- 1Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Co-trimoxazole is used as a prophylaxis for human immunodeficiency virus (HIV) patients to prevent opportunistic infections. Its widespread use results in the emergence of co-trimoxazole resistance, which is a significant problem. This systematic review and meta-analysis determined the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
Methods: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was applied to report this study. The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the assigned number CRD42024532240. Article search was performed using electronic databases such as PubMed, Medline, EMBASE, Google Scholar, Hinari, Web of Science, Science Direct, and African Journals Online. Data were extracted using a Microsoft Excel spreadsheet and analyzed using STATA version 11.0 software. A random-effects model was used to estimate the pooled effect size of co-trimoxazole resistance across studies with a 95% confidence interval. The heterogeneity was checked using I2 statistic. The presence of publication bias was determined using a funnel plot and Egger’s test with a p-value <0.05 evidence of statistically significant bias. Subgroup and sensitivity analyses were performed.
Results: Twenty-two studies with 5,788 HIV-infected individuals were included. The pooled prevalence of co-trimoxazole resistance was 61.73% (95% CI: 53.10–70.37%), with heterogeneity (I2 = 87.7%) and statistical significance (p < 0.001). A higher co-trimoxazole resistance was observed in HIV-infected individuals with urinary tract infection; 82.10% (95% CI: 75.03–89.17%). Among the bacterial spp., higher resistance to co-trimoxazole was observed in Escherichia coli; 70.86% (95% CI: 53.44–88.27%) followed by Salmonella spp.; 67.66% (95% CI: 41.51–93.81%) and Proteus spp.; 66.23% (95% CI: 34.65–97.82%).
Conclusion: There is a higher prevalence of co-trimoxazole resistance in HIV-infected individuals in Ethiopia. This alarms WHO’s recommendation of co-trimoxazole prophylaxis guidelines to review and update it. Additionally, a nationwide assessment of co-trimoxazole resistance in Ethiopia as a whole is required.
Systematic review registration: identifier: CRD42024532240.
Introduction
Human immunodeficiency virus (HIV), which later causes acquired immunodeficiency syndrome (AIDS), is one of the global health threats (1). According to the Joint United Nations Programme on HIV/AIDS, 2022 Global HIV and AIDS Statistics fact sheet, 84.2 million people have become infected with HIV since the HIV epidemic began, and 40.1 million have died from AIDS-related illnesses. Besides, around 650,000 people died from AIDS-related illnesses worldwide in 2021 (2). Ethiopia has made remarkable strides in controlling the HIV/AIDS epidemic over the past decade. However, the prevalence remains relatively high in urban areas where estimates indicate a 3 % rate compared to under 1 % nationally (3).
Co-trimoxazole, a combination of trimethoprim and sulfamethoxazole antibiotics, is recommended as a prophylaxis for HIV-infected individuals to prevent opportunistic infections; however, the indication may vary according to CD4 cell count, age, geographical setting, and prior medical history (4, 5). The use of co-trimoxazole prophylaxis in low-income settings is recommended by the World Health Organization (WHO) for all children with HIV and for adults with advanced HIV infection or who are living in areas where bacterial infections are prevalent, irrespective of antiretroviral therapy (ART) (4, 6).
Previously, research had shown that co-trimoxazole prophylaxis decreases morbidity and death in HIV-infected individuals (7). However, the current scenario might be different with over 80% of bacteria causing opportunistic infections are resistant to co-trimoxazole (8). The widespread use of co-trimoxazole prophylaxis in HIV patients is thought to be the cause of an increasing burden of antimicrobial resistance throughout the AIDS era. The emergence of co-trimoxazole resistance among HIV-infected individuals has become a significant problem (8). A study in Tanzania reported a 75% bacterial resistance to co-trimoxazole from HIV patients and 81.3% resistance in patients with prophylaxis (8).
In Ethiopia, different individual studies were conducted on the pattern of co-trimoxazole resistance to bacterial infections or colonizations in HIV-infected individuals. This systematic review and meta-analysis report is necessary to provide valuable data on the status of co-trimoxazole resistance among HIV-infected individuals in Ethiopia, which is crucial for revising the recent guideline of co-trimoxazole prophylaxis. Therefore, this systematic review and meta-analysis determined the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
Methods
Study design and protocol registration
This systematic review and meta-analysis were performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9) (Supplementary Table S1). The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the assigned number CRD42024532240.
Database search strategy
The co-trimoxazole resistance in bacterial pathogens that infect or colonize HIV-positive people was the main focus of this meta-analysis. The appropriateness of the included studies for this meta-analysis was assessed using the COCOPOP (Condition, Context, and Population) paradigm. Ethiopia served as the context (CO), HIV-infected individuals as the population (POP), and the study’s condition (CO) was the prevalence of co-trimoxazole resistance. Studies published before 2024 were included in the search, and it was last conducted until March 25, 2024. Electronic databases such as PubMed, Medline, EMBASE, Google Scholar, Hinari, Web of Science, Science Direct, and African Journals Online were searched to identify articles reporting the prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia. We used search terms alone and in combination with Boolean operators such as “OR” or “AND.” An example of a PubMed search strategy used was as follows: (((((bacteria) OR (infection)) OR (colonization))) AND ((((((antimicrobial resistance) OR (antibiotic resistance)) OR (co-trimoxazole resistance)) OR (trimethoprim-sulfamethoxazole resistance)) OR (antibiogram))) AND ((((human immunodeficiency virus) OR (immunocompromised)) OR (HIV/AIDS)) OR (HIV))) AND (Ethiopia). A manual search was conducted for relevant papers in the references of the included studies and other reviews. The articles retrieved were imported into EndNote X9 bibliographic software manager (Clarivate Analytics, Philadelphia, PA, United States).
Outcome of interest
The main finding in this systematic review and meta-analysis was the national assessment of the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals. Co-trimoxazole resistance based on the type of bacteria spp. was the second outcome of this study.
Study selection and eligibility criteria
Two independent reviewers (MA and GG) screened the titles and abstracts of the identified studies to determine their eligibility. Full-text articles were then assessed for eligibility, and any disagreements between the reviewers were resolved through discussion. Articles published in the English language with a cross-sectional study design that included the prevalence of co-trimoxazole resistance in HIV-infected individuals in the results, studies conducted in Ethiopia and without limit on specimen type, type of infection or colonization, and study period. Studies not reporting co-trimoxazole resistance profile, case reports, review articles, meta-analysis studies, conducted on tuberculosis, sexually transmitted infections (viral and protozoa), or parasites, studies not conducted in Ethiopia, and study populations other than HIV-infected individuals were excluded from the study.
Quality assessment
After removing duplicated papers, all potentially eligible papers were reviewed. Full-text papers were retrieved for review and relevant information was extracted. The Joana Briggs Institute (JBI) critical appraisal checklist for prevalence study was used to assess the quality of included studies (10). Accordingly, articles with high and medium quality (fulfilling 50% of quality assessment) were included in the final analysis (Supplementary Table S2). Two independent authors (MA and GG) assessed the quality of the studies, and any disagreement was solved by discussion.
Data extraction
Data were extracted from each study using the designed tool in Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, United States) by two independent authors (MA and GG). Any ambiguity and difference during extraction were resolved through discussion. The data extracted from eligible studies were author name, year of publication, area in which the study was conducted, study design, type of sampling method, number of HIV-infected individuals, sex (male), age group, type of infection or colonization, number of total isolates, bacterial group as Gram-positive or Gram-negative, number of bacterial species, co-trimoxazole resistance, and co-trimoxazole prophylaxis.
Data analysis and synthesis
The relevant data were extracted from the eligible studies into a Microsoft Excel spreadsheet and then exported to the STATA version 11.0 software for meta-analysis. The pooled prevalence of co-trimoxazole resistance and 95% confidence intervals were visually displayed using a forest plot. Subgroup analysis was performed based on region, sampling technique, year of study, type of infection or colonization, and bacterial group. The heterogeneity of the included studies was evaluated using the inverse variance index (I2 statistic). No, low, moderate, and high levels of heterogeneity were considered when the values of I2 were 0, 25, 50, and 75%, respectively (11). In all pooled analyses, heterogeneity resulting from differences in effects across different studies was accounted for using a random-effects model. A sensitivity analysis of each study’s impact on the overall prevalence was also conducted. Publication bias was statistically investigated using Egger’s test (12) and visual inspection of funnel plots. A p-value <0.05 in Egger’s test was considered as evidence of statistically significant publication bias.
