- 1Unit of Anaesthesia and Intensive Care, Department of Human Pathology in Adulthood and Childhood, University of Messina, Messina, Italy
- 2Department of Medical Sciences and Public Health, Faculty of Medicine and Surgery, University of Cagliari, Cagliari, Italy
- 3Section of Radiological Sciences, Department of Biomedical, Dental and Morphological and Functional Imaging Sciences, University of Messina, Messina, Italy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has put enormous pressure on healthcare systems worldwide. While the majority of severe cases present with respiratory failure, thrombosis or bleeding have also been reported at unusual sites. Major bleeding, particularly in patients treated with therapeutic anticoagulation, has been observed between the second and third week after the onset of SARS-CoV-2 infection. This article describes three cases of patients admitted to the hospital with severe SARS-CoV-2 pneumonia who had spontaneous arterial bleeding from the thoracic and subscapular regions during treatment with helmet continuous positive airway pressure (H-CPAP) in the intensive care unit (ICU), requiring a percutaneous embolization procedure. A possible correlation with helmet-supported ventilation is hypothesized.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a coronavirus strain that caused the coronavirus disease 2019 (COVID-19) pandemic (1, 2).
During SARS-CoV-2 infection, the virus primarily targets the lungs, causing severe inflammation and damage to the alveoli, leading to respiratory distress. This can develop into pneumonia, acute respiratory distress syndrome (ARDS), and, in severe cases, lung failure. In addition, SARS-CoV-2 triggers abnormal blood clotting, which increases the risk of thrombosis. Microthrombi can form in the blood vessels of the lungs, further impairing oxygen exchange and contributing to the severity of the disease. These effects on both the lungs and the coagulation system are key features of severe COVID-19 cases (3).
Patient management primarily focuses on supportive care, which includes oxygenation, fluid management, and treatment with various drugs, such as antiviral therapies, antibiotics, steroids, non-steroidal anti-inflammatory drugs, and immunosuppressive drugs. Patients with COVID-19 pneumonia may develop acute hypoxemic respiratory failure (AHRF) and require positive end-expiratory pressure (PEEP) (2–4).
COVID-19 patients have also shown alterations in coagulation tests, with a significant increase in D-dimer levels associated with the severity of illness and adverse outcomes (4). In addition, a high risk of venous thromboembolism has been identified, with a high prevalence of symptomatic acute pulmonary embolism and deep vein thrombosis (4).
In patients requiring respiratory support, non-invasive ventilation (NIV) administered through a helmet has been widely used, even for prolonged periods. This approach reduces the need for intubation, although its effect on outcomes can vary (1–4). The helmet is an alternative interface for delivering NIV, consisting of a clear plastic hood mounted on a hard-plastic ring with a multi-size silicon-polyvinyl chloride (PVC) soft collar to fit a wide range of neck dimensions and to surround the entire head of the patient (1–4).
The World Health Organization conducted a systematic review of ventilation strategies for coronaviruses, including MERS, SARS, and COVID-19, and concluded that while NIV may reduce mortality and the need for intubation, it also has the potential to increase the risk of transmission to healthcare workers (3).
Traditional fixation systems used to anchor the helmet during CPAP can worsen the discomfort or pain experienced by patients and cause device-related pressure ulcers (2, 3). The most commonly used solutions for securing helmet non-invasive ventilation (H-NIV) setups are armpit straps and counterweight systems (5).
In a recent meta-analysis of trials involving adults with AHRF, treatment with non-invasive respiratory support, including H-NIV, was associated with a lower risk of death compared with standard oxygen therapy (6). H-NIV reduces air leaks compared to face mask interfaces, potentially decreasing viral transmission when used to treat patients with AHRF caused by COVID-19 (6, 7).
In various forms of AHRF, H-NIV may enhance the recruitment of non-aerated alveoli in dependent pulmonary regions, thereby increasing lung functional residual capacity and decreasing shunting (2–4).
The most commonly used solutions for fixing H-NIV setups are axillary straps and counterweight systems. However, this traditional securing system may compress the lateral thoracic wall and subscapular region, worsening the pain and discomfort experienced by the patient and causing device-related local complications, such as hematomas, edema, and pressure ulcers (6–8).
