- 1School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 2Student Research Committee, Islamic Azad University Tehran Medical Sciences, Tehran, Iran
- 3Student Research Committee, Islamic Azad University Tehran Medical Sciences, Mashhad, Iran
- 4School of Medicine, Shahroud Azad University of Medical Sciences, Shahroud, Iran
- 5Student Research Committee, School of Medicine, Alborz University of Medical Sciences, Alborz, Iran
- 6School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 7School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran
- 8Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran
- 10School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 11Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Preclinical research has identified the mechanisms via which bacteria influence cancer treatment outcomes. Clinical studies have demonstrated the potential to modify the microbiome in cancer treatment. Herein, we systematically analyze how gut microorganisms interact with chemotherapy and immune checkpoint inhibitors, specifically focusing on how gut bacteria affect the pharmacokinetics and pharmacodynamics of cancer treatment.
Method: This study searched Web of Science, Scopus, and PubMed until August 2023. Studies were screened by their title and abstract using the Rayyan intelligent tool for systematic reviews. Quality assessment of studies was done using the JBI critical appraisal tool.
Result: Alterations in the gut microbiome are associated with gastric cancer and precancerous lesions. These alterations include reduced microbial alpha diversity, increased bacterial overgrowth, and decreased richness and evenness of gastric bacteria. Helicobacter pylori infection is associated with reduced richness and evenness of gastric bacteria, while eradication only partially restores microbial diversity. The gut microbiome also affects the response to cancer treatments, with higher abundances of Lactobacillus associated with better response to anti-PD-1/PD-L1 immunotherapy and more prolonged progression-free survival. Antibiotic-induced gut microbiota dysbiosis can reduce the anti-tumor efficacy of 5-Fluorouracil treatment, while probiotics did not significantly enhance it. A probiotic combination containing Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus can reduce inflammation, enhance immunity, and restore a healthier gut microbial balance in gastric cancer patients after partial gastrectomy.
Conclusion: Probiotics and targeted interventions to modulate the gut microbiome have shown promising results in cancer prevention and treatment efficacy.
Systematic review registration: https://osf.io/6vcjp.
Introduction
Gastric cancer (GC) is among the most critical health issues worldwide. Beyond improvements in diagnosis, surveillance, and treatment over the past years, it is ranked as the fifth cancer in incidence and fourth in cancer mortality cause. The last report from 2020 shows the incidence of over 1 million new cases per year and more than 768/000 deaths annually. However, the incidence varies worldwide, with the highest cases occurring in Asia, with more than 820/000 new cases and 576/000 mortality (1). It is estimated that the statistics will rise with all efforts, and in recent years, the incidence of young adults has been increasing. The effect of Helicobacter pylori (Hp) is known already. Moreover, other risk factors such as genetics, tobacco use, alcohol consumption, and other lifestyle elements also play an essential role in the pathogenesis of the disease in both high-income and low-income countries. This may explain the possible rise in the disease incidence in the future (2, 3).
Patients at early stages are mostly asymptomatic or may have non-specific symptoms such as dyspepsia. At advanced stages, the tumor might demonstrate abdominal pain, weight loss, anorexia, or might exhibit complications of the tumor, such as hematemesis for ulcerated tumors or persistent vomiting in gastric outlet obstruction (4). This is why, in many countries, patients may present with advanced disease without efficient screening systems for GC, making the treatment more complicated.
More than 90% of the tumors arise sporadically, and the remnants have hereditary origins (5). According to Lauren’s classification of tumors, which is still a widely known histopathological classification besides the World Health Organization (WHO), there are three subtypes of tumor, including Intestinal, Diffuse (signet ring), and indeterminate (6). WHO also divides GC tumors into groups based on their origin: Adenocarcinomas, squamous cell carcinoma, non-Hodgkin lymphoma, Gastrointestinal stromal tumor (GIST), and Neuroendocrine tumors, which tend to occur less frequently. Adenocarcinomas are also poorly cohesive, Mucinous, Papillary, and tubular. Intestinal and Tubular subgroups are the most common types among the patients (7).
As we mentioned above, due to the late diagnosis in advanced stages, GC is mostly recognized at advanced stages with metastasis, decreasing the survival rate and complicating the treatment plan. Over the past decades, many therapeutic approaches have been introduced based on surgical, medical, and a combination. Adjuvant or neo-adjuvant therapies, along with radical surgery, improve the survival of resectable tumors (8). Despite the progress, the challenge of unresectable advanced-stage or progressive metastatic tumors remains a primary concern (9, 10). In recent years, targeted therapies based on mononuclear antibodies have also been applied in this field (11, 12). Beyond all of these efforts, because of the complexity of the disease and heterogeneity of tumors related to the genetics of patients and environmental factors, the overall survival is still not hopeful and hardly achieves one year (13). In an attempt to find the reasons for failure in chemotherapy regimens and targeted therapies, several studies have demonstrated that the arrangement of the gut microbiome can impact the host’s reaction to therapy (14, 15).
The role of gut microbiota, the collection of colonized microorganisms in the gastrointestinal lumen, on a healthy immune system has been studied in past years. It has been shown to have an essential effect on human health and regulates various aspects of it, such as host immunological responses, energy metabolism, elimination of pathogens, and oncogenesis (16). The dysbiosis of this system may affect several diseases, including GC (17, 18). Previous research has proven that infection with helicobacter pylori, a known and significant risk factor of the condition, can cause alterations in the microbiome. These changes have been found to contribute not only to gastric atrophy but also to an increased likelihood of developing gastric cancer, as observed in both animal and human models (19, 20). According to the study conducted by Miao et al. (21), it has been observed that the population of organisms changes at the various stages of the disease, thus presenting potential opportunities for focused treatment interventions. Although the impact of gut microbiota on chemotherapy and targeted immune therapy has been investigated, its precise mechanisms and effects remain uncertain.