Results
Search results
A total of 1761records were retrieved during an electronic database search. The 155 were screened after removing records for several reasons such as studies with irrelevance of topics, not published in the English language, studies on tuberculosis, sexually transmitted infections, or intestinal parasites, studies not conducted in Ethiopia, and study populations other than HIV-infected individuals. Moreover, 61 duplicates were removed and 94 full-text articles were assessed for eligibility. Seventy-two full-text articles were excluded because of no co-trimoxazole resistance data, not fulfilling study quality criteria (Q-score), case reports, review articles, and meta-analysis studies. Finally, 22 studies were included in the systematic review and meta-analysis (Figure 1).
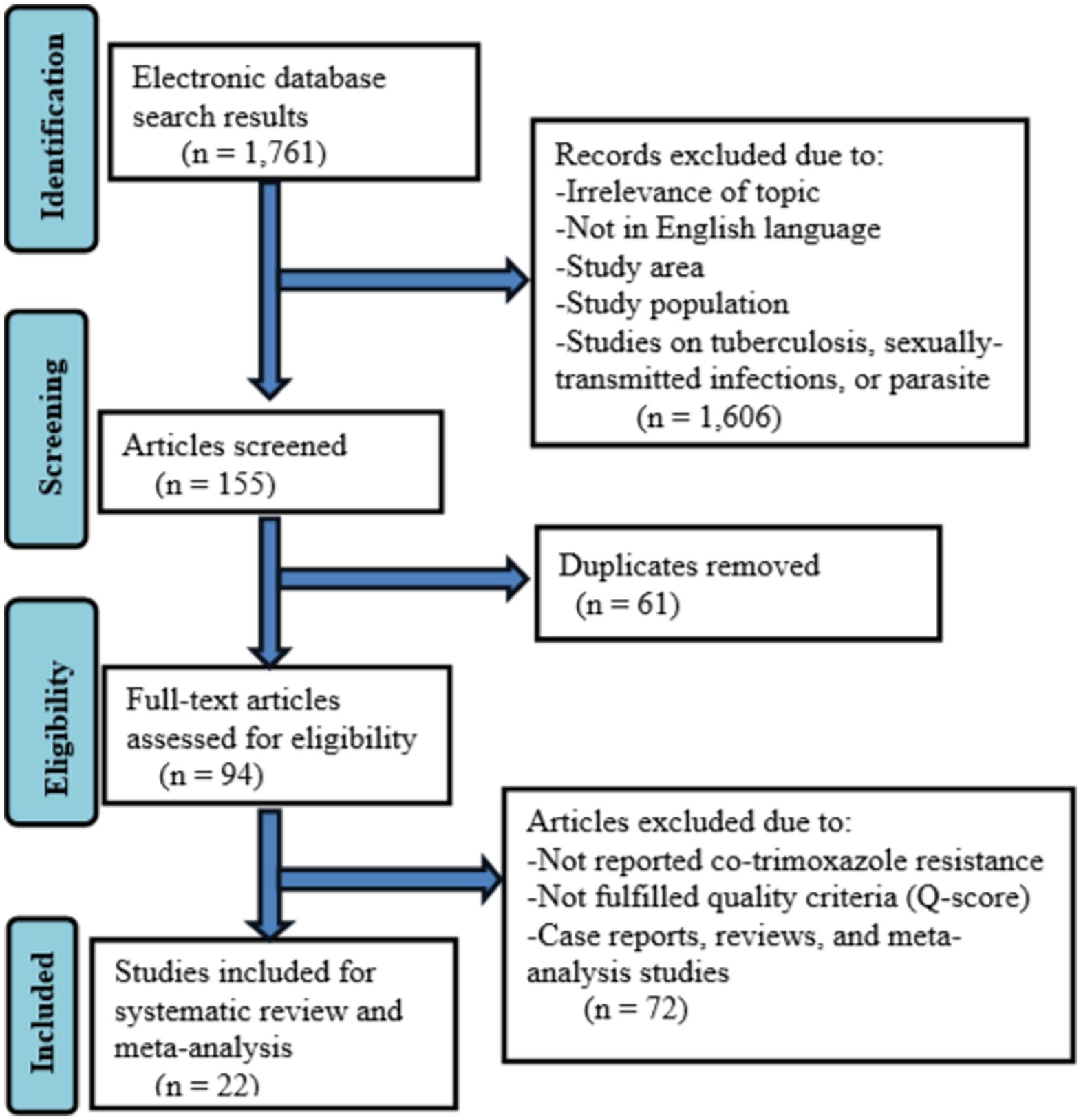
Figure 1. Flow diagram describing the selection of studies for the systematic review and meta-analysis on the prevalnce of co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
Studies characteristics
A total of 22 studies (13–34) comprising 5788HIV-infected individuals were included in this systematic review and meta-analysis. All of these studies used a cross-sectional study design. Regarding the sampling technique, 11 studies used systematic random sampling, 8 studies used convenient sampling, and the remaining 3 