- 1Anesthesiology Research Laboratory, Tianjin Medical University General Hospital, Tianjin, China
- 2Cardiovascular Disease Research Laboratory, Tianjin Medical University General Hospital, Tianjin, China
- 3Anesthesiology Research Laboratory, Erdos Central Hospital, Ordos, China
- 4Center for Translational Pain Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, NC, United States
Background: Propofol and midazolam are commonly used sedative drugs in mechanically ventilated patients in the Intensive Care Unit (ICU). However, there is still a lack of relevant studies exploring the influence of midazolam and propofol on the prognosis of patients with Sepsis-associated Acute Kidney Injury (S-AKI).
Patients and methods: A statistical analysis was conducted on 3,745 patients with S-AKI in the Medical Information Mart for Intensive Care IV database. The patients’ baseline characteristics were grouped based on the use of either propofol or midazolam as sedatives. Cox proportional hazards models, logistic regression models, and subgroup analyses were used to compare the effects of propofol and midazolam on the short-term prognosis of S-AKI patients, including 30-day mortality, ICU mortality, and duration of mechanical ventilation.
Results: In the statistical analysis, a total of 3,745 patients were included, with 649 patients using midazolam and 3,096 patients using propofol. In terms of the 30-day mortality, compared to patients using midazolam, S-AKI patients using propofol had a lower ICU mortality (hazard ratio = 0.62, 95% confidence interval: 0.52–0.74, p < 0.001), lower 30-day mortality (hazard ratio = 0.56, 95% confidence interval: 0.47–0.67, p < 0.001), and shorter mechanical ventilation time (odds ratio = 0.72, 95% confidence interval: 0.59–0.88, p < 0.001). Kaplan–Meier curves showed lower survival probabilities in the midazolam group (p < 0.001). Subgroup analyses showed that propofol was strongly protective of short-term prognosis in older, male, smaller SOFA score CCI score, no heart failure, and comorbid chronic kidney disease patients with S-AKI.
Conclusion: Compared to midazolam, propofol was considered a protective factor for short-term mortality risk and ICU mortality risk in S-AKI patients. Additionally, S-AKI patients using propofol had a lower risk of requiring prolonged mechanical ventilation. Overall, propofol may be more beneficial for the short-term prognosis of S-AKI patients compared to midazolam.
Introduction
Sepsis, a systemic inflammatory response syndrome triggered by infection, significantly impacts global health (1). S-AKI is a form of acute kidney injury that arises within the context of sepsis. This kidney injury typically results from the systemic inflammatory response and hemodynamic alterations induced by sepsis, leading to insufficient renal perfusion and/or direct renal cellular damage (2). In 2017, there were an estimated 48.9 million sepsis cases globally, resulting in 11 million sepsis-related deaths, accounting for 19.7% of all worldwide fatalities (3). Sepsis remains the primary cause of morbidity and mortality in ICUs globally, coupled with significant economic repercussions (4, 5). A multicenter prospective cohort study revealed an AKI incidence of 51% among 1,177 sepsis patients (6–9). Consequently, S-AKI is a prevalent complication among critically ill ICU patients. The mortality rate is markedly higher in sepsis patients who develop AKI compared to those without AKI (10).
Appropriate sedation management using sedative drugs is nearly universal for mechanically ventilated ICU patients, enhancing their tolerance to mechanical ventilation and effectively reducing psychological stress in critically ill ICU patients (11). Propofol and midazolam are commonly used sedatives for mechanically ventilated ICU patients, including those with sepsis (12–14). The 2013 Pain, Agitation, and Delirium Guidelines recommend propofol over midazolam for mechanically ventilated adult ICU patients due to its association with reduced mechanical ventilation duration, ICU length of stay, and delirium (15). The same meta-analysis indicated that, compared with midazolam, propofol use can shorten intubation time in critically ill patients (16). Additionally, studies have found that compared to midazolam, critically ill patients receiving propofol have a lower risk of AKI in the first 7 days in the ICU and a reduced rate of renal replacement therapy potentially linked to propofol (17). The beneficial effects of propofol on renal ischemia–reperfusion injury are associated with its inhibition of pro-inflammatory cytokines (18, 19).
Thus, the critical importance of appropriate sedation for critically ill patients, significant gaps remain in the existing literature, especially regarding the comparative effects of midazolam and propofol on the prognosis of S-AKI patients. This study for the first time compared the short-term prognostic impacts of propofol and midazolam, focusing on critical outcomes such as 30-day mortality, ICU mortality, and the duration of invasive mechanical ventilation in patients diagnosed with S-AKI.
Methods
Study population
This study is a single-center, retrospective cohort analysis utilizing data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. The MIMIC-IV database is an extensive, publicly accessible resource, developed and maintained by the Massachusetts Institute of Technology (MIT) Computational Physiology Laboratory. It contains a substantial collection of medical records for ICU patients at Beth Israel Deaconess Medical Center from 2008 to 2019 (20). Utilization of the MIMIC-IV database was sanctioned by the Institutional Review Board of both MIT and Beth Israel Deaconess Medical Center. This project adhered to the principles outlined in the Declaration of Helsinki. The ethics committee approved the utilization of the data, citing the anonymized nature of the participants and the standardized formatting of the dataset.
One of the authors, Zhenkun Yang, secured access to the MIMIC-IV database and acquired the necessary certifications (ID: 57121385). Sepsis was diagnosed using the Sepsis-3.0 criteria (1), Among these sepsis patients, those with acute kidney injury were identified based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria (21). Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Diagnosed with S-AKI (sepsis and AKI onset within 0–48 h of ICU admission); (3) Sedation with propofol or midazolam during ICU stay; (4) Availability of survival data. Exclusion criteria were: (1) Absence of mechanical ventilation; (2) ICU stay less than 48 h. Ultimately, 3,745 patients met the inclusion criteria for statistical analysis (Figure 1).