Hence, this study aims to evaluate the impact of explicitly targeting the gut microbiota on the treatment results of gastric cancer through a systematic review and meta-analysis conducted for the first time.
Methods
This systematic review follows the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA2020) statement (22). The study protocol has been registered on the Open Science Framework (OSF) (registration doi: 10.17605/OSF.IO/6VCJP).
Search strategy
We collected original articles in this field by searching through PubMed, Google Scholar, Web of Science, and Scopus databases for English language literature published up to the 12th of August, 2023. The search was conducted based on “Gastric or stomach cancer,” “Gastric or stomach neoplasm,” AND “Gut microbiota” or “Microbiome” as keywords. The search strategy is mentioned in Table 1. Furthermore, duplicated records were omitted using EndNote ver.21. To identify other suitable studies, we also reviewed the references of relevant papers and reviews on the topic (see Table 2).
Inclusion criteria
After excluding the animal studies, the remaining studies were included for the review if the study follows the PICOS:
P: population: gastric cancer patients.
I: intervention: using gut microbiota in treatment regimen.
C: the control group: gastric cancer patients who receive treatments regardless of gut microbiota.
O: outcome: patient’s progression-free survival and responsiveness to treatment.
S: study design: English language RCTs
Study selection and quality assessment
Two reviewers (SH, SM), Using the RAYYAN intelligent tool for systematic reviews, analyzed and screened titles and abstracts to identify similar papers in a blinded manner. Full texts of them were obtained to assess the qualification of the “Yes” and “Maybe” groups. In case of conflicts, a third reviewer was involved and then reached an agreement to overcome differences and disagreements. Conflicts have been resolved through discussion between them. For each included study, the quality assessment and risk of bias were performed using JBI’s critical appraisal tools.1
Result
Study characteristics
Our search strategies yielded 2,999 studies across databases (Figure 1). After removing duplicates and excluding articles that did not meet our criteria, 15 studies were included in this systematic review, comprising eight observational studies (4 cross-sectional studies, two case–control studies, and 2 cohort studies), five experimental studies, and two randomized controlled trials. The studies were conducted in various countries, including China (7 studies), South Korea (3 studies), the United States (1 study), Japan (1 study), and multinational collaborations (2 studies). Sample sizes ranged from 19 to 1,600 participants, with some studies involving animal models or in vitro experiments.
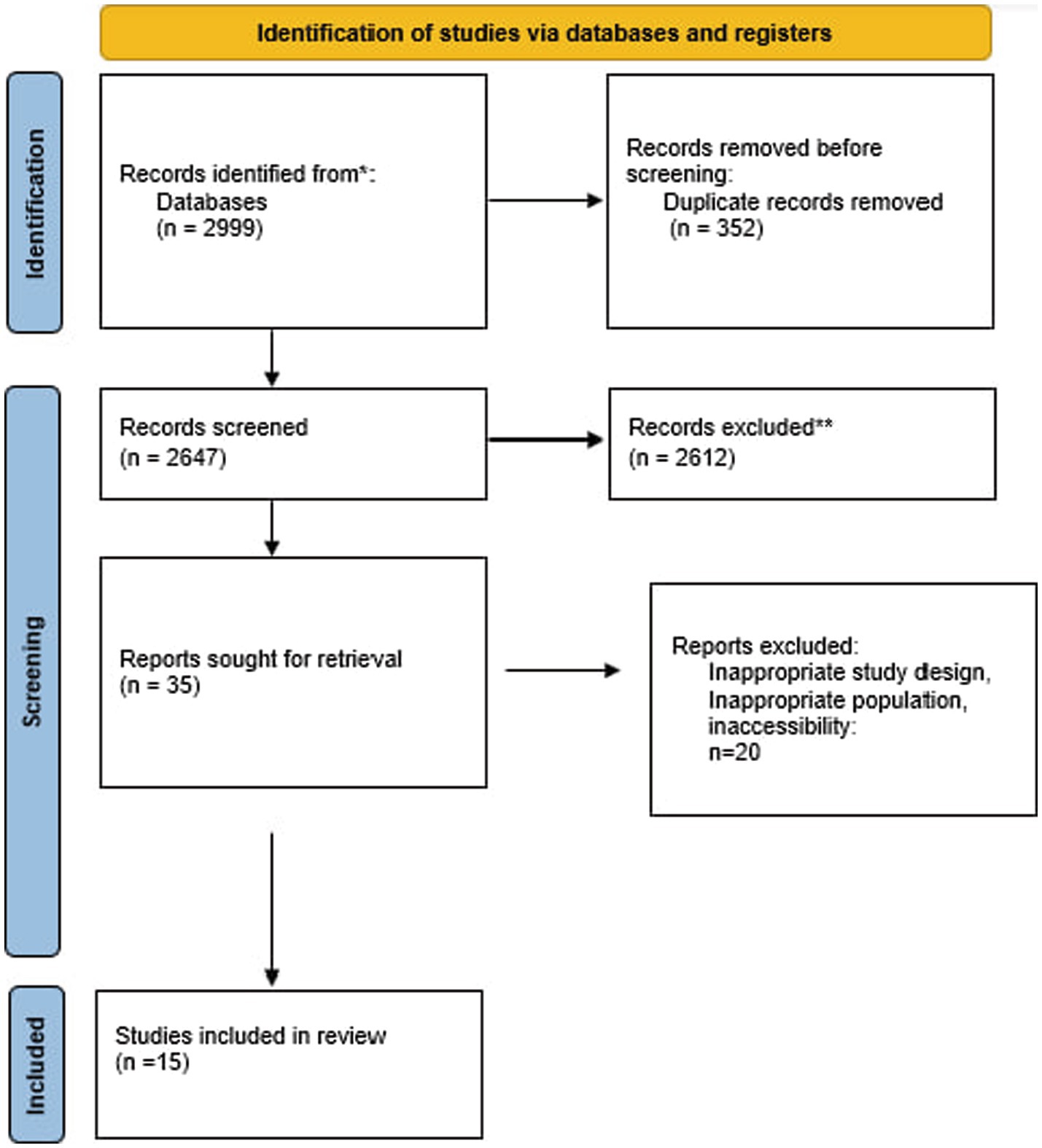
Figure 1. Flow diagram of the study selection procedure. The PRISMA approach to meta-analysis’s flow diagram template was used.