studies did not report their sampling techniques. Studies included 9 from Amhara, 5 from South Ethiopia, 3 from Addis Ababa, 2 from Sidama, and 1 from (Oromia, Harari, and Tigray). The minimum and maximum numbers of study participants were 100 in Gondar (19) and 450 in Addis Ababa (16), respectively. Of the 5788HIV-infected individuals, 3,318 (57.3%) were females. The proportion of HIV-infected individuals on co-trimoxazole prophylaxis was not consistently reported in published articles; only 40.9% of studies have reported the status of prophylaxis. Studies reported that a total of 859 HIV-infected individuals were given the prophylaxis (Table 1).
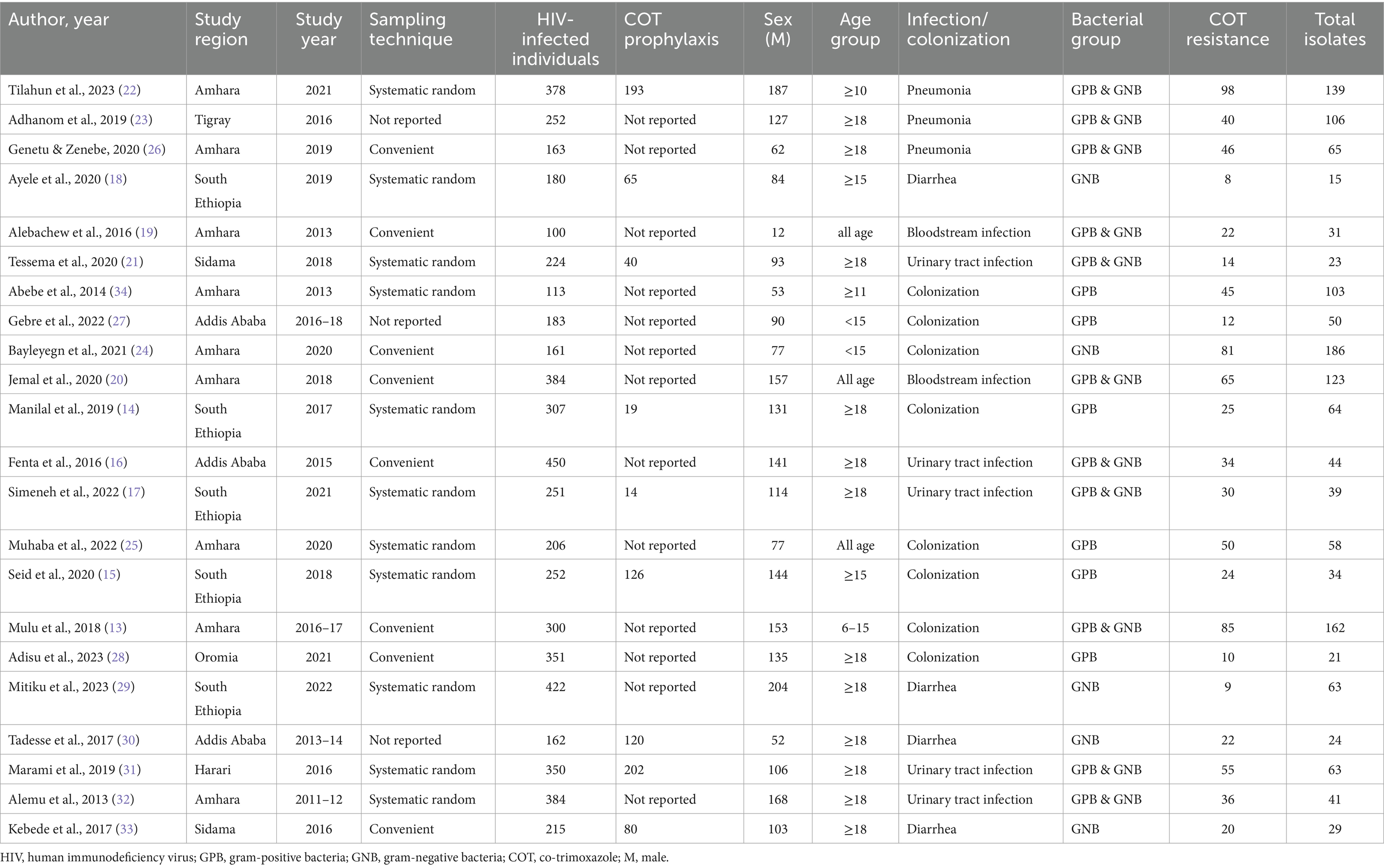
Table 1. Summary of studies on the prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
Pooled prevalence of co-trimoxazole resistance among HIV-infected individuals
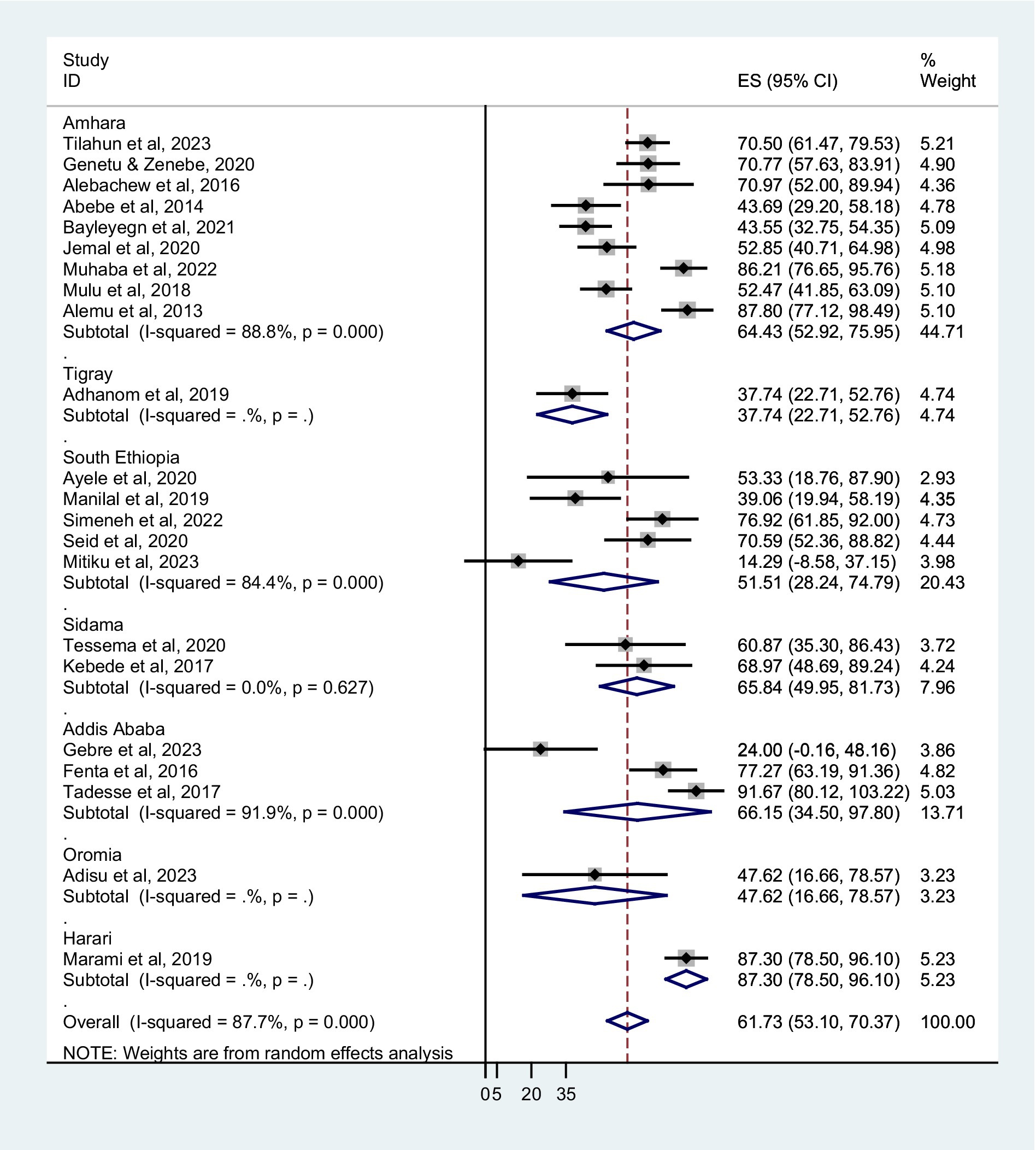
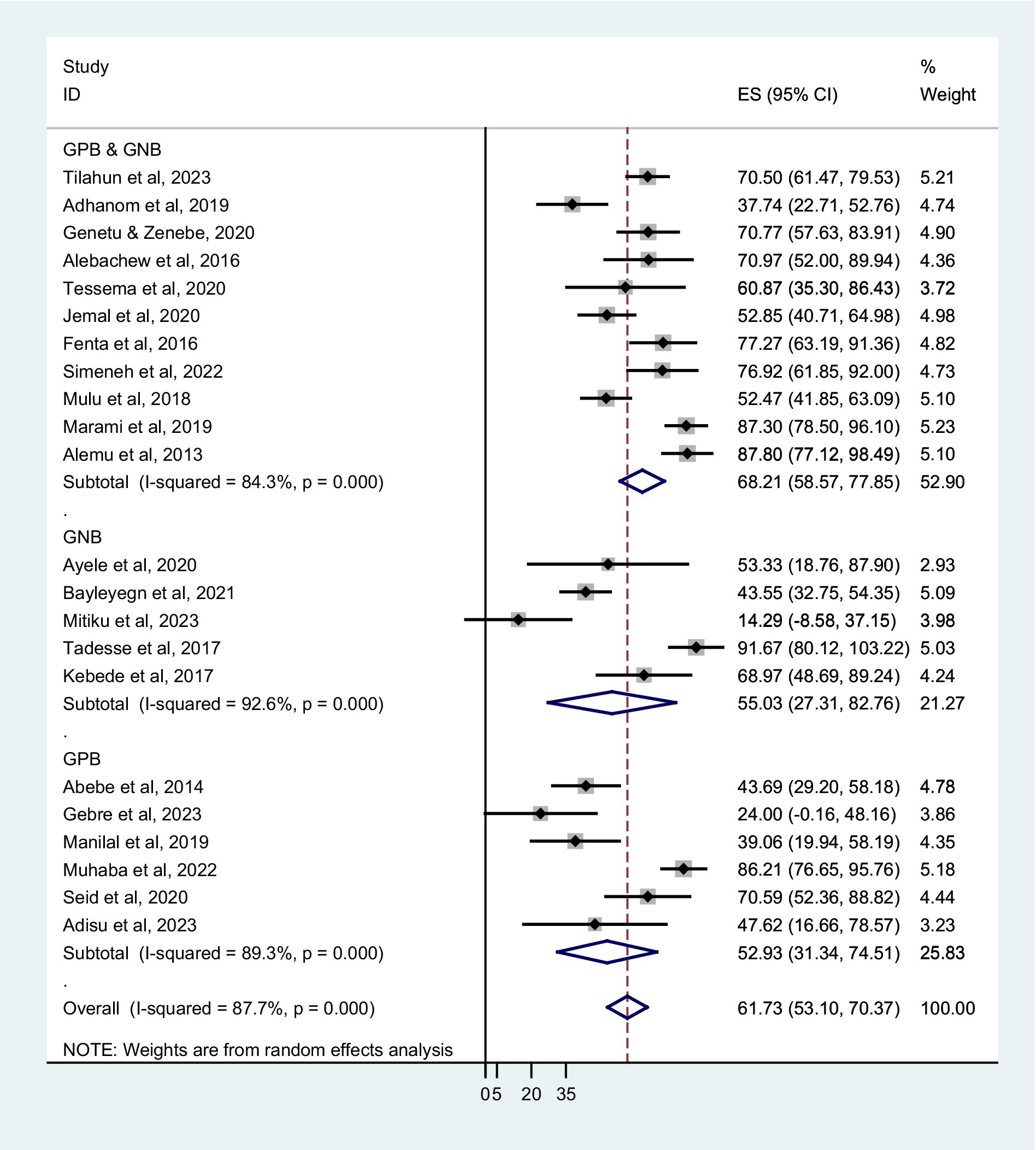
The prevalence of co-trimoxazole resistance reported by the studies ranged from 14.3% in Dilla (29) to 91.7% in Addis Ababa (30). Among 1,483 bacterial isolates identified from the eligible studies, 831 were co-trimoxazole-resistant (Table 1). Accordingly, the pooled prevalence of co-trimoxazole resistance was 61.73% (95% CI: 53.10–70.37%), with heterogeneity (I2 = 87.7%) and statistical significance (p < 0.001) (Figure 2).
