- 1Department of Nephrology, Shaoxing Second Hospital, Shaoxing, Zhejiang, China
- 2Department of Traditional Chinese Medicine, The First Affiliated Hospital of Zhejiang University, Hangzhou, Zhejiang, China
- 3Department of Dermatology, Shaoxing Second Hospital, Shaoxing, Zhejiang, China
Background: Icodextrin is a type of peritoneal dialysis (PD) osmolyte that can be extended retention times (8–16 h) and may offer a viable alternative to conventional glucose dialysis solutions for PD patients. Nonetheless, prolonged use of icodextrin may lead to allergic rash, and rarely severe skin lesions.
Case presentation: In February 2024, a 45-year-old male was admitted to the Department of Nephrology at Shaoxing Second Hospital presenting with a 3-day history of intense generalized pruritic erythematous rash. Physical examination revealed diffuse erythematous pruritic rash and exfoliative rash, particularly on the abdominal. Abnormal laboratory findings included elevated eosinophil count and total IgE levels, indicative of an allergic rash. Standard anti-allergic regim was initiated. However, on the third day in the hospital, the patient developed new pustules on his neck and arms. Subsequent historical investigation uncovered that the individual had previously administered icodextrin 2 weeks prior due to volume overload, and the last intraperitoneal administration time was second day of hospitalization. The dermatologist rendered a diagnosis of generalized exfoliative rash and acute localized exanthematous pustulosis (ALEP) induced by icodextrin, and initiated prophylactic antimicrobial therapy accordingly. Furthermore, the patient declined to undergo a skin biopsy. Noteworthy is the observation that the rash ameliorated and the pustules resolved by the seventh day post-admission. Presently, the patient is still under clinical follow-up.
Conclusion: This article aims to report the first case of severe allergic rash caused by icodextrin in Chinese PD patients and highlight the potential for icodextrin to trigger ALEP. A literature review of similar cases found that severe allergic rash induced by icodextrin is rare, the underlying mechanism remains poorly understood, and the prognosis is positive with proper treatment.
1 Introduction
Icodextrin, the water-soluble glucose polymer derived from starch and linked by α-1 and α-4 glycosidic bonds, exhibits isotonic properties, low glucose content, and minimal metabolite presence (1). Since its introduction to the European market in 1997, icodextrin peritoneal dialysis (PD) solution has been extensively utilized in over 80 countries globally and has demonstrated favorable clinical efficacy and safety (2, 3). In developed nations, the utilization rate of icodextrin among PD patients exceeds 50% (4). Nonetheless, prolonged use of icodextrin may lead to allergic rash, and rarely severe skin lesions. The risk of rash induced by icodextrin is reported to be approximately 2–3 times higher than that associated with glucose-based dialysate (5). This article outlines the first documented case of a severe skin allergy in China following the use of icodextrin, leading to generalized exfoliative rash and acute localized exanthematous pustular (ALEP).