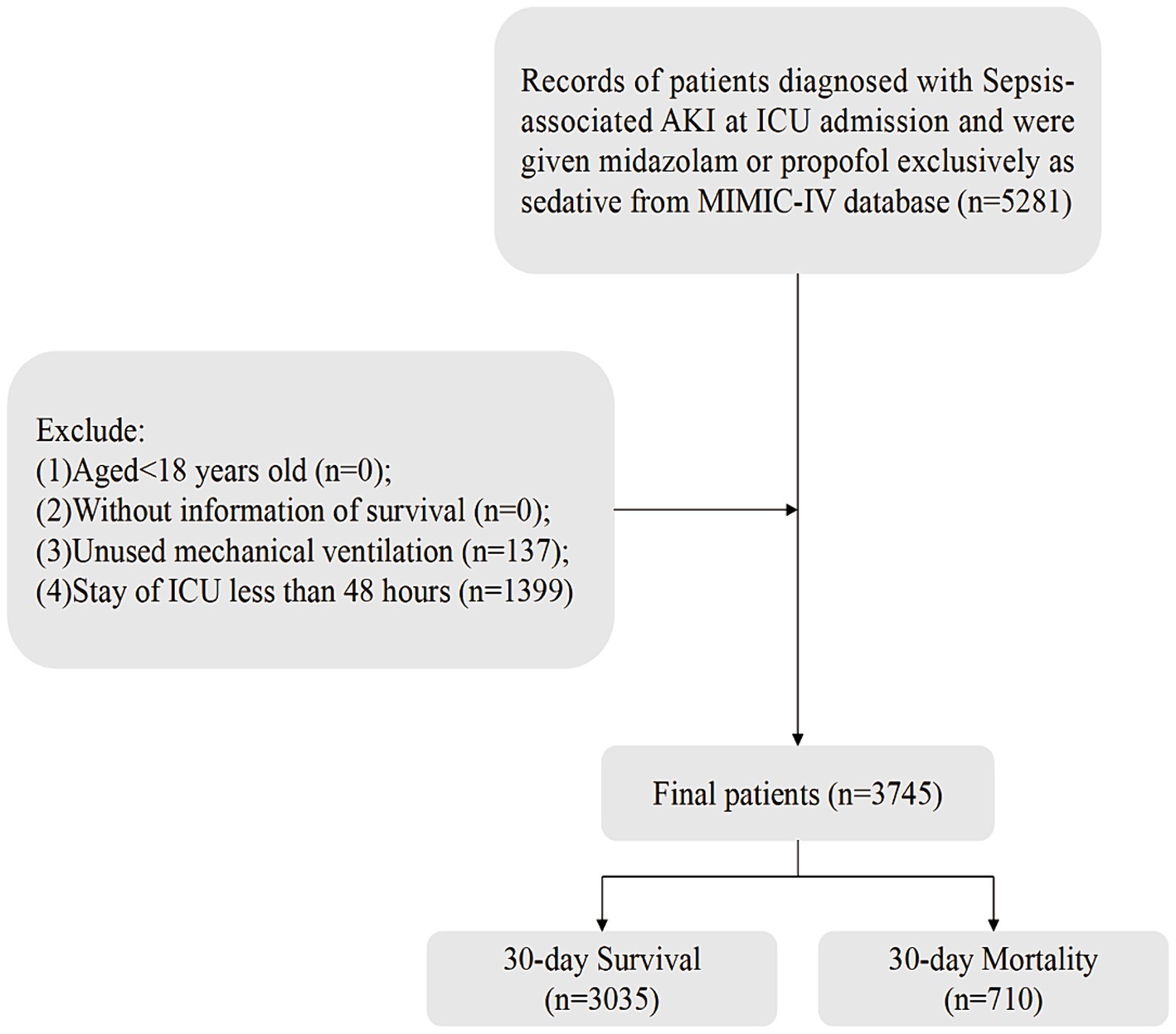
Figure 1. Flow of included patients through the trial. AKI, acute kidney injury; ICU, intensive care unit; MIMIC-IV, Medical Information Mart for Intensive Care-IV.
Variables extraction
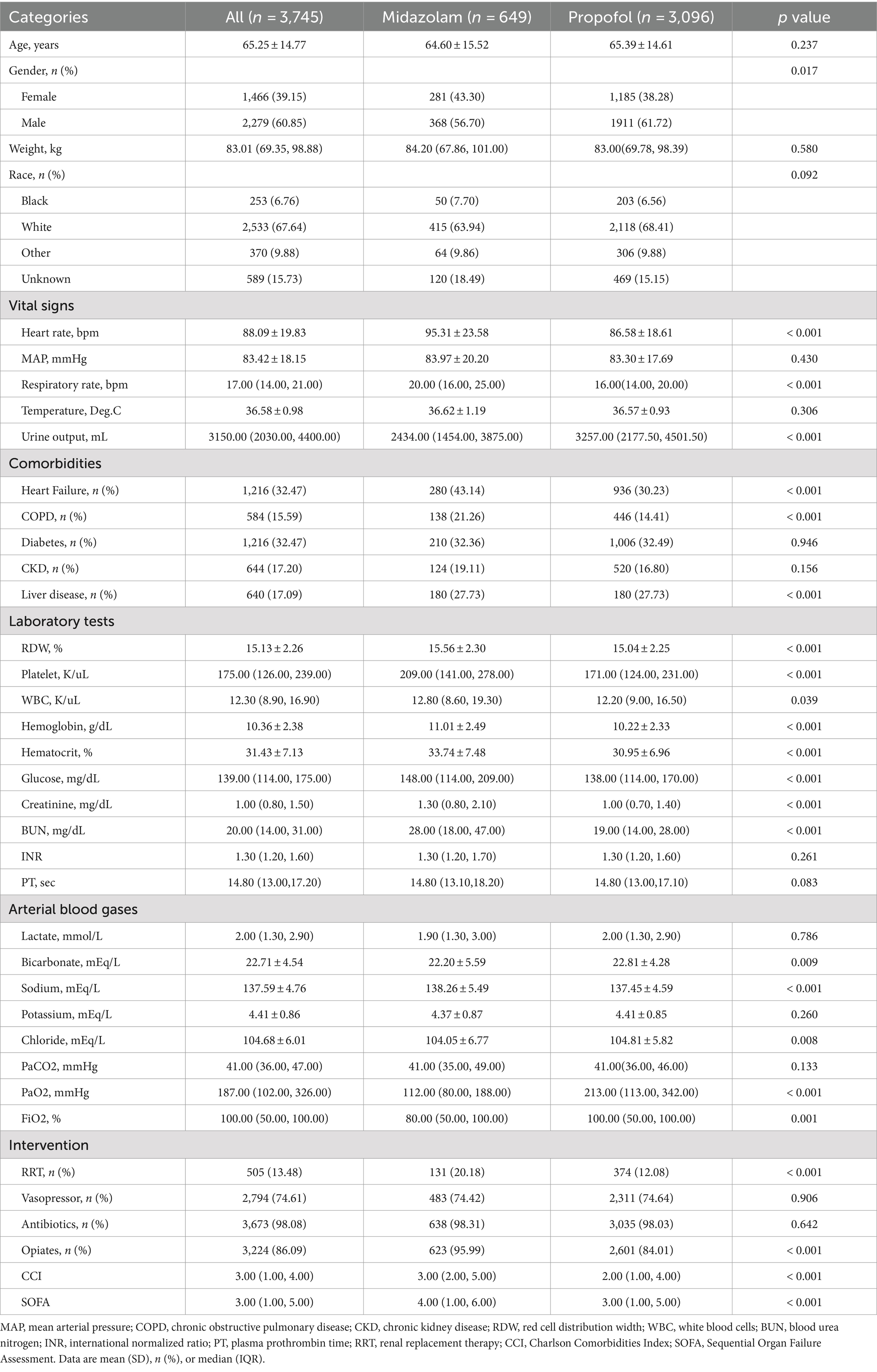
We extracted data information by using Structured Query Language running with the software Navicat Premium (version 16). Such as demographic characteristics: age, gender, race, weight; vital signs: mean arterial pressure (MAP), heart rate, temperature, respiratory rate, urine output, comorbidities: heart failure, chronic obstructive pulmonary disease (COPD), diabetes, chronic kidney disease (CKD), liver disease; laboratory tests: red blood cell distribution width (RDW), glucose, creatinine, platelet, white blood cell counts (WBC), hemoglobin, hematocrit, blood urea nitrogen (BUN), lactate, bicarbonate, sodium, potassium, chloride, PaCO2, PaO2, FiO2, international normalized ratio (INR), plasma prothrombin time (PT); hospitalization treatment measures: opiates, vasopressor, antibiotics, and renal replacement therapy (RRT); scoring systems: Sepsis-Related Organ Failure Assessment Score (SOFA), Charlson Comorbidity Index (CCI) score. Where MAP = diastolic blood pressure(DBP) + 1 / 3 [systolic blood pressure (SBP)—DBP]. For those with multiple admission records, only the first admission data is extracted. For those with multiple values, the first measurement value within 24 h after admission to the ICU is selected. Concerning missing values, as illustrated in Supplementary Table S1, the highest percentage of missing data was approximately 8.8%. Sensitivity analyses before and after interpolation of missing variables are shown in Supplementary Table S2.