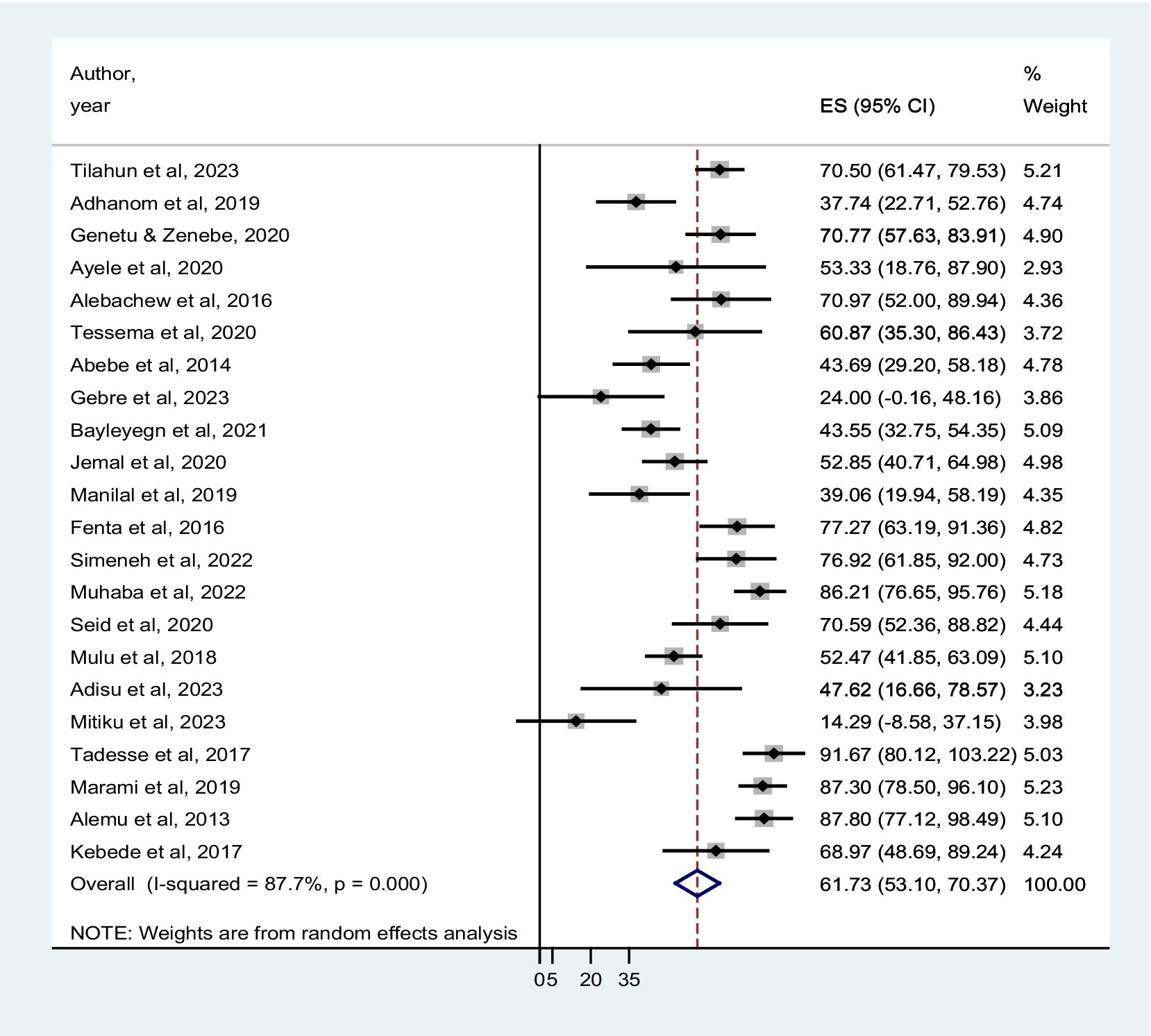
Figure 2. Forest plot showed the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
Subgroup analysis
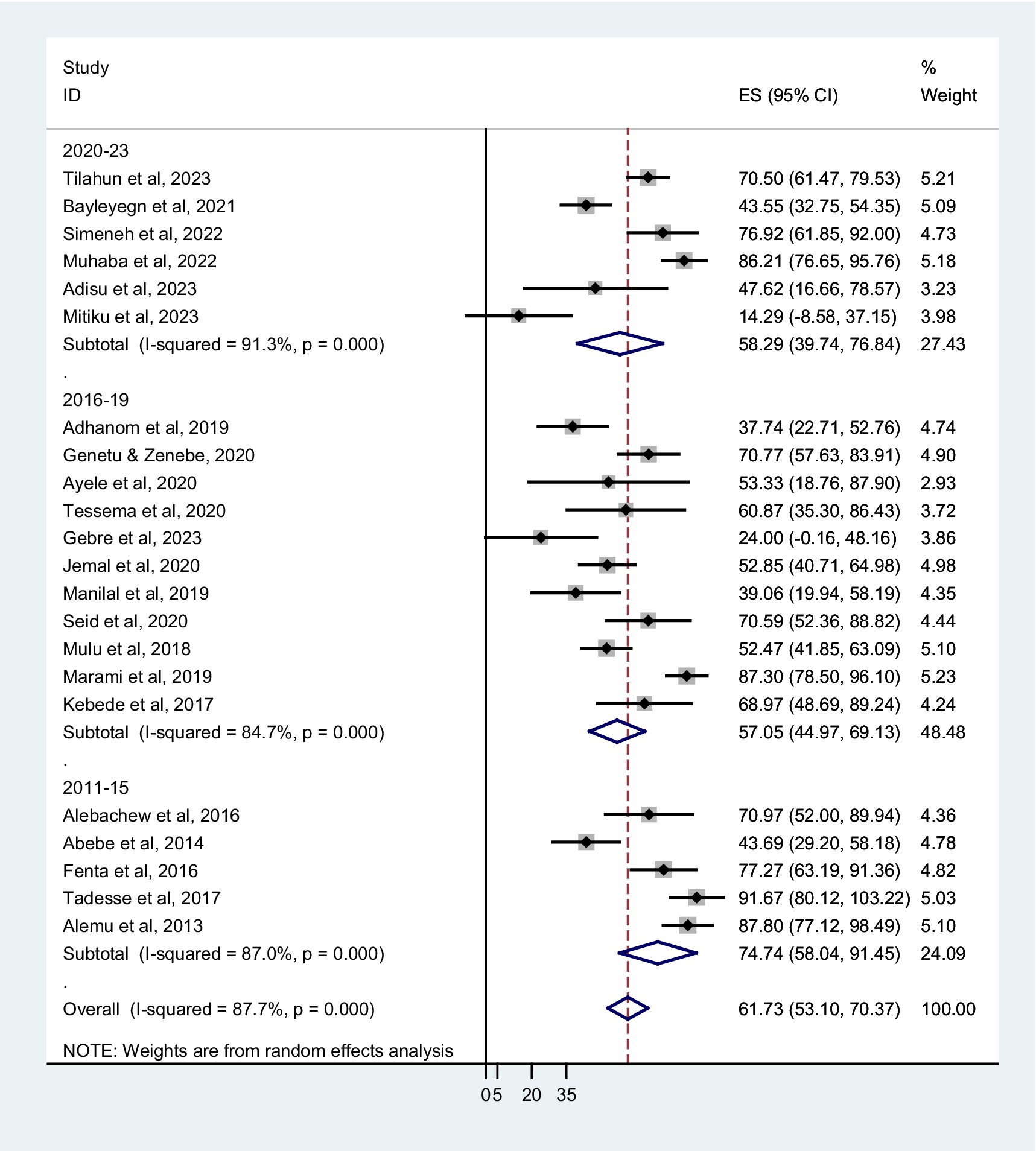
The prevalence of co-trimoxazole resistance among regions of the studies performed, years of the study, sampling techniques, different types of infection or colonization, and bacterial groups were analyzed by subgroup analysis. The combined prevalence of co-trimoxazole resistance across the Ethiopian regions showed that the highest co-trimoxazole resistance was reported in Harari; 87.30% (95% CI: 78.50–96.10%), followed by Addis Ababa; 66.15% (95% CI: 34.50–97.80%), Sidama; 65.84% (95% CI: 49.95–81.73%), and Amhara; 64.43% (52.92–75.9%) (Figure 3). The pooled prevalence of co-trimoxazole resistance per year of study was as follows: 2011–15; 74.74% (95% CI: 58.04–91.45%), 2016–19; 57.05% (95% CI: 44.97–69.13%), and 2020–23; 58.29% (95% CI: 39.74–76.84%) (Figure 4).