Gut microbiome and gastric cancer
Several studies reported alterations in the gut microbiome composition and diversity associated with gastric cancer or precancerous lesions. Miao et al. (21) found reduced microbial alpha diversity and altered community structure in patients with gastric mucosal atypical hyperplasia and gastric cancer compared to those with superficial gastritis. Specific bacterial genera, such as Enterococcus, Fusicatenibacter, Faecalibacterium, Roseburia, Lachnoclostridium, Tyzzerella_3, Butyricicoccus, and Dorea, were enriched at different stages of gastric carcinogenesis.
Wang et al. (33) observed increased bacterial overgrowth and diversification of the microbial communities in gastric cancer patients, with enrichment of Lactobacillus, Escherichia-Shigella, Nitrospirae, Burkholderia fungorum, and Lachnospiraceae uncultured genera. Park et al. (26, 27) identified two microbial modules (pink and brown) positively correlated with intestinal metaplasia (a precancerous lesion), containing bacteria like Neisseriaceae, Pasteurellaceae, Veillonellaceae, Pseudomonadaceae, and Staphylococcaceae.
Watanabe et al. (28) found that Helicobacter pylori (H. pylori) infection was associated with reduced richness and evenness of gastric bacteria, and eradication of H. pylori only partially restored microbial diversity. Genera like Blautia, Ralstonia, Faecalibacterium, Methylobacterium, and Megamonas were depleted in H. pylori-positive patients.
Gut microbiome and treatment response
Multiple research studies have investigated the correlation between gut microbiota and the effectiveness of cancer therapies. Han et al. (23) found that the gut microbiota composition influenced the effectiveness of several therapies (chemotherapy, immunotherapy, and combination therapy) in HER2-negative advanced gastric cancer patients. Increased levels of Lactobacillus were linked to improved outcomes with anti-PD-1/PD-L1 immunotherapy and extended progression-free survival (PFS). Yuan et al. (30) discovered that antibiotic-induced changes in gut bacteria lowered the effectiveness of 5-Fluorouracil (5-FU) treatment for colorectal cancer. Probiotics did not notably improve the treatment’s efficiency compared to 5-FU alone. Zheng et al. (29) found that a probiotic mix with Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus decreased inflammation, boosted immunity, and improved gut microbial balance in gastric cancer patients following partial gastrectomy.
Gut microbiome and cancer prevention
Jones et al. (24) demonstrated that the H. pylori virulence factor CagA disrupted the gut microbiota balance and promoted the proliferation of epithelial cells in a Drosophila gut model. Addressing the microbial imbalance may minimize the risk of stomach cancer during H. pylori infection. Oh et al. (38) discovered that administering probiotics with H. pylori eradication treatment reduced the disturbance of gut microbiota induced by antibiotics, potentially lowering the risk factors linked to the onset of gastric cancer. Song et al. (39) demonstrated that pretreatment with the probiotic mixture Bifico improved colitis and decreased tumor development in a mouse model of colitis-associated colorectal cancer (CAC) by changing the gut microbiota composition and lowering the expression of pro-inflammatory genes. In a case–control study, Turati et al. (35) found that consuming a high amount of Galactooligosaccharide raffinose was linked to a decreased likelihood of developing stomach cancer.
Biomarkers and prediction models
Qi et al. (37) developed a random forest model using differential gut bacteria that could predict poorly differentiated colorectal cancer with 100% accuracy, with Pseudoramibacter, Megamonas, and Bifidobacterium being the most critical bacterial predictors.
Yu et al. (31) described the development of gastric acid-responsive ROS nanogenerators that effectively eliminated H. pylori infection in mice without disrupting the normal gut microbiota, suggesting a potential approach for treating H. pylori infection and preventing gastric cancer development.
In summary, this systematic review highlights the crucial role of the gut microbiome in gastric and gastrointestinal cancers. Dysbiosis and altered microbial composition were associated with gastric cancer development, precancerous lesions, and treatment response. Specific bacterial genera, such as Lactobacillus, Bifidobacterium, and Akkermansia, were linked to favorable outcomes, while others, like Streptococcus and Escherichia-Shigella, were associated with poorer prognosis. Probiotics and targeted interventions to modulate the gut microbiome showed promising cancer prevention results and enhanced treatment efficacy. Additionally, gut microbial signatures could serve as potential biomarkers for cancer diagnosis, patient stratification, and prediction of treatment response.
Discussion
Gastric cancer is ranked as the fifth cancer in incidence and fourth in cancer mortality cause. The last report from 2020 shows the incidence of over 1 million new cases per year and more than 768/000 deaths annually. It is estimated that the statistics will rise with all efforts, and in recent years, the incidence of young adults has been increasing (2). Despite the progression of therapeutic methods, targeted immunotherapy, and combinations of chemotherapy or radiotherapy with surgery, the challenge with unresectable advanced-stage or progressive metastatic tumors remains a primary concern. Beyond all of these efforts, because of the complexity of the disease and heterogeneity of tumors, the efforts to overcome the therapy failures and find the underlying causes are still ongoing. Among the factors known so far, some studies have investigated the relationship between gut microbiome and the development, course, treatment, and prognosis of gastric cancer.
