- 1School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 2Department of Gastroenterology, The People’s Hospital of Linyi, Linyi, Shandong, China
Hypermagnesemia commonly occurs in patients with renal dysfunction. Diagnosing hypermagnesemia represents a challenge due to its rarity and the absence of routine monitoring of magnesium levels. Furthermore, the lack of awareness among clinicians regarding this uncommon condition frequently leads to delayed diagnoses. Few patients survive with a serum magnesium level exceeding 7 mmol/L. This article presents a case study of near-fatal hypermagnesemia resulting from the oral administration of Epsom salts in a patient with normal renal function. A 60-year-old female presented to the gastroenterology department on Oct. 6, 2023, with a 3-day history of black stools. She underwent subtotal gastrectomy in 2005 and has a stable history of nephrotic syndrome. To investigate the cause of her bleeding, electronic gastroscopy and colonoscopy were scheduled for Oct. 11, 2023. She experienced a sudden loss of consciousness 30 min after the ingestion of Epsom salts. The attending physician suspected a severe magnesium poisoning. She was promptly administered calcium gluconate, underwent tracheal intubation with ambu bag ventilation, and received early continuous renal replacement therapy (CRRT). Swift diagnosis and CRRT contributed to a reduction in her serum magnesium levels from an initial 8.71 mmol/L to 1.35 mmol/L, leading to a remarkable improvement in the toxic symptoms associated with hypermagnesemia. Subsequently, she was managed in the gastroenterology department, with gastroscopy revealing bleeding from the gastrointestinal anastomotic ulcer. Following conservative treatments including acid suppression, stomach protection, and hemostasis, her symptoms improved, and she was successfully discharged. This study aims to alert clinicians to the possibility of hypermagnesemia in individuals with normal renal function. Physicians should exercise caution when prescribing Epsom salts to patients with underlying gastrointestinal conditions. If necessary, alternative drug therapies may be considered to mitigate the risk of hypermagnesemia. Timely intervention is pivotal in averting life-threatening complications linked to hypermagnesemia.
1 Introduction
Magnesium salts play a pivotal role in various clinical applications. The pharmacological effects of different types of magnesium salts are specific to treating a wide range of diseases and symptoms. For instance, magnesium sulfate is predominantly utilized in obstetrics for the prevention of seizures in pre-eclampsia and recurrence in eclampsia (1, 2). It also acts as a neuroprotective agent in cases of preterm labor and in critically ill patients to manage severe hypomagnesemia (3–6). Intravenous magnesium sulfate stands as the mainstay treatment for torsades de pointes, while nebulized magnesium sulfate is advocated for severe asthma exacerbations (7–10). Magnesium sulfate is also utilized topically to treat sprains and hemorrhoids (11). Moreover, magnesium salts such as magnesium sulfate, magnesium citrate, magnesium hydroxide, and magnesium oxide are commonly used as physiological laxatives to relieve symptoms of constipation (6, 12, 13). Additionally, magnesium oxide, magnesium chloride, magnesium lactate, and magnesium carbonate are employed in the treatment of mild hypomagnesemia (14).
Hypermagnesemia is a rare and typically iatrogenic condition (15). It commonly arises in patients receiving excessive infusions for severe pre-eclampsia/eclampsia (16–18). Individuals with chronic kidney disease (CKD) or acute kidney injury face an elevated risk of hypermagnesemia (19). However, recent studies indicate that hypermagnesemia can also manifest in individuals with normal kidney function, particularly in elderly patients with specific gastrointestinal conditions (11, 20–26). Few patients survive with a serum magnesium level exceeding 7 mmol/L (27, 28). This article presents a unique case study of a patient who developed near-fatal hypermagnesemia, despite maintaining normal renal function and not surpassing the recommended upper limit of laxative salt intake.