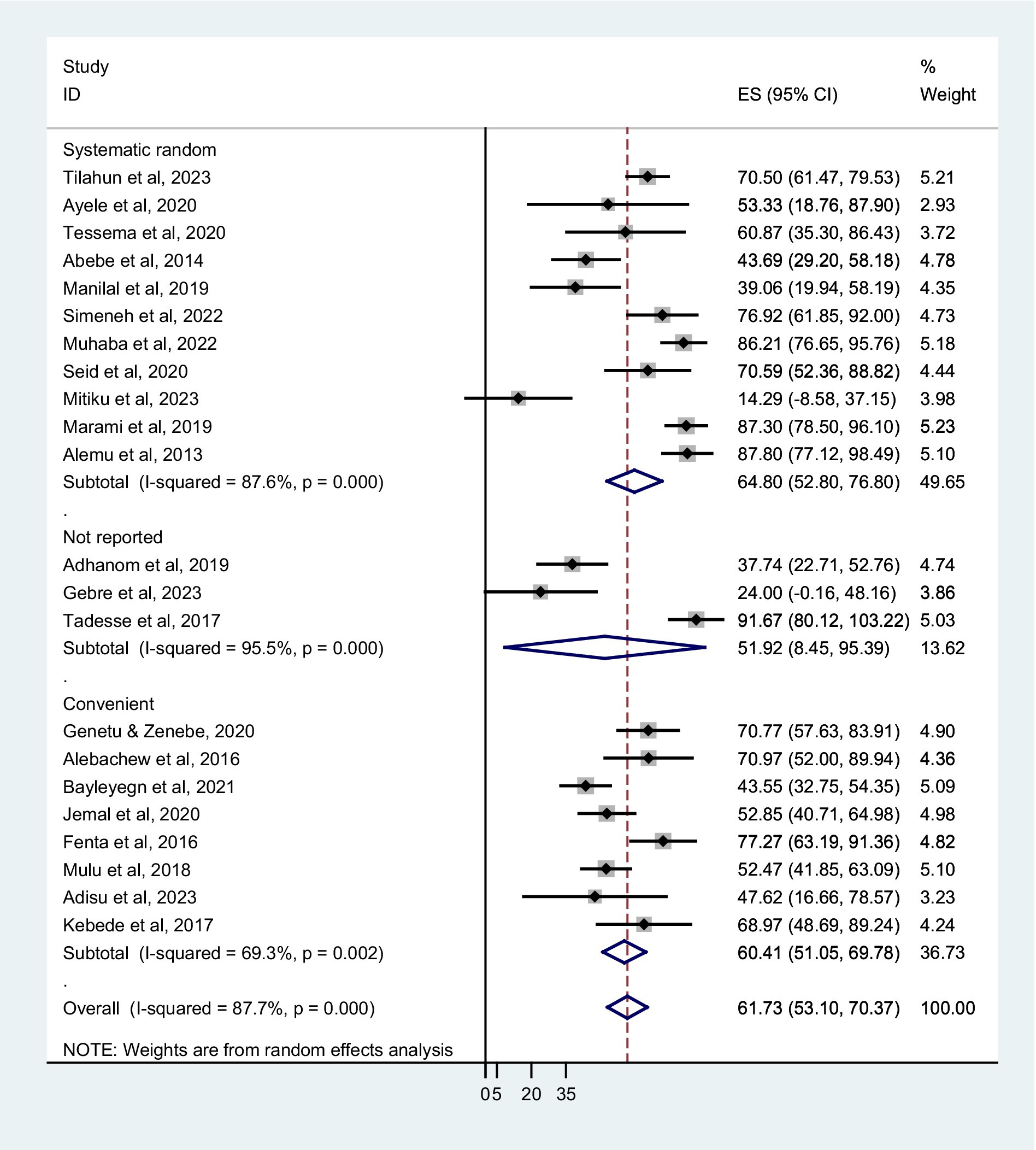
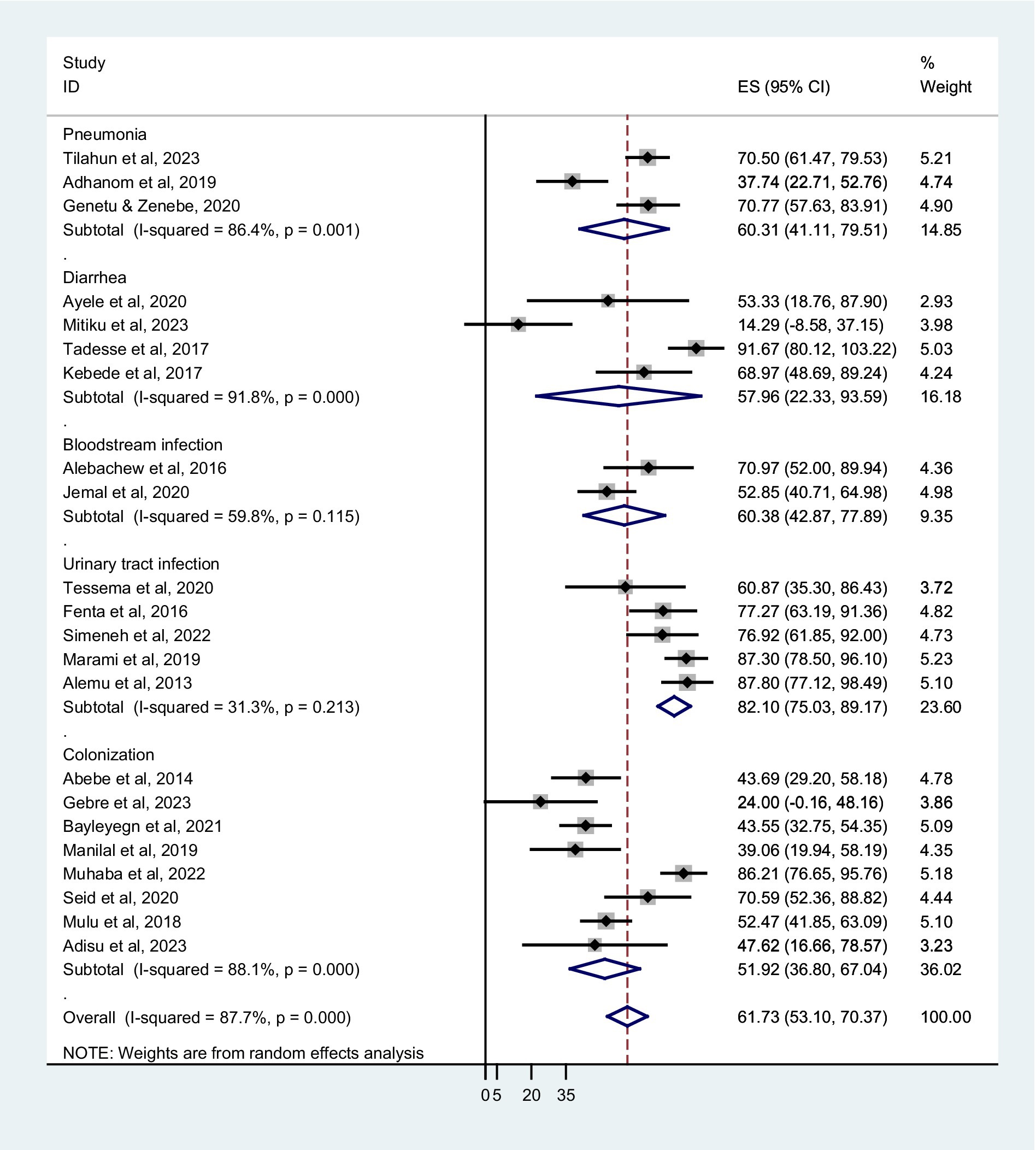
Based on sampling techniques, the co-trimoxazole resistance was described as systematic random; 64.80% (95% CI: 52.80–76.80%), convenient sampling; 60.41% (95% CI: 51.05–69.78%), and studies have not reported the sampling method; 51.92% (95% CI: 8.45–95.39%) (Figure 5). According to the type of infection, a higher co-trimoxazole resistance was observed in HIV-infected individuals with urinary tract infection; 82.10% (95% CI: 75.03–89.17%), bloodstream infection; 60.38% (95% CI: 42.87–77.89%), and pneumonia; 60.31% (95% CI: 41.11–79.51%). The pooled prevalence of co-trimoxazole resistance in bacterial colonization was 51.92% (95% CI: 36.80–67.04%) (Figure 6). Furthermore, the pooled prevalence of co-trimoxazole resistance in HIV-infected individuals per bacterial group was high in studies identifying both Gram-positive and Gram-negative bacterial isolates; 68.21% (95% CI: 58.57–77.85%) (Figure 7).