2 Case presentation
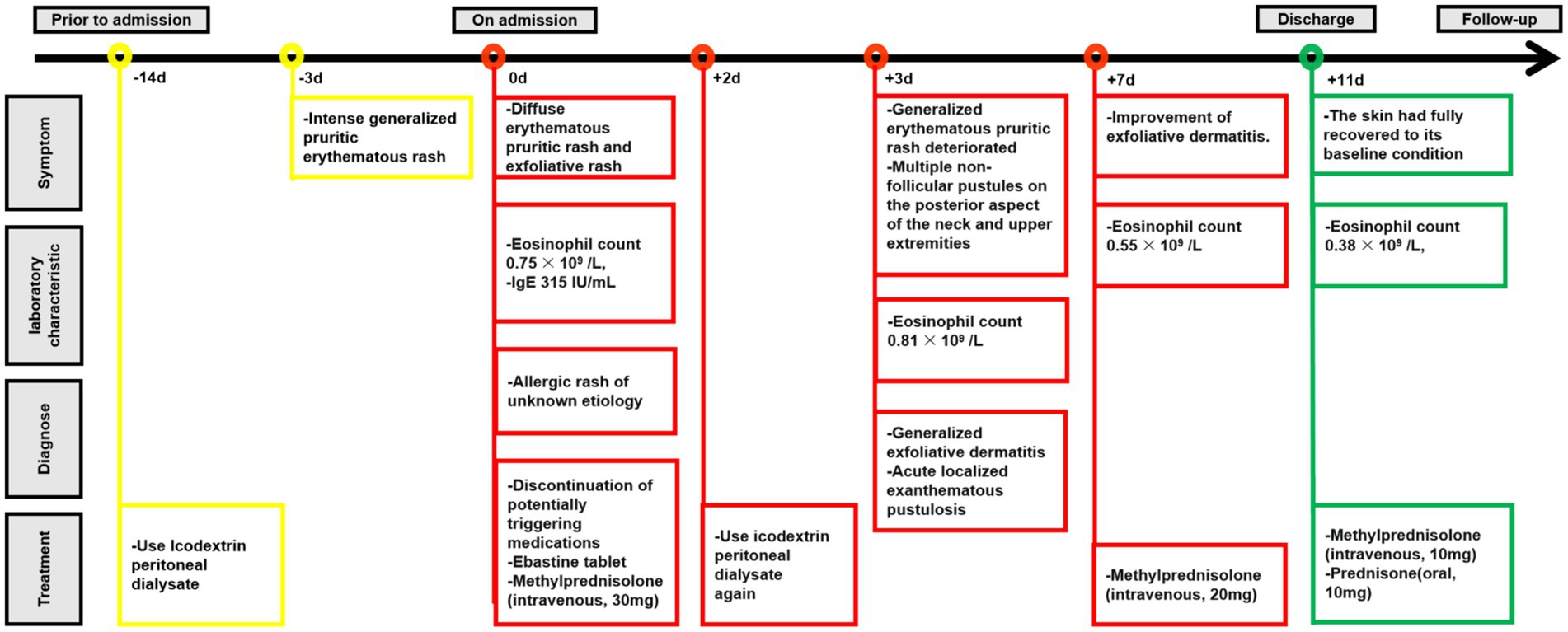
On February 2, 2024, a 45-year-old male undergoing PD was admitted to the Nephrology Department of Shaoxing Second Hospital with a 3-day history of intense generalized pruritic erythematous rash (Figure 1A). Routine physical examination on admission revealed blood pressure of 141/85 mmHg, body temperature of 37.3°C, diffuse erythematous pruritic rash, and exfoliative rash, particularly on the abdominal (Figure 1B), without oral and mucosal lesions.
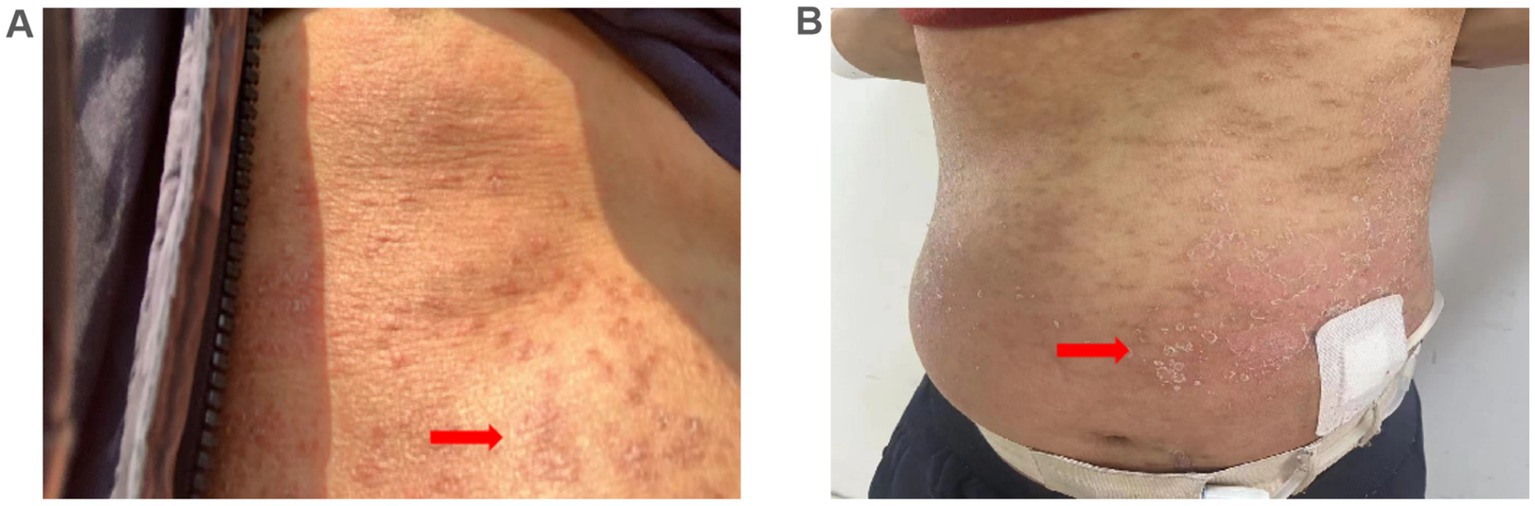
Figure 1. Skin manifestations. (A) Pruritic erythematous rash developed on the abdomen 3 days prior to admission (arrows); (B) Generalized pruritic erythematous rash and exfoliative rash developed on admission (arrows).
The patient’s daily oral medication regimen consisted of sacubitril/valsartan sodium (200 mg, once daily), nifedipine controlled-release tablet (60 mg, once daily), roxadustat (120 mg, three times a week), and Calcicort D tablet (600 mg, once daily). He consistently underwent a standard continuous ambulatory peritoneal dialysis (CAPD) protocol, which involved three exchanges of 2,000 mL of 1.5% PD solution and one exchange of 2,000 mL of 2.5% PD solution per day. No changes were made to the treatment regimen from the initial PD to the onset of the rash. Additionally, neither recent travels nor allergies were noted in the patient’s medical history.
Laboratory tests include elevated non-specific allergy indicators (eosinophil count 0.75 × 109/L, IgE 315 IU/mL), decreased nutritional markers (hemoglobin 9.9 g/dL, albumin 31 g/L), disordered electrolytes (serum potassium 3.7 mmol/L, serum sodium 135 mmol/L, serum calcium 1.96 mmol/L, and serum phosphorus 1.34 mmol/L) and normal inflammatory markers [white blood cell (WBC) count of 8.68 × 109 cells/L, C-reactive protein (CRP) 3.6 mg/L, procalcitonin (PCT) 0.01 ng/mL, WBC count in peritoneal dialysate of 0/mm3]. CT scan of the chest and abdomen showed no obvious abnormalities.