2 Case presentation
A 60-year-old female presenting with a 3-day history of melena was hospitalized on Oct. 6, 2023. She underwent subtotal gastrectomy in 2005. She has a medical history of nephrotic syndrome, currently in a stable condition. Her renal function was within normal limits, with a creatinine level of 42.0 μmol/L, blood urea level of 6.6 mmol/L, and consistently undetectable urine protein levels. During examination, her heart rate was recorded at 109 beats per minute (bpm), blood pressure at 123/69 mmHg, respiratory rate at 18 bpm, and body temperature at 35.8°C. Physical assessment indicated slightly pale conjunctiva. Furthermore, an old longitudinal surgical scar, measuring approximately 15 cm in length, was observed in the midline of the abdominal wall. Laboratory analysis revealed the following: severe anemia (hemoglobin: 64.0 g/L, hematocrit: 20.10%), hypoproteinemia (serum albumin: 28.8 g/L), hypocalcemia (2.01 mmol/L), and hypomagnesemia (0.65 mmol/L). Liver function and other electrolyte parameters showed no abnormalities. A computed tomography (CT) scan of the entire abdomen showed postoperative gastric changes with a patent anastomotic site, low-density nodules in the quadrate hepatic lobe, a calcified lesion in the right hepatic lobe, and small kidney stones or calcified lesions bilaterally. Electrocardiography (ECG) displayed sinus rhythm (94 bpm), left ventricular high voltage (Rv5 + Sv1 = 4.17 mV), and abnormal T waves. Following symptomatic supportive interventions such as acid suppression, gastric protection, hemostasis, fluid resuscitation, and blood transfusion regimen, her condition stabilized with no recurrent bleeding. Considering her mild hypomagnesemia diagnosis, we refrained from initiating treatment with medications like magnesium oxide, magnesium chloride, magnesium lactate, or magnesium carbonate.
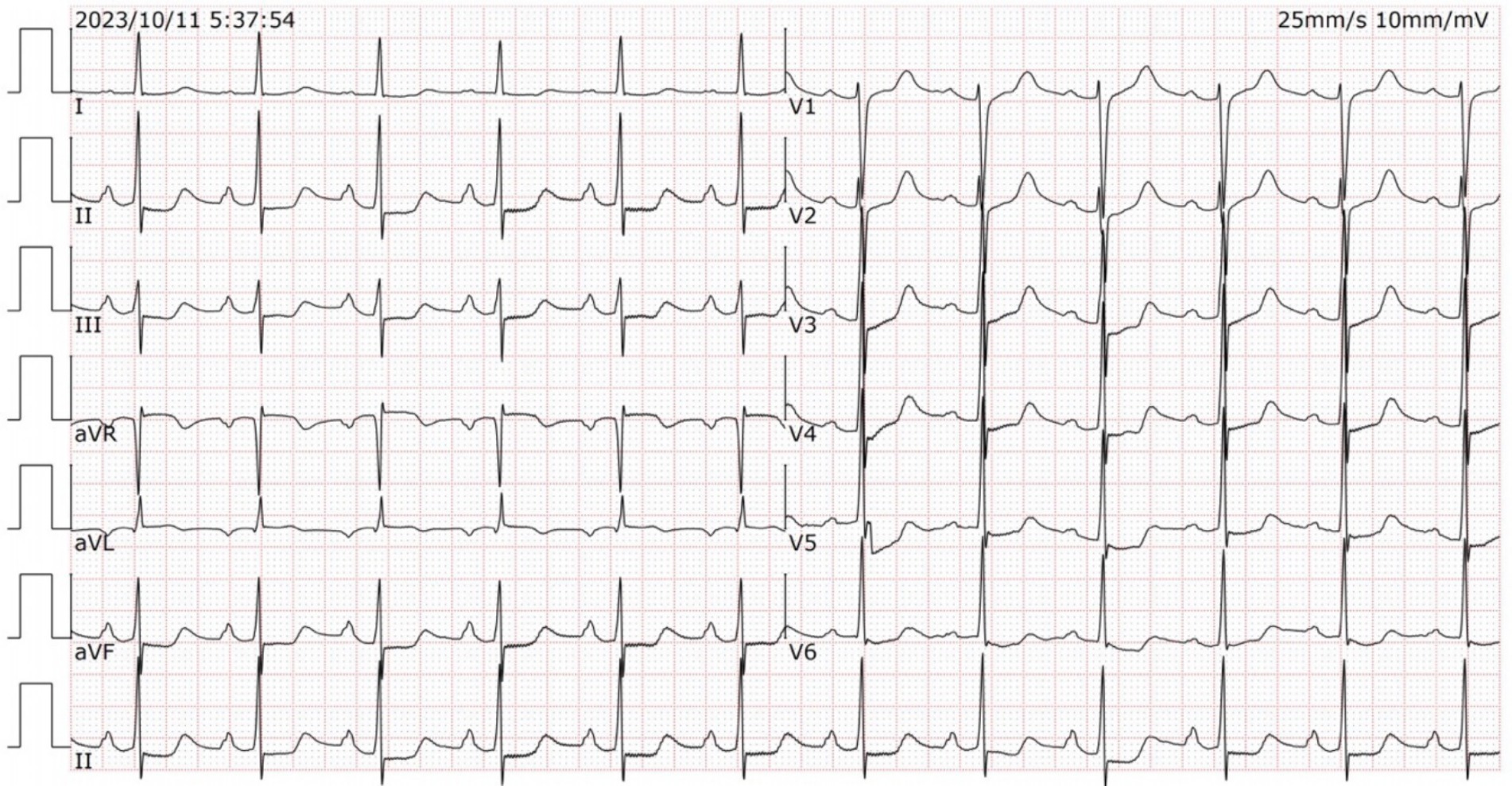
To investigate the root cause of the hemorrhage, she was scheduled for electronic gastroscopy and colonoscopy on Oct. 11, 2023. A solution made of 50 g of magnesium sulfate dissolved in 150 mL of warm water was administered for intestinal preparation. Approximately half an hour after ingestion, she suddenly lost consciousness. Electrocardiogram monitoring revealed a rapid elevation in heart rate (90–100 bpm), while oxygen saturation gradually declined to approximately 75%. She was uncooperative during the physical examination. The attending physician suspected magnesium poisoning as the preliminary diagnosis. She received immediate treatment with intravenous calcium gluconate (2 g) and a 500-mL intravenous infusion of 0.9% sodium