- 1Department of Respiratory and Critical Care Medicine, Hebei Medical University Third Hospital, Shijiazhuang, China
- 2Department of Clinical Laboratory, Hebei Medical University Third Hospital, Shijiazhuang, China
- 3Hebei Key Laboratory of Intractable Pathogens, Shijiazhuang Center for Disease Control and Prevention, Shijiazhuang, China
Penicillium digitatum is a common plant pathogen that causes citrus rot, which is extremely rare in humans. We report a case of a 66-year-old man with a history of consuming large amounts of citrus fruits, smoking for 30 years, and a history of emphysema. He had experienced intermittent coughing with sputum for more than 10 years and was admitted to the hospital due to worsening of symptoms over the past month. Despite antibiotic treatment, his condition did not improve. Subsequently, bronchoalveolar lavage fluid (BALF) was detected by metagenomic next-generation sequencing (mNGS), which showed the presence of P. digitatum. The fungal culture of BALF also indicated the presence of the Penicillium genus. The diagnosis was lung infection caused by P. digitatum, and the patient was treated with itraconazole. The lung infection was controlled. This is the third reported case of invasive pulmonary fungal infection caused by P. digitatum worldwide at the genus level, and the first reported case in China. Although human infections caused by P. digitatum are rare, as an emerging opportunistic pathogen, the detection of this fungus in immunocompromised patients should still be clinically important.
Introduction
Penicillium species are among the most ubiquitous fungi in the environment (1) and rarely cause human infections. There have been only two reported cases of invasive pulmonary fungal infections caused by Penicillium digitatum at the genus level worldwide (2, 3). However, numerous reports suggest that Penicillium spp. can cause clinically significant diseases in immunocompromised individuals, such as urinary tract infections, corneal infections, cutaneous endocarditis, peritonitis, pneumonia, paravertebral infections, and brain infections (3–5). Penicillium digitatum belongs to the genus Penicillium and is one of the factors responsible for the decay of citrus fruits (6, 7). Although P. digitatum is a rare opportunistic pathogen causing human infections, we report a case of pulmonary fungal infection caused by P. digitatum, which is the first clinical case reported in China and the third reported worldwide.
Case presentation
A 66-year-old man from Hebei, China, was admitted to the hospital on 4 February 2023 (day 1) due to a worsened cough and phlegm for 1 month. Despite the self-administration of cefuroxime, the symptoms did not improve. The patient had a chronic cough and expectoration for over 10 years. Two months ago, he suffered from pelvic fractures and underwent surgery. Six weeks ago, he was infected with COVID-19, which mainly manifested as a fever for 3 days, accompanied by pharyngalgia, cough, and sputum, without dyspnea. The patient visited a community hospital, where he was advised to isolate himself at home and rest without receiving glucocorticoids or other related treatments. His symptoms were basically under control. The patient had been smoking and drinking for over 30 years but had quit both 2 months prior to the visit.
On admission, the physical examination revealed that the patient was emaciated (body mass index = 16.53), with a barrel chest and low respiratory sounds in both lungs, especially in the right lower lung. A computed tomography (CT) scan revealed inflammatory lesions and local consolidation in the right lower lobe, with a small amount of pleural effusion on the right side and bilateral lung emphysema (Figures 1A1, B1). The laboratory results were as follows: WBC was 5.87 × 109/L, but the lymphocyte count (LYM) was decreased to 0.7 × 109/L. Additionally, the patient had mild anemia. The erythrocyte sedimentation rate (ESR) was significantly increased at 118.00 mm/h, and the high-sensitivity C-reactive protein (hsCRP) was also significantly elevated at 136.85 mg/L. Furthermore, the patient had significantly decreased levels of plasma albumin (ALB) at 26.63 g/L, while the liver and kidney function and arterial blood gas analysis indicators were essentially normal.