The patient was hospitalized with an initial diagnosis of allergic rash of unknown etiology and was treated with a standard anti-allergic regimen, consisting of discontinuation of potentially triggering medications (excluding antihypertensive medications and dialysis fluids), administration of ebastine tablet (oral, 10 mg/dose, once daily), and methylprednisolone (intravenous, 30 mg/dose, once daily). On the third day post-admission, the patient’s generalized erythematous pruritic rash deteriorated, with the emergence of multiple non-follicular pustules on the posterior aspect of the neck and upper extremities (Figures 2A,B). Upon further investigation, he initiated the use of icodextrin on an alternate day schedule 2 weeks prior due to volume overload and the last intraperitoneal administration time was second day of hospitalization. The dermatologist, who took into account patient’s allergic history and the manifestation of skin rash, rendered a diagnosis of icodextrin-induced generalized exfoliative dermatitis and ALEP. As a prophylactic measure against infection, mupirocin ointment (external application, once daily) and piperacillin-tazobactam (intravenous, 4.5 g/dose, twice a day) were incorporated into the therapeutic regimen. Despite a suggestion for a skin biopsy, the patient opted not to proceed any invasive procedures. By the seventh day following admission, the patient exhibited improvement in exfoliative rashes, accompanied by a reduction in pustules (Figures 3A,B). By the 11th day post admission, the patient’s skin had fully recovered to its baseline condition. Upon discharge, this patient remained free from allergic rash and continues to be under clinical follow-up. Figure 4 illustrates the timeline for diagnosis and treatment.