Study outcomes
The primary endpoints of our study were: (1) 30-day mortality, defined as death within 30 days of ICU admission; (2) ICU mortality, defined as death occurring ICU admission; (3) duration of invasive mechanical ventilation, limited to patients receiving this intervention, categorized by median duration into two groups: < 3 days and ≥ 3 days.
Statistical analysis
Normality of measurement data was assessed using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (Mean ± SD). Group comparisons were conducted using the independent sample T-test; non-normal data were represented as median. Count data and interquartile range [M (Q1, Q3)] were reported, with group comparisons made using the Mann–Whitney U test. Enumeration data were summarized by number of cases and composition ratio N (%), with comparisons conducted via chi-square test. The rank sum test was utilized for ordinal data. All statistical analyses were two-sided, with a significance level of α = 0.05. A two-sided p < 0.05 was deemed statistically significant. Data management and analyses were conducted using SPSS Statistics (version 27) and R (version 4.3.2).
Patients receiving midazolam and propofol were categorized into respective groups, and their baseline characteristics were compared. The midazolam group served as the reference group. Univariate and multivariate analyses were then employed to investigate the effects of midazolam and propofol on various outcomes. To assess the independent associations of midazolam and propofol in S-AKI patients, we utilized the Cox proportional hazards model and Logistic regression model, adjusting for potential confounders. Results were expressed as odds ratios (OR) or hazard ratios (HR) with 95% confidence intervals (CI). Model I: unadjusted; Model II: adjusted for demographic variables; Model III: adjusted for demographic variables and variables with p < 0.05 in univariate analysis. Kaplan–Meier survival analysis was conducted to evaluate the impact of the two sedative drugs on 30-day and ICU mortality, respectively. Differences between the two groups were assessed using the log-rank test. After controlling for multiple confounders using propensity score matching, the effects of the two drugs on different outcomes were again explored. Further stratified analyses were performed based on age (≥ 65 years vs. < 65 years), gender, SOFA score (≥ 3 vs. < 3), CCI (≥ 3 vs. < 3), heart failure, and chronic kidney disease. The objective is to evaluate the consistency of the prognostic values of the two sedative drugs.
Results
Baseline characteristics
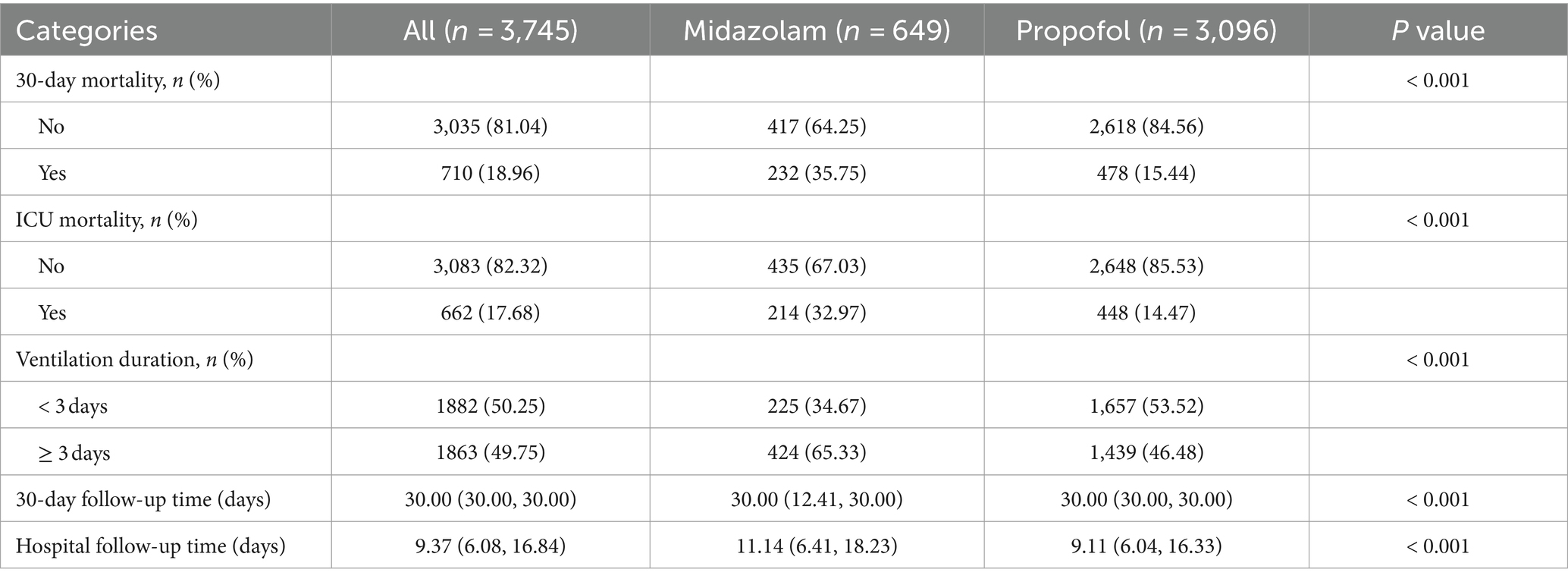
This study involved 3,745 patients with no loss to follow-up. They were divided into propofol and midazolam groups for sedation (Table 1). Patients had an average age of 65.25 ± 14.77 years; 1,466 (39.15%) were male, and 67.64% were White. The midazolam group showed higher heart and respiratory rates, lower urine output on admission, and higher incidences of heart failure, COPD, and liver disease, with elevated SOFA and CCI scores. Except for lactate, potassium, PaCO2, INR, and PT, other lab parameters differed significantly. Additionally, the midazolam group used more RRT and opiates, excluding vasopressors and antibiotics. In S-AKI patients, midazolam sedation correlated with higher 30-day and ICU mortality rates, and longer mechanical ventilation durations (≥ 3 days) compared to propofol (p < 0.001, Table 2). Propofol group had shorter median ICU follow-up durations (9.11 vs. 11.14 days for midazolam).