The effect of gut microbiota on immune system response is already known (40). Beyond this critical role in the immune system hemostasis, alteration in the normal composition of microbiomes taxa proved to be a predisposing factor in many diseases such as colorectal cancer, Cardiovascular disease, and inflammatory bowel disease (41). This alteration is called dysbiosis. The process may promote hyperplasia and inflammation of the human tissues and lead to neoplasia. H. pylori, as a known etiological factor for gastric cancer and precancerous lesions, H. pylori can produce dysbiosis in the gastric and intestinal mucosa (42). However, the extent of this change and its probable effect on the development of neoplasia has not been studied well. Jones et al. (24) studied the effect of the cagA protein factor on the midgut microbiome of the germ-free adult Drosophila model. Cytotoxin-associated gene A (cagA) is a virulence protein that, by injection in the cytoplasm of gastric cells arising from H. pylori infection, may start the oncogenic potency of this bacteria. Drosophila cagA transgenic models and the protein expression were found to be enough for starting the excessive proliferation process and may contribute to dysbiosis of gut microbiota. Also, this phenomenon elicits the expression of innate immune components, Diptericin and Duox.
The effect of H. pylori on dysbiosis was studied on human patients with gastric cancer or precancerous lesions, too. Wang et al. (34) proved the alteration of human gut microbiota by investigating the 313 feces of patients by metagenomics sequencing. The infected patients with H. Pylori showed the colonization of P. corpi and that Vitamine B 12 levels decreased among the group. P. corpi was known before as a microorganism associated with rheumatoid arthritis onset and severity (43). The authors did not investigate the inflammation or histological changes in gastric mucosa, and the patients were checked by breathing test. To improve the data showing the relationship between dysbiosis and GC formation, Turati et al. (35) they investigated the effect of prebiotics on the risk of gastric and upper digestive tract cancers. Prebiotics are substrates that are selectively utilized by microbiota to maintain the health of the digestive system. In a case–control study of nearly 17 years, among several prebiotics used by participants, only high raffinose (a kind of galactooligosaccharide) intake reduced the risk of GC formation. The authors declared that because of the low data, the relationship between the two factors is not strong and needs more investigation. These data showed that there should be a relationship between microbiota in the digestive system environment and the carcinogenesis process. In addition, Song et al. (36) test the idea of using probiotics to decrease inflammation in mice models of IBD.
Bifico, an approved over-the-counter drug in China, previously showed relief from gastritis induced by H. pylori in mice and experimental colitis in mice (44, 45). The article examines the impact of this probiotic on malignancies linked with colitis. The results showed that Bifico reduced intestinal inflammation and inhibited tumor formation. The 16 s rRNA sequencing revealed that the supplementation reduced the prevalence of Desulfovibrio, Mucispirillum, Odoribacter, and Lactobacillus. In addition to that impact, the Bifico target taxa may engage with CXCL1, CXCL2, CXCL3, and CXCL5, all ligands of CXC motif receptor 2 (CXCR2). All these effects reduced the development of colorectal cancer in mice with an inflammatory bowel disease model. If the idea is investigated in the field of GC, the findings might be positive, even if such research has not been published yet (46).
Moving further, Wang et al. (33) Indicated that changes in gut flora occur in gastric cancer. Changes involve diversifying microorganism populations, increasing bacterium number, and enriching bacteria with possible cancer-promoting actions. Proteobacteria, Bacillota, Bacteroidota, Fusobacteria, and Actinobacteria were the predominant bacterial phyla in the microbiome of cancer patients, as revealed by compositional analysis. Supporting the idea, Park et al. (26) investigated the hypothesis with the same method on patients with gastritis, intestinal metaplasia, and gastric cancer. The abundance of Rhizobiales increased as H. pylori-infected patients advanced from gastritis to intestinal metaplasia, alongside changes in microbiota across all patient groups. T4SS genes were prominently detected in the metagenome of patients in the metaplasia stage using RNA sequencing and analysis. The T4SS gene encodes proteins that facilitate the injection of cagA protein into the cytoplasm of infected gastric epithelium. The results indicate that elevated levels of these variables might stimulate the development of cancer. The study’s findings on the alterations in microbiota following the eradication of H. pylori are significant. While the microbiome makeup of individuals with successfully eradicated H. pylori and those without H. pylori showed similarities, but there was no difference in the relative abundance of T4SS genes between the two groups. This gene is crucial in establishing H. pylori colonization and the progression of severe outcomes in individuals infected with virulent strains. Patients with gastritis or metaplasia were examined to analyze the correlation between H. pylori and other T4SS gene-contributing bacteria such as Rhizobiales and Neisseriaceae. The relative quantity of these two species grew as the abundance of H. pylori dropped. The latest study’s results corroborate Jones et al.’s research on the drosophila model. Miao et al. (21) similarly found the changes during the stages of formation of gastric neoplasms.
In contrast with the recently mentioned article, they analyze the feces of patients with superficial gastritis (SG), atrophic gastritis (AG), gastric mucosal atypical hyperplasia (GMAH), and advanced gastric cancer (GC). Through 16 s rRNA gene sequencing, researchers identified six species and two metabolic pathways that were more prevalent in the cancer group compared to the non-cancer group. The six genera with a high GC content are Porphyromonas, Scardovia, Halomonas, Actinobacteria_unclassified, Bergeyella, and Enterococcus.
GC has not yet studied the relationship between gut microbiota-specific organisms and the type of neoplasms. Despite this fact, the study of Qi et al. (37) on colorectal cancer (CRC) patients showed the differences between flora in poorly and well-differentiated CRC. The fecal samples from patients were tested and compared using sequencing. Blautia, Escherichia-Shigella, Streptococcus, Lactobacillus, and Bacteroides bacteria were prevalent in several colorectal cancer individuals with low to moderate differentiation. Nine bacteria were found to have a high abundance in the weakly differentiated group, including Bifidobacterium, norank_f__Oscillospiraceae, and Eisenbergiella. This term might be used for upcoming research on the subject of GC.
