as a long-term sequela of COVID-19. SN Compr Clin Med. (2022) 5:9. doi: 10.1007/s42399-022-01352-5
60. Sowerby, LJ, Almubarak, Z, Biadsee, A, Rocha, T, and Hopkins, C. Coronavirus disease 2019 related parosmia: an exploratory survey of demographics and treatment strategies. J Laryngol Otol. (2023) 137:1256–60. doi: 10.1017/S0022215123000713
61. Toussaint, LL, and Bratty, AJ. Amygdala and insula retraining (AIR) significantly reduces fatigue and increases energy in people with long COVID. Zahiruddin S, editor. Evid Based Complement Alternat Med. (2023) 2023:1–8. doi: 10.1155/2023/7068326
62. Pearson, L, Maina, A, Compratt, T, Harden, S, Aaroe, A, Copas, W, et al. Stellate ganglion block relieves long COVID-19 symptoms in 86% of patients: a retrospective cohort study. Cureus. (2023) 15:e45161. doi: 10.7759/cureus.45161
63. Liu, LD, and Duricka, DL. Stellate ganglion block reduces symptoms of long COVID: a case series. J Neuroimmunol. (2022) 362:577784. doi: 10.1016/j.jneuroim.2021.577784
64. Kirkpatrick, K, Khan, MH, Deng, Y, and Shah, KB. A review of stellate ganglion block as an adjunctive treatment modality. Cureus. (2023) 15:e35174. doi: 10.7759/cureus.35174
65. Goel, V, Patwardhan, AM, Ibrahim, M, Howe, CL, Schultz, DM, and Shankar, H. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. (2019) 44:669–78. doi: 10.1136/rapm-2018-100127
66. Pieniak, M, Oleszkiewicz, A, Avaro, V, Calegari, F, and Hummel, T. Olfactory training – thirteen years of research reviewed. Neurosci Biobehav Rev. (2022) 141:104853. doi: 10.1016/j.neubiorev.2022.104853
67. Hummel, T, Rissom, K, Reden, J, Hähner, A, Weidenbecher, M, and Hüttenbrink, K. Effects of olfactory training in patients with olfactory loss. Laryngoscope. (2009) 119:496–9. doi: 10.1002/lary.20101
68. Isman, A, Nyquist, A, Strecker, B, Harinath, G, Lee, V, Zhang, X, et al. Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav Immun Health. (2024) 36:100733. doi: 10.1016/j.bbih.2024.100733
69. Younger, J, Parkitny, L, and McLain, D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. (2014) 33:451–9. doi: 10.1007/s10067-014-2517-2
70. Bonilla, H, Tian, L, Marconi, VC, Shafer, R, McComsey, GA, Miglis, M, et al. Low-dose naltrexone use for the management of post-acute sequelae of COVID-19. Int Immunopharmacol. (2023) 124:110966. doi: 10.1016/j.intimp.2023.110966
71. Partridge, S, Quadt, L, Bolton, M, Eccles, J, Thompson, C, Colasanti, A, et al. A systematic literature review on the clinical efficacy of low dose naltrexone and its effect on putative pathophysiological mechanisms among patients diagnosed with fibromyalgia. Heliyon. (2023) 9:e15638. doi: 10.1016/j.heliyon.2023.e15638
72. De Domenico, M. Prevalence of long COVID decreases for increasing COVID-19 vaccine uptake. SR Mutheneni, editor. PLOS Glob Public Health. (2023);3:e0001917. doi: 10.1371/journal.pgph.0001917
73. Watanabe, A, Iwagami, M, Yasuhara, J, Takagi, H, and Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis. Vaccine. (2023) 41:1783–90. doi: 10.1016/j.vaccine.2023.02.008
74. Sebők, S, and Gyires, K. Long COVID and possible preventive options. Inflammopharmacology. (2023) 31:2807–17. doi: 10.1007/s10787-023-01204-1
75. Tannous, J, Pan, AP, Potter, T, Bako, AT, Dlouhy, K, Drews, A, et al. Real-world effectiveness of COVID-19 vaccines and anti-SARS-CoV-2 monoclonal antibodies against postacute sequelae of SARS-CoV-2: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. (2023) 13:e067611. doi: 10.1136/bmjopen-2022-067611
76. Lam, ICH, Zhang, R, Man, KKC, Wong, CKH, Chui, CSL, Lai, FTT, et al. Persistence in risk and effect of COVID-19 vaccination on long-term health consequences after SARS-CoV-2 infection. Nat Commun. (2024) 15:1716. doi: 10.1038/s41467-024-45953-1
77. Razzaghi, H, Forrest, CB, Hirabayashi, K, Wu, Q, Allen, AJ, Rao, S, et al. Vaccine effectiveness against long COVID in children. Pediatrics. (2024) 153:e2023064446. doi: 10.1542/peds.2023-064446
78. Taquet, M, Dercon, Q, and Harrison, PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. (2022) 103:154–62. doi: 10.1016/j.bbi.2022.04.013
79. Ayoubkhani, D, Bermingham, C, Pouwels, KB, Glickman, M, Nafilyan, V, Zaccardi, F, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. (2022) 377:e069676. doi: 10.1136/bmj-2021-069676
80. Notarte, KI, Catahay, JA, Velasco, JV, Pastrana, A, Ver, AT, Pangilinan, FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. (2022) 53:101624. doi: 10.1016/j.eclinm.2022.101624
81. Krishna, BA, Metaxaki, M, Wills, MR, and Sithole, N. Reduced incidence of long coronavirus disease referrals to the Cambridge university teaching hospital long coronavirus disease clinic. Clin Infect Dis. (2023) 76:738–40. doi: 10.1093/cid/ciac630
82. Kuodi, P, Gorelik, Y, Zayyad, H, Wertheim, O, Wiegler, KB, Abu Jabal, K, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020–21, Israel. NPJ Vaccines. (2022) 7:101. doi: 10.1038/s41541-022-00526-5
83. Komaroff, AL, and Lipkin, WI. ME/CFS and long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med. (2023) 10:1187163. doi: 10.3389/fmed.2023.1187163
84. Domingo, JC, Battistini, F, Cordobilla, B, Zaragozá, MC, Sanmartin-Sentañes, R, Alegre-Martin, J, et al. Association of circulating biomarkers with illness severity measures differentiates myalgic encephalomyelitis/chronic fatigue syndrome and post-COVID-19 condition: a prospective pilot cohort study. J Transl Med. (2024) 22:343. doi: 10.1186/s12967-024-05148-0
Keywords: long COVID, chronic COVID-19, post-acute sequelae of SARS-CoV-2, post COVID, management, low-dose naltrexone, stellate ganglion block
Citation: Dietz TK and Brondstater KN (2024) Long COVID management: a mini review of current recommendations and underutilized modalities. Front. Med. 11:1430444. doi: 10.3389/fmed.2024.1430444
Edited by:
Shisan Bao, The University of Sydney, AustraliaReviewed by:
Benjamin Anthony Krishna, University of Cambridge, United KingdomCopyright © 2024 Dietz and Brondstater. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiffany K. Dietz, dGlmZmFueS5kaWV0ekBzdS5lZHU=
 Tiffany K. Dietz
Tiffany K. Dietz Kirsten N. Brondstater
Kirsten N. Brondstater