Impact of sedatives on 30-day mortality in S-AKI patients
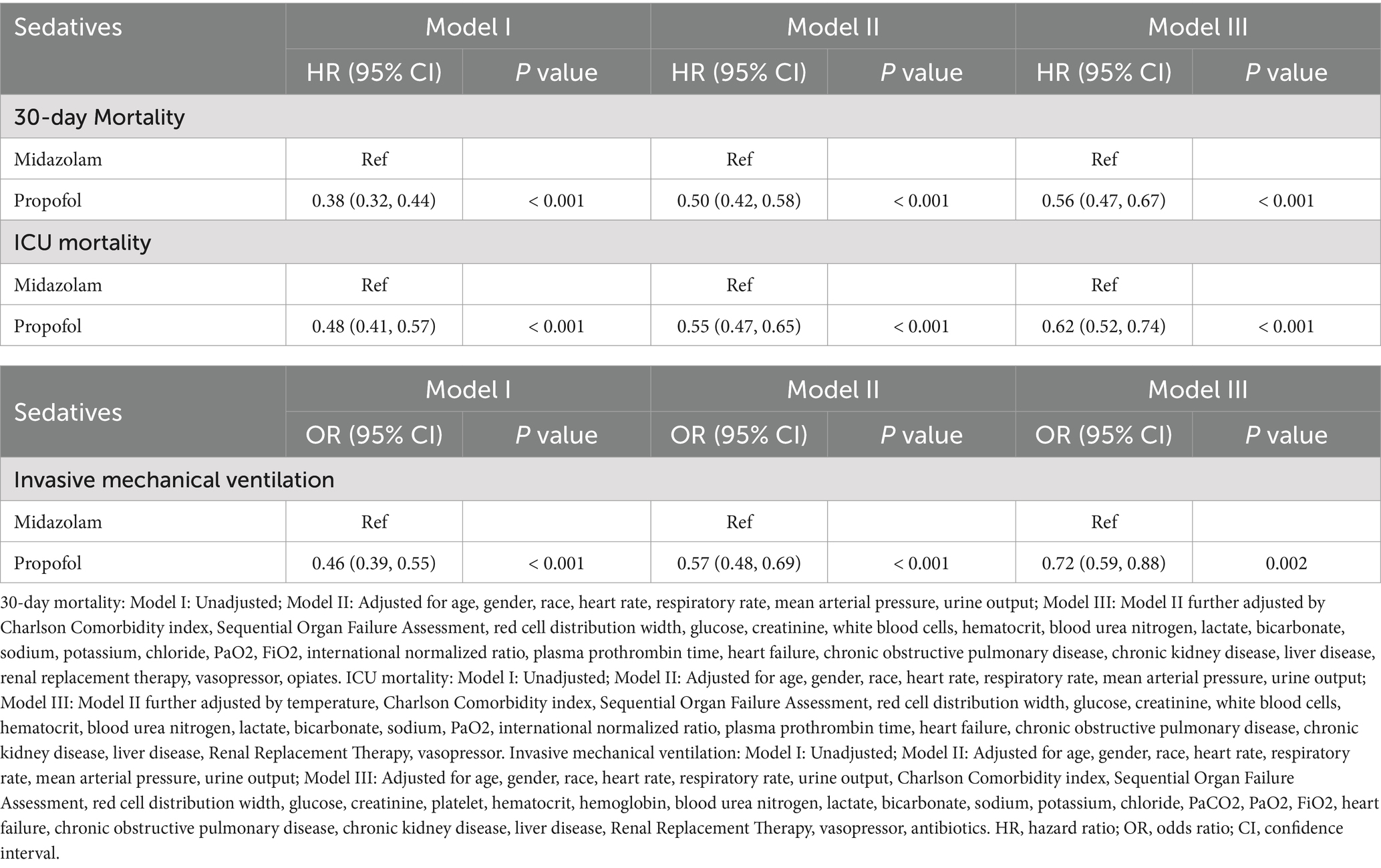
According to Table 3, after adjusting for confounders, exclusive propofol sedation reduced the 30-day mortality risk in S-AKI patients by 44% (HR = 0.56, 95% CI: 0.47–0.67, p < 0.001), indicating it is an effective protective factor. Kaplan–Meier curves showed lower 30-day survival probabilities in the midazolam group (Figure 2A).
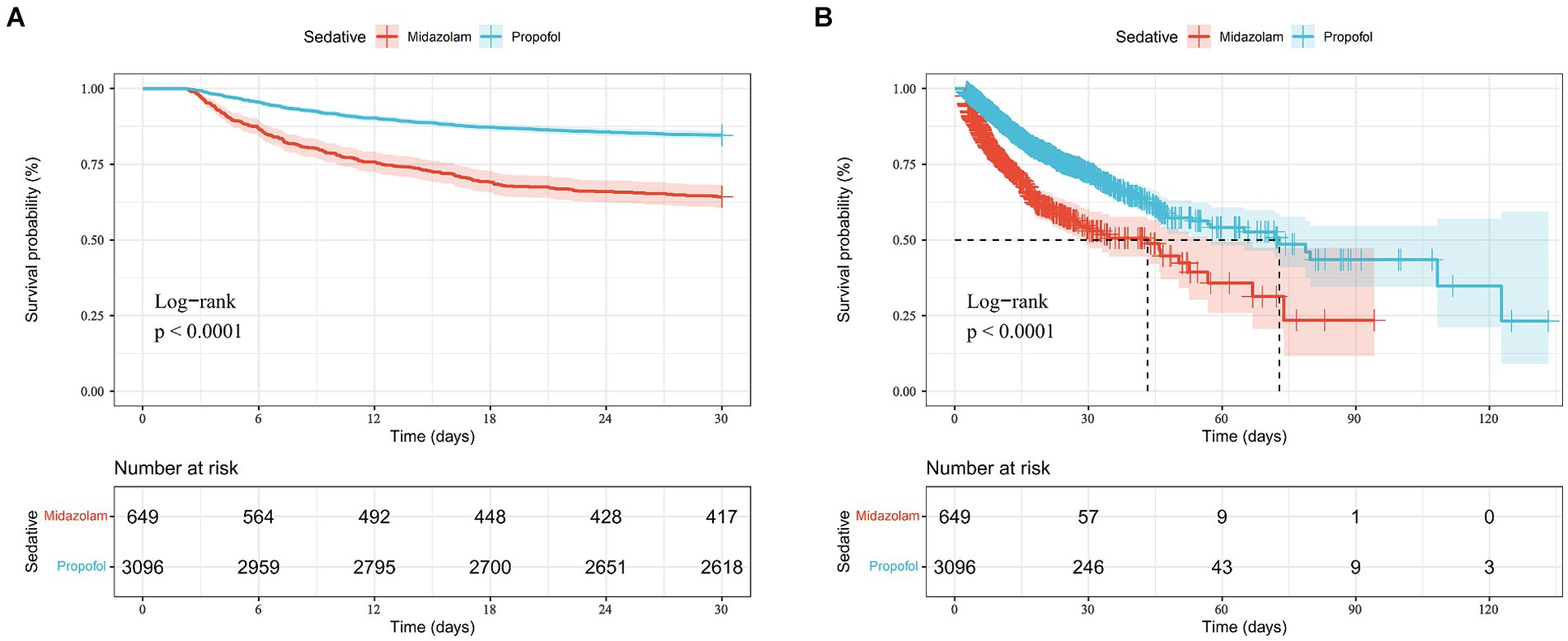
Figure 2. Kaplan-Mill survival analysis of different drugs in sepsis-associated acute kidney injury patients with 30-day mortality (A) and ICU mortality (B).