chloride. A series of laboratory tests were promptly requested, with the results of the arterial blood gas analysis beginning to be available within about 20 min. The findings revealed a combination of respiratory acidosis, metabolic acidosis, and respiratory failure (pH: 7.225, pO2: 68.60 mmHg, pCO2: 57.1 mmHg, bicarbonate: 20.6 mmol/L). The remaining laboratory workup results were available within approximately 1 h after arrival. The laboratory analysis indicated mild anemia (hemoglobin: 100.0 g/L, hematocrit: 31.30%), hypermagnesemia (8.71 mmol/L), hypokalemia (2.75 mmol/L), and hypercalcemia (2.70 mmol/L). There were no abnormalities found in liver and renal function, myocardial enzyme spectrum, other electrolytes and random blood glucose (10.2 mmol/L) levels. The ECG (Figure 1) demonstrated normal sinus rhythm at a rate of 79 bpm, along with first-degree atrioventricular block (210 ms), ST segment depression, and prolonged QTc intervals (542 ms). The ECG did not exhibit any changes suggestive of acute myocardial infarction.
The intensive care unit (ICU) service was consulted for the treatment of hypermagnesemia. She was intubated and ventilated using a balloon, resulting in an increase in oxygen saturation to 94%. Subsequently, she was transferred to the ICU for further care. During the treatment, diprophylline injection was continuously infused to improve respiratory function and metaraminol bitartrate injection to maintain blood pressure. In addition, she was subsequently treated with 2 g of i.v. calcium gluconate to prevent arrhythmia, 20 mg of i.v. furosemide to promote renal excretion of magnesium.
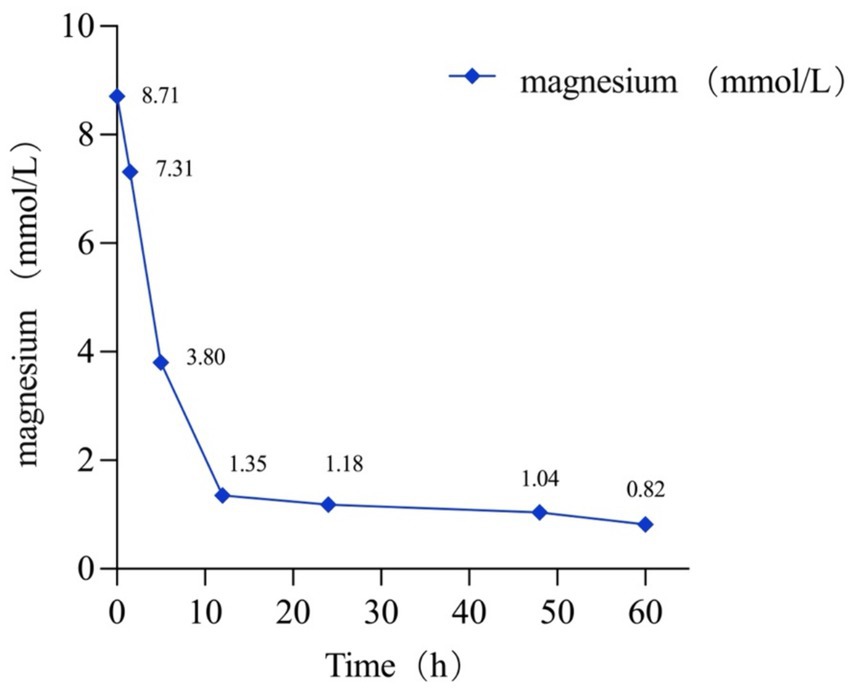
However, her serum magnesium level remained >3.8 mmol/L in the ICU 5 h post-arrival. Consequently, she underwent emergent continuous renal replacement therapy (CRRT), resulting in a significant reduction in her serum magnesium level from >3.8 mmol/L to 1.35 mmol/L after a 7 h hemodialysis session. Subsequent monitoring showed further improvement, with her serum magnesium level decreasing to 1.18 mmol/L 12 h later. After being monitored for 36 h, she was successfully extubated the following afternoon. By day 3, her serum magnesium level had normalized, at 0.82 mmol/L (Figure 2). As her magnesium levels decreased, her level of consciousness gradually returned. Subsequently, she was managed in the gastroenterology department, with gastroscopy revealing bleeding from the gastrointestinal anastomotic ulcer. Following conservative treatments including acid suppression, stomach protection, and hemostasis, her symptoms improved, and she was successfully discharged.
