
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 July 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1420951
This article is part of the Research Topic Advancing Precision Medicine in Acute Stroke Care: Personalized Treatment Strategies and Outcomes View all 20 articles
 Masahiro Kashiura1*
Masahiro Kashiura1* Chisato Nakajima1
Chisato Nakajima1 Yuki Kishihara1
Yuki Kishihara1 Keiichiro Tominaga1
Keiichiro Tominaga1 Hiroyuki Tamura1
Hiroyuki Tamura1 Hideto Yasuda1
Hideto Yasuda1 Masashi Ikota2,3
Masashi Ikota2,3 Kenji Yamada2,3
Kenji Yamada2,3 Yoshikazu Yoshino2,3
Yoshikazu Yoshino2,3 Takashi Moriya1
Takashi Moriya1Introduction: Hybrid emergency room systems (HERSs) have shown promise for the management of severe trauma by reducing mortality. However, the effectiveness of HERSs in the treatment of acute ischemic stroke (AIS) remains unclear. This study aimed to evaluate the impact of HERSs on treatment duration and neurological outcomes in patients with AIS undergoing endovascular therapy.
Materials and methods: This single-center retrospective study included 83 patients with AIS who were directly transported to our emergency department and underwent endovascular treatment between June 2017 and December 2023. Patients were divided into the HERS and conventional groups based on the utilization of HERSs. The primary outcome was the proportion of patients achieving a favorable neurological outcome (modified Rankin Scale score 0–2) at 30 days. The secondary outcomes included door-to-puncture and door-to-recanalization times. Univariate analysis was performed using the Mann–Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables, as appropriate.
Results: Of the 83 eligible patients, 50 (60.2%) were assigned to the HERS group and 33 (39.8%) to the conventional group. The median door-to-puncture time was significantly shorter in the HERS group than in the conventional group (99.5 vs. 131 min; p = 0.001). Similarly, the median door-to-recanalization time was significantly shorter in the HERS group (162.5 vs. 201.5 min, p = 0.018). Favorable neurological outcomes were achieved in 16/50 (32.0%) patients in the HERS group and 6/33 (18.2%) in the conventional group. The HERS and conventional groups showed no significant difference in the proportion of patients achieving favorable neurological outcomes (p = 0.21).
Conclusion: Implementation of the HERS significantly reduced the door-to-puncture and door-to-recanalization times in patients with AIS undergoing endovascular therapy. Despite these reductions in treatment duration, no significant improvement in neurological outcomes was observed. Further research is required to optimize patient selection and treatment strategies to maximize the benefits of the HERS in AIS management.
Acute ischemic stroke (AIS) caused by large-vessel occlusion is associated with poor outcomes. In cases of ischemic stroke, mortality within 3–6 months was higher after large-vessel occlusion than after non-large-vessel occlusion (26.2% vs. 1.3%, odds ratio: 4.1, 95% confidence interval: 2.5–6.7) (1). The penumbra, the tissue surrounding the infarction area that is at risk of cell death, can be salvaged by prompt recanalization (2). Numerous randomized trials have demonstrated the advantages of endovascular treatment, including thrombectomy following intravenous thrombolysis with a tissue plasminogen activator (tPA), in achieving recanalization and favorable neurological outcomes (3). Early successful recanalization after symptom onset remains a critical factor for optimal outcomes (4).
The implementation of hybrid emergency room (ER) systems (HERSs) in Japan has shown promising results in the management of cases involving severe trauma. An HERS is equipped with advanced diagnostic modalities, such as X-ray fluoroscopy and computed tomography (CT), enabling complete diagnosis and immediate treatment without the need for patient transfer (5, 6). The consequent elimination of transfer time has led to a decrease in mortality rates in patients with trauma (7). Timely reperfusion is crucial in AIS caused by large-vessel occlusion (8). Given the critical importance of time in both severe trauma and AIS management, the HERS could plausibly provide similar benefits for stroke care (9). However, the effectiveness of the HERS in the management of AIS remains unclear.
