- Department of Respiratory Medicine, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, Zhejiang, China
Background: Chlamydia abortus causes abortions in ruminants; it can also cause miscarriages and stillbirths in pregnant women. However, it rarely causes pneumonia in humans. Here, we report a case of severe community-acquired pneumonia caused by C. abortus.
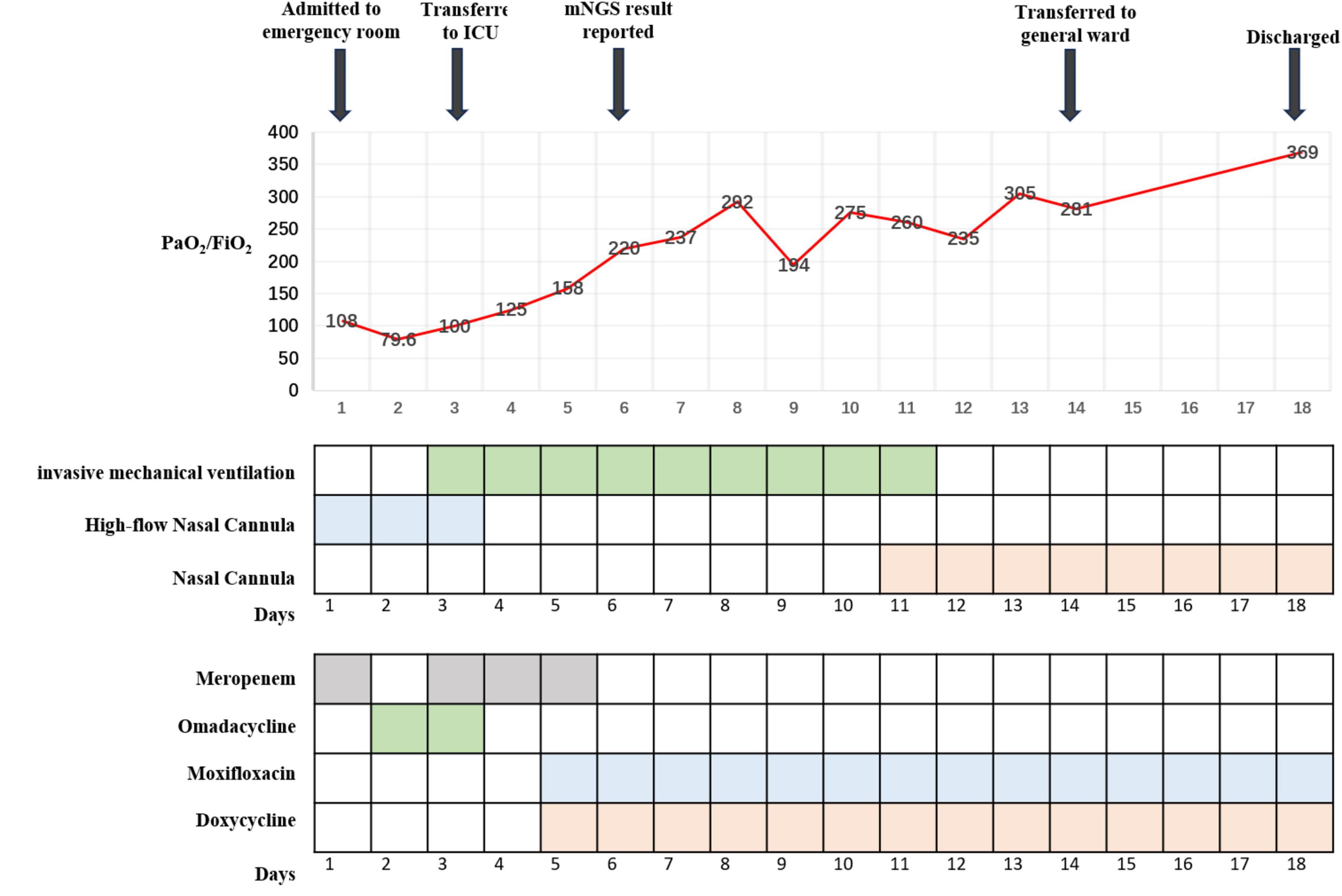
Case presentation: On admission to our hospital, a 74-year-old woman reported that she had had a fever, cough, phlegm in her throat, and shortness of breath for 10 days. In the local hospital, she was initially diagnosed with community-acquired pneumonia and treated with piperacillin–tazobactam for 4 days. However, her condition worsened, and she was therefore transferred to our hospital. On arrival at our emergency department, she was diagnosed with severe community-acquired pneumonia and treated with a high-flow nasal cannula and meropenem; she was then transferred to the Department of Respiratory Medicine. There, her condition continued to worsen despite continued treatment with the high-flow nasal cannula and omadacycline. After 24 h and emergency tracheal intubation, the patient was sent to the intensive care unit (ICU) for further treatment. The doctors in the ICU again adjusted the treatment, this time to meropenem along with mechanical ventilation; they also instituted