Case presentation
We describe three cases of adult patients admitted to the intensive care unit (ICU) of the University Hospital “Gaetano Martino” of Messina between 2021 and 2022. These patients were diagnosed with COVID-19 pneumonia and required helmet continuous positive airway pressure (H-CPAP). They also developed spontaneous arterial bleeding in the thoracic region, necessitating embolization. During hospitalization, the patients were treated with enoxaparin and initially received high-flow nasal cannula (HFNC) oxygen. As their respiratory failure worsened, H-NIV was initiated. Within the first 48–96 h from the start of the H-NIV treatment, arterial bleeding from vessels located in the area compressed by the helmet straps was documented.
The first patient was an 82-year-old woman weighing 62 kg with a BMI of 21.97. Her clinical history included arterial hypertension, chronic ischemic heart disease, diabetes mellitus, and pulmonary emphysema. She was admitted for bilateral interstitial pneumonia due to SARS-CoV-2, complicated by pulmonary embolism. The patient received 8,000 UI of enoxaparin twice a day. She experienced spontaneous bleeding in the right pectoral and subscapular regions 72 h after the start of the treatment. Figure 1 shows a large hematoma in the right pectoral region measuring more than 10 cm. She underwent successful embolization using detachable coils and n-butyl cyanoacrylate glue. At the time of the onset of bleeding, her platelet count was 197.000 /mmc. She died of septic shock 39 days after being admitted to the ICU (Table 1).
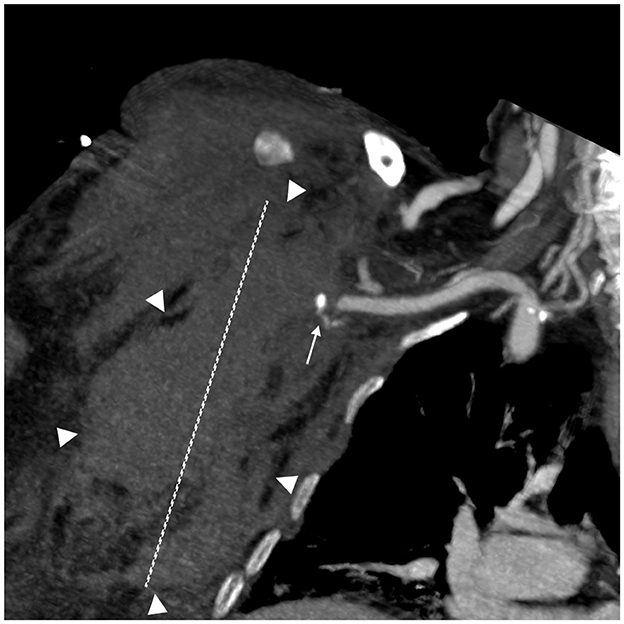
Figure 1. Spontaneous bleeding in the right pectoral region. A coronal Multiplanar reconstruction (MPR) CT angiography image; arterial phase. A small pseudoaneurysm (white arrow) arising from a minor branch of the subscapular artery is visible near the subclavian artery. A large hematoma in the right pectoral and thoracic regions (maximum diameter 10 cm—white dashed line).

Table 1. Summary of the clinical characteristics, laboratory values, management, and outcomes of the three patients.
The second patient was a 78-year-old man weighing 78 kg with a BMI of 26.6. He had a history of chronic obstructive pulmonary disease, arterial hypertension, carotid vasculopathy, and hypercholesterolemia and was admitted for SARS-CoV-2 bilateral interstitial pneumonia and sepsis. He received 4,000 UI of enoxaparin twice a day, and his platelet count was 224.000/mmc. After 3 days in the ICU and 2 days of H-NIV treatment (Table 1), the patient developed hemorrhagic shock, severe hypotension, and anemia caused by spontaneous bleeding in the right thoracic and humeral regions. Figure 2 shows active bleeding (white arrow) from the fourth anterior intercostal artery, a branch of the internal mammary artery, and a 13-cm hematoma. After 34 days of hospitalization, he was discharged to a respiratory rehabilitation facility and is currently in good health.
