- 1Department of Computer Science and Engineering, Prasad V Potluri Siddhartha Institute of Technology, Vijayawada, India
- 2Faculty of Information Science and Technology, University Kebangsaan Malaysia, Bangi, Selangor, Malaysia
- 3Department of Computer Science and Engineering, Sir C R Reddy College of Engineering, Eluru, India
- 4Institute of Computer Science and Digital innovation, UCSI University, Kuala Lumpur, Malaysia
- 5Computer Science Department, College of Computer Engineering and Science, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 6Computer Science Department, College of Science and Humanities-Jubail, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 7Department of Surgery, Medical College For Women and Hospital, Dhaka, Bangladesh
- 8Faculty of Information and Communication Studies, University of the Philippines Open University, Los Baños, Philippines
- 9Center for Computational Imaging and Visual Innovations, De La Salle University, Manila, Philippines
- 10Centre for Cyber Physical Systems, Computer Science Department, Khalifa University, Abu Dhabi, United Arab Emirates
Introduction: Global Cardiovascular disease (CVD) is still one of the leading causes of death and requires the enhancement of diagnostic methods for the effective detection of early signs and prediction of the disease outcomes. The current diagnostic tools are cumbersome and imprecise especially with complex diseases, thus emphasizing the incorporation of new machine learning applications in differential diagnosis.
Methods: This paper presents a new machine learning approach that uses MICE for mitigating missing data, the IQR for handling outliers and SMOTE to address first imbalance distance. Additionally, to select optimal features, we introduce the Hybrid 2-Tier Grasshopper Optimization with L2 regularization methodology which we call GOL2-2T. One of the promising methods to improve the predictive modelling is an Adaboost decision fusion (ABDF) ensemble learning algorithm with babysitting technique implemented for the hyperparameters tuning. The accuracy, recall, and AUC score will be considered as the measures for assessing the model.
Results: On the results, our heart disease prediction model yielded an accuracy of 83.0%, and a balanced F1 score of 84.0%. The integration of SMOTE, IQR outlier detection, MICE, and GOL2-2T feature selection enhances robustness while improving the predictive performance. ABDF removed the impurities in the model and elaborated its effectiveness, which proved to be high on predicting the heart disease.
Discussion: These findings demonstrate the effectiveness of additional machine learning methodologies in medical diagnostics, including early recognition improvements and trustworthy tools for clinicians. But yes, the model’s use and extent of work depends on the dataset used for it really. Further work is needed to replicate the model across different datasets and samples: as for most models, it will be important to see if the results are generalizable to populations that are not representative of the patient population that was used for the current study.
1 Introduction
Many communities are affected by heart disease, a major global health problem that is responsible for many cases of sickness and death. There is an increasing need to understand the complexity of heart diseases as our understanding of cardiovascular health expands. Think about this: someone dies of cardiovascular issues every 37 s in America, which highlights the urgency to quell this unseen epidemic (American Heart Association, 2022). This mind-boggling figure shows how huge numbers of people, families, and societies are affected by cardiac diseases (1).
The human heart is one fantastic example of biologically engineered machinery that coordinates life’s intricate workings by driving vital energy through a network of complex vessels. However, repercussions can be disastrous when this symphony gets disrupted. Heart problems include conditions like coronary artery disease, heart failure, arrhythmias and congenital malformations. Their etiology is multifactorial involving genetic predispositions, behavioral factors and countless sophisticated biochemical pathways (2). Beyond the confines of medical practice, heart diseases contain a rich assortment of stories—chronicles of courage, sadness and hope. Every heartbeat affects those whose lives are touched by it and every diagnosis carries along its own path for each of them which are distinct and personal.
A major global health issue, cardiovascular disease, and cardiovascular disorders. Coronary artery disease (CAD), the most common, causes narrowing or blockage of the coronary arteries, leading to angina or myocardial infarction. Heart failure reduces oxygen delivery because the heart cannot pump blood properly. Mild exercise causes an abnormal heart rate that can impair circulation. Valvular heart disease damages the heart muscles and limits blood flow. Cardiomyopathy occurs when the heart muscle contracts or stiffens, reducing its ability to carry blood (3).