3 Discussion
Magnesium is an indispensable mineral that serves as a vital cofactor in over 300 enzymatic reactions within the human body (15). It plays a critical role in DNA, RNA, and protein synthesis, as well as in adenosine triphosphate (ATP) metabolism (29). Furthermore, magnesium is essential for regulating a diverse array of physiological functions, including blood pressure, cardiac excitability, vasomotor tone, insulin metabolism, muscular contraction, nerve transmission, and neuromuscular conduction (30). The maintenance of magnesium homeostasis hinges on the coordinated interplay of renal excretion and reabsorption, gastrointestinal absorption and bones (23, 31).
The human body predominantly absorbs magnesium through the paracellular pathway in the jejunum and ileum, accounting for 80–90% of total absorption (32, 33). The majority of magnesium in the human body is stored in bones. Less than 1% of magnesium is present in extracellular fluid, with less than 0.3% found in serum (34, 35). The normal reference range for serum magnesium concentration is typically between 0.7–1.10 mmol/L (1.7–2.4 mg/dL) (30, 36). The kidneys play a crucial role in maintaining magnesium homeostasis (15). Under normal magnesium levels, the kidneys filter a daily amount of magnesium ranging from 2000 to 2,400 mg, with approximately 96% of this being reabsorbed through paracellular mechanisms, primarily in the thick ascending limb of the loop of Henle. Only 3–5% of magnesium is excreted in the urine (37). In cases of hypermagnesemia, healthy kidneys can completely inhibit magnesium reabsorption from the loop of Henle, leading to potent magnesium excretion by the kidneys (38). Consequently, in individuals with normal renal function, hypermagnesemia is exceedingly rare.
Hypermagnesemia is a potentially life-threatening complication often associated with renal insufficiency (GFR < 30 mL/min), intravenous magnesium sulfate administration, excessive oral intake of magnesium-containing medications, high-dose magnesium sulfate enemas, and the use of antacids. In addition to these common causes, less frequent contributors include conditions such as hypothyroidism, hyperparathyroidism, adrenal insufficiency, rhabdomyolysis, and tumor lysis syndrome (39, 40). Additionally, constipation and the administration of specific medications, such as vitamin D, anticholinergics, and opiates, may elevate the risk of hypermagnesemia in individuals with normal renal function (21, 23, 39). A retrospective review of the patient’s medical history, conducted after symptom resolution, did not reveal any of the risk factors for hypermagnesemia. Patients with hypermagnesemia may experience a progressive inhibition of neuromuscular, cardiovascular, and respiratory system functions (41). The clinical manifestations of hypermagnesemia are dependent on serum magnesium concentrations. Symptoms such as fatigue, nausea, vomiting, and decreased tendon reflexes typically occur when serum levels reach 5 mg/dL (2.06 mmol/L). Prolongation of the Q-T intervals, loss of deep tendon reflexes, and somnolence are observed at serum levels between 5–12 mg/dL (2.06–4.94 mmol/L). Severe symptoms including coma, cardiac arrest, respiratory failure, and death can occur at serum levels exceeding 10–15 mg/dL (4.12–6.17 mmol/L) (22, 42–44).