Therefore, this study aimed to retrospectively analyze patients who underwent endovascular treatment for AIS at our institution and evaluate the effectiveness of the HERS in this context. We hypothesized that the use of the HERS, which enables immediate CT diagnosis and rapid endovascular intervention without patient transfer, would result in shorter time intervals from hospital arrival to recanalization and, consequently, yield improved neurological outcomes in comparison with conventional stroke management. By addressing this gap in the current understanding of HERS applications, we aimed to contribute to the advancement of stroke care and potentially offer insights into the optimization of ER systems for the management of AIS.
This retrospective study was conducted at a single academic medical center located in an urban area of Kanto, Japan. The HERS, which was equipped with a sliding CT scanner system featuring interventional radiology capabilities, was established adjacent to the conventional ER in 2016 (Figure 1). The distance from the ambulance parking lot to the conventional ER and the hybrid ER was approximately 50 m. Acute AIS management using the HERS commenced in 2017, following the assignment of neuroendovascular specialists.
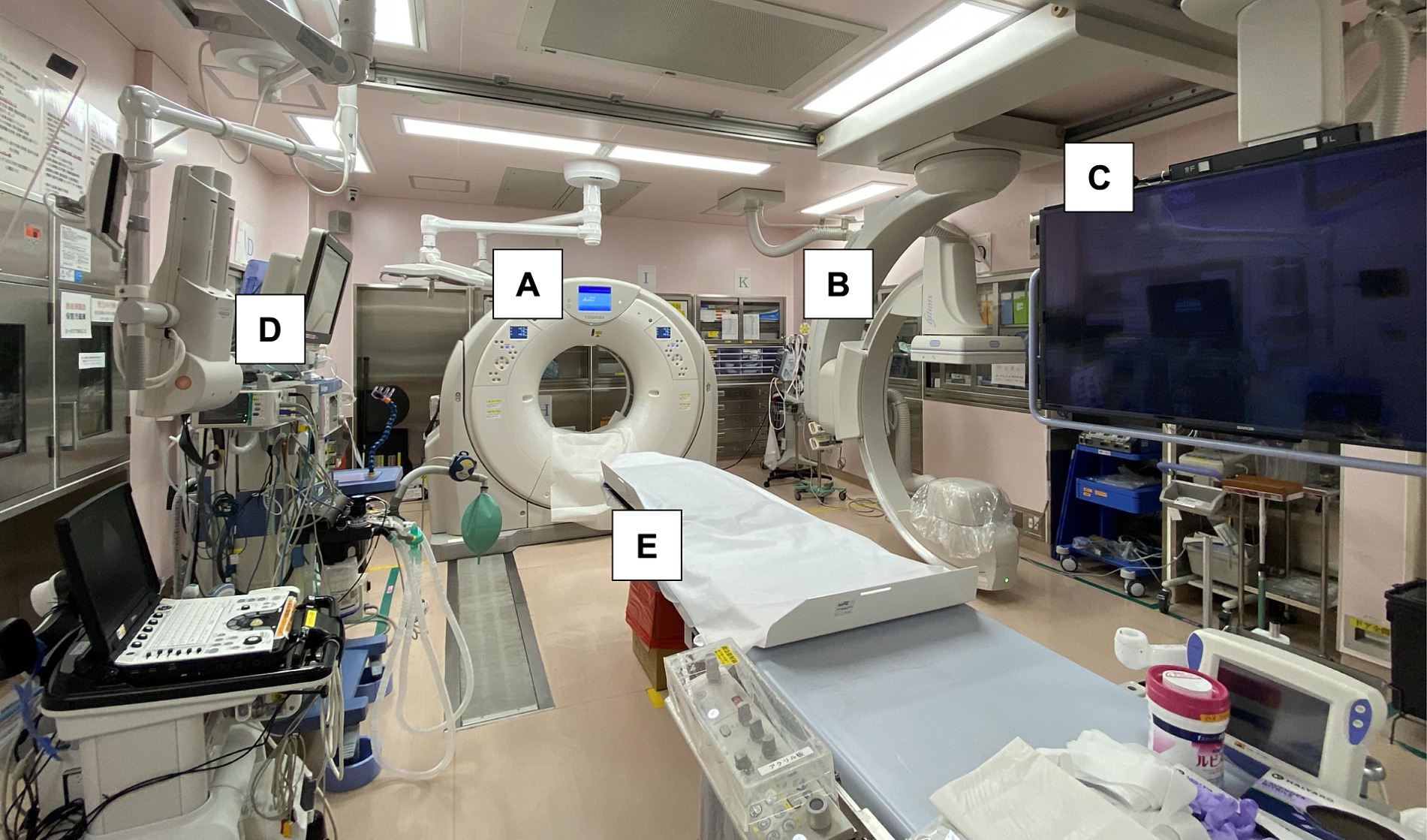
Figure 1. Photograph showing a sliding computed tomography scanner system with interventional radiology features in the emergency room. Acute procedures for ischemic stroke, including airway management, and angiography can be performed on the table without transferring the patient. (A) Sliding computed tomography (CT) scan device, (B) movable C-arm, (C) monitoring screen, (D) mechanical ventilator with anesthesia apparatus, and (E) CT examination and intervention table.
The HERS was preferentially employed when a patient with suspected stroke was transported within 6 h of symptom onset. All patients who were transported to our emergency department with suspected acute stroke underwent an initial evaluation by an emergency physician; if the hybrid ER was available, the patients were treated using the hybrid ER immediately after transport, and if the hybrid ER was being used to treat other patients, the patients were treated in the conventional ER. AIS was diagnosed using head CT, including perfusion imaging, or head magnetic resonance imaging in patients with renal dysfunction. Thrombolytic therapy was administered by the emergency physicians in consultation with a stroke specialist. Endovascular treatment was administered to patients with onset within 6 h or an unknown onset time showing occlusion of the internal carotid artery, M1 or M2 portion of the middle cerebral artery, or basilar artery along with a relatively large penumbra compared to the ischemic core, as judged by the emergency physician. An endovascular physician performed all endovascular treatments.