methylprednisolone, ulinastatin, nadroparin calcium, and human immunoglobulin. In addition, bronchoalveolar lavage fluid was sent for metagenomic next-generation sequencing (mNGS). Subsequent mNGS suggested the presence of C. abortus, sequence number 5072; we therefore discontinued the meropenem and implemented a combination of doxycycline and moxifloxacin. After 8 days of treatment in the ICU, the patient’s condition improved; she was then extubated and, 3 days later, transferred back to the respiratory medicine department. The respiratory physician continued to administer doxycycline and moxifloxacin for 4 days, after which the patient was discharged with medication. A month later, a repeat computed tomography (CT) scan of the chest suggested that the lesions in both lungs had been largely absorbed.
Conclusion: C. abortus can occasionally cause pneumonia in humans and, rarely, severe, life-threatening pneumonia. mNGS is uniquely suited for the early detection of this unusual infection. The combination of doxycycline and quinolones has been shown to be effective in severe pneumonia caused by C. abortus.
Introduction
Chlamydia, as an obligate intracellular Gram-negative bacterium, is known to be responsible for several serious global healthcare challenges. Among these bacteria, Chlamydia abortus is an especially important zoonotic pathogen; it mainly causes infections in ruminants but, less frequently, may cause pneumonia in humans. Even more rarely, it can initiate an extremely severe pneumonia (1). Thus far, only six cases of pneumonia due to C. abortus have been reported worldwide; of these, five were not severe (2–6) and only one was severe (7). To further raise awareness of this rare disease, we here describe a case of severe pneumonia caused by C. abortus.
Case presentation
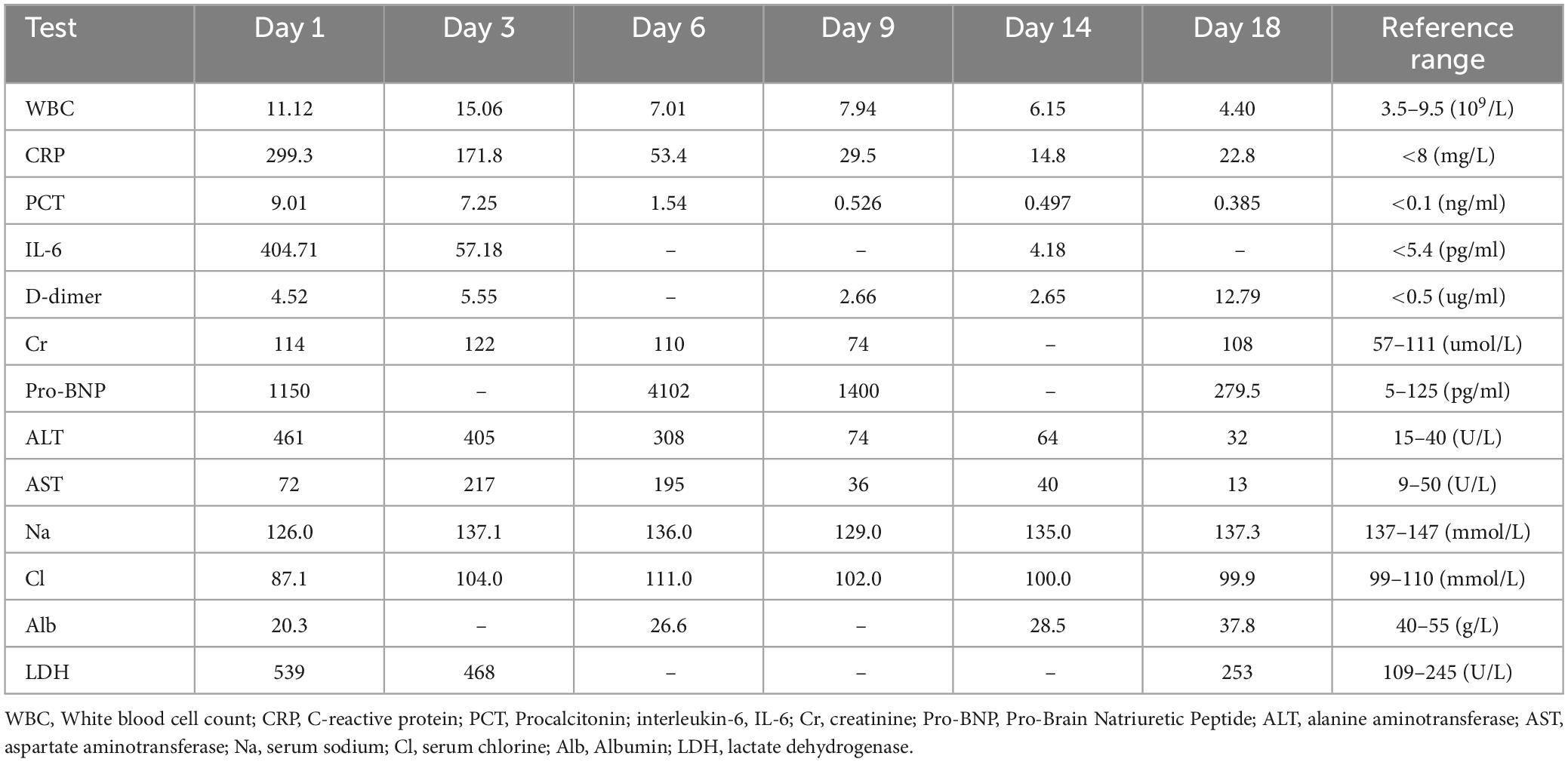
A 72-year-old woman was admitted to our emergency department on December 1, 2023, with complaints of fever as well as cough, phlegm in the throat, and shortness of breath, all of which had been present for 10 days. She had had a cerebral infarction a year earlier as well as an aneurysm of the left internal carotid artery but did not take medication regularly. Nevertheless, she said that she was able to take care of herself. Ten days earlier, she had developed a cold and a fever with a temperature of about 38.0°C along with a cough, phlegm in her throat, dyspnea, generalized muscle aches and pains, and intermittent diarrhea. Her symptoms were not relieved by cold medicine. Thus, after 5 days of illness, she went to her local hospital. There, after computed tomography (CT) of the chest, she was diagnosed with community-acquired pneumonia. The CT pointed to an infection in the upper lobe of the left lung (Figure 1: 1A–C), and she was given piperacillin–tazobactam (4.5 g q8h as an intravenous infusion) for 4 days. However, her condition did not improve. She was then admitted to the emergency department of our hospital.
