- 1Chemistry and Chemical Engineering Institute, Taishan University, Tai'an, Shandong, China
- 2Department of Pharmacy, Zhongda Hospital, Southeast University, Nanjing, Jiangsu, China
- 3Institute of Nephrology, Zhongda Hospital, Southeast University, Nanjing, Jiangsu, China
Editorial on the Research Topic
Pathogenic mechanisms, injury biomarkers, prophylaxis and treatment strategy of drug-induced nephrotoxicity
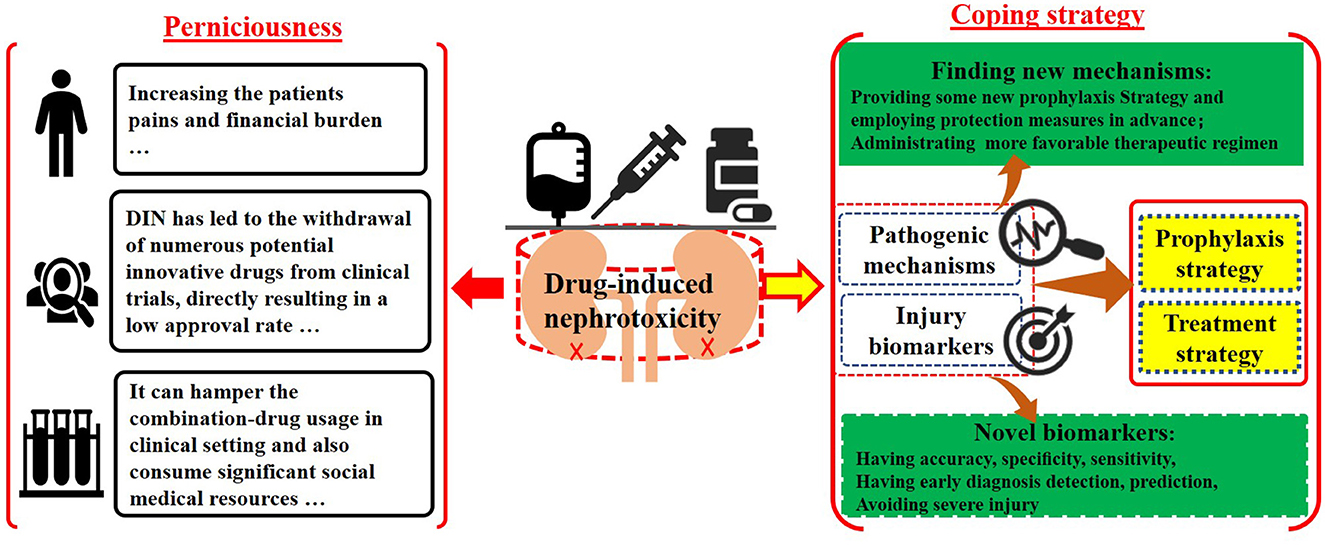
Drug-induced nephrotoxicity (DIN) exhibits high morbidity rate among global adverse reactions, posing a long-term potential burden on patients and society (1). In the United State, the annual number of DIN sufferers exceeds 1.5 million (2). Furthermore, in special groups such as older patients, and those with comorbid conditions, the incidence of drug-associated kidney damage is estimated to be increased further (1, 3, 4). Additionally, in intensive care unit (ICU), DIN events is not infrequently encountered (5). However, in specific clinical settings, such as those involving antiviral, antibacterial, and antitumor drugs (5), nephrotoxic agents may be necessary in the absence of alternative or supplementary agents (6). This may lead to potential kidney damage, which can worsen without adequate protective measures, ultimately resulting in kidney failure (7). Previous researches have indicated that DIN has led to the withdrawal of numerous potential innovative drugs from clinical trials, directly resulting in a low approval rate. Consequently, DIN has significantly hindered the research and development of new drug since healthy kidney tissue plays a crucial role in maintaining water-electrolyte balance, acid-base homeostasis, and overall physiological stability (2, 5). In clinical practice, apart from conventional tests such as serum creatinine (SCr), blood urea nitrogen (BUN) (6), and ultrasonography, there is a lack of highly sensitive and specific analytical methods currently available to assess kidney injury (8). When nephrotoxicity from various sources, including single or combination drug therapies, is identified, limited options exist beyond dosage adjustments or discontinuation (3).
The irreversible kidney damage caused by DIN presents a significant challenge, leading to substantial economic burdens, physical suffering for patients, restrictions on medication use, delays in drug development, and extensive utilization of healthcare resources. Consequently, DIN remains a pressing global health issue that warrants further investigation and understanding.