Impact of sedatives on ICU mortality in S-AKI patients
According to Table 3, after adjusting for confounders, exclusive propofol sedation reduced ICU mortality risk in S-AKI patients by 38% (HR = 0.62, 95% CI: 0.52–0.74, p < 0.001), indicating propofol also was an effective protective factor against ICU mortality in S-AKI. Kaplan–Meier survival curves showed a less pronounced decline in in-hospital survival probabilities over time in the propofol group (Figure 2B).
Impact of sedatives on duration of invasive mechanical ventilation in S-AKI patients
According to Table 3, after adjusting for confounders, exclusive propofol sedation reduced the risk of invasive mechanical ventilation lasting more than 3 days in S-AKI patients by 28% (OR = 0.72, 95% CI: 0.59–0.88, p = 0.002). Figure 3 illustrated the effects of the two drugs on duration of invasive mechanical ventilation. The propofol group exhibited lower OR values and shorter duration of mechanical ventilation in S-AKI patients.
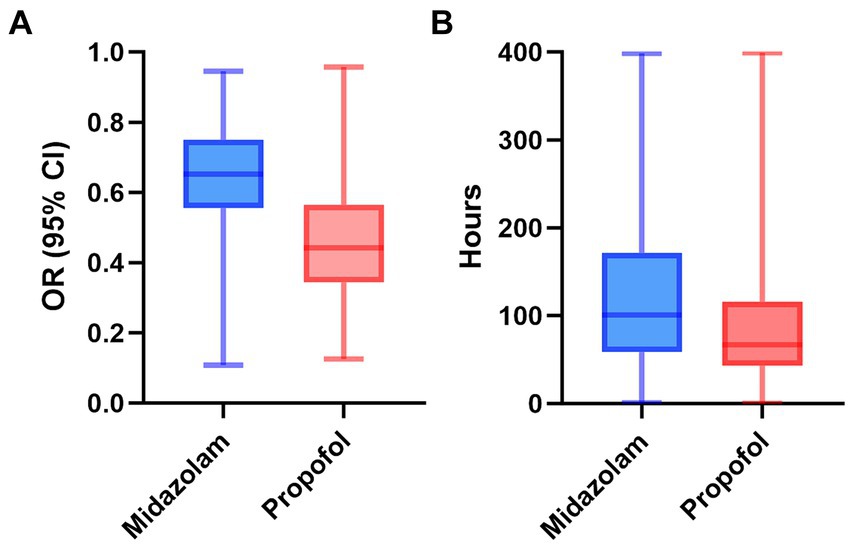
Figure 3. The distribution of two drugs on odds ratio (A) and duration of invasive mechanical ventilation (B) in patients with sepsis-related acute kidney. OR, odds ratio; CI, confidence interval.
Impact of sedatives on three outcomes after propensity score matching analysis
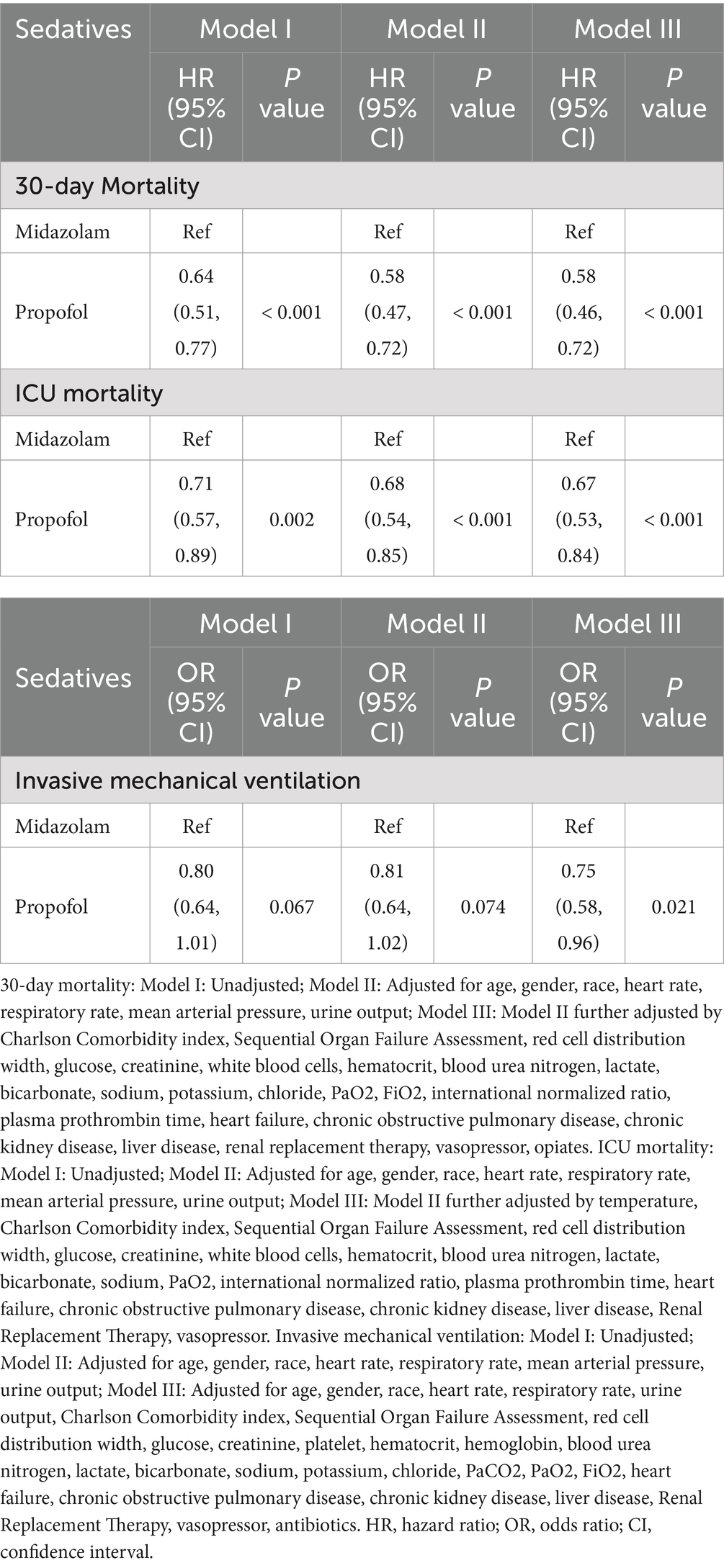
According to Table 4, after propensity score matching analysis, multifactorial Logistic regression and COX regression showed that propofol remained more protective than midazolam against ICU death (HR = 0.67, CI: 0.53–0.84, p < 0.001), 30-day death (HR = 0.58, CI; 0.46–0.72, p < 0.001), and invasive mechanical ventilation (HR = 0.75, CI: 0.58–0.96, p = 0.021) in patients with S-AKI.