Poor diet, lack of physical activity, tobacco use, alcohol abuse and obesity are major risk factors. Heart disease prevention includes healthy eating, exercise, weight control, and smoking cessation. Treatment options range from medical to surgical, depending on the severity. Routine inspections detect and address them quickly (4). Knowing the risk factors and prioritizing cardiovascular health helps reduce the impact of cardiovascular disease.
Risk factors for cardiovascular disease include smoking and alcohol misuse. Coronary artery disease (5), hypertension, decreased oxygen saturation, and accelerated blood clotting are all consequences of smoking. Consuming alcohol raises the risk of hypertension, heart disease (6, 7), and cholesterol. When smoked and drunk at the same time, oxidative stress rises, the immune system is weakened, and blood arteries and cholesterol are damaged. Heart disease, particularly myocardial, cerebral, and cardiac insufficiency, is greatly increased by this lethal combination. It is vital to quit smoking, restrict alcohol intake, and maintain cardiovascular health since these habits add up to a lot of harm. Although beating addiction could be difficult, the rewards in terms of heart health are substantial.
Adaptive enhanced decision fusion is crucial for disease prediction, especially in cardiovascular health. Combining numerous models and adjusting to changing data patterns enhances early disease detection and prediction. The ABDF educates doctors on cardiac illnesses to help them choose the best treatments and improve patient outcomes. In the complex realm of cardiovascular diseases, its versatility allows quick risk assessment and appropriate intervention. ABDF is a cutting-edge ensemble learning approach that enhances cardiovascular health patient care and predictive analytics.
