- 1Department of Gynecology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Clinical Laboratory, Qingdao Central Hospital, University of Health and Rehabilitation Sciences (Qingdao Cancer Hospital), Qingdao, Shandong, China
Krukenberg tumor is a relatively uncommon metastatic ovarian cancer, typically presenting with abdominal pain and distension, primarily due to bilateral ovarian involvement. Pseudo-Meigs' syndrome, caused by a Krukenberg tumor originating from gastric cancer, is extremely rare. In this study, we report the case of a 39-year-old woman who presented with unusual manifestations of a Krukenberg tumor, where abdominal distension and dyspnea were the primary symptoms. After surgical treatment, a histopathological examination of the ovary revealed the presence of signet ring cell carcinoma. We concluded that this case coincided with Pseudo-Meigs' syndrome. Clinicians should note that Pseudo-Meigs' syndrome should be considered in patients with Krukenberg tumor, ascites, and pleural effusion, as resection of the tumor may provide long-term palliation.
1 Introduction
Meigs' syndrome refers to the presence of benign ovarian tumors accompanied by pleural effusion and ascites. Pseudo-Meigs' syndrome, on the other hand, is a clinical manifestation where malignant ovarian tumors are associated with similar benign ascites and pleural effusion. Krukenberg tumor was first reported by the German gynecologic pathologist Friedrich Ernst Krukenberg in 1896 (2). It is a rare metastatic signet ring cell carcinoma of the ovary, accounting for ~1% of all ovarian tumors (3). In most cases, the stomach is the primary site of Krukenberg tumor (5). Abdominal mass, abdominal pain, and abdominal distension are common symptoms in patients with Krukenberg tumor (6). However, symptoms of the primary tumor are often subtle, and the lack of distinctive phenotype in tests can lead to missed diagnosis or misdiagnosis.
In this report, we present a case of gastric cancer with Krukenberg tumor manifesting as Pseudo-Meigs' syndrome, with dyspnea being the first symptom. We also review similar cases to better understand the clinical features of such presentations. This case report also highlights the importance of maintaining a broad diagnostic perspective when evaluating patients with symptoms that may suggest a specific pathological process to avoid missed diagnosis or misdiagnosis.
2 Case description
A 39-year-old female patient presented with symptoms of cough and fever that persisted for 2 months before treatment. Over time, she developed chest pain, chest tightness, and shortness of breath. She initially went to the respiratory department, and a computed tomography (CT) scan revealed pleural effusion (Figure 1). However, anti-infective treatment with cefaclor proved ineffective.
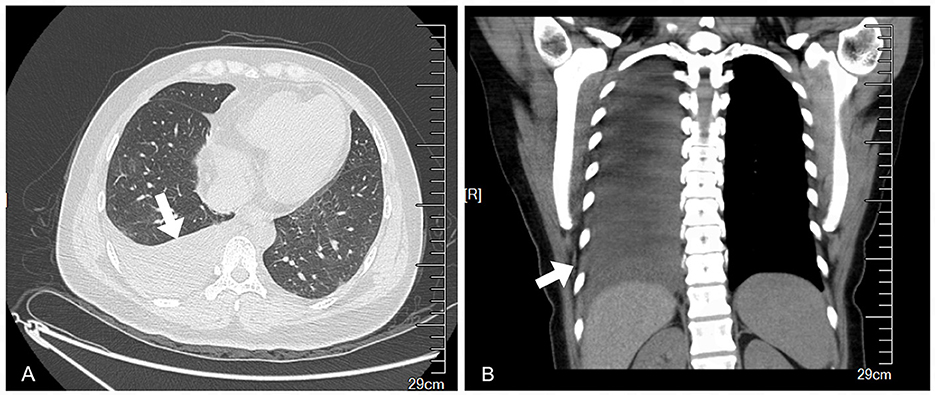
Figure 1. Preoperative chest CT showed massive pleural effusion on the right side and partial dilatation of the lower lobe. (A) Transverse plane. (B) Coronal plane.