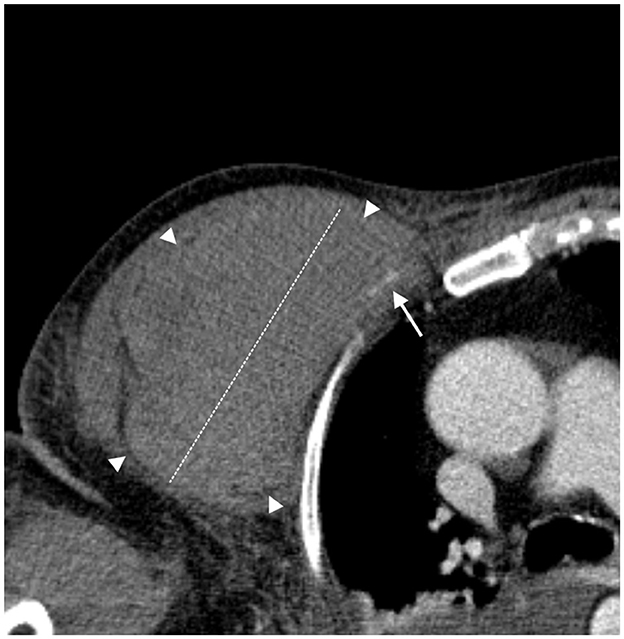
Figure 2. Spontaneous bleeding in the right thoracic and humeral region. Axial CT angiography image; arterial phase. A slightly hyperdense intramuscular pectoral hematoma (diameter 13 cm—white dashed line) due to recent bleeding (borders are marked with white arrowheads). The CT angiography shows active bleeding as a slight linear hyperdensity (white arrow) of the fourth anterior intercostal artery, a branch of the internal mammary artery.
The third patient was a 79-year-old man weighing 74 kg, with a BMI of 27.18. He was admitted to the ICU from the pneumology department. He was diagnosed with SARS-CoV-2 ARDS and sepsis, and he had a clinical history of arterial hypertension, diabetes mellitus, peripheral vascular disease, and prostate cancer. The patient received 4,000 UI of enoxaparin twice a day and was treated with H-NIV using a helmet from the time of admission to the ICU. On day 2, anuria and severe anemia (hemoglobin 6.3 g/dl; hematocrit 18%; platelets 191.000/mmc) prompted an emergency CT scan, which revealed spontaneous bleeding from the subscapular artery. The bleeding was diagnosed 48 h after starting the treatment (Table 1).
Figure 3 shows spontaneous bleeding caused by a pseudoaneurysm in a small branch of the subscapular artery. The patient underwent emergency angiography and successful embolization using detachable microcoils. The patient was mechanically ventilated for 21 days and received continuous renal replacement therapy (CRRT) using continuous veno-venous hemofiltration with dialysis filtration (CVVHDF) for 6 days after the acute bleeding problem was resolved. He died 34 days after ICU admission due to complications from acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI).
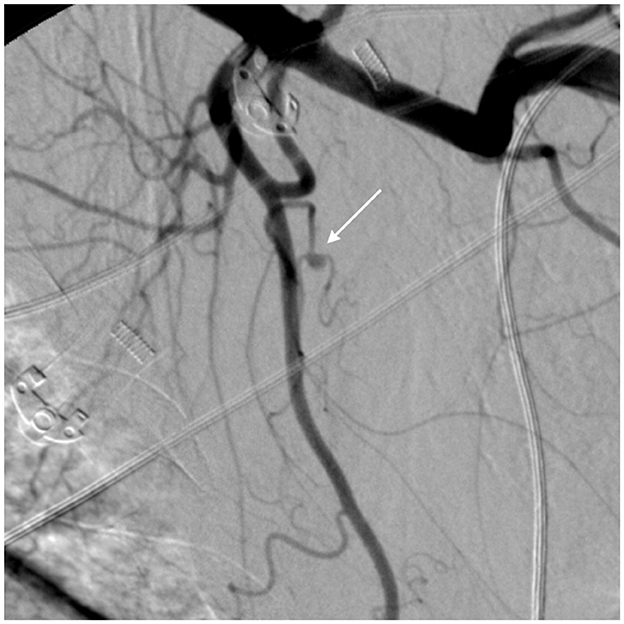
Figure 3. Large left pectoral hematoma due to spontaneous bleeding—digital subtraction angiography (DSA) frame. The DSA shows a 3 mm pseudoaneurysm (white arrow) arising from a small branch of the subscapular artery. The pseudoaneurysm represents one of the most common signs of vascular damage and requires embolization.
Discussion
Helmet non-invasive ventilation (H-NIV) has been used in patients with COVID-19 based on the assumption that the helmet interface is more effective than the mask interface for delivering prolonged treatments with high positive airway pressure. However, data on its effectiveness are limited (7).