Pooled prevalence of co-trimoxazole resistance among bacterial species
Both Gram-positive and Gram-negative bacterial spp. that were reported by at least three studies were included in this meta-analysis. The pooled proportion of co-trimoxazole resistance for each bacterial spp. was computed from the total isolates tested for co-trimoxazole antibiotic. Among the bacterial spp., higher resistance to co-trimoxazole was observed in Escherichia coli; 70.86% (95% CI: 53.44–88.27%) followed by Salmonella spp.; 67.66% (95% CI: 41.51–93.81%), Proteus spp.; 66.23% (95% CI: 34.65–97.82%), Citrobacter spp.; 63.78% (95% CI: 41.08–86.48%), H. influenzae; 62.50% (95% CI: 34.21–90.79%), S. pneumoniae; 62.45% (95% CI: 44.64–80.27%), and S. aureus; 61.71% (95% CI: 48.43–75.00%) (Supplementary Figure S3).
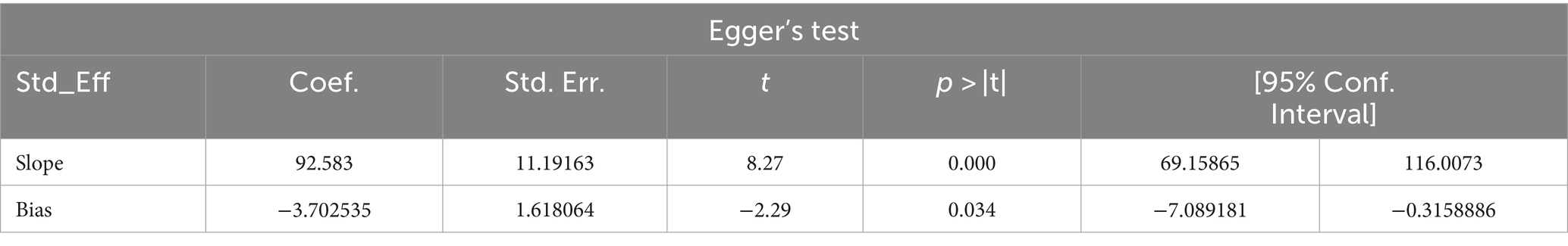
Publication bias
Studies were assessed for potential publication bias with a subjective examination of funnel plot and statistics using Egger’s test and funnel plot (p-value = 0.028), indicating the presence of publication bias (Table 2; Figure 8).

Figure 8. Funnel plot indicated publication bias in the studies on the co-trimoxazole resistance among HIV-infected individuals in Ethiopia.
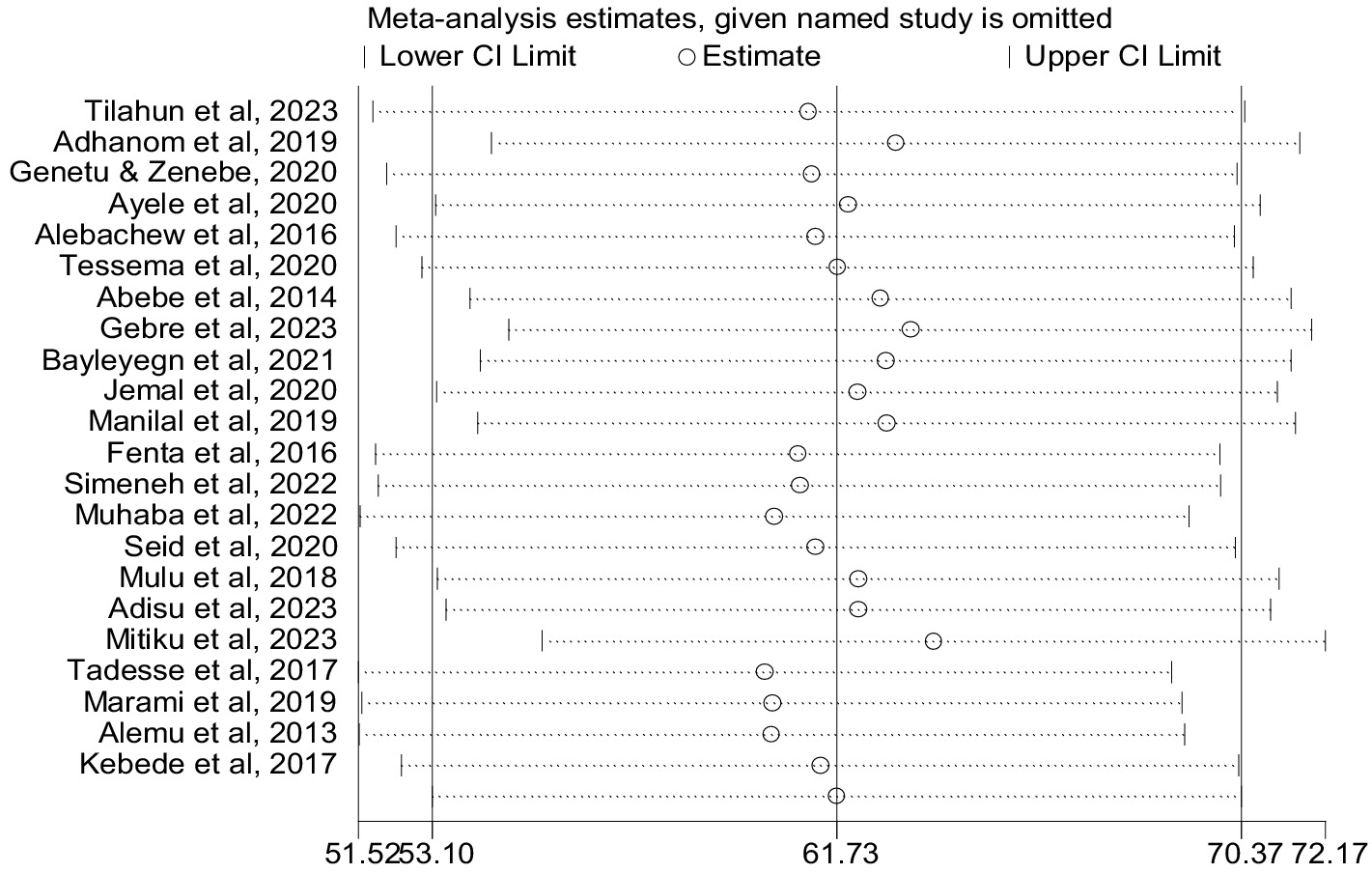
Sensitivity analysis
We conducted a sensitivity analysis for the prevalence of co-trimoxazole resistance using a random effects model by step-by-step removal of each study. The results showed that the exclusion of studies does not have a significant effect on the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia (Figure 9).
Discussion
This systematic review and meta-analysis is the first to determine the pooled prevalence of co-trimoxazole resistance in bacteria causing different types of infection or colonization among HIV-infected individuals in Ethiopia. The study included all available articles published from 2013 to 2023. Our study also determined the prevalence of co-trimoxazole resistance across the different regions in Ethiopia, the study period, sampling technique, types of infection or colonization, and bacterial group in the subgroup analysis to show whether there is a variation in the prevalence of co-trimoxazole resistance. In addition, this study excludes reports related to tuberculosis, sexually transmitted infections (viral and protozoa), and parasite-causing opportunistic infections in HIV-infected individuals. Moreover, this study provides information on co-trimoxazole resistance based on specific bacterial spp. in HIV patients.
According to recent studies from the African continent, Gram-negative pathogenic bacteria show significant rates of co-trimoxazole resistance, ranging from 50 to 97% (35–38). In this study, the pooled prevalence of co-trimoxazole resistance among HIV-infected individuals was 61.73% in all types of bacteria. In addition, the prevalence of co-trimoxazole resistance was higher in E. coli, followed by Salmonella and Proteus spp. This finding is consistent with a 50.3% Ethiopian report on the pooled prevalence of co-trimoxazole resistance in enteric bacteria among children (39). Another systematic review and meta-analysis in Iran revealed the prevalence of co-trimoxazole resistance among different uropathogenic bacterial spp. such as E. coli, Klebsiella, Staphylococcus, and Enterobacter (40). A meta-analysis by Olaru et al. also reported the resistance of co-trimoxazole to S. aureus, S. pneumoniae, E. coli, and K. pneumoniae that were identified in HIV-infected individuals (41).