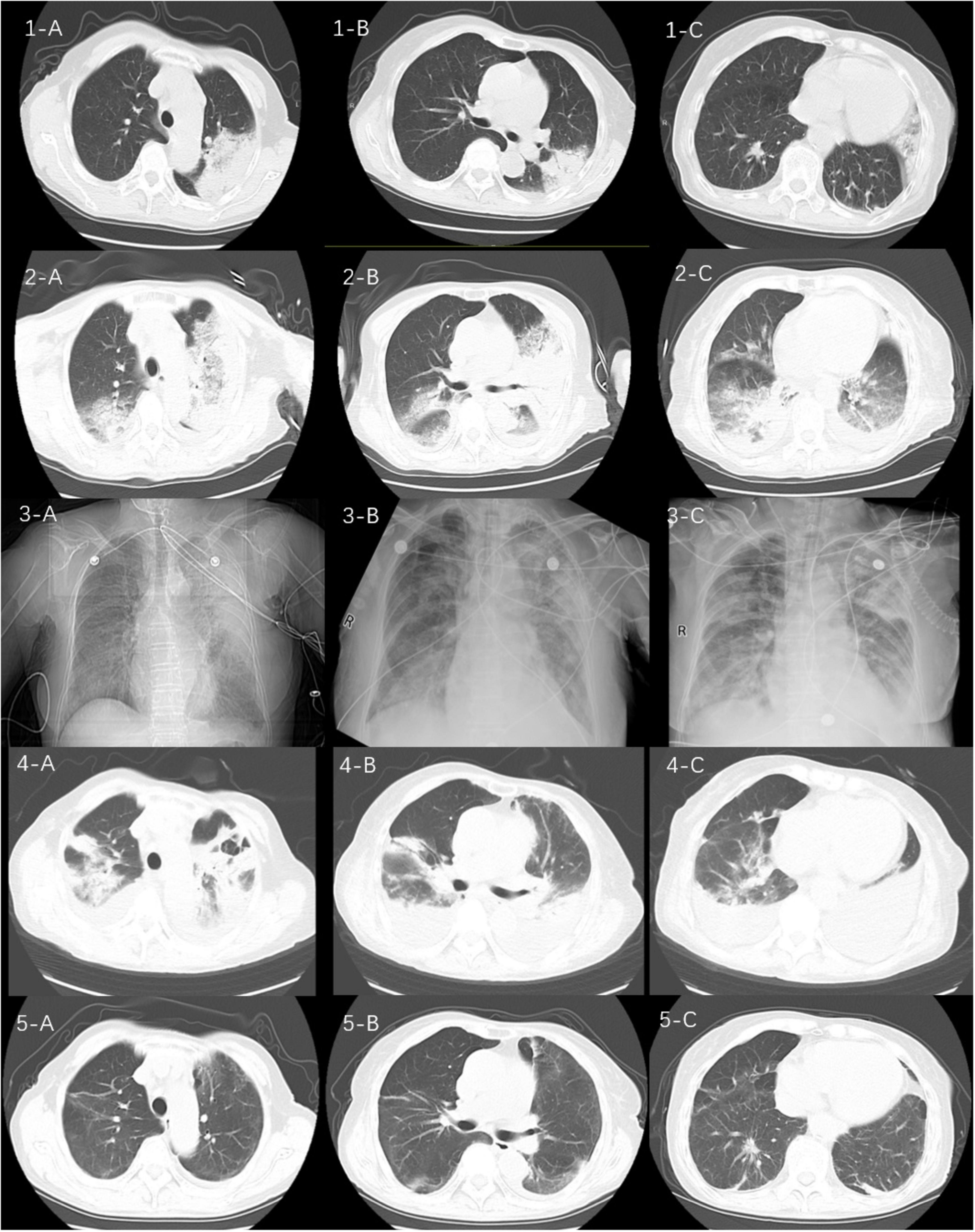
Figure 1. Sequential computed tomograms (CTs) of the patient’s chest. CT at a local hospital (1A–C) appeared to show a solid shadow in the upper lobe of the left lung. CT on admission (2A–C) showed multiple exudative lesions in both lungs. The next images (3A–C) represent the patient’s chest x-ray findings at the time of admission to our hospital, on the 5th and 10th days, respectively. CTs on the 15th day of admission (4A–C) suggested infection in both lungs with partially absorbed and partially enlarged lesions as well as an enlarging pleural effusion on the left side. CTs 1 month after discharge (5A–C) show substantial resorption of the lesions in both lungs.
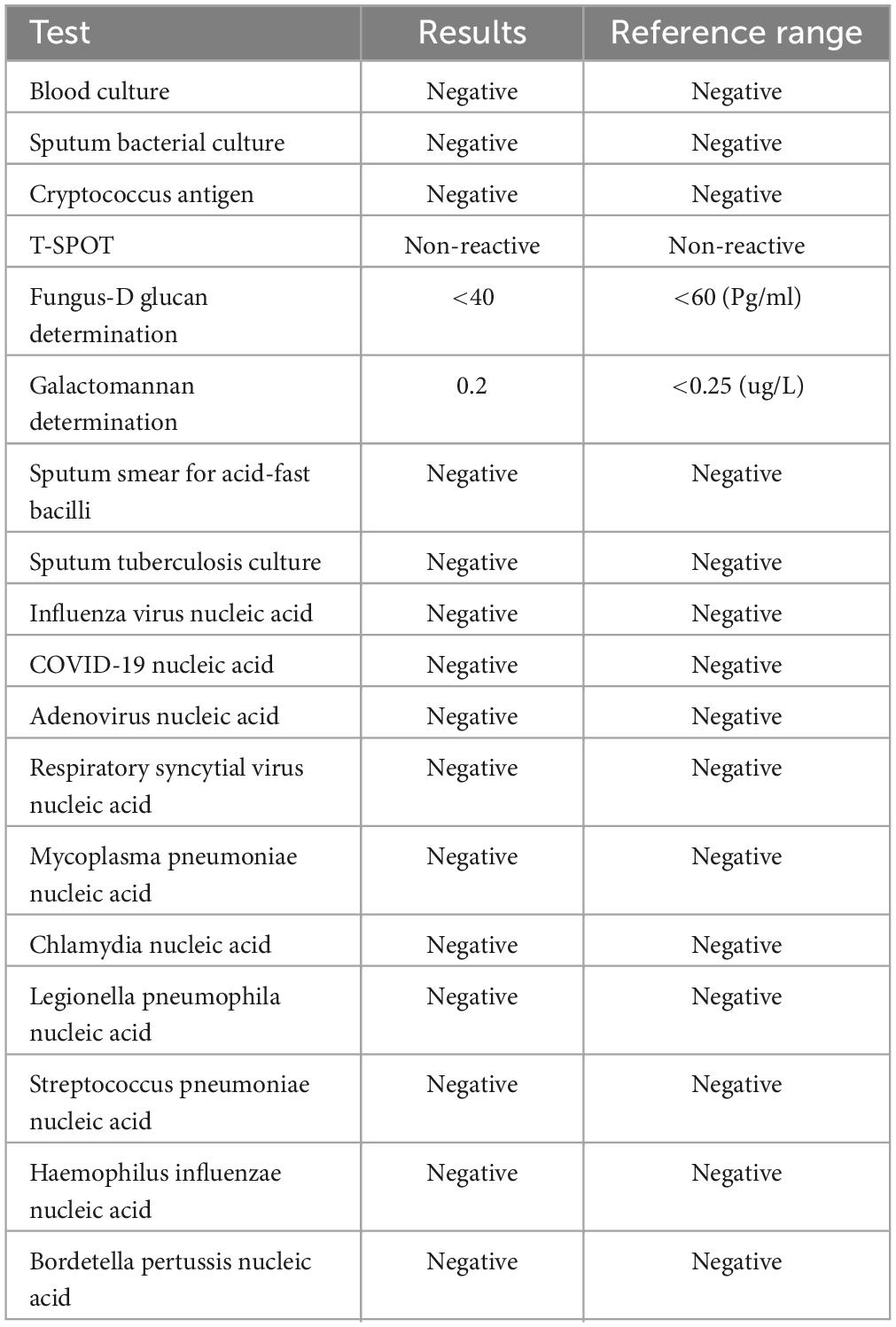
On the patient’s arrival at our emergency department, physical examination showed the following: temperature, 38.9°C; respirations, 26/min; pulse, 102/min; blood pressure, 130/75 mmHg; oxygen saturation, 72% (under mask oxygen of 4 L/min); clear consciousness; tachypnea; rhythmic dry and wet rales audible in both lungs; abdominal tenderness; and no swelling of the limbs. Laboratory tests showed the following: calcitonin, 9.0 ng/mL; C-reactive