The primary focus of initial treatment for hypermagnesemia involves continuous cardiac monitoring and airway management (25). In cases of life-threatening complications, the administration of intravenous calcium salts serves as emergency therapy. Calcium not only reverses cardiac dysrhythmias and respiratory depression but also alleviates hypocalcemia resulting from hypermagnesemia (40). It is worth noting that the patient displays hypermagnesemia and hypercalcemia, a presentation significantly divergent from previous research findings. Our speculation is that this divergence could be attributed to the on-duty physician’s initial administration of calcium gluconate therapy relying on clinical experience, contributing to subsequent alterations in laboratory test results. This underscores the importance of continuous monitoring of serum magnesium and calcium levels throughout the entire therapeutic course. Moreover, the administration of intravenous normal saline is crucial in the management and prophylaxis of hypermagnesemia in high-risk patients, particularly individuals with intestinal dysfunction who use magnesium as a laxative. The sodium chloride content in normal saline is advantageous in preserving appropriate urine output and facilitating the renal excretion of excess magnesium (26). Additionally, intravenous normal saline can aid in averting the buildup of magnesium in the blood, diminishing the serum magnesium levels and thereby decreasing the likelihood of hypermagnesemia in such patients. Furosemide may be administered to enhance the renal excretion of magnesium. For individuals with renal impairment, prompt hemodialysis should be initiated (21). In this study, the decision to proceed with hemodialysis was made due to the patient’s escalating serum magnesium levels and critical clinical state despite normal renal function. Following treatment, her symptoms of poisoning have shown significant improvement.
Following an in-depth literature review, we discovered limited case reports of patients who survived severe hypermagnesemia (27, 28, 45). In this study, the patient with normal renal function experienced near-fatal hypermagnesemia while using standard doses of magnesium sulfate for intestinal preparation. We considered that several possible factors could account for the occurrence of hypermagnesemia in this case. Initially, hydration status plays a pivotal role in regulating the levels of ionized electrolytes in the serum and their impact on organ function. Dehydration secondary to bowel preparation could potentially elevate the likelihood of relative hypovolemia, thereby predisposing the individual to hypermagnesemia (27, 46). Moreover, the patient’s history of subtotal gastrectomy may affect hydration status by influencing gastric emptying and the elimination of magnesium sulfate. Typically, it takes 4–6 h for the stomach to fully empty medication. Once magnesium sulfate enters the intestinal cavity, minimal absorption occurs, with most of it remaining in the intestines. The presence of retained magnesium sulfate elevates osmotic pressure in the intestines, leading to water retention and the absorption of water from surrounding tissues into the intestinal cavity. This increased volume stimulates the intestinal wall, enhancing peristalsis, facilitating fecal excretion, and completing the intestinal preparation process. Following a subtotal gastrectomy, the pylorus loses its normal function, resulting in a notable delay in gastric emptying. Undiluted magnesium sulfate swiftly moves from the stomach into the intestinal cavity. This rapid influx causes the jejunum to expand quickly, leading to a substantial transfer of extracellular fluid into the intestinal cavity, ultimately culminating in dehydration and a decrease in circulating blood volume. This further aggravates the imbalance in hydration status, making patients more susceptible to developing hypermagnesemia. Furthermore, the patient experienced gastrointestinal bleeding, with subsequent gastroscopy revealing bleeding from the gastrointestinal anastomotic ulcer. Given the presence of active gastric ulcer disease, which can increase magnesium absorption, the likelihood of an elevated magnesium concentration is significantly higher (20). Additionally, the elderly female patient with sluggish intestinal peristalsis faces an increased risk. In cases of hypermagnesemia, interference and blockage of myenteric neuronal function can disrupt the excitation-contraction coupling of smooth muscle cells, further exacerbating intestinal peristalsis issues (47). This disturbance allows for the continuous uptake of ingested magnesium. Magnesium remains in the intestinal cavity for an extended period and is continuously absorbed, resulting in persistent hypermagnesemia.