As data reveals, the cardiovascular problem percentage among people in India as diagnosed in the year 2020 is shown in Figure 1, using the breakdown by age group. In cardiovascular matters, most often, the older age group was seen having more frequent problems than the younger age group. The rate of cardiovascular disease found among the teenagers of the age group below 19 is about 2.98%, which is comparably lower compared to that of the young people of the age group 20–29, which registers about 5%. Investigators have been able to ascertain that the 45- to 59-year-old population group had an illness rate of cardiovascular problems of about 11.9%, while that of the 30- to 44-year-old group was about 6.28%. At a rate of 18.7%, the above-60-year-old succession group accounts for the highest prevalence of cardiovascular diseases. Given the existence of age disparities, policymakers should focus on the development of auxiliary policies, early detection, effective healthcare delivery, and educational campaigns that will help in the ongoing battle against the rising frequency of cardiovascular diseases among the aging population (8–12).
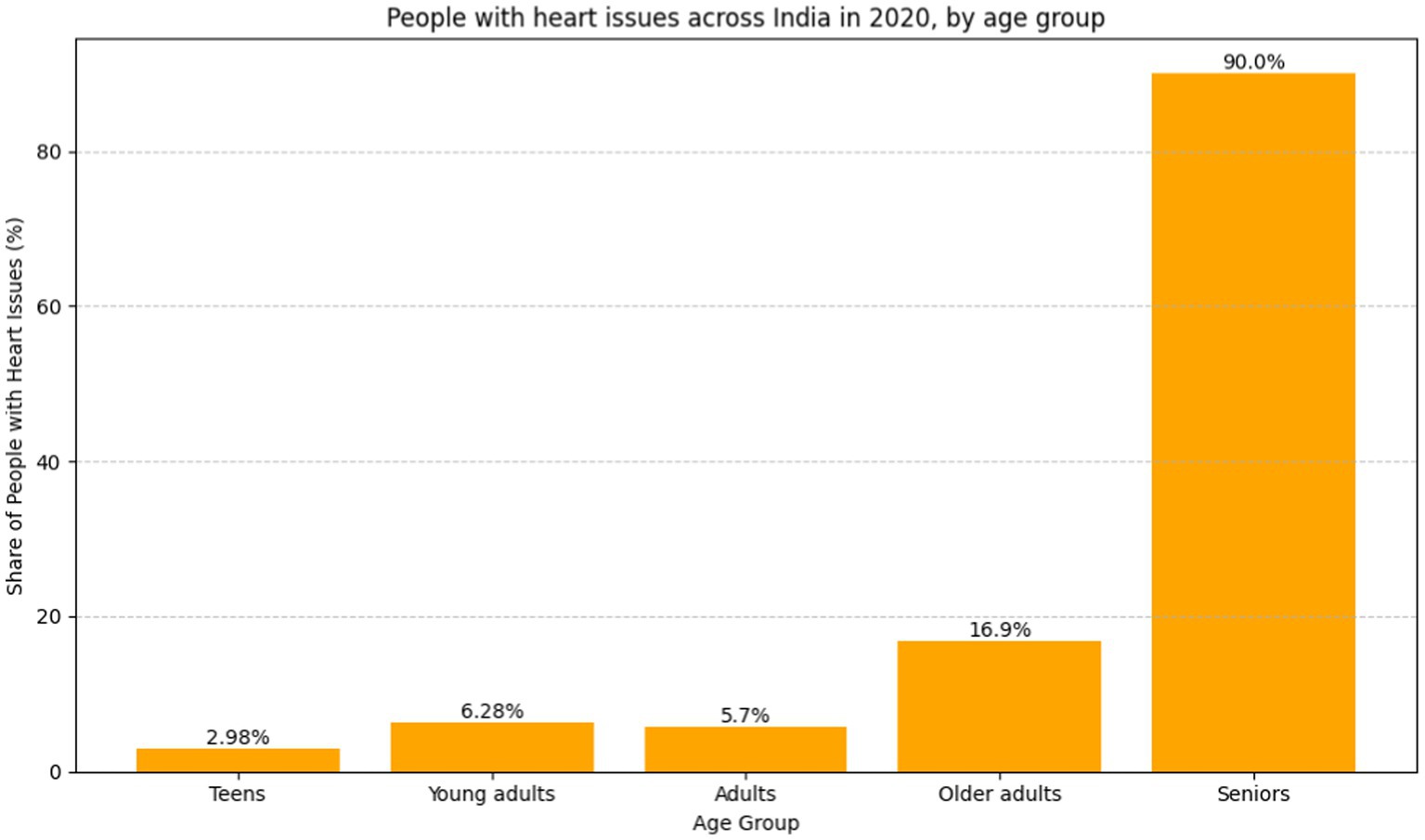
Figure 1. Bar graph people with heart issues across India in 2020, by age group (8) (https://www.statista.com).
2 Literature review
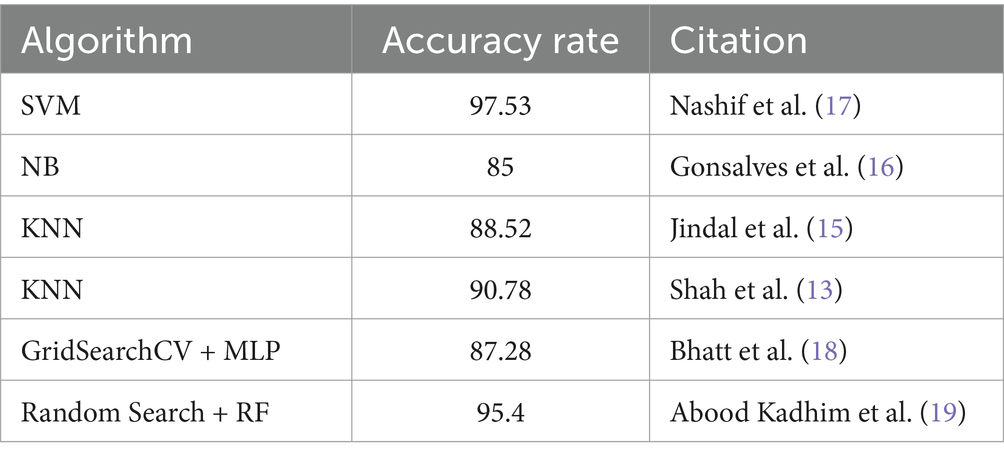
In 2020, Shah et al. (13) examine data mining and machine learning for heart disease prediction. The study stresses the need of precise and timely identification of heart disease, a top worldwide mortality. Using the enormous Cleveland database of UCI repository, 303 cases and 76 characteristics are rigorously condensed to 14 important elements. The study compares popular algorithms including Naïve Bayes, decision tree, K-nearest neighbor (KNN), and random forest for heart disease prediction. KNN was the most accurate algorithm, demonstrating predictive modeling potential. The finding agrees with earlier research that many algorithms are needed for complete findings. Future data mining approaches such time series analysis, clustering, association rules, support vector machines, and evolutionary algorithms are suggested to improve predicted accuracy. While insightful, the paper admits its limits and advocates for further research to improve early and accurate heart disease prediction algorithms.