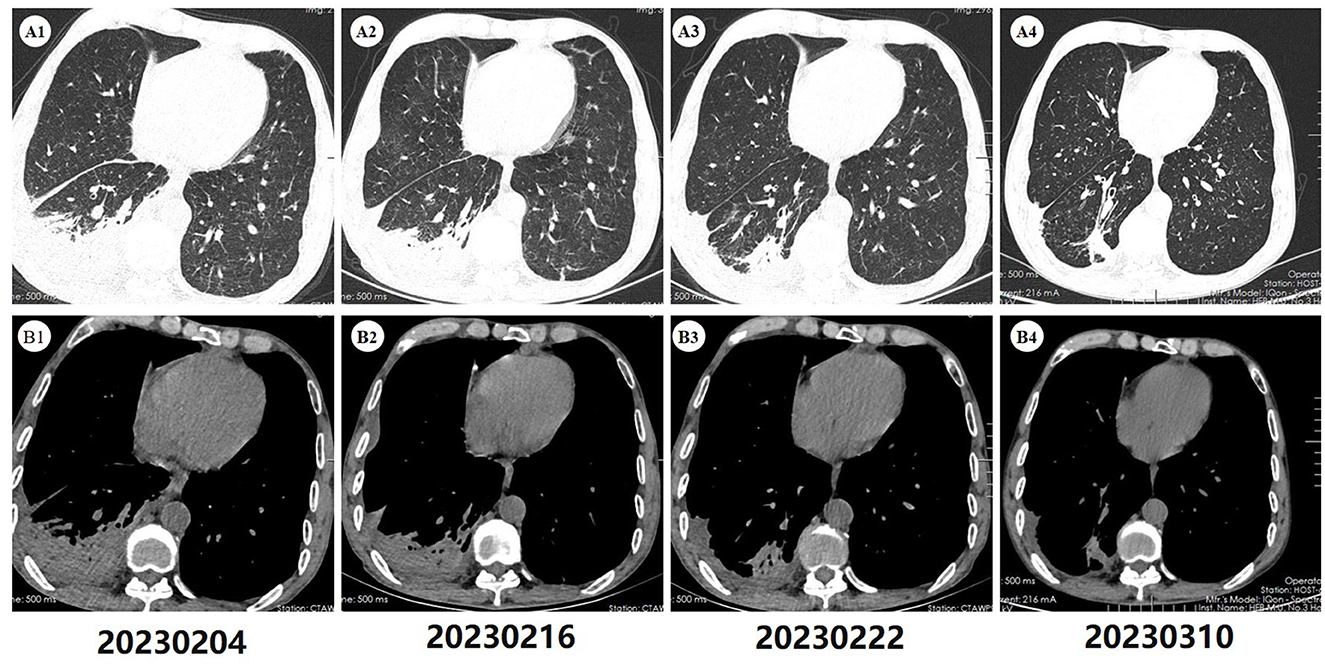
Figure 1. Computed tomography of the chest. (A1, B1) Upon admission, the scan (2023-02-04) revealed inflammatory lesions in the right lower lobe of the lung, along with localized consolidation. Furthermore, minimal pleural effusion was noted in the right pleural cavity and interlobar fissure. Bilateral pulmonary emphysema was also present. (A2, B2) A subsequent scan (2023-02-16), conducted after 12 days of antibiotic therapy, demonstrated persistent pulmonary lesions similar to those observed on the initial admission CT scan, with no apparent improvement. (A3, B3) A follow-up scan (2023-02-22), conducted after 6 days of antifungal therapy, exhibited improvement of pulmonary inflammation and pleural effusion compared to previous scans. (A4, B4) After 22 days of antifungal treatment, a follow-up scan (2023-03-10) demonstrated further absorption of the lung lesions, with only a small amount of residual fibrous streaky changes remaining.
The initial diagnosis suggested that the patient might have community-acquired pneumonia, thus benzylpenicillin and levofloxacin were administered intravenously. After 7 days of treatment, the patient's symptoms did not improve, and repeated chest CT scans revealed no improvement in the pulmonary infiltrates or pleural effusion. The initial treatment was evaluated as ineffective, and three sputum smears, namely, sputum bacterial, fungal cultures, and acid-fast staining, were all negative. Therefore, the patient received an empirical upgrade of antimicrobial therapy with cefoperazone-sulbactam sodium and levofloxacin. Then, the bronchoalveolar lavage fluid (BALF) was collected and sent for metagenomic next-generation sequencing (mNGS), bacterial and fungal cultures, and acid-fast staining. The mNGS (SimcereDx, Nanjing, China) results were reported on the 2nd day of sequencing (day 11), showing the presence of Penicillium digitatum (36 reads with a relative abundance value of 67.92%) (Figure 2). These sequences were submitted to the SRA database at NCBI with mNGS with the accession number PRJNA1102665 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1102665). The BALF smear and acid-fast staining were negative. On day 12, ESR and hsCRP did not significantly decrease, the patient's symptoms did not improve, and repeated chest CT showed no significant improvement (Figures 1A2, B2). Further inquiry of the patient revealed that he had regularly consumed “Shatangju” mandarin, a citrus fruit, in large quantities for the past 3 months. The patient was started on oral itraconazole capsules (200 mg each time, twice a day, taken immediately after meals) for antifungal therapy (day 12) without the above-mentioned antibiotics. Bronchoscopy (day 13) was performed again, and the BALF was collected for fungal and bacterial cultures. On day 16, Penicillium species were found in the fungal culture of the first BALF, and on day 19, the second BALF fungal culture also supported the growth of the same type of Penicillium species (Figure 3). The internal transcribed spacer (ITS) identification of the species was performed. The colony was identified as P. digitatum.