Subgroup analysis
We conducted risk stratification analyses for 30-day mortality in S-AKI patients based on age, gender, SOFA scores, CCI scores, heart failure, and chronic kidney disease history (Figure 4). Propofol was linked to lower 30-day mortality risks in patients aged ≥65 years (HR = 0.51, 95% CI: 0.41–0.64), male (HR = 0.53, 95% CI: 0.42–0.67), with SOFA scores <3 (HR = 0.54, 95% CI: 0.40–0.74), CCI scores <3 (HR = 0.49, 95% CI: 0.36–0.68), without heart failure (HR = 0.51, 95% CI: 0.40–0.64), and with chronic kidney disease (HR = 0.37, 95% CI: 0.25–0.53). The effects of propofol on ICU mortality mirrored those on 30-day mortality. However, propofol did not show significant effects on the duration of mechanical ventilation in patients aged <65 years, female, with CCI scores <3 and without heart failure.
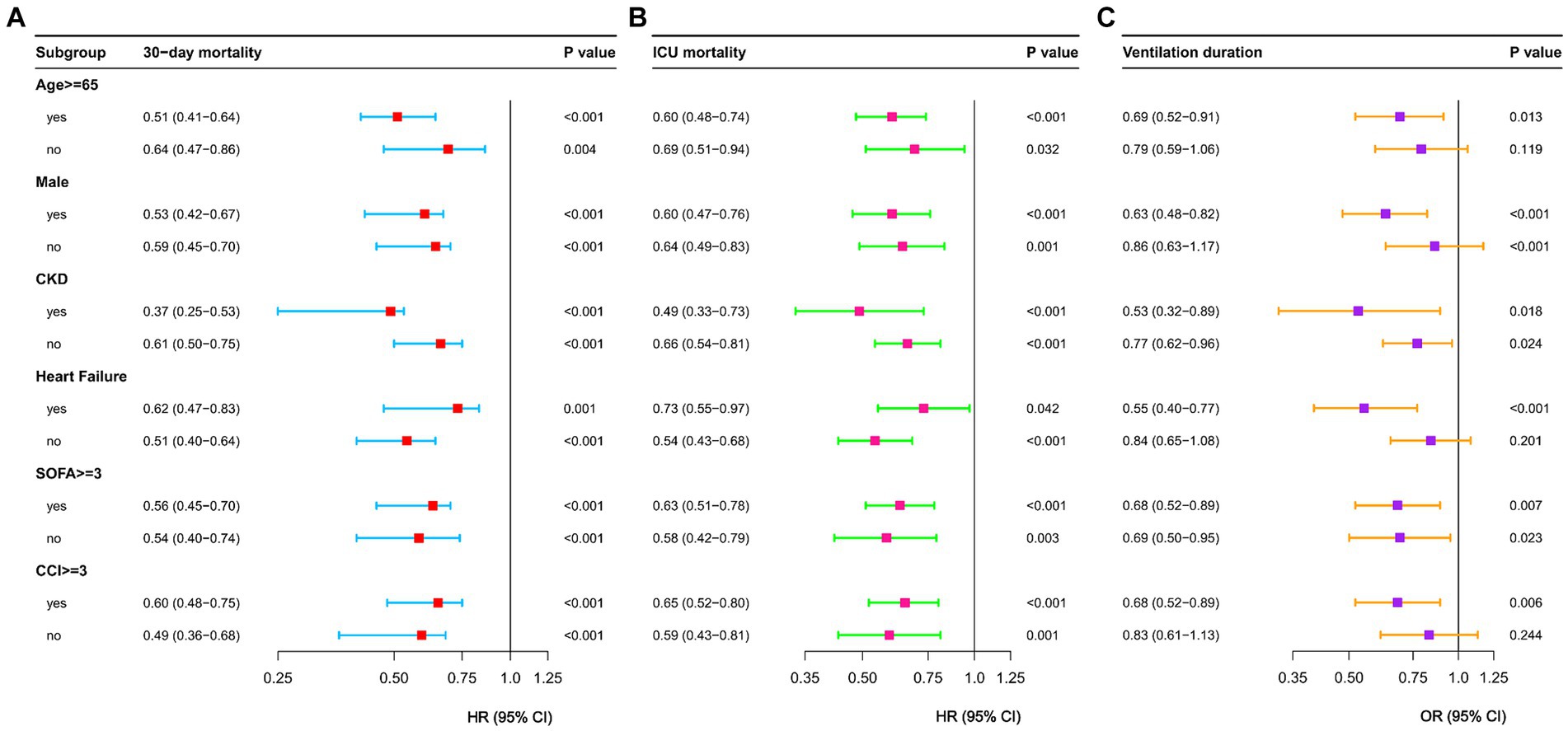
Figure 4. Forest plots of 30-day mortality (A) and ICU mortality (B) and ventilation duration (C) in different subgroups. HR, hazard ratio; OR, odds ratio; CI, confidence interval; CCI, charlson comorbidity index; CKD, chronic kidney disease; SOFA, Sequential Organ Failure Assessment.
Discussion
This study was a single-center, retrospective cohort analysis designed to elucidate the risks associated with propofol and midazolam sedation. Utilizing both univariate and multivariate analyses, we demonstrated that in patients with sepsis-associated acute kidney injury (S-AKI), midazolam sedation, compared to propofol, was linked to higher 30-day mortality, increased ICU mortality, and prolonged durations of invasive mechanical ventilation. Sedatives are routinely administered to critically ill patients requiring invasive mechanical ventilation to minimize patient-ventilator asynchrony and alleviate anxiety and stress (15). Both propofol and midazolam have the potential to suppress respiratory drive, induce immunosuppression, and lead to profound sedation (22, 23).