This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplementary material File 1) (10). This study was approved by the Institutional Review Board of Jichi Medical University Saitama Medical Center. Participants were provided with the opportunity to opt out of the study at any time by withdrawing permission to use their data.
This investigation included a cohort of patients with AIS who were directly transported from prehospital locations to our emergency department by emergency medical service personnel and received endovascular treatment between June 2017 and December 2023. Patients who developed AIS at our hospital or who were transferred from other hospitals or clinics were excluded.
The study population was divided into two groups: the HERS group, in which patients received continuous care from initial evaluation to endovascular treatment using the HERS, and the conventional group, in which the HERS was not used.
The following data were collected from electronic medical records: age, sex, modified Rankin Scale (mRS) score before admission (11), onset-to-door time (interval from symptom onset to hospital arrival), National Institutes of Health Stroke Scale (NIHSS) score on admission (12), door-to-picture time (interval from hospital arrival to CT imaging), Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on admission (13), etiology of AIS (atrial fibrillation, atherothrombotic, left-to-right shunt, or cryptogenic), culprit lesion (M1 or M2 segment of middle cerebral artery, internal carotid artery, basilar artery), presence of tandem lesions, tPA administration, door-to-needle time (interval from hospital arrival to tPA administration), endovascular treatment procedure (fragmentation, mechanical thrombectomy, or carotid artery stenting and mechanical thrombectomy), door-to-puncture time (interval from hospital arrival to arterial puncture), door-to-recanalization time (interval from hospital arrival to recanalization), onset-to-recanalization time (interval from symptom onset to recanalization), Thrombolysis in Cerebral Infarction (TICI) grade, intracranial hemorrhage after procedure (symptomatic or asymptomatic), ASPECTS 30 days after admission, length of hospital stay, NIHSS score 30 days after admission, and mRS score 30 days after admission.