In a randomized controlled trial (RCT) involving 322 patients, Arabi et al. compared the use of H-NIV with standard respiratory support. The primary outcome was 28-day all-cause mortality. The results of this study suggested that H-NIV did not significantly reduce 28-day mortality compared with usual respiratory support in patients with acute hypoxemic respiratory failure caused by COVID-19 pneumonia (7).
In a systematic review and meta-analysis that included 16 RCTs and eight observational studies, Chauduri et al. examined the effect of H-NIV compared to facemask NIV or high-flow nasal cannula (HFNC) in cases of acute respiratory failure (9). The authors concluded that H-NIV, compared to facemask NIV, may reduce mortality and intubation; however, the effect of H-NIV compared to HFNC remains uncertain (9).
While AHRF is the main manifestation of severe COVID-19, the most appropriate form of respiratory support and the optimal timing remain to be defined. A well-seated helmet is generally better tolerated than an oronasal or full-face mask, especially for extended CPAP therapy over several days (2, 6). Tolerance to non-invasive support, ability to clear secretions, worsening of lung injury from large tidal volumes during inspiratory pressure support, and self-induced lung injury are among the main concerns associated with NIV. Additionally, potential harm resulting from skin breakdown, arm edema, or thrombosis has also been reported (6).
In a multicenter randomized clinical trial conducted in four intensive care units (ICUs) in Italy between October and December 2020, 109 patients with moderate to severe hypoxemic respiratory failure caused by COVID-19 were randomly assigned to receive continuous treatment with H-NIV for at least 48 h or high-flow oxygen alone (10). The primary outcome was the number of days free from respiratory support within 28 days after enrollment. The study showed that there were no significant differences between the two groups of patients in terms of the number of days free from respiratory support within 28 days (10).
We have described three cases of acute severe bleeding in the lateral thoracic and subscapular regions, associated with the straps of the device used, in patients admitted to the ICU for severe HARF due to COVID-19 treated with H-NIV.
The risk of bleeding in coronavirus disease is primarily related to thromboembolic or vascular fragility complications that may develop. Therefore, managing the balance between thrombosis prevention and bleeding risk is crucial for effective treatment.
An axillary hematoma is also dangerous because it can lead to neurological deficits. The brachial plexus within the axillary sheath contains the median, ulnar, radial, and musculocutaneous nerves. Therefore, any compression must be treated promptly. Direct compression of a nerve can cause demyelination of axons, resulting in a transient conduction block. Prolonged compression can lead to Wallerian degeneration, resulting in motor nerve impairment (11). The hypothesis is that the straps of the device used have obstructed vascular flow (venous and arterial), progressively resulting in extravasation from the vessel walls, despite preventive measures. One possibility is that increased intrathoracic pressure may have caused hypertension in the scapular and pectoral arteries, which could have consequently promoted bleeding. From another perspective, it is possible that traumatic damage to the vessels occurred during the placement or removal of the device in the ICU. Therapeutic anticoagulation for COVID-19, aimed at addressing local microvascular and macrovascular thrombosis, may have also contributed to increased bleeding (6).
Previous extensive studies, including those by Arabi et al. (7), Colaianni-Alfonso et al. (8), and Al Hashim et al. (12), reported no association between H-CPAP and H-NIV and lateral chest wall bleeding.
Significant bleeding at unusual sites can occur in COVID-19 patients undergoing anticoagulation treatment, but a possible correlation with H-NIV has not been previously reported (7, 13, 14). It is difficult to demonstrate a correlation between the bleeding experienced and the use of the device; however, the impact and decubitus of devices used in bedridden patients, and the importance of nursing procedures in the ICU, should not be underestimated.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
VT: Writing – original draft, Conceptualization. SSa: Writing – original draft. SSi: Writing – review & editing, Investigation. AS: Writing – review & editing, Investigation. AN: Writing – review & editing, Investigation. AB: Writing – review & editing, Investigation. AM: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by PRIN_20223TWA55_001 (P.I.: Anna Teresa Mazzeo).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; H-CPAP, helmet continuous positive airway pressure; H-NIV, helmet non-invasive ventilation; PEEP, positive end-expiratory pressure; NIV, Non-invasive ventilation; ICU, intensive care unit; PVC, polyvinyl chloride; AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; AKI, Acute kidney injury.