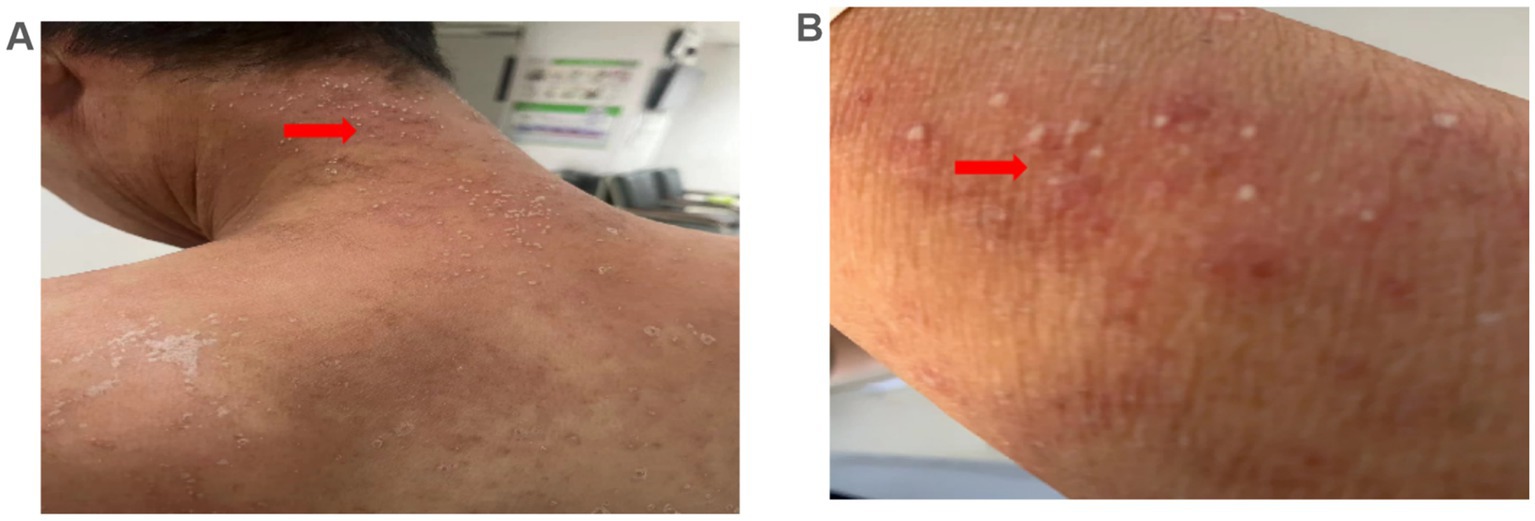
Figure 2. Microbiological examination results. (A,B) Multiple non-follicular pustules on an erythematous base were observed on the neck and arm 3 day after admission (arrows).
Figure 3. Skin manifestations. (A,B) Exfoliative rash on the abdomen and neck improved 7 days after admission (arrows).
3 Discussion and conclusion
Icodextrin, a polymer derived from starch composed of differing glucose chain lengths, functions as an osmotic agent capable of substituting conventional glucose dialysis solutions and has benefits that encompass heightened ultrafiltration, maintenance of peritoneal integrity and functionality, enhanced biocompatibility, and improved survival outcomes (6, 7). In addition, the utilization of icodextrin in individuals undergoing PD with concurrent diabetes or refractory congestive heart failure has demonstrated efficacy in the management of blood glucose levels and improvement of cardiac performance (8–10).