protein, 299 mg/L; leukocyte count, 11.12 × 109/L; neutrophil ratio, 96.4% (which was significantly higher than normal). Arterial blood gas analysis showed type I respiratory failure and an oxygenation index of 108 mmHg. Blood biochemistry suggested hyponatremia and hypochlorhydria. Blood creatinine, liver enzymes, and lactate dehydrogenase were significantly higher than normal. The levels of plasma pro-brain natriuretic peptide (pro-BNP) were 1150 pg/mL (Table 1). A repeat CT scan of the chest suggested infectious lesions in both lungs and a small pleural effusion on each side (Figure 1: 2A–C). Color Doppler ultrasound of the vasculature of both lower extremities revealed atherosclerosis and intermuscular venous thrombosis of both lower legs. Color Doppler ultrasound of the heart showed aortic arteriosclerosis. Tricuspid valve regurgitation was mild; the ejection fraction was 65%. The patient was diagnosed with severe community-acquired pneumonia and was ventilated by a high-flow nasal cannula (oxygen concentration, 80%; flow rate, 55 L/min) with the oxygen saturation maintained at about 95%; she was also medicated with meropenem (0.5 g q8h by intravenous infusion) and methylprednisolone (40 mg q12h by intravenous infusion). At one point during this procedure, her blood pressure dropped to 90/52 mmHg; however, it returned to the normal level after active fluid replacement.