The primary outcome was a favorable neurological outcome at 30 days, defined as an mRS score of 0–2. The secondary outcomes were door-to-puncture and door-to-recanalization times as direct measures of time reduction using the HERS, in addition to the distribution of the mRS score at 30 days.
Descriptive statistics were calculated for all the variables of interest. Continuous variables were presented as median and interquartile range (IQR), whereas categorical variables were presented as count and percentage. Univariate analysis was performed using the Mann–Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables, as appropriate.
All statistical tests were two-sided, and p-values <0.05 were considered statistically significant. Statistical analyses were performed using R Statistical Software.
During the study period, 115 patients with AIS underwent endovascular treatment. Of these, 14 patients (12.2%) were transferred from other hospitals, and 18 (15.7%) developed AIS at our hospital (Figure 2). After excluding these patients, 83 patients with AIS who were directly transported from prehospital locations to the emergency department and underwent endovascular treatment were included in the study. Of these, 50 patients (60.2%) were treated with the HERS (HERS group), whereas 33 (39.8%) were treated in the conventional ER (conventional group).
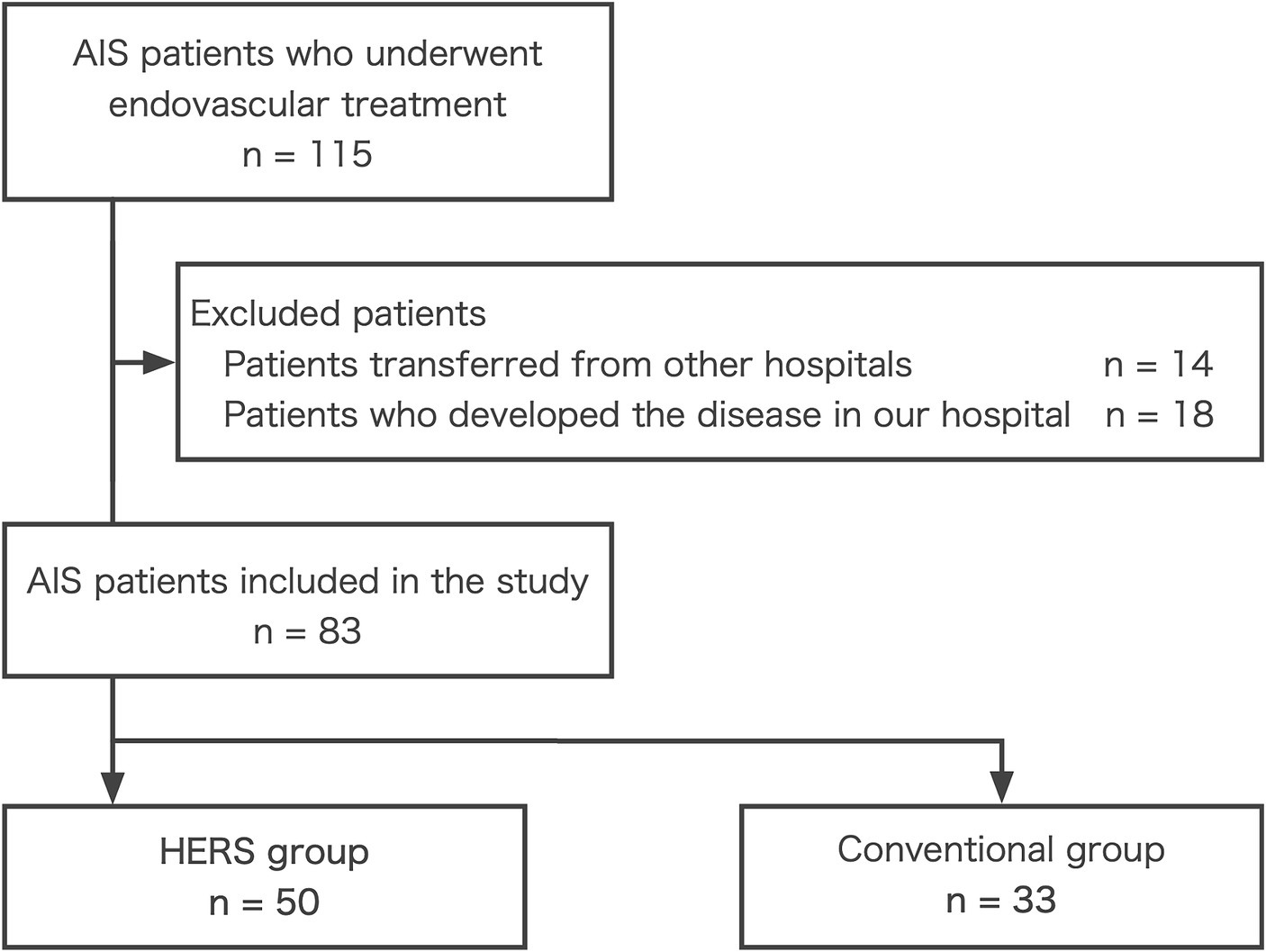
Figure 2. Patient selection flowchart. AIS, acute ischemic stroke; HERS, hybrid emergency room system.