Subsequently, the clinician performed thoracocentesis and drained approximately 450 ml of pleural fluid. Analysis of the pleural effusion revealed an exudate mainly composed of mononuclear cells, with no evidence of tumor cells (Figure 2A). Her serum tumor marker levels were as follows: Alpha-Fetoprotein (AFP) 6.26 ng/ml, Carcinoembryonic Antigen (CEA) 1.46 ng/ml, Cancer Antigen 125 (CA-125) 270.9U/ml, Carbohydrate Antigen 19-9 (CA19-9) 38.56U/ml, Squamous Cell Carcinoma Antigen (SCC) 1.40 ng/ml, Human Epididymis Protein 4 (HE4) 52.67 pmol/L, and a Roma index of 9.8%.
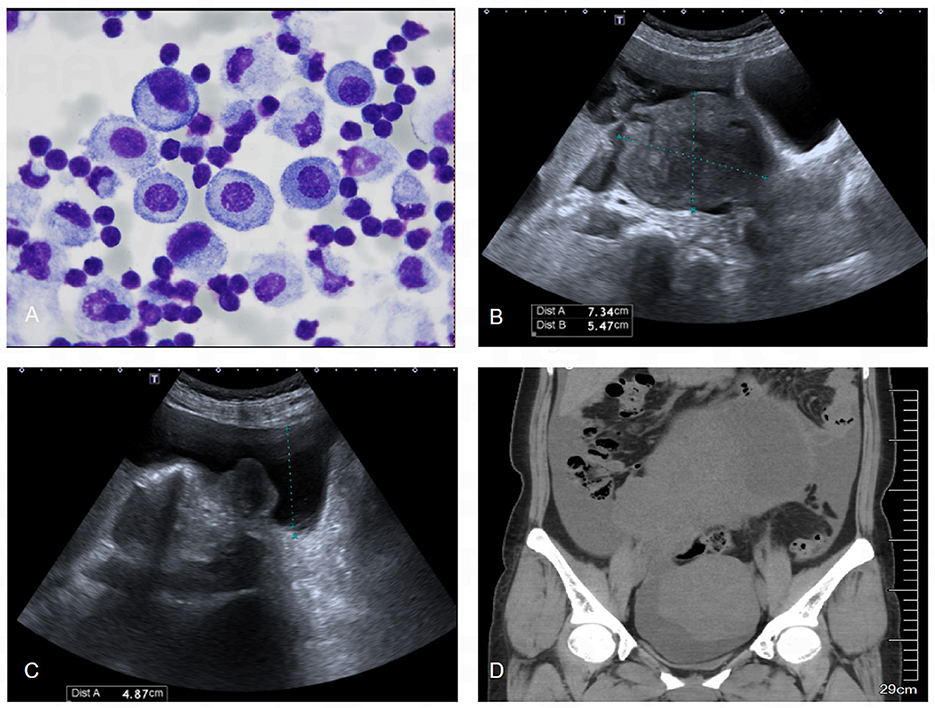
Figure 2. Results of abdominal ultrasound, pelvic CT and pleural effusion cytology. (A) Cytological examination of pleural effusion showed that the cells were mainly lymphocytes, and no malignant cells were found. (B) Abdominal ultrasound showed a heterogeneous echogenic mass about 16.5 × 15.2 × 10.7 cm in size in the pelvis, which was isoechoic, mainly solid, regular in shape, and clear in boundary. (C) Abdominal ultrasound showed a large number of fluid dark areas in the pelvic cavity, which were deep in the pelvic cavity, about 1.8 cm. (D) CT of the pelvic cavity showed a large cystic and solid mass in the pelvis, with a larger cross-sectional area of about 190 × 116 mm.
An ultrasound examination revealed a cystic-solid mass (17.3*19.4*9.7 cm) in the right adnexal region, which was suspected to originate from the right ovary (Figures 2B, C). Further pelvic CT imaging revealed a large mass in the pelvic and abdominal cavities, with a large amount of effusion in both the pelvic and abdominal cavities as well as the right pleural cavity (Figure 2D). Given these findings, the clinician suspected Pseudo-Meigs syndrome and referred her to the gynecology department for surgery.