The next day the patient was admitted to the respiratory ward, and ventilation by high-flow nasal cannula (oxygen concentration 75%, flow rate 50 L/min) was continued to maintain oxygen saturation at about 95%. The retest of her blood inflammation index was still significantly higher than normal (Table 1), and it was felt that the atypical pathogen infection had to be covered. The treatment was therefore adjusted to omadacycline (0.1 g qd by intravenous infusion with 0.2 g being given on the first day), methylprednisolone (40 mg q12h by intravenous infusion), ambroxol hydrochloride (30 mg bid by intravenous infusion) for phlegm, and nadroparin calcium (4100 U qd by hypodermic injection) for anticoagulant and symptom-supportive therapy.
To detect the etiology of her pneumonia, the patient underwent a series of microbiologic diagnostic tests for bacteria, fungi, and viruses, and including staining and culture, serologic testing, and others. However, all of the tests were negative (Table 2). Thus the patient’s clinical symptoms continued to deteriorate, with oxygen saturation maintained between 89 and 95%. She experienced shortness of breath and—after being given an emergency tracheal intubation for mechanical ventilation 24 h after admission—was transferred to the ICU.
At the ICU, her treatment was adjusted to meropenem (1.0 g q8h by intravenous infusion) with continuing methylprednisolone, ambroxol hydrochloride, and nadroparin calcium plus the addition of thymopeptide (1.6 mg qd by hypodermic injection) and immunoglobulin (10 g qd by intravenous infusion) to improve the immunity. On the next day, bedside bronchoscopy showed that there was more white mucous sputum in both bronchi, and the lumen was clear after aspiration. The bronchoalveolar lavage fluid was then sent for mNGS examination. Two days later, the result suggested C. abortus, with 5072 sequence numbers and a relative abundance of 95.03%. A repeat bedside radiograph of the chest showed infection in both lungs, with greater infection in the right lung and partial absorption of infection in the left lung by comparison with the previous (December 1, 2023) CT localization film of the chest (Figure 1: 3B). Therefore the patient was given moxifloxacin (0.4 g qd by intravenous infusion) combined with doxycycline (0.1 g bid PO), and the meropenem was stopped. The patient’s condition gradually stabilized under these treatments; methylprednisolone was reduced; and eventually—after 8 days of mechanical ventilation—the tracheal tube was removed to be replaced by a nasal cannula for the administration of oxygen. The bedside chest radiograph was then reviewed again; it pointed to infections in both lungs, similar to the previous one (December 6, 2023), with a small pleural effusion on the left side (Figure 1: 3C). After extubation, the patient appeared to have blood in her sputum. A review of the color Doppler ultrasound of her lower extremity vessels suggested that there was no increase in thrombus, so the nadroparin calcium was stopped. Three days after extubation, the patient was stable and transferred back to respiratory medicine.
In the respiratory medicine department, moxifloxacin and doxycycline were continued up to 14 days. During this period, CT pulmonary angiography did not indicate a thrombus. A repeat CT scan of the chest suggested partial resorption of the lesions whereas others had increased, as had the bilateral pleural effusions (Figure 1: 4A–C). After 14 days of antichlamydial treatment, the patient’s blood inflammatory index had returned to normal; therefore, the antibiotics were stopped. Because the serum D-dimer level increased to 12.79 μg/mL, after communicating with the patient’s family members, the patient was discharged with a prescription for rivaroxaban 10 mg qd PO. A month after discharge, the patient was reexamined by CT of the chest, indicating significant absorption of both lung lesions and pleural effusions (Figure 1: 5A–C). The full timeline of hospitalization and clinical treatment is shown in Figure 2.
Discussion
Chlamydia abortus used to be classified as a subspecies of Chlamydia psittaci, which caused mainly animal infections, with abortion and stillbirth being common among pregnant cows and sheep (8) and could also lead to miscarriage in pregnant women, sepsis in pregnancy, and pelvic inflammatory disease (3, 9, 10). However, thus far, only six cases of human pneumonia due to C. abortus have been found worldwide. C. abortus organisms can be excreted through urine, feces, lactated milk, amniotic fluid, placenta, aborted fetus, and other routes in diseased animals (11). Based on the findings of previously reported cases, most patients infected with C. abortus appear to have a history of direct or indirect contact with infected animals (2–7). This suggests that humans can become ill through direct contact with infected animals or secondary owing to contamination of the environment. The patient in this case was a farmer who fed small animals such as chickens, ducks, and pigs at home and had regular contact with these animals. Therefore we suspected that the patient might have developed pneumonia through direct contact with animals infected with C. abortus. No one else in the family was known to have had a similar infection.