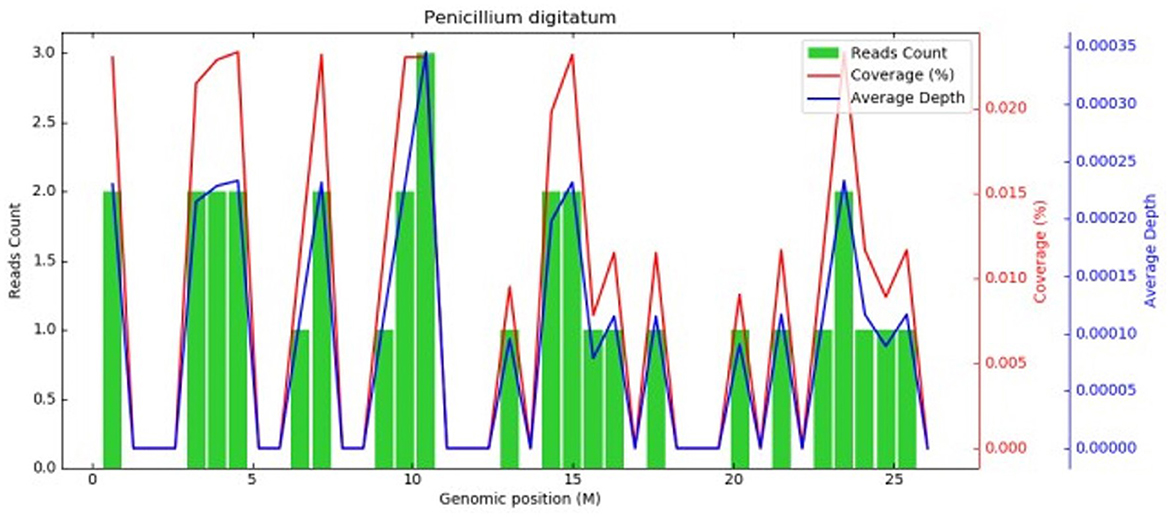
Figure 2. Metagenomic next-generation sequencing. Coverage graph of P.digitatum (36 reads, relative abundance value of 67.92%). The abscissa represents the genome position, the left ordinate is the number of reads of the pathogen aligned on the genome location (The number of map reads shown in green), and the right ordinate is the coverage and average sequencing depth at the corresponding position (coverage and depth, the red and blue lines in the figures).
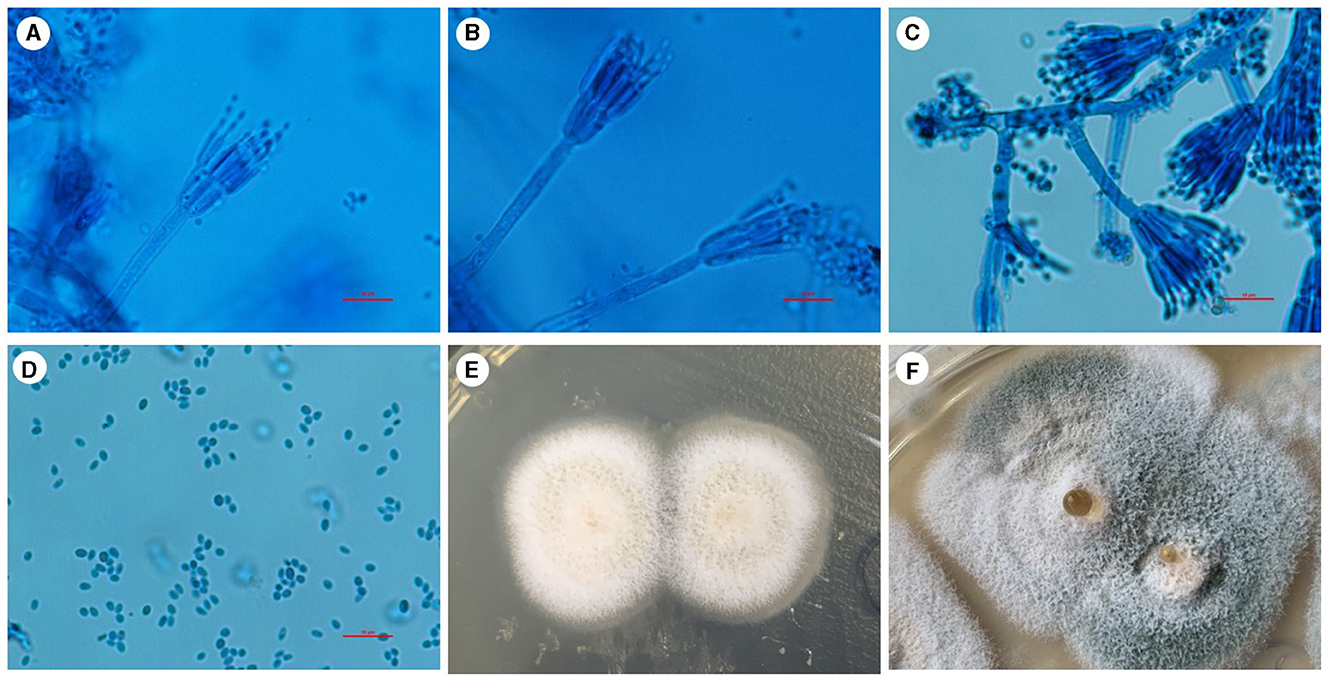
Figure 3. Penicillium digitatum was cultured for fungi in Sabouraud agar medium in a BALF sample at 28°C. (A–C) Positive colonies were cultured and stained with a lactophenol cotton blue staining solution. Under the microscope, the colonies showed a range of branching patterns, from simple to complex. The conidiophores had undergone multiple branchings and produced several rounds of symmetrical or asymmetrical small branches resembling brooms. (D) The spores of this Penicillium are typically elliptical under the microscope. (E) After 5 days of culture, the initial growth of Penicillium was observed, with white colonies exhibiting a velvety texture. (F) With the prolongation of growth time, the color of the colony gradually changes to light green or green, and liquid droplets appear in the center. Scale bars: 10 μm.