During laparotomy, ~2,000 ml of clear brown-yellow ascites were found in the abdominal cavity. Scattered miliary nodules were observed on the peritoneum and intestinal canal, along with a 20*18*10 cm cystic-solid mass in the right ovary. An intraoperative pathology report showed a poorly differentiated carcinoma in the right adnexa, with a high probability of signet cell carcinoma.
The patient underwent radical lymphadenectomy, total hysterectomy, and bilateral salpingo oophorectomy. Postoperative pathological examination confirmed the presence of signet ring cells filled with mucin in the right ovarian stroma, which was characteristic of a Krukenberg tumor. The results of immunohistochemistry and special staining were as follows: vimentin (interstitial +), CD10 (partial parenchymal cell +), a-inhibin (partial stromal cell +), Her-2 (1+, positive control 3 +), CK7 (+), CK20 (+), CK (+), CDX-2 (+), Villin (+), CLDN18.2 (+), SATB2 (-), CA-125(-), AB-PAS (PAS+), and reticulation (+). These findings suggest that the Krukenberg tumor involving the ovary originated from the gastrointestinal tract. Ascites examination showed scattered atypical cells, possibly tumor cells, with PAS (+) suggesting a likely origin from the digestive system.
She was diagnosed with poorly differentiated adenocarcinoma of the cardia and gastric angles, with the majority of the tumor composed of signet ring cell carcinoma, based on gastroscopy and pathology examinations conducted after surgery. This confirmed the diagnosis of Pseudo-Meigs' syndrome caused by a Krukenberg tumor. After the surgery, the pleural effusion resolved, her respiratory symptoms improved, and the ascites significantly decreased. She was then transferred to an oncology hospital for chemotherapy.
3 Discussion
Many clinicians broadly classify primary metastatic ovarian tumors originating from the gastrointestinal tract as Krukenberg tumors (10). However, the diagnosis of Krukenberg tumor is based on the WHO diagnostic criteria, which were established from the pathological descriptions by Novak et al.
These criteria include the presence of ovarian stromal infiltration, mucin-producing signet ring cells, and sarcomatous hyperplasia of the ovarian stromal tissue. The presence of signet ring cells is the key diagnostic feature of Krukenberg tumor (11). Krukenberg tumor is a selectively metastatic tumor, most commonly forming along the gastric-ovarian metastatic axis.
Interestingly, gastric cancer can metastasize selectively to the ovary without involving other tissues. However, the exact pathway and mechanism of gastrointestinal tumor metastasis to the ovary remain unclear. At present, three possible metastatic pathways exist: lymphatic metastasis, hematogenous (blood) metastasis, and transcoelomic (abdominal implant) metastasis (12).
Due to the low incidence of Krukenberg tumors, they are often misdiagnosed as primary gynecological tumors. Compared with primary ovarian cancer, the clinical manifestations of Krukenberg tumor are less specific, with common symptoms including abdominal pain and distension, often accompanied by ascites. These symptoms are followed by clinical symptoms related to the primary tumor, such as nausea and hematemesis (6, 13).
However, in our case, the patient initially presented with dyspnea and symptoms resembling a respiratory infection, leading her to first seek treatment in the respiratory department, where she was treated for inflammation. It was only later, after a pelvic mass was identified, that she was referred to the gynecology department. She had obvious respiratory symptoms and a large amount of pleural effusion, resulting in a few weeks of hospitalization before the gynecologist considered that the patient's symptoms might be consistent with Meigs' syndrome or Pseudo-Meigs' syndrome.
Preoperative ultrasound showed a solid-cystic mixed ovarian mass with malignant characteristics that differed from the typical presentation of Meigs' syndrome, usually caused by benign ovarian tumors. This finding made us more inclined to diagnose Pseudo-Meigs' syndrome before surgery, although the primary site of the tumor in the digestive tract had not been confirmed at that time.
As is well known, Meigs' syndrome is rare and is characterized by benign ovarian tumors, including fibroma, theca cell tumor, Brenner tumor, and granulosa cell tumor, accompanied by pleural and peritoneal effusion, with fibroma being the most common type. A key feature of Meigs' syndrome is that ascites and pleural effusion typically resolve following tumor resection (14). In contrast, Pseudo-Meigs' syndrome presents similarly but involves different types of tumors, including primary malignant tumors, metastases, or other benign ovarian tumors (15). The specific nature of the pelvic mass can only be confirmed through surgical resection and histological examination.