C. abortus infection initially presents with flulike symptoms, causing fever, headache, and weakness in the limbs. Due to the lack of specificity of the clinical symptoms and clinicians’ low level of knowledge of the disease, it is easy to miss or misdiagnose this disease. Chlamydial infection can also cause serious visceral complications; therefore, clinicians need to be alert to this possibility (12, 13). In this case, the patient presented with typical influenza-like symptoms (such as fever and generalized muscle pain) at the beginning of the disease, followed by respiratory symptoms (such as cough, phlegm, and dyspnea). These were accompanied by extrapulmonary manifestations such as diarrhea as well as laboratory tests suggesting hyponatremia and hypochlorhydria, impaired hepatic and renal function, and elevated lactate dehydrogenase. These are similar to the clinical features of Legionella pneumonitis (14) and C. psittaci pneumonia (15).
This patient’s lung imaging showed rapid progression from a solid lesion in the upper lobe of the left lung to solid lesions in multiple lobes, with air bronchial signs at the site of the solid lesion accompanied by bilateral pleural effusions. These imaging features are nonspecific and are also seen in streptococcal and other atypical infections, making early diagnosis of the pathogen critical.
Pneumonia caused by C. abortus is very rare and cannot be detected by current detection methods, such as specimen culture, serologic detection, and the polymerase chain reaction. mNGS, as an unbiased method—with its high detection speed, high accuracy, wide coverage, and high throughput (16)—theoretically detects all pathogens in clinical samples and is particularly suitable for rare and complex infectious diseases with atypical etiology (17). In this case—after blood culture, sputum culture, and chlamydial nucleic acid testing were all found to be negative—we were able to diagnose C. abortus by mNGS.
C. abortus is unaffected by beta-lactam antibiotics because it lacks a cell wall. Chlamydia is usually sensitive to antibiotics that inhibit DNA and protein synthesis, including tetracyclines, macrolides, and quinolones. Therefore, tetracyclines are used as first-line therapeutic agents, whereas other approaches include macrolides and quinolones (18). The length of antibiotic administration is usually 10–14 days. In this case, immediately after confirmation of C. abortus infection, the antibiotics were adjusted to doxycycline combined with moxifloxacin, which had an immediate effect. Repeat CT of the chest 1 month later showed substantial resorption of both lung lesions.
In summary, ruminants can transmit Chlamydia abortus to humans. Its early diagnosis is low owing to nonspecific clinical signs and imaging features, making it hard to detect by conventional tests. Therefore, if early β-amide antibiotics are ineffective in patients with pneumonia who have a history of ruminant exposure, the possibility of infection with Chlamydia abortus must be considered. mNGS of the bronchoalveolar lavage fluid is an important method for diagnosing this disease.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Q-FY: Writing – original draft, Writing – review and editing. C-MS: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the nurses, and residents from the department of respiratory (Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China), who were involved in the management of the patient. We would also like to thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ICU, intensive care unit; mNGS, metagenomic next-generation sequencing; CT, computed tomography; CRP, C-reactive protein; pro-BNP, pro-brain natriuretic peptide.