The case of the patient described here draws parallels to a 2020 report by Philip et al. on hypermagnesemia resulting from an Epsom salts overdose (44). In both instances, elderly patients displayed symptoms of magnesium toxicity, such as hypotension, shock, and respiratory failure, post magnesium sulfate administration. Treatment for both cases involved similar interventions, including calcium administration to counteract myocardial toxicity, respiratory support via endotracheal intubation, fluid support, and furosemide to enhance magnesium excretion. Notably, both patients exhibited positive outcomes with prompt hypermagnesemia correction through timely CRRT. However, significant differences exist between the two cases. Compared to Philip et al.’s report, the Epsom salt quantity was lower, onset was swifter, serum magnesium levels were higher, and symptoms were more pronounced in the current patient. The condition’s etiology may be influenced by factors like gender, prior gastric surgery, and active bleeding peptic ulcers, impacting magnesium metabolism and excretion, potentially leading to more severe symptoms. A comparative analysis of these cases offers a deeper understanding of hypermagnesemia’s etiology, clinical signs, and treatment strategies, aiding in more effective management of similar clinical scenarios.
4 Conclusion
This study has provided us with a valuable clinical insight. Hypermagnesemia can occur in patients who have undergone subtotal gastrectomy or peptic ulcer, even when the magnesium sulfate dosage is within the recommended limits and renal function is normal. It is imperative for physicians to exercise caution and monitor vital signs and serum magnesium levels when prescribing or increasing magnesium sulfate for such patients to avert symptomatic hypermagnesemia. Physicians are encouraged to recommend alternative laxatives, such as sodium picosulfate or polyethylene glycol electrolyte powder, as viable options for these patients. Early intervention is paramount in preventing life-threatening complications associated with hypermagnesemia. Upon diagnosing hypermagnesemia, prompt supportive measures should be initiated, including intravenous calcium supplementation, loop diuretics, fluid replacement, and emergency hemodialysis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Science and Technology Ethics Committee Linyi People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
G-FS: Conceptualization, Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Y-XG: Investigation, Writing – review & editing. X-PL: Writing – review & editing. Y-QL: Writing – review & editing. X-MC: Data curation, Writing – review & editing. X-MY: Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the general project of the Development Fund of the Affiliated Hospital of Xuzhou Medical University (XYFM2021032), Linyi Key R&D Program (2022YX0028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diaz, V, Long, Q, and Oladapo, OT. Alternative magnesium sulphate regimens for women with pre-eclampsia and eclampsia. Cochrane Database Syst Rev. (2023) 10:CD007388. doi: 10.1002/14651858.CD007388.pub3
2. Laskowska, M, and Bednarek, A. Optimizing delivery strategies in eclampsia: a comprehensive review on seizure management and birth methods. Med Sci Monit. (2023) 29:e941709. doi: 10.12659/msm.941709
3. Shepherd, ES, Goldsmith, S, Doyle, LW, Middleton, P, Marret, S, Rouse, DJ, et al. Magnesium Sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. (2024) 5:CD004661. doi: 10.1002/14651858.CD004661.pub4
4. Brookfield, KF, and Vinson, A. Magnesium sulfate use for fetal neuroprotection. Curr Opin Obstet Gynecol. (2019) 31:110–5. doi: 10.1097/gco.0000000000000529
5. Hellström, S, Jonsdotter, A, Jonsson, M, Pettersson, K, Saltvedt, S, Herbst, A, et al. A follow up on the feasibility after National Implementation of magnesium sulfate for neuroprotection prior to preterm birth. Acta Obstet Gynecol Scand. (2023) 102:1741–8. doi: 10.1111/aogs.14673