While the efficacy and safety of icodextrin are well established, adverse reactions like peritonitis and allergic rash can still occur during its use. The incidence of allergic rash from icodextrin ranges from 2.3 to 18.9%, with severe cases being rare (6, 11, 12). A multicenter, randomized, double-blind study involving 92 PD patients found a significantly higher rate of maculopapular eruptions in the icodextrin group (4.6%) compared to the glucose dialysate group (0%) (5). However, a 2013 meta-analysis that included 11 randomized controlled trials with 1,222 participants did not find a statistically significant increase in eruption risk associated with icodextrin vs. glucose dialysate (6). Thus, the debate over whether long-term use of icodextrin raises the risk of allergic rash continues. The precise pathophysiological process by which allergic rash induced by icodextrin is not yet fully understood. One proposed mechanism suggests that icodextrin is metabolized within patients, leading to the formation of maltose molecules with a glucan-like configuration, and maltose molecules may accumulate in the skin and peripheral nerves, combining with immunoglobulin G molecules to form immune complexes, ultimately provoking sustained allergic responses, which is analogous to the anaphylactic response elicited by glucan (13, 14).
To the best of our knowledge, a total of 11 cases documenting severe allergic rash induced by icodextrin have been comprehensively reported in the existing literature. These cases include three from France (15), two from the United States (16, 17), and one each from Saudi Arabia (18), Greece (14), Turkey (19), Canada (20), South Korea (21), and the United Kingdom (22). This case is the first reported icodextrin-induced severe allergic rash in China, which may be related to the time of icodextrin’s market approval. Icodextrin was only officially approved for the Chinese market in August 2021, while it has been used in Europe and the United States for more than 20 years. Table 1 provides a comprehensive summary of 11 cases. Icodextrin-induced severe allergic rash primarily impacted female patients (8/11), ranging in age from 23 to 91 years. Research has indicated that female is a significant risk factor for allergic rash resulting from icodextrin exposure, however, there is no observed correlation between gender and prognosis (23). The time interval between the use of icodextrin and the onset of severe rash in almost all cases (10/11), including our case, varied from a few days to 4 weeks. Only one PD patient documented an immediate allergic response following exposure to icodextrin, as detailed by Lee (21), which is exceptionally uncommon. The rash types were primarily categorized as generalized exfoliative rash (7/11) (14–18, 21, 24) and purulent erythematous rash (3/11) (14–18, 21, 24), with only the patient described by Valance et al. being diagnosed with Acute Generalized Exanthematous Pustulosis (AGEP) (15). The rash type in our case is rare, presenting as a generalized exfoliative rash and ALEP, with no previous reports of ALEP induceded by icodextrin. ALEP is a unique form of Acute Generalized Exanthematous Pustulosis (AGEP), marked by nonfollicular, pinhead-sized pustules in localized skin areas (25). Research indicates that about 90% of ALEP cases are due to systemic drug use, often affecting the face, neck, and other regions (26). This ailment typically resolves spontaneously with prompt cessation of the medication (25). Nevertheless, the exact pathological mechanism of ALEP is not fully understood and may bear resemblance to ADEP, primarily characterized by T cell-mediated drug-specific mechanisms that trigger delayed-type allergic responses. In our case, ALEP presented on the third day of hospitalization; icodextrin was promptly ceased and corticosteroid therapy was commenced, leading to a positive clinical outcome. Regrettably, a positive patch test could not be conducted due to the patient’s acute skin lesions and continued anti-allergic therapy. Additionally, the overall prognosis of icodextrin-induced rash was favorable, with the exception of specific cases such as the patient described by Alotaibi who necessitated transfer to hemodialysis due to refractory peritonitis (16), and the elderly patient reported by Liakopoulos who tragically passed away as a result of an accident (colon rupture) during treatment for the rash (14).