It is suggested that co-trimoxazole prophylaxis may cause resistance in HIV-infected individuals for a variety of reasons, including prior exposure to co-trimoxazole or other sulphonamide-based drugs, non-adherence to co-trimoxazole prophylaxis, mutation, and patient self-medication, even though it lowers the risk of antimicrobial resistance by preventing infections and minimizing hospitalizations (42, 43). According to a study, there is evidence of cross-resistance between co-trimoxazole and penicillin antibiotics, and co-trimoxazole prophylaxis finally leads to the selection of co-trimoxazole resistance and MDR in pneumococci (15).
Additionally, co-trimoxazole is used for the prevention of Pneumocystis jiroveci (previously carinii) pneumonia and bacterial opportunistic infections in HIV-infected individuals. In this study, the subgroup analysis revealed a higher co-trimoxazole resistance in bacterial uropathogens, followed by bloodstream infection and pneumonia. Our finding is supported by a systematic review and meta-analysis that reported a high prevalence of co-trimoxazole resistance in uropathogens such as E. coli, Klebsiella, Staphylococcus, and Enterobacter (40). The selection of the appropriate antibiotics for treatment has become difficult and is primarily based on the information obtained from determining the pattern of antimicrobial resistance in the given area. This is because bacteria have acquired antibiotic resistance genes over time in different geographical areas and because there has been a change in the susceptibility patterns of bacteria to antibiotics (44).
Moreover, there was a significant heterogeneity among studies (I2 = 87.7%), which is not surprising given the variation in study settings, study period, type of bacterial infection or colonization, and type of bacterial species identified in the studies. A meta-analysis of co-trimoxazole resistance for most bacteria could not be performed because of the small number of studies reported, and we included bacteria reported in at least three studies. The presence of high heterogeneity across studies and limited number of studies in certain regions may impact the generalizability of findings.
Limitations of the study
As a strength, this study is the first report of pooled prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia. Due to the absence of published articles, we could not include all regions in Ethiopia, which resulted lack of generalizability for the problem. In addition, viral load, CD4 count, and co-trimoxazole prophylaxis were not fully reported in our systematic review and meta-analysis.
Conclusion and recommendations
According to our study, there is a higher prevalence of co-trimoxazole resistance to bacterial species causing opportunistic infections or colonization in HIV-infected individuals. This provides information for the WHO recommendation of co-trimoxazole prophylaxis to consider the need for modification to the prophylaxis agent and a detailed review of current guidelines. It can include medical care and regular follow-up to monitor compliance and side effects during co-trimoxazole administration. Moreover, epidemiologic surveillance on the extent of co-trimoxazole resistance is required in Ethiopia as a whole by including regions not described in this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MA: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GG: Data curation, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the scientific researchers of the included studies in this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1418954/full#supplementary-material
Abbreviations
HIV, Human Immunodeficiency Virus; AIDS, Acquired immunodeficiency syndrome; ART, Antiretroviral Therapy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International Prospective Register of Systematic Reviews; WHO, World Health Organization.
References
1. Prabhu, S, and van Wagoner, N. Human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS): an overview. Sexually transmissible Oral diseases. Prabhu, SR, van Wagoner, N, Hill, J, and Sawleshwarkar, S. (Editors). John Wiley & Sons (2023):51–71.
2. The Joint United Nations Programme on HIV/AIDS (UNAIDS) . (2022). Global HIV & AIDS statistics — Fact sheet. Available at: https://www.unaids.org/en/resources/fact-sheet.
3. USAID . HIV/AIDS. Available at: https://www.usaid.gov/ethiopia/hivaids.
4. Suthar, AB, Vitoria, MA, Nagata, JM, Anglaret, X, Mbori-Ngacha, D, Sued, O, et al. Co-trimoxazole prophylaxis in adults, including pregnant women, with HIV: a systematic review and meta-analysis. Lancet HIV. (2015) 2:e137–50. doi: 10.1016/S2352-3018(15)00005-3
5. Mermin, J, Lule, J, Ekwaru, JP, Malamba, S, Downing, R, Ransom, R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. (2004) 364:1428–34. doi: 10.1016/S0140-6736(04)17225-5
6. Takuva, S, Nabyoma, JM, Dawood, H, Black, A, Maartens, G, Parrish, A, et al. Eligibility for co-trimoxazole prophylaxis among adult HIV-infected patients in South Africa. S Afr Med J. (2019) 109:198–9. doi: 10.7196/SAMJ.2019.v109i4.13877
7. Wedderburn, CJ, Evans, C, Slogrove, AL, Rehman, AM, Gibb, DM, Prendergast, AJ, et al. Co-trimoxazole prophylaxis for children who are HIV-exposed and uninfected: a systematic review. J Int AIDS Soc. (2023) 26:e26079. doi: 10.1002/jia2.26079
8. Marwa, KJ, Mushi, MF, Konje, E, Alele, PE, Kidola, J, and Mirambo, MM. Resistance to Cotrimoxazole and other antimicrobials among isolates from HIV/AIDS and non-HIV/AIDS patients at Bugando medical Centre, Mwanza, Tanzania. AIDS Res Treat. (2015) 2015:103874. doi: 10.1155/2015/103874
9. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGGroup P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
10. Joanna Briggs Institute . The Joanna Briggs institute critical appraisal tools for use in JBI systematic reviews checklist for analytical cross sectional studies. North Adelaide, Australia: The Joanna Briggs Institute (2017).
11. Ruppar, T . Meta-analysis: how to quantify and explain heterogeneity? Eur J Cardiovasc Nurs. (2020) 19:646–52. doi: 10.1177/1474515120944014
12. Page, MJSJ, Higgins, JP, and Egger, M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. (2021) 12:248–59. doi: 10.1002/jrsm.1468
13. Mulu, W, Yizengaw, E, Alemu, M, Mekonnen, D, Hailu, D, Ketemaw, K, et al. Pharyngeal colonization and drug resistance profiles of Morraxella catarrrhalis, Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae among HIV infected children attending ART Clinic of Felegehiwot referral hospital, Ethiopia. PLoS One. (2018) 13:e0196722. doi: 10.1371/journal.pone.0196722
14. Manilal, A, Shewangizaw, M, Mama, M, Gezmu, T, and Merdekios, B. Methicillin-resistant Staphylococcus aureus colonization in HIV patients of Arba Minch province, Ethiopia: carriage rates, antibiotic resistance, and biofilm formation. Acta Microbiol Immunol Hung. (2019) 66:469–83. doi: 10.1556/030.66.2019.014
15. Seid, M, Beyene, G, Alemu, Y, Workalemahu, B, Delbo, M, Taddesse, D, et al. Does cotrimoxazole prophylaxis in HIV patients increase the drug resistance of pneumococci? A comparative cross-sectional study in southern Ethiopia. PLoS One. (2020) 15:e0243054. doi: 10.1371/journal.pone.0243054
16. Fenta, G, Legese, M, and Weldearegay, G. Bacteriuria and their antibiotic susceptibility patterns among people living with HIV attending Tikur Anbessa specialized and Zewditu memorial hospital ART clinics, Addis Ababa, Ethiopia. J Bacteriol Parasitol. (2016) 7. doi: 10.4172/2155-9597.1000292
17. Simeneh, E, Gezimu, T, Woldemariam, M, and Alelign, D. Magnitude of multidrug-resistant bacterial uropathogens and associated factors in urinary tract infection suspected adult HIV-positive patients in southern Ethiopia. Open Microbiol J. (2022) 16. doi: 10.2174/18742858-v16-e2208180
18. Ayele, A, Tadesse, D, Manilal, A, Yohanes, T, Seid, M, and Mekuria, MS. Prevalence of enteric bacteria and enteroparasites in human immunodeficiency virus-infected individuals with diarrhoea attending antiretroviral treatment clinic, Arba Minch general hospital, southern Ethiopia. New Microbes New Infect. (2020) 38:100789. doi: 10.1016/j.nmni.2020.100789
19. Alebachew, G, Teka, B, Endris, M, Shiferaw, Y, and Tessema, B. Etiologic agents of bacterial sepsis and their antibiotic susceptibility patterns among patients living with human immunodeficiency virus at Gondar University teaching hospital, Northwest Ethiopia. Biomed Res Int. (2016) 2016:1–8. doi: 10.1155/2016/5371875
20. Jemal, M, Deress, T, Belachew, T, and Adem, Y. Antimicrobial resistance patterns of bacterial isolates from blood culture among HIV/AIDS patients at Felege Hiwot referral hospital, Northwest Ethiopia. Int J Microbiol. (2020) 2020:1–8. doi: 10.1155/2020/8893266
21. Tessema, NN, Ali, MM, and Zenebe, MH. Bacterial associated urinary tract infection, risk factors, and drug susceptibility profile among adult people living with HIV at Haswassa university comprehensive specialized hospital, Hawassa, southern Esthiopia. Sci Rep. (2020) 10:10790. doi: 10.1038/s41598-020-67840-7
22. Tilahun, M, Gebretsadik, D, Seid, A, Gedefie, A, Belete, MA, Tesfaye, M, et al. Bacteriology of community-acquired pneumonia, antimicrobial susceptibility pattern and associated risk factors among HIV patients, Northeast Ethiopia: cross-sectional study. SAGE Open Med. (2023) 11:205031212211455. doi: 10.1177/20503121221145569
23. Adhanom, G, Gebreegziabiher, D, Weldu, Y, Gebreyesus Wasihun, A, Araya, T, Legese, H, et al. Species, risk factors, and antimicrobial susceptibility profiles of bacterial isolates from HIV-infected patients suspected to have pneumonia in Mekelle zone, Tigray, northern Ethiopia. Biomed Res Int. (2019) 2019:1–9. doi: 10.1155/2019/8768439
24. Bayleyegn, B, Fisaha, R, and Kasew, D. Fecal carriage of extended spectrum beta-lactamase producing Enterobacteriaceae among HIV infected children at the University of Gondar Comprehensive Specialized Hospital Gondar, Ethiopia. AIDS Res Ther. (2021) 18:1–9. doi: 10.1186/s12981-021-00347-x
25. Muhaba, H, Fenta, GM, and Gebretsadik, D. Methicillin resistance Staphylococcus aureus nasal carriage and its associated factors among HIV patients attending art clinic at Dessie comprehensive specialized hospital, Dessie, north East Ethiopia. PLOS Glob Public Health. (2022) 2:e0000838. doi: 10.1371/journal.pgph.0000838
26. Genetu, DE, and Zenebe, Y. Bacterial profile and their antibiotic resistance pattern among HIV patients diagnosed with pneumonia in Felege-Hiwot referral hospital. Northwest Ethiopia: Bahir Dar (2020).