In 2020, Katarya et al. (14) conducted a survey saying that heart disease is a global issue with rising treatment expenses, therefore early detection is essential. Alcohol, tobacco, and inactivity are essential heart disease indicators. The paper recommends using machine learning, particularly supervised methods, for healthcare decision-making and prediction to address this essential issue. Several algorithms, including as ANN, DT, RF, SVM, NB, and KNN, being investigated for heart disease prediction. The research summarizes these algorithms’ performance to reveal their efficacy. In conclusion, automated technologies to anticipate cardiac disease early on help healthcare professionals diagnose and empower patients to monitor their health. Feature selection is critical, and hybrid grid search and random search are suggested for optimization. Search algorithms for feature selection and machine learning will improve cardiac disease prediction, leading to better healthcare treatments, according to the report.
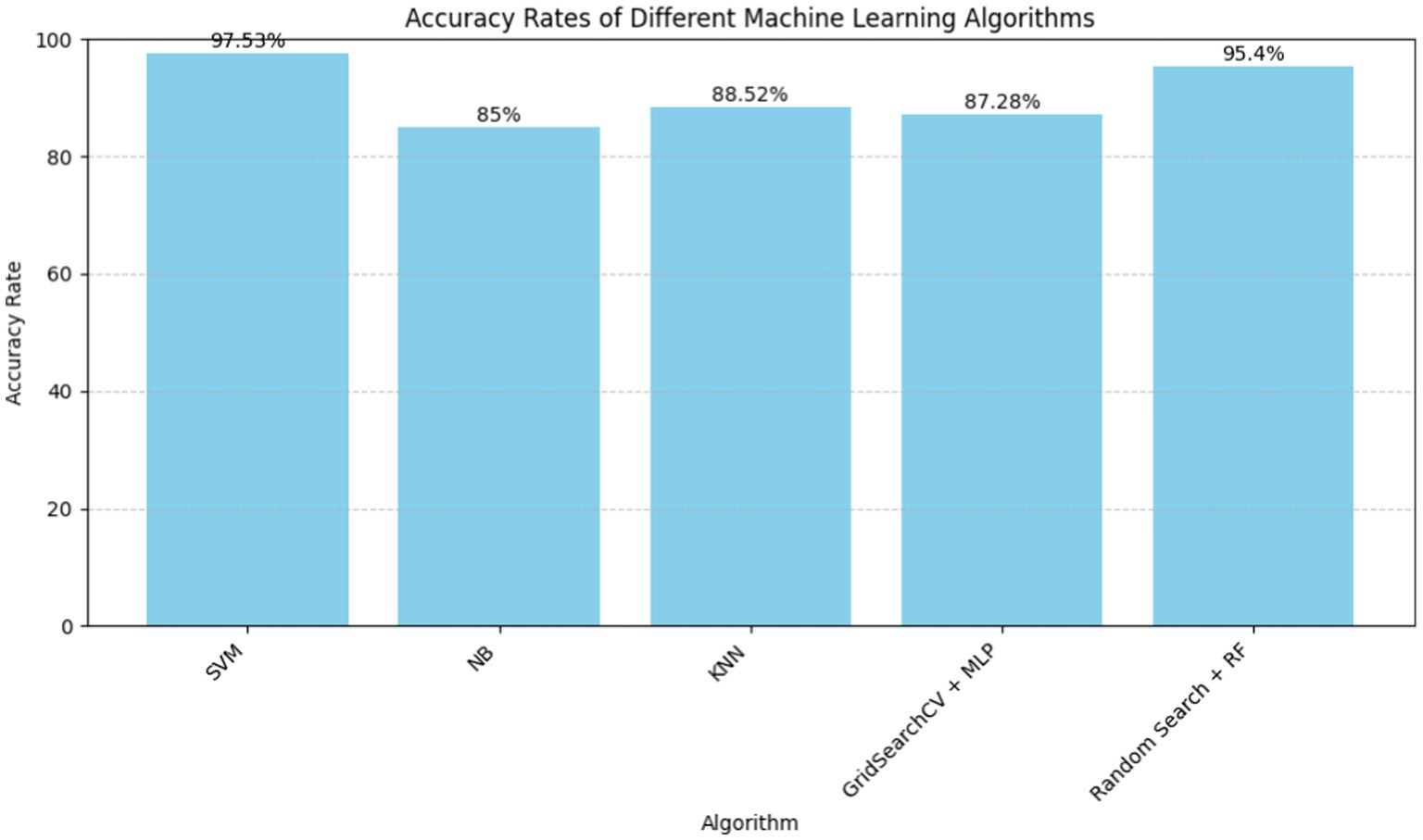
In 2021, Jindal et al. (15) highlights the increasing number of heart diseases and the need for prediction models. The declaration acknowledges the challenge of correct diagnosis and promotes machine learning techniques for accurate projections. Logistic regression and KNN are compared to naive Bayes in the research. The proposed heart disease prediction system reduces costs and improves medical care. The research also includes a Logistic Regression, Random Forest Classifier, and KNN cardiovascular disease detection model. The model’s accuracy is 87.5%, up from 85% for previous models. The literature shows that the KNN method outperforms other algorithms with an accuracy rate of 88.52%. The article claims that machine learning can predict cardiac issues more accurately than conventional techniques, improving patient care and lowering costs.
In 2019, Gonsalves et al. (16) uses Machine Learning (ML) approaches such as Naïve Bayes (NB), Support Vector Machine (SVM), and Decision Tree (DT) to predict Coronary Heart Disease (CHD). Coronary heart disease (CHD) is a major cause of death around the world, highlighting the need of early detection. The work uses historical medical data and three