6. Kupetsky-Rincon, EA, and Uitto, J. Magnesium: novel applications in cardiovascular disease--a review of the literature. Ann Nutr Metab. (2012) 61:102–10. doi: 10.1159/000339380
7. Garner, O, Ramey, JS, and Hanania, NA. Management of Life-Threatening Asthma: severe asthma series. Chest. (2022) 162:747–56. doi: 10.1016/j.chest.2022.02.029
8. Rovsing, AH, Savran, O, and Ulrik, CS. Magnesium sulfate treatment for acute severe asthma in adults-a systematic review and Meta-analysis. Front Allergy. (2023) 4:1211949. doi: 10.3389/falgy.2023.1211949
9. Siraj, TZ, Ganim, I, Barker, W, Abraham, J, and Landa, E. Torsades De pointes with a Normal magnesium level in the setting of short bowel syndrome. Cureus. (2021) 13:e16743. doi: 10.7759/cureus.16743
10. Tzivoni, D, Banai, S, Schuger, C, Benhorin, J, Keren, A, Gottlieb, S, et al. Treatment of torsade De pointes with magnesium sulfate. Circulation. (1988) 77:392–7. doi: 10.1161/01.cir.77.2.392
11. Nordt, SP, Chen, J, and Clark, RF. Severe Hypermagnesemia after enteral Administration of Epsom Salts. Am J Health Syst Pharm. (2011) 68:1384–5. doi: 10.2146/ajhp100625
12. Philichi, L, and Yuwono, M. A retrospective study comparing polyethylene glycol-electrolyte solution with magnesium citrate for treatment of fecal Disimpaction. Gastroenterol Nurs. (2018) 41:141–4. doi: 10.1097/sga.0000000000000315
13. Mori, H, Tack, J, and Suzuki, H. Magnesium oxide in constipation. Nutrients. (2021) 13:2. doi: 10.3390/nu13020421
14. Rosner, MH, Ha, N, Palmer, BF, and Perazella, MA. Acquired disorders of hypomagnesemia. Mayo Clin Proc. (2023) 98:581–96. doi: 10.1016/j.mayocp.2022.12.002
15. Aal-Hamad, AH, Al-Alawi, AM, Kashoub, MS, and Falhammar, H. Hypermagnesemia in clinical practice. Medicina (Kaunas). (2023) 59:7. doi: 10.3390/medicina59071190
16. Al Alawi, AM, Al Badi, A, Al Huraizi, A, and Falhammar, H. Magnesium: the recent research and developments. Adv Food Nutr Res. (2021) 96:193–218. doi: 10.1016/bs.afnr.2021.01.001
17. Omer, MS, Latif, S, and Grisson, R. Iatrogenic hypermagnesemia in a patient with preeclampsia caused by misinterpretation of the magnesium reporting unit following magnesium sulfate administration. Cureus. (2022) 14:e32446. doi: 10.7759/cureus.32446
18. Elasy, AN, and Nafea, OE. Critical hypermagnesemia in preeclamptic women under a magnesium sulfate regimen: incidence and associated risk factors. Biol Trace Elem Res. (2023) 201:3670–8. doi: 10.1007/s12011-022-03479-x
19. Adomako, EA, and Yu, ASL. Magnesium disorders: core curriculum 2024. Am J Kidney Dis. (2024) 83:803–15. doi: 10.1053/j.ajkd.2023.10.017
20. Clark, BA, and Brown, RS. Unsuspected morbid hypermagnesemia in elderly patients. Am J Nephrol. (1992) 12:336–43. doi: 10.1159/000168469
21. Shoaib Khan, M, Zahid, S, and Ishaq, M. Fatal hypermagnesemia: an acute ingestion of Epsom salt in a patient with normal renal function. Caspian J Intern Med. (2018) 9:413–5. doi: 10.22088/cjim.9.4.413
22. Nishikawa, M, Shimada, N, Kanzaki, M, Ikegami, T, Fukuoka, T, Fukushima, M, et al. The characteristics of patients with hypermagnesemia who underwent emergency hemodialysis. Acute Med Surg. (2018) 5:222–9. doi: 10.1002/ams2.334
23. Yamaguchi, H, Shimada, H, Yoshita, K, Tsubata, Y, Ikarashi, K, Morioka, T, et al. Severe hypermagnesemia induced by magnesium oxide ingestion: a case series. CEN Case Rep. (2019) 8:31–7. doi: 10.1007/s13730-018-0359-5
24. Weber, CA, and Santiago, RM. Hypermagnesemia. A potential complication during treatment of theophylline intoxication with Oral activated charcoal and magnesium-containing cathartics. Chest. (1989) 95:56–9. doi: 10.1378/chest.95.1.56
25. Nordt, SP, Williams, SR, Turchen, S, Manoguerra, A, Smith, D, and Clark, RF. Hypermagnesemia following an acute ingestion of Epsom salt in a patient with Normal renal function. J Toxicol Clin Toxicol. (1996) 34:735–9. doi: 10.3109/15563659609013838
26. Ishida, Y, and Tabuchi, A. Severe hypermagnesemia with Normal renal function can improve with symptomatic treatment. Case Rep Emerg Med. (2020) 2020:2918249–4. doi: 10.1155/2020/2918249