In the past, there have been a few reported cases of Pseudo-Meigs' syndrome resulting from secondary ovarian tumors associated with gastrointestinal cancer, but Pseudo-Meigs' syndrome caused by Krukenberg tumors is extremely rare. In 1950, Dick et al. reported the first case of Pseudo-Meigs' syndrome resulting from a Krukenberg tumor of gastric cancer (1). However, only a limited number of cases have been reported since then. To the best of our knowledge, this case is the first report of Pseudo-Meigs' syndrome caused by a Krukenberg tumor of gastric cancer in China. Table 1 summarizes the reported cases of this disease, including our case and those reported locally in Spain and Japan.
One notable feature across these cases is the younger age of onset in the patients. Krukenberg tumors are indeed more common in younger women than primary ovarian cancer, possibly due to the fact that functioning ovaries, with their abundant blood supply, are more prone to metastatic disease. Another key observation is that the primary site of the tumor in these patients is consistently the stomach, suggesting that gastric cancer may have a particular propensity to cause Pseudo-Meigs' syndrome when it metastasizes as a Krukenberg tumor (16).
Moreover, nearly all of the patients in these cases presented with symptoms of abdominal distension and dyspnea. Kiyokawa et al. studied 120 cases of Krukenberg tumor and found that abdominal swelling was the most common clinical manifestation, with only five patients reporting symptoms of dyspnea, chest pain, or cough (17). This finding indicates that dyspnea may be a more common symptom in cases of Pseudo-Meigs' syndrome caused by Krukenberg tumors.
In clinical practice, especially in respiratory departments, it is crucial not to overlook the possibility of abdominal pathology in patients presenting with dyspnea and pleural effusion. When symptoms such as abdominal pain, distension, or palpable lumps or effusions are present, further digestive and gynecological examinations should be conducted to improve the accuracy of the diagnosis.
In addition, in all the cases we reviewed, the primary tumors were consistently identified as poorly differentiated adenocarcinoma. The ovarian tumors in these cases were typically bilateral, and the onset of tumor metastasis was predominantly simultaneous, which are common characteristics of Krukenberg tumors (18). However, what sets Pseudo-Meigs' syndrome caused by a Krukenberg tumor of gastric cancer apart is that pleural effusion is more frequently observed on the right side, and most patients exhibit only mildly or moderately elevated levels of CA125.
Interestingly, in the reviewed cases of Pseudo-Meigs' syndrome caused by gastric cancer, including our case, CA-125 levels were elevated to < 500 U/ml, even in the presence of massive ascites, suggesting that CA-125 may not be a reliable marker for distinguishing between benign and malignant tumors and for assessing the extent of dissemination in such cases. In addition, it has been reported that Meigs' syndrome caused by benign tumors can lead to a significant increase in CA-125 levels (19, 20), reaching up to 1,835 U/ml. Therefore, when making a differential diagnosis, more attention should be paid to imaging findings and clinical symptoms, with pathological results serving as the definitive standard.
Finally, all of these patients underwent ovariectomy. Massive ascites and pleural effusion are characteristic manifestations of Pseudo-Meigs' syndrome, although the precise mechanism of their formation remains unclear. It is speculated that fluid from the tumor infiltrates the abdominal cavity through the serosa surface and then enters the pleural cavity, either unilaterally or bilaterally, via lymphatic vessels that connect the pleural cavity with the abdominal cavity (21). This process likely explains why ascites and pleural effusion typically resolve after the surgical removal of ovarian tumors.
However, in this particular case, while the pleural effusion quickly disappeared after surgery, the ascites persisted. The most likely explanation is that we found a large number of metastatic lesions in the peritoneum during the operation, contributing to both ascites associated with Pseudo-Meigs' syndrome and malignant ascites caused by these metastatic lesions. This persistence of ascites may indicate a poor prognosis for the patient.