An experimental method for in vitro antifungal susceptibility testing
1. The surface of bacterial colonies is rinsed with sterile physiological saline to create a spore suspension with a concentration of 0.5–1 McFarland.
2. A sterile swab is dipped into the bacterial suspension and squeezed to dry. Then, the agar surface is gently and evenly streaked in three directions, ensuring that the moisture is absorbed by the agar in <15 min.
3. An Etest strip is placed on the agar surface (ensuring the agar surface is dry and uniformly smooth before applying) and then incubated at 35°C for 48–72 h until clear inhibition zones appear.
Result interpretation
The interpretation of results varies based on the different antimicrobial mechanisms of various drugs.
1. For amphotericin B, the MIC value is interpreted at the point where 100% of bacterial growth is inhibited.
2. For 5-fluorocytosine, the MIC value is interpreted at the point where 90% of bacterial growth is inhibited.
3. For azoles and echinocandins, the MIC value is interpreted at the point where 80% of bacterial growth is inhibited.
In vitro antifungal susceptibility testing by broth microdilution methods showed the following minimum inhibitory concentrations: amphotericin B at 2 μg/ml, voriconazole at 0.06 μg/ml, micafungin at ≤ 0.25 μg/ml, and itraconazole at 0.125 μg/ml. After antifungal therapy, the patient's ESR and hsCRP levels significantly decreased. The chest CT scan on day 18 showed improvement in pulmonary inflammation and pleural effusion compared to before (Figures 1A3, B3). The patient's infection was evaluated as being under control, and he was discharged to continue with outpatient treatment, with oral itraconazole (200 mg each time, twice a day).
After 15 days, considering the patient experienced mild gastrointestinal discomfort while the pulmonary condition was well controlled, the itraconazole dosage was reduced to 200 mg once daily for 1 month. A follow-up chest CT scan on 10 March 2023 showed further absorption of pulmonary lesions (Figures 1A4, B4). There has been no recurrence during the 1 year follow-up period. Although no further CT scans were performed during this time, the patient remained in good health with no worsening of respiratory symptoms.
Discussion
Penicillium is a ubiquitous genus of mold, with many soil-borne saprophytic species, thriving in environments with moisture and decaying vegetation (8). In clinical samples, it is often isolated as a contaminant and overlooked, but it has become an opportunistic pathogen in immunocompromised patients. Except for Talaromyces marneffei (formerly known as P. marneffei), Penicillium infections are associated with allergic pneumonia and immunosuppressive diseases (9, 10) and, in severe cases, it can lead to systemic disseminated infections (11). P. digitatum is one of the most destructive pathogens of decaying citrus fruits, causing up to 90% of postharvest losses (12).
We searched for case reports and case series of pulmonary infections due to Penicillium species during 1995–2024 in PubMed using the keywords “(lung) AND (Penicillium),” except Talaromyces marneffei. Our search yielded 29 case reports that are summarized in Table 1. It summarizes data from 37 patients, of whom 15 (41%) were men and 13 (35%) were women. A total of 16 patients (43%) were over 50 years old, and 18 patients had their fungal species identified at the species level, with the following species being most common: Penicillium citrinum (4 patients, 11%), P. digitatum (2 patients, 5%), Penicillium chrysogenum (2 patients, 5%), Penicillium brevicompactum (2 patients, 5%), and Penicillium purpurogenum (2 patients, 5%). Regarding predisposing factors, 23 patients (62%) had occupational exposure, which mostly resulted in allergic pneumonia. Moreover, four patients (11%) had underlying pulmonary diseases, and seven patients (19%) were immunocompromised, including five (14%) with a history of cancer. In terms of treatment, 14 patients (38%) received steroid therapy, mainly for occupational exposure-related hypersensitivity pneumonitis. Penicillium infections were mostly treated with amphotericin B and itraconazole. Specifically, four patients (11%) were treated with amphotericin B, and nine patients (24%) received azole medications, with four out of these nine patients (4%) choosing itraconazole. Ultimately, 22 patients (59%) showed good recovery after treatment.