It is essential to consider specific characteristics while examining the research on the relationship between gut microbiota and GC. The T4SS gene’s presence increased in organisms as the sickness progressed, regardless of the variety of bacteria examined. Future research should comprehensively investigate the pathways and mechanisms of the cagA protein and T4SS gene. Park et al. (27) used weighted correlation network analysis to identify two modules linked to advanced gastric carcinogenesis (group C [intestinal metaplasia with H. pylori infection] or group D [intestinal metaplasia without H. pylori infection]): the pink and brown modules. The two modules included nitrosating/nitrate-reducing bacteria and T4SS protein gene-contributing bacteria from different families such as Acidobacteriaceae, Burkholderiaceae, Neisseriaceae, Pasteurellaceae, Veillonellaceae, Bartonellaceae, Brucellaceae, unclassified Rhizobiales, Pseudomonadaceae, Sphingomonadaceae, Staphylococcaceae, and Xanthomonadaceae. Group C patients saw reduced H. pylori levels and increased intragastric acidity compared to group B, which had H. pylori infection without intestinal metaplasia. Bacteria other than H. pylori from the pink or brown modules can be identified in group C patients. In group D, the presence of H. pylori decreases, but non-H. pylori bacteria may increase. Bacteria, not H. pylori from the pink or brown modules, are more easily identified in groups C and D than in groups A or B. Both groupings may be associated with the pink and brown modules. Individuals harboring pink- or blue-module bacteria are at a higher risk of developing stomach cancer compared to those in groups A and B. They suggested that using these components might streamline patient screening for endoscopy. Researchers recommend doing more research to determine the specific bacterial taxa associated with the development of stomach cancer due to their presence in the samples.
We believe it is crucial to note that neither research had a substantial sample size. Furthermore, the investigations do not account for distinctions in ethnicity and race. Differences in H. pylori prevalence among nations may influence the outcomes of similar investigations. Furthermore, in addition to identifying H. pylori as a carcinogenic predisposing factor, future research may uncover other bacteria responsible for this process.
The process of dysbiosis may occur after the eradication of H. pylori via antibiotics. Watanabe et al. (32) showed that dysbiosis may persist long-term after eradicating H. pylori. In a trial, they compared patients in the early stages of GC who undergo endoscopic submucosal dissection. The changes beyond the H. pylori positive group stand longer than negative ones, and they declare that this can involve the development of primary and metachronous GCs. Several genera, including Blautia, Ralstonia, Faecalibacterium, Methylobacterium, and Megamonas, are deleted among H. pylori-positive patients and not restored fully after the eradication and application of the antibiotic regimens. Yu et al. (31) designed an experimental study to prevent this event and decrease dysbiosis during H. pylori eradication. Bacteria are vulnerable to oxidative damage caused by external reactive oxygen species (ROS). By creating a ROS nanogenerator, they are testing this theory. The nanogenerators demonstrated the ability to eradicate multi-drug resistant H. pylori in mice with little adverse effects. The alterations in the natural flora due to antibiotic therapy were minimal.
Zheng et al. (29) studied patients who underwent partial gastrectomy for the treatment of gastric cancers. Patients who received probiotics supplementation in a randomized controlled experiment had a substantial improvement in immune responses and a decrease in inflammation. The probiotic mixture notably increased the populations of beneficial bacteria such as Bacteroides, Faecalibacterium, and Akkermansia while reducing the abundance of Streptococcus, a pathogenic bacterium associated with cancer development. The study utilized a probiotic blend of Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus. This combination reduced physiological issues resulting from gastrectomy by monitoring blood levels, index, and microbiological diversity through high-throughput sequencing.
We are in the early stages of using gut microbiota to treat resistant gastric cancers. Chemotherapies have been the most effective therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma for many years. The therapy’s outcomes are restricted. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of gastric cancer. The limited use of this technology remains a difficulty (47). As far as we know, In melanoma, the gut microbiota can influence the response to anti-PD-1/PD-L1 immunotherapy by infiltrating CD8+ T-cells (48). Based on this theory and to find new methods to overcome the resistance in therapy, Han et al. (23) investigated the relationship between gut microbiota and the response to treatment in HER2-negative advanced gastric cancer.
Patients in stages III or IV according to TNM staging were included in a cohort study. Patients were categorized into three groups based on their treatment method: chemotherapy alone, immunotherapy, and a combination of both. The result data is categorized into responders and non-responders. The study revealed that the gut microbiota can impact the response to therapy in HER-2 negative advanced gastric cancer based on the treatment method. The combo treatment yielded better results. Patients with a greater prevalence of Lactobacillus had increased microbial diversity and showed enhanced responsiveness to anti-PD-1/PD-L1 therapy.
Moreover, they frequently encounter enhanced progression-free survival. This might serve as a focal point for developing novel therapeutic approaches to enhance patient outcomes and longevity in the future. Like this study, Yuan et al. (30) revealed the influence of dysbiosis on the anti-tumor efficacy of 5-Fluorouracil (5-FU) treatment in mice models of colorectal cancer. The results suggested that using antibiotics not only proceeds to dysbiosis but can also decrease the treatment efficacy of 5-FU. As we mentioned before, the changes in gut microbiota in this study, after using antibiotics, to the pathogenic microorganisms like backichia, shigella, and Enterobacter mentioned. The mice that received probiotics with antibiotics showed better anti-tumor activity of 5-FU.