27. Gebre, HA, Wami, AA, Kebede, ES, Yidnekachew, M, Gebre, M, and Negash, AA. Nasopharyngeal Staphylococcus aureus colonization among HIV-infected children in Addis Ababa, Ethiopia: antimicrobial susceptibility pattern and association with Streptococcus pneumoniae colonization. Access Microbiol. (2023) 5:acmi000557.v3. doi: 10.1099/acmi.0.000557.v3
28. Adisu, M, Amankwah, S, Tamrat, R, Alemu, Y, Akeba, T, and Tadesse, M. Colonization and associated risk factors of methicillin-resistant Staphylococcus aureus among people living with HIV at Jimma medical center, Southwest Ethiopia. Int J Clin Exp Med Sci. 9:65–74. doi: 10.11648/j.ijcems.20230904.12
29. Mitiku, A, Solomon, Z, Gidisa, B, Gebeyhu, K, Tewabe, H, Shenkute, D, et al. Prevalence, antibiotic susceptibility pattern, and associated factors of enteric bacterial pathogens among HIV infected patients with diarrhea attending the ART Clinic of Dilla University referral hospital, southern Ethiopia. Infect Drug Resist. (2023) 16:4227–36. doi: 10.2147/IDR.S410759
30. Tadesse, M, Dagne, Z, Tadesse, B, and Diriba, G. Drug resistance pattern of Diarrheogenic Enterobacteriaceae among HIV seropositive individuals on Cotrimoxazole prophylaxis at St. Paul’s hospital, Addis Ababa. Ethiop J Public Health Nutr. (2017) 1:86–92.
31. Marami, D, Balakrishnan, S, and Seyoum, B. Prevalence, antimicrobial susceptibility pattern of bacterial isolates, and associated factors of urinary tract infections among HIV-positive patients at Hiwot Fana specialized university hospital, eastern Ethiopia. Can J Infect Dis Med Microbiol. (2019) 2019:1–8. doi: 10.1155/2019/6780354
32. Alemu, A, Dagnew, M, Alem, M, and Gizachew, M. Uropathogenic bacterial isolates and their antimicrobial susceptibility patterns among HIV/AIDS patients attending Gondar University specialized hospital, Gondar, Northwest Ethiopia. J Microbiol Res Rev. (2013) 1:42–51. doi: 10.4172/2327-5073.1000134
33. Kebede, A, Aragie, S, and Shimelis, T. The common enteric bacterial pathogens and their antimicrobial susceptibility pattern among HIV-infected individuals attending the antiretroviral therapy clinic of Hawassa university hospital, southern Ethiopia. Antimicrob Resist Infect Control. (2017) 6:128. doi: 10.1186/s13756-017-0288-7
34. Abebe, W, Endris, M, Tiruneh, M, and Moges, F. Prevalence of vancomycin resistant enterococci and associated risk factors among clients with and without HIV in Northwest Ethiopia: a cross-sectional study. BMC Public Health. (2014) 14:185. doi: 10.1186/1471-2458-14-185
35. Langendorf, C, Le Hello, S, Moumouni, A, Gouali, M, Mamaty, AA, Grais, RF, et al. Enteric bacterial pathogens in children with diarrhea in Niger: diversity and antimicrobial resistance. PLoS One. (2015) 10:e0120275. doi: 10.1371/journal.pone.0120275
36. Ntirenganya, C, Manzi, O, Muvunyi, CM, and Ogbuagu, O. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg. (2015) 92:865–70. doi: 10.4269/ajtmh.14-0607
37. Isendahl, J, Manjuba, C, Rodrigues, A, Xu, W, Henriques-Normark, B, Giske, CG, et al. Prevalence of community-acquired bacteraemia in Guinea-Bissau: an observational study. BMC Infect Dis. (2014) 14:3859. doi: 10.1186/s12879-014-0715-9
38. Manyahi, J, Matee, MI, Majigo, M, Moyo, S, Mshana, SE, and Lyamuya, EF. Predominance of multi-drug resistant bacterial pathogens causing surgical site infections in Muhimbili National Hospital, Tanzania. BMC Res Notes. (2014) 7:500. doi: 10.1186/1756-0500-7-500
39. Embaye, SM, Amaha, ND, Muthoka, EN, Abebe, A, Mesfin, T, Kazambe, D, et al. Prevalence of co-trimoxazole resistance against bacterial isolates from children with diarrhea in Ethiopia: systematic review and meta-analysis. (2023) (preprint).
40. Rezaei-Tavirani, M, Ghafourian, S, Sayehmiri, F, Pakzad, R, Safiri, S, and Pakzad, I. Prevalence of cotrimoxazole resistance uropathogenic bacteria in Iran: a systematic review and meta-analysis. Arch Clin Infect Dis. (2018) 13. doi: 10.5812/archcid.63256
41. Olaru, ID, Tacconelli, E, Yeung, S, Ferrand, RA, Stabler, RA, Hopkins, H, et al. The association between antimicrobial resistance and HIV infection: a systematic review and meta-analysis. Clin Microbiol Infect. (2021) 27:846–53. doi: 10.1016/j.cmi.2021.03.026
42. Mwambete, K, and Kamuhabwa, A. Resistance of commensal intestinal Escherichia coli and other enterics to co-trimoxazole and commonly used antibiotics in HIV/AIDS patients. Clin Microbiol. (2013) 3:134.
43. Manyahi, J, Moyo, S, Aboud, S, Langeland, N, and Blomberg, B. High rate of antimicrobial resistance and multiple mutations in the dihydrofolate reductase gene among Streptococcus pneumoniae isolated from HIV-infected adults in a community setting in Tanzania. J Glob Antimicrob Resist. (2020) 22:749–53. doi: 10.1016/j.jgar.2020.06.026
Keywords: co-trimoxazole resistance, human immunodeficiency virus, systematic review, meta-analysis, Ethiopia
Citation: Assefa M and Girmay G (2024) Prevalence of co-trimoxazole resistance among HIV-infected individuals in Ethiopia: a systematic review and meta-analysis. Front. Med. 11:1418954. doi: 10.3389/fmed.2024.1418954
Edited by:
Faris Lami, University of Baghdad, IraqReviewed by:
Manal Younus, Ministry of Health, IraqHemant Khuntia, Siksha O Anusandhan University, India
Copyright © 2024 Assefa and Girmay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muluneh Assefa, mulunehassefa2010@gmail.com; muluneh.assefa@uog.edu.et
 Muluneh Assefa
Muluneh Assefa Getu Girmay
Getu Girmay