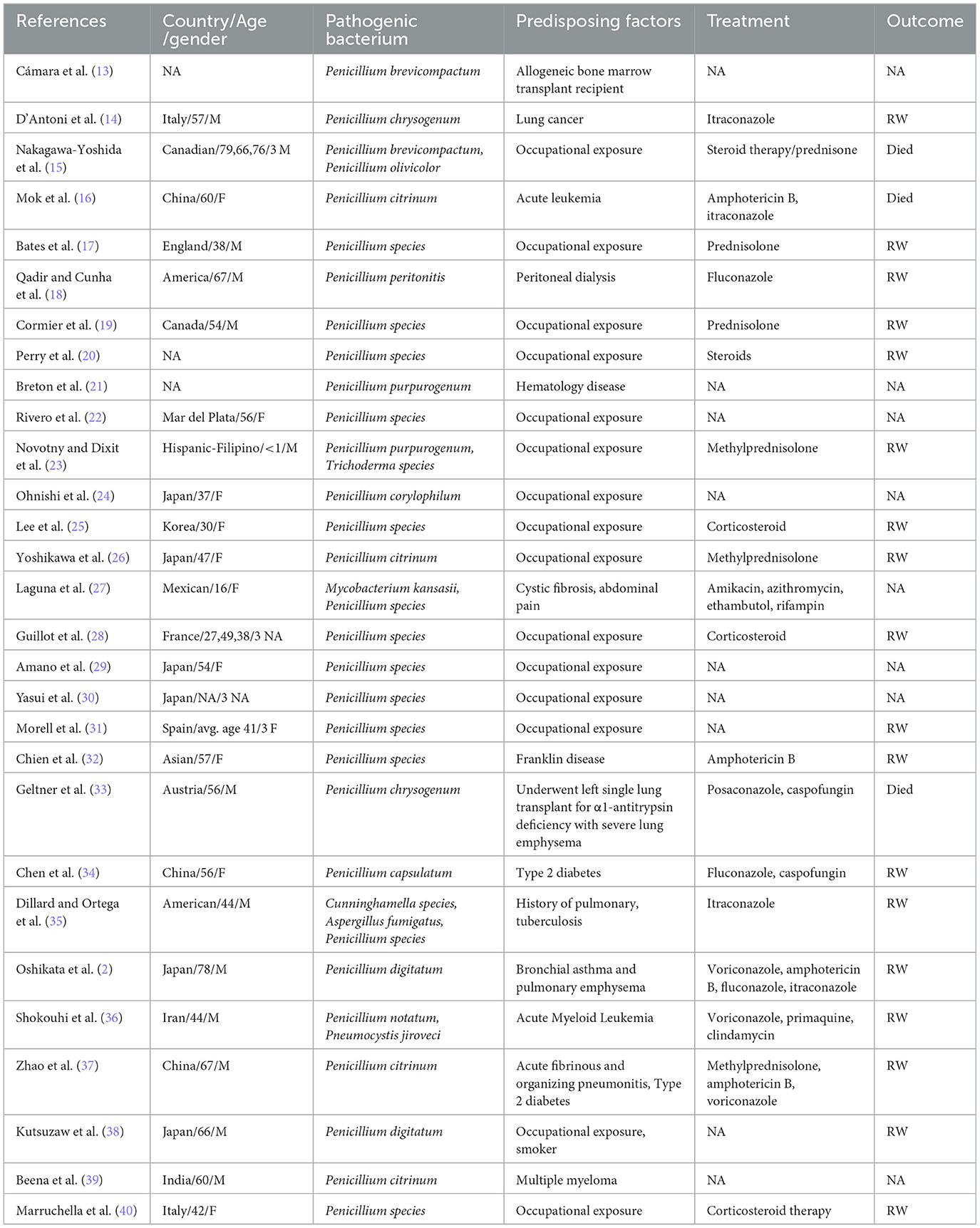
Table 1. Case reports and case series of Penicillium species pulmonary infection during 1995–2024 in PubMed (M, male; F, female; RW, recover well).
To the best of our knowledge, only two cases of pulmonary infection caused by P. digitatum have been reported (2, 3). Oshikata (2) reported the first case of pulmonary infection caused by P. digitatum in an elderly Japanese man who had emphysema and was undernourished. The second case was reported by Iturrieta-González et al. (3) in a late-term pregnant young woman who developed new nodular lesions in the lungs after being infected with SARS-CoV-2 and underwent termination of pregnancy, received treatment with corticosteroids, antibiotics, and mechanical ventilation. This infection was ultimately identified as P. digitatum.