Immunotherapeutic targets for GC have been identified, leading to the development of drugs that specifically target Human Epidermal Growth Factor Receptor 2 (HER2), Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), PD-1, and programmed cell death ligand 1 (PD-L1). These drugs are currently either available in the market or undergoing clinical trials. Microorganisms have the ability to impact the way medicines are metabolized by undergoing chemical alterations (48) and accumulating them in their bodies. Several studies have verified that the microbiota and their metabolites can significantly influence anti-GC immunotherapy through the release of cytokines and the promotion of T cell infiltration (49). Thus, the use of antibiotics can hinder the effectiveness of cancer immunotherapy due to their impact on the gut microbiota, which plays a crucial role in enhancing the body’s immune response against tumors. Scientists utilized fecal sample sequencing techniques to distinguish between individuals who responded to ICIs and those who did not. They demonstrated that certain microorganisms could potentially be associated with enhanced immunity and the infiltration of immune cells in malignancies (50). For instance, research has shown that gut microorganisms are more abundant in patients who have a robust response to anti-PD-1 therapy. These microbes also stimulate the production of memory CD8+ T cells and natural killer cells in advanced non-small cell lung cancer (NSCLC) patients in China (51). The gut microbiota has the ability to affect the response rate to immunotherapy through several methods, therefore playing a role in the gut microbiota-immune system axis. Gastric cancer (GC) can be categorized into four distinct types: EBV-positive, microsatellite instability (MSI), genomically stable, and chromosomal instability (52). An extensive analysis of microbial profiles in gastric cancer (GC) from two different cohorts has revealed that Selenomonas, Bacteroids, and Porphyromonas are the three most prevalent microorganisms in patients with high microsatellite instability (MSI-high) GC. A clinical experiment aimed to establish a connection between molecular characterisation and the effectiveness of immunotherapy using pembrolizumab, a PD-1 inhibitor. The trial showed that having a high degree of microsatellite instability or being positive for EBV can be used as a predictive marker. Moreover, besides the presence of high-level microsatellite instability and positive EBV status, Hp infection serves as both an indicator of elevated PD-L1 expression and a predictor of unfavorable outcomes following immunotherapy. This is due to its ability to hinder innate and adaptive immune responses, suggesting that Hp infection could be utilized as a measure to assess the effectiveness of immunotherapy in patients with GC. Nevertheless, the specific mechanisms by which Hp regulates immunotherapy are still unclear due to the conflicting findings reported in the aforementioned research (47).
This systematic review conducted a comprehensive analysis of the alterations in the gut microbiota that are associated with the onset of gastric cancer. The text emphasized the complex connection between microbial diversity and cancer, the potential of medicines focusing on the microbiome, and the importance of rigorous methodology in future study. The constraints of this study, such as insufficient data, potential bias, and the inability to incorporate all pertinent components, underscore the necessity for extensive-scale studies. These limitations emphasize the necessity of doing extensive research to validate these findings and delve deeper into the involvement of the microbiome in gastric cancer. Future research should prioritize doing long-term cohort studies to investigate the dynamic alterations in the gut microbiota throughout the development and advancement of gastric cancer. Simultaneously, it is imperative to perform investigations on the pathogenic mechanisms to gain insight into how certain bacteria contribute to or impact the progression of gastric cancer. Furthermore, interventional studies could be carried out to assess the effectiveness of particular microorganisms in preventing and treating stomach cancer. By doing this research, we can get a more comprehensive comprehension of the microbiome’s function in stomach cancer and offer direction for focused preventive and therapy methods in the clinical context.
To conclude, the effect of dysbiosis of gut microbiota on carcinogenesis of GC and the effect of H. pylori on gut microbiota should considered. However, as we mentioned, the knowledge is incomplete, and the exact mechanisms have not been studied well. However, knowing the microorganisms that perhaps co-related with H. Pylori in GC formation can make them a suitable target for therapy in the future. There is a long way to go.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Writing – original draft, Writing – review & editing. SH: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. RK: Writing – original draft, Writing – review & editing. SMA: Writing – original draft, Writing – review & editing. YD: Writing – original draft, Writing – review & editing. PL: Writing – original draft, Writing – review & editing. SV: Writing – original draft, Writing – review & editing. FJ: Writing – original draft, Writing – review & editing. ShA: Writing – original draft, Writing – review & editing. KN: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. NH: Writing – original draft, Writing – review & editing. ArA: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. AiA: Methodology, Investigation, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. MA: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GC, gastric cancer; Hp, Helicobacter pylori; WHO, World Health Organization; GIST, gastrointestinal stromal tumor; PRISMA2020, preferred reporting items for systematic reviews and meta-analyses; OSF, the open science framework; PFS, progression-free survival; 5-FU, 5-Fluorouracil; CAC, colitis-associated colorectal cancer; cagA, cytotoxin-associated gene A; CXCR2, CXC motif receptor 2; GMAH, gastric mucosal atypical hyperplasia; SG, superficial gastritis; CRC, colorectal cancer; ICI, immune checkpoint inhibitors.