There were no missing data. The patient demographics, clinical characteristics, and outcomes are summarized in Table 1. The median age of the study population was 79 years (IQR: 73–85 years), and 62.7% of the patients were male. The median onset-to-door time was 55 min (IQR, 41–111 min), and the median NIHSS score at admission was 20 (IQR: 13.5–28). The most common etiology of AIS was atrial fibrillation (67.5%), and the culprit lesions were most frequently present in the M1 segment of the middle cerebral artery (53.0%). Tandem lesions were present in 4.8% of the patients. tPA was administered in 67.5% of the cases.
The median door-to-puncture time was significantly shorter in the HERS group than in the conventional group (99.5 [IQR: 75.75–117.5] min vs. 131 [IQR: 98–166] min, p = 0.001). Similarly, the median door-to-recanalization time was significantly shorter in the HERS group (162.5 [IQR: 141.75–184.5] min vs. 201.5 [IQR: 154–251] min, p = 0.018). However, the onset-to-recanalization time was not significantly different between the two groups (225 [IQR: 188.25–269.75] min vs. 274 [201–369] min, p = 0.10).
The primary outcome, i.e., an mRS score of 0–2 at 30 days after admission, was achieved in 32.0% of the patients in the HERS group and 18.2% of those in the conventional group; however, this difference was not statistically significant (p = 0.21). The distribution of the mRS scores 30 days after admission is shown in Figure 3.
This retrospective study aimed to evaluate the efficacy of the HERS in managing patients with AIS undergoing endovascular treatment. Our findings demonstrate that the implementation of the HERS significantly reduced the median door-to-puncture and door-to-recanalization times in comparison with the conventional approach. However, despite the observed reduction in treatment times, the HERS and conventional groups showed no significant difference in the proportion of patients achieving favorable neurological outcomes. The significant reduction in door-to-puncture and door-to-recanalization times associated with the HERS highlights the potential of this novel system for streamlining the care pathway for patients with AIS.
By enabling rapid diagnosis and treatment initiation without the need for patient transfer, the HERS can minimize the delays that often compromise the effectiveness of endovascular therapy (9). The significant reduction in the treatment time observed in the HERS group in this study was consistent with the findings of previous studies demonstrating the benefits of the HERS in the management of acute conditions (14, 15). Kinoshita et al. found that the use of a hybrid ER significantly reduced the time required for CT examinations and emergency surgery in patients with severe traumatic brain injury (16). These findings support the notion that the integrated imaging and intervention capabilities of the HERS can streamline the care pathway for acute conditions and eliminate delays associated with patient transfer (6). Because early successful recanalization is a critical factor for favorable outcomes in AIS (2), the time savings afforded by the HERS may have important implications for patient prognosis.