The patient, in this case, had a clear history of consuming a large number of citrus fruits, including those with signs of mild rot, during the disease-onset period. There is also a possibility of inhaling spores of the Penicillium genus present in the air. Additionally, the patient was elderly and malnourished, had long-term smoking history and emphysema, had recently undergone major surgery, and was infected with COVID-19. The patient was in the recovery phase of COVID-19 infection, and his blood lymphocyte count remained below normal upon hospital admission. This indicates that the patient's cellular immune function has not yet fully recovered from the COVID-19 infection. These factors may have collectively contributed to the immunocompromise in the patient and facilitated the occurrence of lung infection due to P. digitatum.
Only individual case reports have documented successful treatment of Penicillium infections with itraconazole, amphotericin B, or fluconazole (4, 41). In the report by Oshikata et al. (2), the patient finally received a multidrug combination therapy of itraconazole, amphotericin B, and fluconazole, but the patient's condition did not improve. In the report by Iturrieta-González et al. (3), antifungal susceptibility testing showed in vitro activity of amphotericin B, voriconazole, and itraconazole, and the patient received itraconazole 400 mg/day, and the condition was effectively controlled after 10 days. The patient in our case also received itraconazole and showed a good therapeutic response. Currently, there are no relevant guidelines regarding the treatment of Penicillium. The preferred treatment involves administering Amphotericin B at a dosage of 0.5–1 mg/kg/day for 2 weeks, followed by maintenance therapy with Itraconazole at a dosage of 200 mg twice daily for 10 weeks (12–14). More research and reports are needed to explore the antifungal treatment regimens for Penicillium.
Conclusion
To the best of our knowledge, this is the first reported case of pulmonary infection caused by P. digitatum in China and the third case reported worldwide. The patient was an elderly man, undernourished, had emphysema, was in the recovery period of COVID-19 infection and surgery, and had a history of exposure to citrus fruit pathogens, which may have contributed to the occurrence of lung infection caused by P. digitatum. The patient's pulmonary fungal infection was effectively controlled with the use of itraconazole antifungal therapy. Although human infection with P. digitatum is considered rare, as an emerging opportunistic pathogen, clinical attention is still needed when it is isolated from immunocompromised patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant for the publication of this case report.
Author contributions
XS: Conceptualization, Writing – original draft. JY: Methodology, Writing – original draft. PL: Data curation, Writing – original draft. WG: Formal analysis, Writing – original draft. ZF: Supervision, Writing – review & editing. CZ: Writing – original draft. YH: Writing – original draft. YG: Formal analysis, Writing – original draft. LZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CH, Perrone G, et al. Identification and nomenclature of the genus Penicillium. Stud Mycol. (2014) 78:343–71. doi: 10.1016/j.simyco.2014.09.001
2. Oshikata C, Tsurikisawa N, Saito A, Watanabe M, Kamata Y, Tanaka M, et al. Fatal pneumonia caused by Penicillium digitatum: a case report. BMC Pulm Med. (2013) 13:16. doi: 10.1186/1471-2466-13-16
3. Iturrieta-González I, Giacaman A, Godoy-Martínez P, Vega F, Sepúlveda M, Santos C, et al. Penicillium digitatum, First Clinical Report in Chile: Fungal Co-Infection in COVID-19 Patient. J Fungi. (2022) 8:961. doi: 10.3390/jof8090961
4. Avilés-Robles M, Gómez-Ponce C, Reséndiz-Sánchez J, Rodríguez-Tovar AV, Ceballos-Bocanegra A, Martínez-Rivera Á. Disseminated penicilliosis due to Penicillium chrysogenum in a pediatric patient with Henoch-Schönlein syndrome. Int J Infect Dis. (2016) 51:78–80. doi: 10.1016/j.ijid.2016.08.026
5. Garg A, Stuart A, Fajgenbaum M, Laidlaw DA, Stanford M. Chronic postoperative fungal endophthalmitis caused by Penicillium citrinum after cataract surgery. J Cataract Refract Surg. (2016) 42:1380–2. doi: 10.1016/j.jcrs.2016.07.025
6. Yang Q, Qian X, Dhanasekaran S, Boateng NAS, Yan X, Zhu H, et al. Study on the infection mechanism of penicillium digitatum on postharvest citrus (citrus reticulata blanco) based on transcriptomics. Microorganisms. (2019) 7:672. doi: 10.3390/microorganisms7120672
7. Ballester AR, González-Candelas L. EFE-mediated ethylene synthesis is the major pathway in the citrus postharvest pathogen penicillium digitatum during fruit infection. J Fungi. (2020) 6:172. doi: 10.3390/jof6030175
8. Bassett IJ, Crompton CW, Parmelee JA. An atlas of airborne pollen grains and common fungus spores of Canada. London: CABI (1978).