In summary, icodextrin has been safe and well-tolerated in Chinese PD patients for the past 3 years. However, rare complications like severe allergic rash require attention. This report documents the first case of icodextrin-induced severe allergic rash in China, identifying icodextrin as the cause of ALEP. While no standardized guidelines exist for diagnosing and treating ALEP, early diagnosis and prompt treatment usually result in positive outcomes for PD patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Shaoxing Second Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Writing – original draft, Writing – review & editing, Data curation, Visualization. TF: Writing – original draft, Methodology, Supervision. YZ: Formal analysis, Supervision, Writing – review & editing. WSh: Writing – review & editing. WSa: Data curation, Writing – review & editing. MH: Investigation, Writing – review & editing. FW: Investigation, Writing – review & editing. FC: Investigation, Writing – review & editing, Conceptualization, Formal analysis.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Zhejiang Medical and Health Science and Technology Program (No. 2023XY059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mistry, CD, and Gokal, R. The use of glucose polymer (icodextrin) in peritoneal dialysis: an overview. Perit Dial Int. (1994) 14:S158–61.
2. Mistry, CD. The beginning of icodextrin. Perit Dial Int. (2011) 31:S49–52. doi: 10.3747/pdi.2009.00217
3. Yoon, HE, Chang, YK, Shin, SJ, Choi, BS, Kim, BS, Park, CW, et al. Benefits of a continuous ambulatory peritoneal dialysis (capd) technique with one icodextrin-containing and two biocompatible glucose-containing dialysates for preservation of residual renal function and biocompatibility in incident capd patients. J Korean Med Sci. (2014) 29:1217–25. doi: 10.3346/jkms.2014.29.9.1217
4. Silver, SA, Harel, Z, and Perl, J. Practical considerations when prescribing icodextrin: a narrative review. Am J Nephrol. (2014) 39:515–27. doi: 10.1159/000363417
5. Finkelstein, F, Healy, H, Abu-Alfa, A, Ahmad, S, Brown, F, Gehr, T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. (2005) 16:546–54. doi: 10.1681/ASN.2004090793
6. Cho, Y, Johnson, DW, Badve, S, Craig, JC, Strippoli, GF, and Wiggins, KJ. Impact of icodextrin on clinical outcomes in peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. (2013) 28:1899–907. doi: 10.1093/ndt/gft050
7. Dousdampanis, P, Musso, CG, and Trigka, K. Icodextrin and peritoneal dialysis: advantages and new applications. Int Urol Nephrol. (2018) 50:495–500. doi: 10.1007/s11255-017-1647-2
8. Bertoli, SV, Ciurlino, D, Maccario, M, Martino, S, Bigatti, G, Traversi, L, et al. Home peritoneal ultrafiltration in patients with severe congestive heart failure without end-stage renal disease. Adv Perit Dial. (2005) 21:123–7.
9. Bertoli, SV, Musetti, C, Ciurlino, D, Basile, C, Galli, E, Gambaro, G, et al. Peritoneal ultrafiltration in refractory heart failure: a cohort study. Perit Dial Int. (2014) 34:64–70. doi: 10.3747/pdi.2012.00290
10. Davies, S, Zhao, J, McCullough, KP, Kim, YL, Wang, AY, Badve, SV, et al. International icodextrin use and association with peritoneal membrane function, fluid removal, patient and technique survival. Kidney. (2022) 3:872–82. doi: 10.34067/KID.0006922021
11. Wolfson, M, Piraino, B, Hamburger, RJ, and Morton, AR. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. (2002) 40:1055–65. doi: 10.1053/ajkd.2002.36344
12. Biblaki, DN, Filiopoulos, VC, and Vlassopoulos, DA. Icodextrin skin rash incidence. Kidney Int. (2015) 87:1258. doi: 10.1038/ki.2015.40
13. Valance, A, Lebrun-Vignes, B, Descamps, V, Queffeulou, G, and Crickx, B. Icodextrin cutaneous hypersensitivity: report of 3 psoriasiform cases. Arch Dermatol. (2001) 137:309–10.