Footnotes
References
1. Ferlay, J, Colombet, M, Soerjomataram, I, Parkin, DM, Piñeros, M, Znaor, A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. (2021) 149:778–89. doi: 10.1002/ijc.33588
2. Lu, L, Mullins, CS, Schafmayer, C, Zeissig, S, and Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41:1137–51. doi: 10.1002/cac2.12220
3. Tan, P, and Yeoh, KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. (2015) 149:1153–62.e3. doi: 10.1053/j.gastro.2015.05.059
4. Correa, P. Gastric cancer: overview. Gastroenterol Clin N Am. (2013) 42:211–7. doi: 10.1016/j.gtc.2013.01.002
5. Conti, CB, Agnesi, S, Scaravaglio, M, Masseria, P, Dinelli, ME, Oldani, M, et al. Early gastric Cancer: update on prevention, diagnosis and treatment. Int J Environ Res Public Health. (2023) 20:2149. doi: 10.3390/ijerph20032149
6. Lauren, P. The two histological main types of gastric carcinoma: DIFFUSE and so-called intestinal-type carcinoma. An attempt at a HISTO-clinical classification. Acta Pathol Microbiol Scand. (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
7. Nagtegaal, ID, Odze, RD, Klimstra, D, Paradis, V, Rugge, M, Schirmacher, P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
8. van den Ende, T, Ter Veer, E, Machiels, M, Mali, RMA, Abe Nijenhuis, FA, de Waal, L, et al. The efficacy and safety of (neo)adjuvant therapy for gastric Cancer: a network Meta-analysis. Cancers (Basel). (2019) 11:80. doi: 10.3390/cancers11010080
9. Fuchs, CS, Doi, T, Jang, RW, Muro, K, Satoh, T, Machado, M, et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction Cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
10. Kang, YK, Boku, N, Satoh, T, Ryu, MH, Chao, Y, Kato, K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
11. Shitara, K, Yatabe, Y, Matsuo, K, Sugano, M, Kondo, C, Takahari, D, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer. (2013) 16:261–7. doi: 10.1007/s10120-012-0179-9
12. De Vita, F, Borg, C, Farina, G, Geva, R, Carton, I, Cuku, H, et al. Ramucirumab and paclitaxel in patients with gastric cancer and prior trastuzumab: subgroup analysis from RAINBOW study. Future Oncol. (2019) 15:2723–31. doi: 10.2217/fon-2019-0243
13. Zhang, XY, and Zhang, PY. Gastric cancer: somatic genetics as a guide to therapy. J Med Genet. (2017) 54:305–12. doi: 10.1136/jmedgenet-2016-104171
14. Yu, T, Guo, F, Yu, Y, Sun, T, Ma, D, Han, J, et al. Fusobacterium nucleatum promotes Chemoresistance to colorectal Cancer by modulating autophagy. Cell. (2017) 170:548–63.e16. doi: 10.1016/j.cell.2017.07.008
15. Yi, Y, Shen, L, Shi, W, Xia, F, Zhang, H, Wang, Y, et al. Gut microbiome components predict response to Neoadjuvant Chemoradiotherapy in patients with locally advanced rectal Cancer: a prospective. Longitudinal Study Clin Cancer Res. (2021) 27:1329–40. doi: 10.1158/1078-0432.CCR-20-3445
16. Thursby, E, and Juge, N. Introduction to the human gut microbiota. Biochem J. (2017) 474:1823–36. doi: 10.1042/BCJ20160510
17. Png, CW, Lee, WJJ, Chua, SJ, Zhu, F, Yeoh, KG, and Zhang, Y. Mucosal microbiome associates with progression to gastric cancer. Theranostics. (2022) 12:48–58. doi: 10.7150/thno.65302
18. Nicholson, JK, Holmes, E, Kinross, J, Burcelin, R, Gibson, G, Jia, W, et al. Host-gut microbiota metabolic interactions. Science. (2012) 336:1262–7. doi: 10.1126/science.1223813
19. Lertpiriyapong, K, Whary, MT, Muthupalani, S, Lofgren, JL, Gamazon, ER, Feng, Y, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. (2014) 63:54–63. doi: 10.1136/gutjnl-2013-305178
20. Coker, OO, Dai, Z, Nie, Y, Zhao, G, Cao, L, Nakatsu, G, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. (2018) 67:1024–32. doi: 10.1136/gutjnl-2017-314281
21. Miao, Y, Tang, H, Zhai, Q, Liu, L, Xia, L, Wu, W, et al. Gut microbiota Dysbiosis in the development and progression of gastric Cancer. J Oncol. (2022) 2022:1–15. doi: 10.1155/2022/9971619
22. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). (2021) 74:790–9. doi: 10.1016/j.recesp.2021.06.016
23. Han, Z, Cheng, S, Dai, D, Kou, Y, Zhang, X, Li, F, et al. The gut microbiome affects response of treatments in HER2-negative advanced gastric cancer. Clin Transl Med. (2023) 13:e1312. doi: 10.1002/ctm2.1312
24. Jones, TA, Hernandez, DZ, Wong, ZC, Wandler, AM, and Guillemin, K. The bacterial virulence factor CagA induces microbial dysbiosis that contributes to excessive epithelial cell proliferation in the Drosophila gut. PLoS Pathog. (2017) 13:e1006631. doi: 10.1371/journal.ppat.1006631
25. Oh, B, Kim, BS, Kim, JW, Kim, JS, Koh, SJ, Kim, BG, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. (2016) 21:165–74. doi: 10.1111/hel.12270
26. Park, CH, Lee, AR, Lee, YR, Eun, CS, Lee, SK, and Han, DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. (2019) 24:e12547. doi: 10.1111/hel.12547
27. Park, CH, Lee, JG, Lee, AR, Eun, CS, and Han, DS. Network construction of gastric microbiome and organization of microbial modules associated with gastric carcinogenesis. Sci Rep. (2019) 9:12444. doi: 10.1038/s41598-019-48925-4
28. Watanabe, M, Otake, R, Kozuki, R, Toihata, T, Takahashi, K, Okamura, A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. (2020) 50:12–20. doi: 10.1007/s00595-019-01952-0
29. Zheng, C, Chen, T, Wang, Y, Gao, Y, Kong, Y, Liu, Z, et al. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer. (2019) 10:568–76. doi: 10.7150/jca.29072
30. Yuan, L, Zhang, S, Li, H, Yang, F, Mushtaq, N, Ullah, S, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Biomed Pharmacother. (2018) 108:184–93. doi: 10.1016/j.biopha.2018.08.165
31. Yu, J, Guo, Z, Yan, J, Bu, C, Peng, C, Li, C, et al. Gastric acid-responsive ROS Nanogenerators for effective treatment of Helicobacter pylori infection without disrupting homeostasis of intestinal Flora. Adv Sci (Weinh). (2023) 10:e2206957. doi: 10.1002/advs.202206957
32. Watanabe, T, Nadatani, Y, Suda, W, Higashimori, A, Otani, K, Fukunaga, S, et al. Long-term persistence of gastric dysbiosis after eradication of Helicobacter pylori in patients who underwent endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. (2021) 24:710–20. doi: 10.1007/s10120-020-01141-w
33. Wang, L, Zhou, J, Xin, Y, Geng, C, Tian, Z, Yu, X, et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol. (2016) 28:261–6. doi: 10.1097/MEG.0000000000000542
34. Wang, D, Li, Y, Zhong, H, Ding, Q, Lin, Y, Tang, S, et al. Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Open Bio. (2019) 9:1552–60. doi: 10.1002/2211-5463.12694
35. Turati, F, Concina, F, Bertuccio, P, Fiori, F, Parpinel, M, Garavello, W, et al. Prebiotics and the risk of upper digestive tract and stomach cancers: the PrebiotiCa study. J Acad Nutr Diet. (2023) 123:1772–80. doi: 10.1016/j.jand.2023.07.008
36. Song, H, Wang, W, Shen, B, Jia, H, Hou, Z, Chen, P, et al. Pretreatment with probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci. (2018) 109:666–77. doi: 10.1111/cas.13497
37. Qi, Z, Zhibo, Z, Jing, Z, Zhanbo, Q, Shugao, H, Weili, J, et al. Prediction model of poorly differentiated colorectal cancer (CRC) based on gut bacteria. BMC Microbiol. (2015) 21:312. doi: 10.1186/s12866-022-02712-w
38. Oh, HY, Kim, B-S, Seo, S-S, Kong, J-S, Lee, S-Y, Park, K-M, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clinical Microbiology and Infection. (2015) 21:674-e1.