In general, the detection of a Krukenberg tumor precedes the diagnosis of the primary tumor, which is often small, asymptomatic, and may not even be detected until several years after an ovariectomy, complicating the initial diagnosis. Since most primary lesions do not cause symptoms, patients are often unaware of the disease until ovarian metastasis has progressed.
Statistics show that only 20% to 30% of cases have a prior history of gastric cancer or other organ cancer (22). By the time the tumor is discovered, it is frequently at an advanced stage. Overall, the prognosis for Krukenberg tumor is poor, with a mean survival period of only 14 months (23). Differentiating between hydrothorax and ascites caused by tumors can be challenging, highlighting the importance of identifying the primary tumor that causes these symptoms before surgery to guide treatment as soon as possible.
There are currently no guidelines for the treatment of Pseudo-Meigs' syndrome caused by a Krukenberg tumor. At present, surgery is generally considered the main treatment approach. In addition to the removal of the primary tumor when feasible, both bilateral and unilateral ovarian metastases should be addressed simultaneously, along with the excision of the uterus, bilateral adnexa, and greater omentum.
Most of the cases we reviewed reported that patients experienced symptom relief, particularly with a reduction in hydrothorax and ascites, following surgical intervention. The survival time for patients who underwent surgery was significantly longer compared to those who only received a biopsy or did not undergo surgery at all. Therefore, for patients who are able to tolerate surgery, it is recommended to remove both the primary tumor and any metastases simultaneously. Even if the resection of the primary cancer is palliative, it can reduce the tumor burden, alleviate pain or obstructions, and ultimately improve the patient's quality of life.
In summary, we present a case of a Krukenberg tumor causing Pseudo-Meigs syndrome, where dyspnea was the first symptom. In this article, we aim to raise awareness of the possible clinical presentations of this tumor. When symptoms cannot be explained by routine examination, it is crucial to examine the patient thoroughly. Although Krukenberg tumor is a rare ovarian tumor, it should be considered in the differential diagnosis, particularly when patients, especially within the relevant age group, present with unusual symptoms such as dyspnea. Recognizing this syndrome can lead to a quicker diagnosis and appropriate treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Tiantan Hospital affiliated to Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SQ: Conceptualization, Data curation, Writing – original draft. HJ: Conceptualization, Data curation, Investigation, Writing – original draft. HD: Investigation, Methodology, Writing – original draft. LF: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by grants from the Beijing Natural Science Foundation of China (No. J200002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dick HJ, Spire LJ, Worboys CS. The association of Meigs's syndrome with krukenberg tumors. N Y State J Med. (1950) 50:1842–3.
2. An-Chieh Liu A, Chen C-H, Liu W-M, Chang C-W. A rare krukenberg tumor arising from a primary adenocarcinoma of the small intestine. Taiwan J Obstet Gynecol. (2018) 57:319–22. doi: 10.1016/j.tjog.2018.02.025
3. Tai H, Yang Q, Wu Z. Sun Sa, Cao R, Xi Y, et al. Pd-L1 expression predicts a distinct prognosis in krukenberg tumor with corresponding origins. J Immunol Res. (2018) 2018:9485285. doi: 10.1155/2018/9485285
4. Cetin B, Aslan S, Akinci M, Atalay C, Cetin A. A long surviving case of Pseudomeigs' syndrome caused by krukenberg tumor of the stomach. Jpn J Clin Oncol. (2005) 35:221–3. doi: 10.1093/jjco/hyi055
5. Zhou Z, Li C, Wang Z, Haybaeck J, Zhang C. Cd44v6 acts as a directional responding factor in the process of transcoelomic metastasis from gastric carcinoma to Krukenberg tumor. Expert Rev Mol Diagn. (2023) 23:583–8. doi: 10.1080/14737159.2023.2223981
6. Agnes A, Biondi A, Ricci R, Gallotta V, D'Ugo D, Persiani R. Krukenberg tumors: seed, route and soil. Surg Oncol. (2017) 26:438–45. doi: 10.1016/j.suronc.2017.09.001
7. Bayod MJH, Carlón ME, Idoate MA. Pseudomeigs syndrome in a patient with Krukenberg's tumor. Rev Med Univ Navarra. (2007) 51:19–22.