9. Deesomchok A, Tanprawate S. A 12-case series of Penicillium marneffei pneumonia. J Med Assoc Thai. (2006) 89:441–7.
10. Yoshikawa S, Tsushima K, Yasuo M, Fujimoto K, Kubo K, Kumagai T, et al. Hypersensitivity pneumonitis caused by Penicillium citrinum, not Enoki spores. Am J Ind Med. (2007) 50:1010–7. doi: 10.1002/ajim.20535
11. Ramírez I, Hidrón A, Cardona R. Successful treatment of pulmonary invasive fungal infection by Penicillium non-marneffei in lymphoblastic lymphoma: case report and literature review. Clin Case Rep. (2018) 6:1153–7. doi: 10.1002/ccr3.1527
12. Holmes GJ, Eckert JW. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology. (1999) 89:716–21. doi: 10.1094/PHYTO.1999.89.9.716
13. de la Cámara R, Pinilla I, Muñoz E, Buendía B, Steegmann JL, Fernández-Rañada JM. Penicillium brevicompactum as the cause of a necrotic lung ball in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. (1996) 18:1189–93.
14. D'Antonio D, Violante B, Farina C, Sacco R, Angelucci D, Masciulli M, et al. Necrotizing pneumonia caused by Penicillium chrysogenum. J Clin Microbiol. (1997) 35:3335–7. doi: 10.1128/jcm.35.12.3335-3337.1997
15. Nakagawa-Yoshida K, Ando M, Etches RI, Dosman JA. Fatal cases of farmer's lung in a Canadian family. Probable new antigens, Penicillium brevicompactum and P olivicolor. Chest. (1997) 111:245–8. doi: 10.1378/chest.111.1.245
16. Mok T, Koehler AP Yu MY, Ellis DH, Johnson PJ, Wickham NW. Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. J Clin Microbiol. (1997) 35:2654–6. doi: 10.1128/jcm.35.10.2654-2656.1997
17. Bates C, Read RC, Morice AH. A malicious mould. Lancet (London, England). (1997) 349:1598. doi: 10.1016/S0140-6736(97)03231-5
18. Qadir MT, Cunha BA. Penicillium peritonitis in a patient receiving continuous ambulatory peritoneal dialysis. Heart Lung. (1998) 27:67–8. doi: 10.1016/S0147-9563(98)90072-3
19. Cormier Y, Israël-Assayag E, Bédard G, Duchaine C. Hypersensitivity pneumonitis in peat moss processing plant workers. Am J Respir Crit Care Med. (1998) 158:412–7. doi: 10.1164/ajrccm.158.2.9712095
20. Perry LP, Iwata M, Tazelaar HD, Colby TV, Yousem SA. Pulmonary mycotoxicosis: a clinicopathologic study of three cases. Modern Pathol. (1998) 11:432–6.
21. Breton P, Germaud P, Morin O, Audouin AF, Milpied N, Harousseau JL. Rare pulmonary mycoses in patients with hematologic diseases. Rev Pneumol Clin. (1998) 54:253–7.
22. Rivero MG, Basile LM, Salvatore AJ, Fridlender H, Maxit M. Salami worker's lung. Medicina. (1999) 59:367–9.
23. Novotny WE, Dixit A. Pulmonary hemorrhage in an infant following 2 weeks of fungal exposure. Arch Pediatr Adolesc Med. (2000) 154:271–5. doi: 10.1001/archpedi.154.3.271
24. Ohnishi T, Yamada G, Tanaka H, Nakajima K, Tanaka S, Morita-Ichimura S, et al. A case of chronic hypersensitivity pneumonia with elevation of serum SP-D and KL-6. Nihon Kokyuki Gakkai Zasshi. (2002) 40:66–70.
25. Lee YM, Kim YK, Kim SO, Kim SJ, Park HS. A case of hypersensitivity pneumonitis caused by Penicillium species in a home environment. J Korean Med Sci. (2005) 20:1073–5. doi: 10.3346/jkms.2005.20.6.1073
26. Yoshikawa S, Tsushima K, Koizumi T, Kubo K, Kumagai T, Yamazaki Y. Hypersensitivity pneumonitis induced by spores of Penicillium citrinum in a worker cultivating Enoki mushroom. Internal Med. (2006) 45:537–41. doi: 10.2169/internalmedicine.45.1646
27. Laguna TA, Sagel SD, Sontag MK, Accurso FJ. The clinical course of a Mexican female with cystic fibrosis and the novel genotype S531P/S531P. J Cystic Fibrosis. (2008) 7:454–6. doi: 10.1016/j.jcf.2008.03.008
28. Guillot M, Bertoletti L, Deygas N, Raberin H, Faure O, Vergnon JM. Dry sausage mould hypersensitivity pneumonitis: three cases. Rev Mal Respir. (2008) 25:596–600. doi: 10.1016/S0761-8425(08)71617-6
29. Amano Y, Enomoto M, Bando M, Kawakami M, Sugiyama Y. Hypersensitity pneumonitis in a greenhouse rose grower. Nihon Kokyuki Gakkai Zasshi. (2009) 47:960–4.