27. Bamgbade, OA . Preoperative bowel preparation complicated by lethal hypermagnesaemia and acute nephropathy. Niger Postgrad Med J. (2017) 24:254–6. doi: 10.4103/npmj.npmj_145_17
28. Kontani, M, Hara, A, Ohta, S, and Ikeda, T. Hypermagnesemia induced by massive cathartic ingestion in an elderly woman without pre-existing renal dysfunction. Intern Med. (2005) 44:448–52. doi: 10.2169/internalmedicine.44.448
29. Dominguez, LJ, Veronese, N, and Barbagallo, M. Magnesium and the hallmarks of aging. Nutrients. (2024) 16:496. doi: 10.3390/nu16040496
30. Al Alawi, AM, Majoni, SW, and Falhammar, H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. (2018) 2018:9041694–17. doi: 10.1155/2018/9041694
31. de Baaij, JHF . Magnesium reabsorption in the kidney. Am J Physiol Renal Physiol. (2023) 324:F227–44. doi: 10.1152/ajprenal.00298.2022
32. Schuchardt, JP, and Hahn, A. Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr Nutr Food Sci. (2017) 13:260–78. doi: 10.2174/1573401313666170427162740
33. Chamniansawat, S, Suksridechacin, N, and Thongon, N. Current opinion on the regulation of small intestinal magnesium absorption. World J Gastroenterol. (2023) 29:332–42. doi: 10.3748/wjg.v29.i2.332
34. de Baaij, JH, Hoenderop, JG, and Bindels, RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. (2012) 5:i15–24. doi: 10.1093/ndtplus/sfr164
35. Gröber, U, Schmidt, J, and Kisters, K. Magnesium in prevention and therapy. Nutrients. (2015) 7:8199–226. doi: 10.3390/nu7095388
36. Workinger, JL, Doyle, RP, and Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients. (2018) 10:1202. doi: 10.3390/nu10091202
37. Blaine, J, Chonchol, M, and Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. (2015) 10:1257–72. doi: 10.2215/cjn.09750913
38. Schelling, JR . Fatal hypermagnesemia. Clin Nephrol. (2000) 53:61–5. doi: 10.2169/internalmedicine.45.1482
39. Bokhari, SR, Siriki, R, Teran, FJ, and Batuman, V. Fatal hypermagnesemia due to laxative use. Am J Med Sci. (2018) 355:390–5. doi: 10.1016/j.amjms.2017.08.013
40. Tofil, NM, Benner, KW, and Winkler, MK. Fatal hypermagnesemia caused by an Epsom salt enema: a case illustration. South Med J. (2005) 98:253–6. doi: 10.1097/01.Smj.0000145307.80421.4b
41. Qureshi, T, and Melonakos, TK. Acute hypermagnesemia after laxative use. Ann Emerg Med. (1996) 28:552–5. doi: 10.1016/s0196-0644(96)70120-8
42. Birrer, RB, Shallash, AJ, and Totten, V. Hypermagnesemia-induced fatality following Epsom salt gargles(1). J Emerg Med. (2002) 22:185–8. doi: 10.1016/s0736-4679(01)00462-0
43. Khairi, T, Amer, S, Spitalewitz, S, and Alasadi, L. Severe symptomatic hypermagnesemia associated with over-the-counter laxatives in a patient with renal failure and sigmoid volvulus. Case Rep Nephrol. (2014) 2014:560746. doi: 10.1155/2014/560746
44. Walker, P, Parnell, S, and Dillon, RC. Epsom salt ingestion leading to severe hypermagnesemia necessitating dialysis. J Emerg Med. (2020) 58:767–70. doi: 10.1016/j.jemermed.2020.04.023
45. Uchiyama, C, Kato, T, Tomida, K, Suzuki, R, Nakata, K, Hamanaka, M, et al. Fatal hypermagnesemia induced by preoperative colon preparation in an elderly woman: report of a case. Clin J Gastroenterol. (2013) 6:105–10. doi: 10.1007/s12328-012-0353-y
46. Hoy, SM, Scott, LJ, and Wagstaff, AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. (2009) 69:123–36. doi: 10.2165/00003495-200969010-00009
Keywords: hypermagnesemia, Epsom salts, magnesium sulfate, subtotal gastrectomy, normal renal function, peptic ulcer
Citation: Si G-F, Ge Y-X, Lv X-P, Li Y-Q, Chen X-M and Yuan X-M (2024) Case report: Near-fatal hypermagnesemia resulting from the use of Epsom salts in a patient with normal renal function. Front. Med. 11:1416956. doi: 10.3389/fmed.2024.1416956
Edited by:
Francesco Corica, University of Messina, ItalyReviewed by:
Luca Soraci, IRCCS INRCA, ItalySang-Hwan Do, Seoul National University Bundang Hospital, Republic of Korea
Copyright © 2024 Si, Ge, Lv, Li, Chen and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Min Yuan, bHl4aHl4bUAxNjMuY29t
 Gui-Fei Si1
Gui-Fei Si1 Xue-Min Yuan
Xue-Min Yuan