References
1. Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, Llontop C, Guerder A, Trosini-Desert V, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. (2020) 56:2001692. doi: 10.1183/13993003.01692-2020
2. Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. (2023) 49:727–59. doi: 10.1007/s00134-023-07050-7
3. Ute Muti-Schüenemann GE, Szczeklik W, Solo K, Khabsa J, Thomas R, Borowiack E, et al. Update alert 3: ventilation techniques and risk for transmission of coronavirus disease, including COVID-19. Ann Intern Med. (2022) 175:W6–7. doi: 10.7326/L21-0424
4. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. (2020) 323:1574–1581. Erratum in: JAMA. (2021) 325:2120. doi: 10.1001/jama.2020.5394
5. Cammarota G, Simonte R, De Robertis E. Comfort during non-invasive ventilation. Front Med. (2022) 9:874250. doi: 10.3389/fmed.2022.874250
6. Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. (2020) 324:57–67. doi: 10.1001/jama.2020.9524
7. Arabi YM, Aldekhyl S, Al Qahtani S, Al-Dorzi HM, Abdukahil SA, Al Harbi MK, et al. Effect of helmet noninvasive ventilation vs usual respiratory support on mortality among patients with acute hypoxemic respiratory failure due to COVID-19: the HELMET-COVID randomized clinical trial. JAMA. (2022) 328:1063–72. doi: 10.1001/jama.2022.15599
8. Colaianni-Alfonso N, Montiel GC, Vega ML, Mazzinari G, Alonso-Íñigo JM, Grieco DL. Helmet vs facemask CPAP in COVID-19 respiratory failure: a prospective cohort study. Chest. (2023) 163:341–4. doi: 10.1016/j.chest.2022.08.2221
9. Chaudhuri D, Jinah R, Burns KEA, Angriman F, Ferreyro BL. Munshi L, et al. Helmet noninvasive ventilation compared to facemask noninvasive ventilation and high-flow nasal cannula in acute respiratory failure: a systematic review and meta-analysis. Eur Respir J. (2022) 59:2101269. doi: 10.1183/13993003.01269-2021
10. Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of helmet noninvasive ventilation vshigh-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemicrespiratory failure: the HENIVOT randomized clinical trial. JAMA. (2021) 325:1731–43. doi: 10.1001/jama.2021.4682
11. Tran DD, Andersen CA. Axillary sheath hematomas causing neurologic complications following arterial access. Ann Vasc Surg. (2011) 25:697.e5–8. doi: 10.1016/j.avsg.2010.12.024
12. Al Hashim AH, Al Reesi A, Al Lawati NM, Burad J, Al Khabori M, Chandwani J, et al. Comparison of noninvasive mechanical ventilation with high-flow nasal cannula, face-mask, and helmet in hypoxemic respiratory failure in patients with COVID-19: a randomized controlled trial. Crit Care Med. (2023) 51:1515–26. doi: 10.1097/CCM.0000000000005963
13. Aliberti S, Radovanovic D, Billi F, Sotgiu G, Costanzo M, Pilocane T, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. (2020) 56:2001935. doi: 10.1183/13993003.01935-2020
Keywords: COVID-19, SARS-CoV-2, continuous positive airway pressure, ARDS, respiratory failure, embolization
Citation: Tripodi VF, Sardo S, Silipigni S, Stagno A, Neri AF, Bottari A and Mazzeo AT (2024) Case report: Spontaneous arterial bleeding in the lateral thoracic region during helmet CPAP treatment: a report of three cases in patients with severe COVID-19. Front. Med. 11:1418029. doi: 10.3389/fmed.2024.1418029
Received: 06 June 2024; Accepted: 21 October 2024;
Published: 29 November 2024.
Edited by:
Mariachiara Ippolito, University of Palermo, ItalyReviewed by:
Valentina Chiavieri, University of Milan, ItalyLev Volkov, Centre Hospitalier Le Mans, France
Stefano Tigano, IRCCS University Hospital of Bologna Sant Orsola Polyclinic, Italy
Copyright © 2024 Tripodi, Sardo, Silipigni, Stagno, Neri, Bottari and Mazzeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Francesco Tripodi, dnRyaXBvZGlAdW5pbWUuaXQ=
 Vincenzo Francesco Tripodi
Vincenzo Francesco Tripodi Salvatore Sardo2
Salvatore Sardo2 Alberto Stagno
Alberto Stagno Antonio Bottari
Antonio Bottari