39. Song, D, Peng, Q, Chen, Y, Zhou, X, Zhang, F, Li, A, et al. Altered gut microbiota profiles in sows and neonatal piglets associated with porcine epidemic diarrhea virus infection. Scientific reports. (2017) 7:17439.
40. Wang, J, Zhu, N, Su, X, Gao, Y, and Yang, R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. (2023) 12:793. doi: 10.3390/cells12050793
41. Holmes, E, Li, JV, Marchesi, JR, and Nicholson, JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. (2012) 16:559–64. doi: 10.1016/j.cmet.2012.10.007
42. Wroblewski, LE, and Peek, RM Jr. Helicobacter pylori, Cancer, and the gastric microbiota. Adv Exp Med Biol. (2016) 908:393–408. doi: 10.1007/978-3-319-41388-4_19
43. Zhang, X, Zhang, D, Jia, H, Feng, Q, Wang, D, Liang, D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. (2015) 21:895–905. doi: 10.1038/nm.3914
44. Yu, HJ, Liu, W, Chang, Z, Shen, H, He, LJ, Wang, SS, et al. Probiotic BIFICO cocktail ameliorates Helicobacter pylori induced gastritis. World J Gastroenterol. (2015) 21:6561–71. doi: 10.3748/wjg.v21.i21.6561
45. Zhao, HM, Huang, XY, Zuo, ZQ, Pan, QH, Ao, MY, Zhou, F, et al. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J Gastroenterol. (2013) 19:742–9. doi: 10.3748/wjg.v19.i5.742
46. Komori, E, Kato-Kogoe, N, Imai, Y, Sakaguchi, S, Taniguchi, K, Omori, M, et al. Changes in salivary microbiota due to gastric cancer resection and its relation to gastric fluid microbiota. Sci Rep. (2023) 13:15863. doi: 10.1038/s41598-023-43108-8
47. Kim, ST, Cristescu, R, Bass, AJ, Kim, KM, Odegaard, JI, Kim, K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. (2018) 24:1449–58. doi: 10.1038/s41591-018-0101-z
48. Gopalakrishnan, V, Spencer, CN, Nezi, L, Reuben, A, Andrews, MC, Karpinets, TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
49. Zimmermann, M, Zimmermann-Kogadeeva, M, Wegmann, R, and Goodman, AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. (2019) 570:462–7. doi: 10.1038/s41586-019-1291-3
50. Jin, Y, Dong, H, Xia, L, Yang, Y, Zhu, Y, Shen, Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol. (2019) 14:1378–89. doi: 10.1016/j.jtho.2019.04.007
51. Oster, P, Vaillant, L, Riva, E, McMillan, B, Begka, C, Truntzer, C, et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. (2022) 71:457–66. doi: 10.1136/gutjnl-2020-323392
Keywords: gastric cancer, gut microbiota, immunotherapy, immune regulation, microbial metabolites
Citation: Marashi A, Hasany S, Moghimi S, Kiani R, Mehran Asl S, Dareghlou YA, Lorestani P, Varmazyar S, Jafari F, Ataeian S, Naghavi K, Sajjadi SM, Haratian N, Alinezhad A, Azhdarimoghaddam A, Sadat Rafiei SK and Asadi Anar M (2024) Targeting gut-microbiota for gastric cancer treatment: a systematic review. Front. Med. 11:1412709. doi: 10.3389/fmed.2024.1412709
Edited by:
Yan-Dong Wang, Beijing University of Chemical Technology, ChinaReviewed by:
Li Zhang, Brown University, United StatesGeorgia Damoraki, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Marashi, Hasany, Moghimi, Kiani, Mehran Asl, Dareghlou, Lorestani, Varmazyar, Jafari, Ataeian, Naghavi, Sajjadi, Haratian, Alinezhad, Azhdarimoghaddam, Sadat Rafiei and Asadi Anar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahsa Asadi Anar, TWFoc2EuYm96QGdtYWlsLmNvbQ==; Arman Alinezhad, YXJtYW4uNzYuYWxpbmVqYWRAZ21haWwuY29t
†ORCID: Mahsa Asadi Anar, orcid.org/0000-0002-5772-2472
 Amir Marashi1
Amir Marashi1 Shirin Varmazyar
Shirin Varmazyar Arman Alinezhad
Arman Alinezhad Aida Azhdarimoghaddam
Aida Azhdarimoghaddam Mahsa Asadi Anar
Mahsa Asadi Anar