The Research Topic group aims to address the adverse effects of DIN by urging researchers to investigate two key factors: potential pathogenic mechanisms and kidney injury biomarkers, and to correlate them with prophylaxis and treatment strategies (Graphical Abstract). Understanding pathogenic mechanisms can reveal fundamental principles of kidney damage and guide potential preventive and therapeutic measures. By delving deeper into nephrotoxicity-related theoretical frameworks, researchers may identify specific components beyond the cellular level, which play significant roles in nephron damage, such as related to organelles, ligands, or second-messenger molecules. Additionally, there is an interest in uncovering how potential nephrotoxins, including drugs, herbs, nanomaterials, complexes, and metal ions, disrupt specific action receptors and elucidating the characteristics of their toxic effects (9). Therefore, researchers are encouraged to utilize cutting-edge technologies such as frozen electron microscopy, advanced imaging techniques, stem cell methodologies, and membrane potential analysis to develop targeted therapeutic interventions based on these specific mechanisms. With the advancement of analytical techniques such as high-resolution magic angle rotating proton NMR spectroscopy, multi-omics approaches, and bioinformatics-related molecular biology, a comprehensive multi-platform testing strategy can be systematically integrated. This integration enables the accurate identification of potential injury biomarkers by precisely tracking minute changes in quality and quantity of trace biomolecules. Traditional biomarkers like BUN, SCr, and urine protein, which are measured using less sensitive and specific instruments, often fail to detect abnormalities until significant damage has occurred, potentially leading to irreversible conditions. In contrast, advanced kidney injury biomarkers offer higher specificity, accuracy, and sensitivity, providing more comprehensive data. Early detection of specific biomarker traits before significant kidney damage occurs can significantly reduce the incidence of kidney injury, as timely interventions can be implemented. Furthermore, integrating advanced analytical results with bioinformatics and stem cell repair techniques can greatly enhance treatment strategies for kidney injuries. Early diagnosis, detection, and treatment of kidney injuries can reduce patient suffering, medical costs, and emotional distress, ultimately lowering mortality and morbidity rates.
Therefore, our group has conceptualized and designed a Research Topic focusing on advancing treatment strategies for DIN, with emphasis on understanding pathogenic mechanisms, identifying injury biomarkers, and developing prophylactic measures. Sun et al. highlighted the role of thioredoxin-interacting protein (TXNIP) in diabetic kidney damage (DKD), noting its upregulation in DKD patients compared to healthy individuals. They suggested TXNIP as a promising therapeutic target. Wang et al. investigated the association between elevated uric acid levels and gouty nephropathy (GN) and proposed relevant animal models that mirror clinical manifestations. By exploring response mechanisms, they identified potential treatment targets for various kidney injuries. Zhou et al. not only reviewed mechanisms of DIN injury but also explored the correlation between DIN and urinary exosomes as potential biomarkers for accurate clinical testing. Zou et al. utilized a mathematical model to assess the therapeutic efficacy of a combination medicine containing sirolimus (SRL) and low-dose extended-release tacrolimus (LER-TAC) in kidney transplant patients. Their findings indicated superior efficacy and safety of the combination therapy, suggesting a promising treatment strategy for kidney transplant recipients, especially those requiring immunosuppression.
Author contributions
GJ: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. YN: Conceptualization, Formal analysis, Investigation, Writing – review & editing, Supervision. BW: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are much grateful to all the submitted manuscripts authors and referees for their diligent contributions to this topic. We are also deeply grateful to the group of Frontiers in Medicine, Penerade Huang, Nicole Deng, and Jing Tang for their sincerest support. Our team would like to express our gratitude to the journal manager for Frontiers in Medicine, Luke Block, for providing us with the advancement opportunity to explore this interesting project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. (2018) 14:607–25. doi: 10.1038/s41581-018-0052-0
2. Connor S, Li T, Qu Y, Roberts RA, Tong W. Generation of a drug-induced renal injury list to facilitate the development of new approach methodologies for nephrotoxicity. Drug Discov Today. (2024) 29:103938. doi: 10.1016/j.drudis.2024.103938
3. Wu H, Huang J. Drug-induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Curr Drug Metab. (2018) 19:559–67. doi: 10.2174/1389200218666171108154419
4. Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm. (2014) 11:1933–48. doi: 10.1021/mp400720w
5. Khajavi Rad A, Mohebbati R, Hosseinian S. Drug-induced nephrotoxicity and medicinal plants. Iran J Kidney Dis. (2017) 11:169–79.
6. Huang J. Drug-induced nephrotoxicity and drug metabolism in renal failure. Curr Drug Metab. (2018) 19:558. doi: 10.2174/138920021907180709121120
7. Gao C, Liu C, Chen Y, Wang Q, Hao Z. Protective effects of natural products against drug-induced nephrotoxicity: a review in recent years. Food Chem Toxicol. (2021) 153:112255. doi: 10.1016/j.fct.2021.112255
8. Chen L, Smith J, Mikl J, Fryer R, Pack F, Williams BJ, et al. A Multiplatform approach for the discovery of novel drug-induced kidney injury biomarkers. Chem Res Toxicol. (2017) 30:1823–34. doi: 10.1021/acs.chemrestox.7b00159
Keywords: drug-induced nephrotoxicity, pathogenic mechanisms, injury biomarkers, prophylaxis and treatment strategy, kidney, thioredoxin-interacting protein (TXNIP)
Citation: Jiao G, Niu Y and Wang B (2024) Editorial: Pathogenic mechanisms, injury biomarkers, prophylaxis and treatment strategy of drug-induced nephrotoxicity. Front. Med. 11:1412795. doi: 10.3389/fmed.2024.1412795
Received: 05 April 2024; Accepted: 15 April 2024;
Published: 29 April 2024.
Edited and reviewed by: Ibrahim Batal, Columbia University, United States
Copyright © 2024 Jiao, Niu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Niu, eWltaW5uaXVAc2V1LmVkdS5jbg==; Bin Wang, d2FuZ2Jpbmhld2VpQDEyNi5jb20=
 Guozheng Jiao
Guozheng Jiao Yimin Niu
Yimin Niu Bin Wang
Bin Wang