30. Yasui H, Matsui T, Yokomura K, Nakano Y, Suda T, Chida K. Three cases of hypersensitivity pneumonitis in citrus farmers. Nihon Kokyuki Gakkai Zasshi. (2010) 48:172–7.
31. Morell F, Cruz MJ, Gómez FP, Rodriguez-Jerez F, Xaubet A, Muñoz X. Chacinero's lung - hypersensitivity pneumonitis due to dry sausage dust. Scand J Work Environ Health. (2011) 37:349–56. doi: 10.5271/sjweh.3151
32. Weng CH, Wang RC, Hsieh TY, Tsai CA, Lin TH. Penicillium pneumonia in a patient with newly diagnosed Franklin disease. Am J Med Sci. (2012) 344:69–71. doi: 10.1097/MAJ.0b013e31824a8927
33. Geltner C, Lass-Flörl C, Bonatti H, Müller L, Stelzmüller I. Invasive pulmonary mycosis due to Penicillium chrysogenum: a new invasive pathogen. Transplantation. (2013) 95:e21–3. doi: 10.1097/TP.0b013e31827ff214
34. Chen M, Houbraken J, Pan W, Zhang C, Peng H, Wu L, et al. Pulmonary fungus ball caused by Penicillium capsulatum in a patient with type 2 diabetes: a case report. BMC Infect Dis. (2013) 13:496. doi: 10.1186/1471-2334-13-496
35. Dillard TA, Ortega I. Multiple endobronchial mycetomas with varied appearances and mixed fungal flora. J Bronchol Interv Pulmonol. (2013) 20:147–9. doi: 10.1097/LBR.0b013e31828ab757
36. Shokouhi S, Tehrani S, Hemmatian M. Mixed pulmonary infection with Penicillium notatum and Pneumocystis jiroveci in a patient with acute myeloid leukemia. Tanaffos. (2016) 15:53–6.
37. Zhao J, Shi Y, Yuan D, Shi Q, Wang W, Su X, et al. case report of fungal infection associated acute fibrinous and organizing pneumonitis. BMC Pulm Med. (2020) 20:98. doi: 10.1186/s12890-020-1145-7
38. Kutsuzawa N, Takihara T, Shiraishi Y, Kajiwara H, Imanishi T, Fukutomi Y, et al. Occupational hypersensitivity pneumonitis in a Japanese citrus farmer. Internal Med. (2021) 60:3581–4. doi: 10.2169/internalmedicine.7588-21
39. Beena H, Gupta M, Kindo AJ. Pulmonary infection with Penicillium citrinum in a patient with multiple myeloma. Indian J Med Microbiol. (2021) 39:259–61. doi: 10.1016/j.ijmmb.2021.03.001
40. Marruchella A, Faverio P, Luppi F. Concurrent features of sarcoidosis and hypersensitivity pneumonitis in two patients exposed to fungal antigens. BMC Pulm Med. (2023) 23:427. doi: 10.1186/s12890-023-02642-x
41. Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. (2021) 21:e246–e57. doi: 10.1016/S1473-3099(20)30784-2
Keywords: Penicillium digitatum, fungal infection, mNGS, pulmonary infection, invasive pulmonary fungal infection
Citation: Shi X, Ye J, Liu P, Gao W, Feng Z, Zheng C, Huang Y, Guo Y and Zhang L (2024) Case report: Rare pulmonary fungal infection caused by Penicillium digitatum: the first clinical report in China. Front. Med. 11:1424586. doi: 10.3389/fmed.2024.1424586
Received: 28 April 2024; Accepted: 28 June 2024;
Published: 17 July 2024.
Edited by:
Uday Kishore, United Arab Emirates University, United Arab EmiratesReviewed by:
Somanon Bhattacharya, Wuxi Advanced Therapeutics, Inc., United StatesJian Zhou, Henan Provincial Cancer Hospital, China
Copyright © 2024 Shi, Ye, Liu, Gao, Feng, Zheng, Huang, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Guo, MzI1MDY5Njc1QHFxLmNvbQ==; Lijie Zhang, emhhbmdsaWppZUBoZWJtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xiaojuan Shi1†
Xiaojuan Shi1† Yumei Guo
Yumei Guo Lijie Zhang
Lijie Zhang