However, the lack of a significant difference in favorable neurological outcomes between the HERS and conventional groups warrants further discussion. Although the use of the HERS significantly reduced treatment time, it did not translate into improved neurological outcomes in our study population. Expanding the application of the HERS to a broader range of patients with AIS may lead to improved outcomes; however, this requires careful consideration and further research to identify optimal patient selection criteria. Furthermore, the median difference in the door-to-recanalization time to reperfusion was <40 min, and the onset-to-recanalization time was not significantly different, suggesting that the prognostic impact may be limited.
Current trends and future directions in AIS treatment emphasize the importance of reducing door-to-groin time and improving outcomes by employing a direct-to-angiography suite approach in the early time window for selected patients with large-vessel occlusions (17). Although our study demonstrated the effectiveness of the HERS in reducing treatment time, the lack of improvement in neurological outcomes highlights the need for further optimization of HERS-based treatment strategies. This may involve refining patient selection criteria, improving device selection and technical aspects of the procedure, and ensuring seamless coordination among multidisciplinary stroke teams.
This study had several limitations. First, the door-to-puncture time was delayed even in the HERS group. Such delays can be attributed to several reasons, including determination of the indications for endovascular treatment by emergency physicians, administration of tPA intravenously before endovascular treatment, and the on-call availability of endovascular physicians at night and on holidays. Second, this was a single-center observational study with a small number of cases and no adjustments for confounding factors. Prospective multicenter studies incorporating a large number of patients will be important to expand the coverage of the HERS and optimize treatment strategies for patients with AIS.
In conclusion, our study demonstrates the potential of the HERS in reducing the treatment time for patients with AIS undergoing endovascular therapy. Although the lack of improvement in neurological outcomes highlights the need for further research and optimization of treatment strategies, the significant reduction in door-to-puncture and door-to-recanalization times suggests that the HERS could be a valuable tool for the management of AIS. As the field of AIS treatment continues to evolve, investigating the effectiveness of the HERS in various patient subgroups and developing comprehensive stroke care protocols that maximize the benefits of this innovative system will become more important.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Jichi Medical University Saitama Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the requirement for informed consent was waived because of the observational study design, which posed minimal risk to the patients and preserved their anonymity. Patients and their respective families were provided with an opportunity to opt out of the study.
MK: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. CN: Data curation, Formal analysis, Supervision, Visualization, Writing – review & editing. YK: Supervision, Writing – review & editing. KT: Writing – review & editing. HT: Supervision, Writing – review & editing. HY: Conceptualization, Supervision, Writing – review & editing. MI: Supervision, Writing – review & editing. KY: Supervision, Writing – review & editing. YY: Supervision, Writing – review & editing. TM: Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant number 20K17905). The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
We thank Editage (www.editage.com) for providing writing support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1420951/full#supplementary-material
1. Malhotra, K, Gornbein, J, and Saver, JL. Ischemic stroke due to large-vessel occlusion contributes disproportionately to stroke-related dependence and death. Front Neurol. (2017) 8:651. doi: 10.3389/fneur.2017.00651
2. Saver, JL, Filip, B, Hamilton, S, Yanes, A, Craig, S, Cho, M, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: Rankin-focused assessment (RFA). Stroke. (2010) 41:992–5. doi: 10.1161/STROKEAHA.109.571364
3. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischemic stroke: a meta-analysis of individual patient data from five randomized trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
4. Khatri, P, Abruzzo, T, Yeatts, SD, Nichols, C, Broderick, JP, Tomsick, TA, et al. Good clinical outcomes after ischemic stroke with successful revascularization are time-dependent. Neurology. (2009) 73:1066–72. doi: 10.1212/WNL.0b013e3181b9c847
5. Founding Members of the Japanese Association for Hybrid Emergency Room System (JA‐HERS) . Hybrid emergency room system: a novel trauma evaluation and care system created in Japan. Acute Med Surg. (2019) 6:247–51. doi: 10.1002/ams2.412
6. Kinoshita, T, Yamakawa, K, Matsuda, H, Yoshikawa, Y, Wada, D, Hamasaki, T, et al. The survival benefit of a novel trauma workflow that includes immediate whole-body computed tomography, surgery, and interventional radiology, all in one trauma resuscitation room: a retrospective historical control study. Ann Surg. (2019) 269:370–6. doi: 10.1097/SLA.0000000000002527
7. Ito, K, Sugimoto, M, Tsunoyama, T, Nagao, T, Kondo, H, Nakazawa, K, et al. A trauma patient care simulation using extended reality technology in the hybrid emergency room system. J Trauma Acute Care Surg. (2021) 90:e108–12. doi: 10.1097/TA.0000000000003086
8. Turc, G, Bhogal, P, Fischer, U, Khatri, P, Lobotesis, K, Mazighi, M, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. (2019) 11:535–8. doi: 10.1136/neurintsurg-2018-014568
9. Kashiura, M, Amagasa, S, Tamura, H, Sanayama, H, Yamashina, M, Ikota, M, et al. Reperfusion therapy of acute ischemic stroke in an all-in-one resuscitation room called a hybrid emergency room. Oxf Med Case Rep. (2019) 2019:omz042. doi: 10.1093/omcr/omz042
10. Vandenbroucke, JP, von Elm, E, Altman, DG, Gotzsche, PC, Mulrow, CD, Pocock, SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
11. Yi, K, Nakajima, M, Ikeda, T, Yoshigai, M, and Ueda, M. Modified Rankin scale assessment by telephone using a simple questionnaire. J Stroke Cerebrovasc Dis. (2022) 31:106695. doi: 10.1016/j.jstrokecerebrovasdis.2022.106695
12. Goldstein, LB, and Samsa, GP. Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke. (1997) 28:307–10. doi: 10.1161/01.str.28.2.307
13. Alexander, LD, Pettersen, JA, Hopyan, JJ, Sahlas, DJ, and Black, SE. Long-term prediction of functional outcome after stroke using the Alberta stroke program early computed tomography score in the subacute stage. J Stroke Cerebrovasc Dis. (2012) 21:737–44. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.010
14. Kashiura, M, Sugiyama, K, Tanabe, T, Akashi, A, and Hamabe, Y. Effect of ultrasonography and fluoroscopic guidance on the incidence of complications of cannulation in extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a retrospective observational study. BMC Anesthesiol. (2017) 17:4. doi: 10.1186/s12871-016-0293-z
15. Umemura, Y, Watanabe, A, Kinoshita, T, Morita, N, Yamakawa, K, and Fujimi, S. Hybrid emergency room shows maximum effect on trauma resuscitation when used in patients with higher severity. J Trauma Acute Care Surg. (2021) 90:232–9. doi: 10.1097/ta.0000000000003020
16. Kinoshita, T, Hayashi, M, Yamakawa, K, Watanabe, A, Yoshimura, J, Hamasaki, T, et al. Effect of the hybrid emergency room system on functional outcome in patients with severe traumatic brain injury. World Neurosurg. (2018) 118:e792–9. doi: 10.1016/j.wneu.2018.07.053
Keywords: cerebral infarction, endovascular procedures, intracranial embolism and thrombosis, radiographic image enhancement, thrombectomy
Citation: Kashiura M, Nakajima C, Kishihara Y, Tominaga K, Tamura H, Yasuda H, Ikota M, Yamada K, Yoshino Y and Moriya T (2024) Effectiveness of a hybrid emergency room system in the management of acute ischemic stroke: a single-center experience. Front. Med. 11:1420951. doi: 10.3389/fmed.2024.1420951
Received: 21 April 2024; Accepted: 26 June 2024;
Published: 04 July 2024.
Edited by:
Mohamed F. Doheim, University of Pittsburgh Medical Center, United StatesReviewed by:
Takehiro Katano, Nippon Medical School, JapanCopyright © 2024 Kashiura, Nakajima, Kishihara, Tominaga, Tamura, Yasuda, Ikota, Yamada, Yoshino and Moriya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Kashiura, a2FzaGl1cmFAbWUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.