A previous systematic review showed that compared to midazolam, propofol sedation improved clinical outcomes in ICU patients, decreased ICU stay and duration of mechanical ventilation in patients undergoing acute surgery, and shortened weaning time in critically ill patients (16). Similarly, in a multi-center observational cohort study, propofol sedation was associated with lower hospital mortality rates, shorter hospital stays, and shorter duration of invasive mechanical ventilation compared to midazolam sedation in patients with acute respiratory distress syndrome (24). A Canadian study also showed faster extubation for mechanically ventilated patients receiving propofol versus those receiving midazolam (25). In another observational propensity score matched cohort study, propofol sedation reduced vasopressor dosing, mortality rates, and bleeding events compared to midazolam in patients with cardiogenic shock (26).
Our data and other experiments above proved that propofol was more friendly to the short-term prognosis of S-AKI patients than midazolam, including 30-day mortality, in-hospital mortality, and mechanical ventilation time. Prolonged mechanical ventilation was associated with adverse outcomes and can increase patient mortality (27, 28). Therefore, we preferred patients to receive a shorter duration of invasive mechanical ventilation. Compared to benzodiazepines like midazolam, propofol had shorter recovery times to arousable mental status, allowing patients to be liberated from the ventilator more quickly with adequate respiratory drive to breathe spontaneously (29, 30), consistent with our conclusions. Additionally, due to pharmacokinetic properties, propofol can rapidly awaken patients. Propofol had a rapid onset, short duration of action, taking effect in seconds to minutes, and is quickly redistributed to peripheral tissues, along with a large volume of distribution, allowing early recovery of consciousness (31). Midazolam was a lipophilic drug not easily metabolized in adipose tissues, leading to its accumulation and longer persistence in the body (32). Prolonged midazolam sedation can also lead to neurological injury (33). Clinical ICU analgesia and sedation practice guidelines (e.g., PADIS guidelines 26) also emphasize shortened time on ventilators and early rehabilitation (34). For S-AKI patients specifically, propofol has been shown to act as a scavenger of oxygen free radicals (OFRs), reducing lipid peroxidation in the kidneys (35), and modulate ischemia/reperfusion injury (IRI) with organ-protective potentials as a measure to improve patient outcomes (36). Therefore, in S-AKI patients, propofol was an effective strategy with superior outcomes compared to midazolam.
In subgroup analyses, the propofol group exhibited protective effects on 30-day mortality compared to the midazolam group across various subgroups, including age, gender, SOFA scores, CCI scores, heart failure, and chronic kidney disease. Comparable outcomes were observed for in-hospital mortality. Specifically, in S-AKI patients aged ≥65 years [HR = 0.51 (95% CI: 0.41–0.64)], propofol was associated with superior short-term outcomes compared to midazolam. In an additional randomized controlled trial involving elderly patients, propofol sedation demonstrated significant advantages over midazolam sedation, particularly in reducing the incidence of post-operative cognitive dysfunction (37). Propofol showed no statistically significant effects compared to midazolam on duration of mechanical ventilation in S-AKI patients aged <65 years [OR = 0.79 (95% CI: 0.56–1.06)], females [OR = 0.86 (95% CI, 0.63–1.17)], without heart failure [OR = 0.83 (95% CI, 0.61–1.13)], and with CCI scores <3 [OR = 0.84 (95% CI, 0.65–1.08)]. These findings underscore the need for further exploration into the optimal selection of first-line sedatives for populations at various stages of illness.
This study presented three significant strengths. Firstly, it was the first to explore the impact of various sedatives on short-term outcomes in patients with S-AKI, offering crucial insights for the optimal selection of sedative agents in this specific population. Secondly, the study’s substantial sample size guaranteed robust statistical power. Lastly, the study encompassed crucial variables pertinent to clinical practice in ICU management of sepsis-related acute kidney injury, including severity, prognosis, complications, and a comprehensive range of laboratory indicators. Nonetheless, our study had certain limitations. First, as a retrospective analysis, the study may have been subject to inherent selection bias. Moreover, the analysis exclusively concentrated on patients’ initial hospital admissions, potentially overlooking the influence of dynamic changes in indicators on the study’s outcomes. Finally, due to the limitations in available sedative data, we were unable to ascertain the impact of sedative duration on the study outcomes. Although a meticulous multi-factorial analysis was employed to mitigate confounding variables, further validation of our findings through large-scale multi-center studies and randomized controlled trials remains imperative.
Conclusion
Propofol has been identified as a protective factor for short-term and ICU mortality in S-AKI patients. Additionally, S-AKI patients receiving propofol had a lower risk of prolonged mechanical ventilation compared to those receiving midazolam. These findings suggested that propofol may offer greater short-term benefits for S-AKI patients than midazolam.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YuL: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. TG: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft. ZY: Investigation, Software, Writing – original draft. RZ: Visualization, Writing – original draft. ZW: Investigation, Writing – original draft. YiL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to extend our gratitude to the investigators at Tianjin Medical University General Hospital who provided assistance that greatly contributed to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1415425/full#supplementary-material
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Bellomo, R, Kellum, JA, and Ronco, C. Acute kidney injury. Lancet. (2012) 380:756–66. doi: 10.1016/s0140-6736(11)61454-2
3. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/s0140-6736(19)32989-7
4. Cecconi, M, Evans, L, Levy, M, and Rhodes, A. Sepsis and septic shock. Lancet. (2018) 392:75–87. doi: 10.1016/s0140-6736(18)30696-2
5. Rello, J, Valenzuela-Sánchez, F, Ruiz-Rodriguez, M, and Moyano, S. Sepsis: a review of advances in management. Adv Ther. (2017) 34:2393–411. doi: 10.1007/s12325-017-0622-8