8. Okazaki Y, Yonezawa K, Shimomatsuya T, Komai Y, Yukioka N. A case of pseudo-Meigs' syndrome caused by metastatic ovarian tumors from gastric cancer. Nihon Shokakibyo Gakkai Zasshi. (2009) 106:529–35.
9. Horimatsu T, Miyamoto Si, Mashimo Y, Okabe H, Mikami Y, Chiba T, et al. Pseudo-Meigs' syndrome caused by a Krukenberg Tumour of gastric cancer. Intern Med. (2015) 54:2595–7. doi: 10.2169/internalmedicine.54.4088
10. Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg's tumor. Gynecol Oncol. (2001) 82:105–9. doi: 10.1006/gyno.2001.6210
11. Scully RE, Sobin LH. Histologic typing of ovarian tumors. Arch Pathol Lab Med. (1987) 111:794–5.
12. Qiu L, Yang T, Shan X-H, Hu M-B, Li Y. Metastatic factors for Krukenberg tumor: a clinical study on 102 cases. Med Oncol. (2011) 28:1514–9. doi: 10.1007/s12032-010-9610-4
13. Bonomi RE, Kovoor J, Zaki M, Szlaczky M, Christensen M, Kupsky W, et al. Glioblastoma metastatic to the ovary, a very different Krukenberg tumor? Pract Radiat Oncol. (2018) 8:373–5. doi: 10.1016/j.prro.2018.04.002
14. Argento AC, Gillespie CT. Pleural disease in women. Semin Respir Crit Care Med. (2019) 40:402–9. doi: 10.1055/s-0039-1695050
15. Tan N, Jin K-Y, Yang X-R, Li C-F, Yao J, Zheng H, et al. Case of death of patient with ovarian fibroma combined with meigs syndrome and literature review. Diagn Pathol. (2022) 17:83. doi: 10.1186/s13000-022-01258-9
16. La Fianza A, Alberici E, Pistorio A, Generoso P. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori. (2002) 88:284–7. doi: 10.1177/030089160208800408
17. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. (2006) 30:277–99. doi: 10.1097/01.pas.0000190787.85024.cb
18. Xie H, Erickson BJ, Sheedy SP, Yin J, Hubbard JM. The diagnosis and outcome of krukenberg tumors. J Gastrointest Oncol. (2021) 12:226–36. doi: 10.21037/jgo-20-364
19. Stabile G, Zinicola G, Romano F, Laganà AS, Pozzolo CD, Ricci G. Pelvic mass, ascites, hydrothorax: a malignant or benign condition? Meigs syndrome with high levels of Ca 125 Prz. Menopauzalny. (2021) 20:103–7. doi: 10.5114/pm.2021.106100
20. Loué VAS, Gbary E, Koui S, Akpa B, Kouassi A. Bilateral ovarian fibrothecoma associated with ascites, bilateral pleural effusion, and marked elevated serum Ca-125. Case Rep Obstet Gynecol. (2013) 2013:189072. doi: 10.1155/2013/189072
21. Hogan ME, Andreotti R. Ovarian fibroma presenting with meigs syndrome. Ultrasound Q. (2015) 31:215–8. doi: 10.1097/RUQ.0000000000000144
22. Holtz F, Hart WR. Krukenberg tumors of the ovary: a clinicopathologic analysis of 27 cases. Cancer. (1982) 50:2438–47.
Keywords: Krukenberg tumor, Pseudo-Meigs' syndrome, ovary, dyspnoea, gastric cancer
Citation: Qiu S, Jiang H, Ding H and Feng L (2024) Krukenberg tumor arising from gastric cancer presenting as Pseudo-Meigs' syndrome: a case report and literature review. Front. Med. 11:1427568. doi: 10.3389/fmed.2024.1427568
Received: 04 May 2024; Accepted: 20 August 2024;
Published: 13 September 2024.
Edited by:
Mattia Dominoni, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Dabao Xu, Central South University, ChinaGuglielmo Stabile, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), Italy
Copyright © 2024 Qiu, Jiang, Ding and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Feng, bHVjeWZlbmcxOTY2QDE2My5jb20=
 Shikang Qiu1
Shikang Qiu1 Limin Feng
Limin Feng