References
1. Xie G, Hu Q, Cao X, Wu W, Dai P, Guo W, et al. Clinical identification and microbiota analysis of Chlamydia psittaci- and Chlamydia abortus- pneumonia by metagenomic next-generation sequencing. Front Cell Infect Microbiol. (2023) 13:1157540. doi: 10.3389/fcimb.2023.1157540
2. Ortega N, Caro M, Gallego M, Murcia-Belmonte A, Álvarez D, Del Río L, et al. Isolation of Chlamydia abortus from a laboratory worker diagnosed with atypical pneumonia. Irish Vet J. (2015) 69:8. doi: 10.1186/s13620-016-0067-4
3. Pichon N, Guindre L, Laroucau K, Cantaloube M, Nallatamby A, Parreau S. Chlamydia abortus in pregnant woman with acute respiratory distress syndrome. Emerg Infect Dis. (2020) 26:628–9. doi: 10.3201/eid2603.191417
4. Zhu C, Lv M, Huang J, Zhang C, Xie L, Gao T, et al. Bloodstream infection and pneumonia caused by Chlamydia abortus infection in China: A case report. BMC Infect Dis. (2022) 22:181. doi: 10.1186/s12879-022-07158-z
5. Imkamp F, Albini S, Karbach M, Kimmich N, Spinelli C, Herren S, et al. Zoonotic Chlamydiae as rare causes of severe pneumonia. Swiss Med Wkly. (2022) 152:w30102. doi: 10.4414/smw.2022.w30102
6. Liu M, Wen Y, Ding H, Zeng H. Septic shock with Chlamydia abortus infection. Lancet Infect Dis. (2022) 22:912. doi: 10.1016/s1473-3099(21)00756-8
7. Huang J, Liu C, Zhou Z, Xia H, Zhu Z, Lu J, et al. Extracorporeal membrane oxygenation in severe acute respiratory distress syndrome caused by Chlamydia abortus: A case report. Infect Drug Resist. (2023) 16:3893–901. doi: 10.2147/idr.S411331
8. Caspe S, Ewing D, Livingstone M, Underwood C, Milne E, Sargison N, et al. The immune response in the uteri and placentae of Chlamydia abortus-infected ewes and its association with pregnancy outcomes. Pathogens. (2023) 12:846. doi: 10.3390/pathogens12060846
9. Pospischil A, Thoma R, Hilbe M, Grest P, Zimmermann D, Gebbers J. [Abortion in humans caused by Chlamydophila abortus (Chlamydia psittaci serovar 1)]. Schweizer Arch Tierheilkunde. (2002) 144:463–6. doi: 10.1024/0036-7281.144.9.463
10. Walder G, Meusburger H, Hotzel H, Oehme A, Neunteufel W, Dierich M, et al. Chlamydophila abortus pelvic inflammatory disease. Emerg Infect Dis. (2003) 9:1642–4. doi: 10.3201/eid0912.020566
11. Everett K, Bush R, Andersen A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. (1999) 49:415–40. doi: 10.1099/00207713-49-2-415
12. Walder G, Hotzel H, Brezinka C, Gritsch W, Tauber R, Würzner R, et al. An unusual cause of sepsis during pregnancy: Recognizing infection with Chlamydophila abortus. Obstetr Gynecol. (2005) 106:1215–7. doi: 10.1097/01.AOG.0000161060.69470.9c
13. Rohde G, Straube E, Essig A, Reinhold P, Sachse K. Chlamydial zoonoses. Deutsches Arzteblatt Int. (2010) 107:174–80. doi: 10.3238/arztebl.2010.0174
14. Muggetti E, Fusi-Schmidhauser T, Schwarzenbach H, Pons M. [CME: Legionella pneumonia]. Praxis. (2020) 109:658–64. doi: 10.1024/1661-8157/a003467
15. Kong C, Zhu J, Lu J, Xu Z. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. (2021) 134:353–5. doi: 10.1097/cm9.0000000000001313
16. Liu H, Zhang Y, Yang J, Liu Y, Chen J. Application of mNGS in the etiological analysis of lower respiratory tract infections and the prediction of drug resistance. Microbiol Spectr. (2022) 10:e0250221. doi: 10.1128/spectrum.02502-21
17. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock G. Making the leap from research laboratory to clinic: Challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. (2015) 6:e1888–1815. doi: 10.1128/mBio.01888-15
Keywords: severe, community-acquired pneumonia, Chlamydia abortus, metagenomic next-generation sequencing, case report
Citation: Yang Q-F and Shu C-M (2024) Severe community-acquired pneumonia caused by Chlamydia abortus in China: a case report. Front. Med. 11:1426577. doi: 10.3389/fmed.2024.1426577
Received: 01 May 2024; Accepted: 08 July 2024;
Published: 22 July 2024.
Edited by:
Giuseppe Fiorentino, Hospital of the Hills, ItalyReviewed by:
Hrishikesh Pandit, National Heart, Lung, and Blood Institute (NIH), United StatesMartina Maritati, University of Ferrara, Italy
Copyright © 2024 Yang and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong-Fang Yang, cWZ5MTk1NTgxQDE2My5jb20=
†ORCID: Qiong-Fang Yang, orcid.org/0000-0003-4576-7734; Cai-Min Shu, orcid.org/0000-0002-6112-1581
 Qiong-Fang Yang
Qiong-Fang Yang Cai-Min Shu†
Cai-Min Shu†