14. Liakopoulos, V, Georgianos, PI, Demirtzi, P, Vaios, V, Kalathas, T, and Zebekakis, PE. Icodextrin-associated generalized exfoliative skin rash in a capd patient: a case-report. BMC Nephrol. (2018) 19:19. doi: 10.1186/s12882-018-1071-6
15. Valance, A, Bénédicte LebrunVignes,, Descamps, V, et al. Icodextrin Cutaneous Hypersensitivity. Archives of Dermatology. (2001). 137:309–10. doi: 10.4161/cbt.9.8.11318
16. Alotaibi, ME, Saggese, S, Tawhari, I, Zheng, L, Nguyen, CV, and Aggarwal, V. A case of drug reaction with eosinophilia and systemic symptoms (dress) syndrome in a patient receiving peritoneal dialysis with icodextrin exposure. Cureus. (2022) 14:e30797. doi: 10.7759/cureus.30797
17. Almiani, MKohn OF. Severe exfoliative skin rash with icodextrin. Kidney Int. (2014) 86:449. doi: 10.1038/ki.2013.511
18. Al-Khatib, N, Assiri, O, Asiri, K, and Asiri, AJ. An icodextrin-related allergic reaction with cutaneous and mucosal involvement. Saudi J Kidney Dis Transpl. (2022) 33:833–8. doi: 10.4103/1319-2442.390262
19. Cevher, SK, Ozkayar, N, and Dede, F. A case report on allergic rash caused by icodextrin. Case Rep Nephrol Dial. (2015) 5:26–9. doi: 10.1159/000368187
20. Ankur, G, and Mohan, B. Icodextrin and skin rash: unusual presentation. Ind J Nephrol. (2012) 22:62–3. doi: 10.4103/0971-4065.86413
21. Lee, SH, Park, HC, Sim, SR, Chung, SJ, Kim, KJ, Park, WI, et al. Severe cutaneous hypersensitivity to icodextrin in a continuous ambulatory peritoneal dialysis patient. J Nephrol. (2006) 19:673–6. doi: 10.1089/end.2006.20.698
22. Fletcher, S, Stables, GA, and Turney, JH. Icodextrin allergy in a peritoneal dialysis patient. Nephrol Dial Transplant. (1998) 13:2656–8. doi: 10.1093/ndt/13.10.2656
23. Wolfson, M, Ogrinc, F, and Mujais, S. Review of clinical trial experience with icodextrin. Kidney Int Suppl. (2011) 2002:S46–52. doi: 10.1046/j.1523-1755.62.s81.7.x
24. Queffeulou, G, Lebrun-Vignes, B, Wheatley, P, Montagnac, R, and Mignon, F. Allergy to icodextrin. Lancet. (2000) 356:75. doi: 10.1016/S0140-6736(05)73411-5
25. Safa, I, Ines, L, Noureddine, L, Meriem, J, Manel, N, Belhajali, H, et al. Acute localized exanthematous pustulosis: clinical features, pathophysiology, and therapy. Dermatol Ther. (2021) 34:e15087. doi: 10.1111/dth.15087
Keywords: icodextrin, severe allergic rash, acute localized exanthematous pustulosis, peritoneal dialysis, peritoneal dialysate
Citation: Huang Y, Fu T, Zhang Y, Shen W, Sang W, Han M, Wang F and Chen F (2024) Severe allergic rash induced by icodextrin: case report and literature review. Front. Med. 11:1421109. doi: 10.3389/fmed.2024.1421109
Edited by:
Yvon Gauthier, Université de Bordeaux, FranceReviewed by:
Xuancheng Zhou, Southwest Medical University, ChinaGuoqiang Zhang, The First Hospital of Hebei Medical University, China
Copyright © 2024 Huang, Fu, Zhang, Shen, Sang, Han, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenjuan Chen, bTE4NzY3NTA5ODU5XzFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yiqi Huang
Yiqi Huang Tianxiao Fu2†
Tianxiao Fu2†