6. Bagshaw, SM, Uchino, S, Bellomo, R, Morimatsu, H, Morgera, S, Schetz, M, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. (2007) 2:431–9. doi: 10.2215/cjn.03681106
7. Bouchard, J, Acharya, A, Cerda, J, Maccariello, ER, Madarasu, RC, Tolwani, AJ, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. (2015) 10:1324–31. doi: 10.2215/cjn.04360514
8. Hoste, EA, Bagshaw, SM, Bellomo, R, Cely, CM, Colman, R, Cruz, DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
9. Uchino, S, Kellum, JA, Bellomo, R, Doig, GS, Morimatsu, H, Morgera, S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. (2005) 294:813–8. doi: 10.1001/jama.294.7.813
10. Morrell, ED, Kellum, JA, Pastor-Soler, NM, and Hallows, KR. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care. (2014) 18:501. doi: 10.1186/s13054-014-0501-5
11. Patel, SB, and Kress, JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. (2012) 185:486–97. doi: 10.1164/rccm.201102-0273CI
12. Hughes, CG, Mailloux, PT, Devlin, JW, Swan, JT, Sanders, RD, Anzueto, A, et al. Dexmedetomidine or Propofol for sedation in mechanically ventilated adults with Sepsis. N Engl J Med. (2021) 384:1424–36. doi: 10.1056/NEJMoa2024922
13. Marler, J, Mohrien, K, Kimmons, LA, Vandigo, JE, Oliphant, CS, Boucher, AN, et al. Effects of propofol on vasopressor use in patients with sepsis and severe sepsis: a pilot study. J Crit Care. (2016) 35:155–60. doi: 10.1016/j.jcrc.2016.05.015
14. Tekwani, KL, Watts, HF, Sweis, RT, Rzechula, KH, and Kulstad, EB. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: a prospective, randomized study. Ann Emerg Med. (2010) 56:481–9. doi: 10.1016/j.annemergmed.2010.05.034
15. Barr, J, Fraser, GL, Puntillo, K, Ely, EW, Gélinas, C, Dasta, JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182783b72
16. Garcia, R, Salluh, JIF, Andrade, TR, Farah, D, da Silva, PSL, Bastos, DF, et al. A systematic review and meta-analysis of propofol versus midazolam sedation in adult intensive care (ICU) patients. J Crit Care. (2021) 64:91–9. doi: 10.1016/j.jcrc.2021.04.001
17. Leite, TT, Macedo, E, Martins Ida, S, Neves, FM, and Libório, AB. Renal outcomes in critically ill patients receiving Propofol or midazolam. Clin J Am Soc Nephrol. (2015) 10:1937–45. doi: 10.2215/cjn.02330315
18. Hsing, CH, Chou, W, Wang, JJ, Chen, HW, and Yeh, CH. Propofol increases bone morphogenetic protein-7 and decreases oxidative stress in sepsis-induced acute kidney injury. Nephrol Dial Transplant. (2011) 26:1162–72. doi: 10.1093/ndt/gfq572
19. Yang, S, Chou, WP, and Pei, L. Effects of propofol on renal ischemia/reperfusion injury in rats. Exp Ther Med. (2013) 6:1177–83. doi: 10.3892/etm.2013.1305
20. Johnson, AEW, Bulgarelli, L, Shen, L, Gayles, A, Shammout, A, Horng, S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
21. Khwaja, A . KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
22. Devlin, JW, and Roberts, RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin. (2011) 29:567–85. doi: 10.1016/j.anclin.2011.09.001
23. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/ccm.0000000000003299
24. Hu, AM, Zhong, XX, Li, Z, Zhang, ZJ, and Li, HP. Comparative effectiveness of midazolam, Propofol, and Dexmedetomidine in patients with or at risk for acute respiratory distress syndrome: a propensity score-matched cohort study. Front Pharmacol. (2021) 12:614465. doi: 10.3389/fphar.2021.614465
25. Hall, RI, Sandham, D, Cardinal, P, Tweeddale, M, Moher, D, Wang, X, et al. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest. (2001) 119:1151–9. doi: 10.1378/chest.119.4.1151
26. Scherer, C, Kleeberger, J, Kellnar, A, Binzenhöfer, L, Lüsebrink, E, Stocker, TJ, et al. Propofol versus midazolam sedation in patients with cardiogenic shock - an observational propensity-matched study. J Crit Care. (2022) 71:154051. doi: 10.1016/j.jcrc.2022.154051
27. Blackwood, B, Alderdice, F, Burns, KE, Cardwell, CR, Lavery, G, and O'Halloran, P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev. (2010) 5:Cd006904. doi: 10.1002/14651858.CD006904.pub2
28. Wolkewitz, M, Palomar-Martinez, M, Alvarez-Lerma, F, Olaechea-Astigarraga, P, and Schumacher, M. Analyzing the impact of duration of ventilation, hospitalization, and ventilation episodes on the risk of pneumonia. Infect Control Hosp Epidemiol. (2019) 40:301–6. doi: 10.1017/ice.2018.360
29. Carson, SS, Kress, JP, Rodgers, JE, Vinayak, A, Campbell-Bright, S, Levitt, J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. (2006) 34:1326–32. doi: 10.1097/01.Ccm.0000215513.63207.7f
30. Kress, JP, O'Connor, MF, Pohlman, AS, Olson, D, Lavoie, A, Toledano, A, et al. Sedation of critically ill patients during mechanical ventilation. A comparison of propofol and midazolam. Am J Respir Crit Care Med. (1996) 153:1012–8. doi: 10.1164/ajrccm.153.3.8630539
31. Sahinovic, MM, Struys, M, and Absalom, AR. Clinical pharmacokinetics and pharmacodynamics of Propofol. Clin Pharmacokinet. (2018) 57:1539–58. doi: 10.1007/s40262-018-0672-3
32. Spina, SP, and Ensom, MH. Clinical pharmacokinetic monitoring of midazolam in critically ill patients. Pharmacotherapy. (2007) 27:389–98. doi: 10.1592/phco.27.3.389
33. McKenzie, CA, McKinnon, W, Naughton, DP, Treacher, D, Davies, G, Phillips, GJ, et al. Differentiating midazolam over-sedation from neurological damage in the intensive care unit. Crit Care. (2005) 9:R32–6. doi: 10.1186/cc3010
34. Chanques, G, Drouot, X, and Payen, JF. 2008-2018: ten years of gradual changes in the sedation guidelines for critically ill patients. Anaesth Crit Care Pain Med. (2018) 37:509–11. doi: 10.1016/j.accpm.2018.10.014
35. Jin, YC, Kim, W, Ha, YM, Shin, IW, Sohn, JT, Kim, HJ, et al. Propofol limits rat myocardial ischemia and reperfusion injury with an associated reduction in apoptotic cell death in vivo. Vasc Pharmacol. (2009) 50:71–7. doi: 10.1016/j.vph.2008.10.002
36. Kato, R, and Foëx, P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. (2002) 49:777–91. doi: 10.1007/bf03017409
Keywords: MIMIC-IV, sepsis-associated acute kidney injury, propofol, midazolam, mortality
Citation: Li Y, Guo T, Yang Z, Zhang R, Wang Z and Li Y (2024) Effect of propofol versus midazolam on short-term outcomes in patients with sepsis-associated acute kidney injury. Front. Med. 11:1415425. doi: 10.3389/fmed.2024.1415425
Edited by:
Harish Ramakrishna, Mayo Clinic, United StatesReviewed by:
Liping Zhuang, The First People Hospital of Mudanjiang City, ChinaJingjing Yuan, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2024 Li, Guo, Yang, Zhang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yize Li, bGl5aXplbGlzYUAxMjYuY29t
†These authors have contributed equally to this work
 Yuanjie Li
Yuanjie Li Taipu Guo1†
Taipu Guo1† Zhenkun Yang
Zhenkun Yang Yize Li
Yize Li