supervised learning approaches to discover CHD data correlations to improve prediction precision. The summary of the literature acknowledges the complexity of medical data and CHD prediction linkages, stressing the challenges of existing techniques. The study’s focus on NB, SVM, and DT matches existing research techniques, highlighting the availability of disease prediction machine learning algorithms. Early screening and identification are crucial for patient well-being, resource allocation, and preventative interventions, according to the research. The discussion of ML model performance, including accuracy, sensitivity, specificity, and other characteristics, sheds light on Naive Bayes, Support Vector Machines, and Decision Trees. Despite not meeting threshold rates, the Naive Bayes (NB) classifier looks to be the best option for the dataset. According to the literature review, unsupervised learning and data imbalance should be studied in the future. This will enhance prediction algorithms and may lead to mobile CHD diagnosis apps.
In 2018, Nashif et al. (17), addresses cardiovascular problems across the globe and highlights the necessity to detect and monitor them early. The cloud-based heart disease prediction system uses powerful machine learning. Interestingly, the Support Vector Machine (SVM) method has 97.53% accuracy. Real-time patient monitoring using Arduino for data collection is presented in the study, focusing on remote healthcare. Comparative evaluations show SVM outperforms other models. The abstract concludes with potential issues including photoplethysmography-based blood pressure monitoring. The literature analysis highlights cloud-based prediction and real-time patient monitoring as a solution to PPG-based system constraints.
In 2023, Bhatt et al. (18) used Machine learning to create a cardiovascular disease prediction model. The study employed 70,000 Kaggle-downloaded real-world samples. Huang initialization improves k-modes clustering classification accuracy. GridSearchCV optimizes random forest, decision tree, multilayer perceptron, and XGBoost models. With 86.37 to 87.28% accuracy, the models are great. Multiple layer perceptron outperforms other models. The study adjusts age, blood pressure, and gender to account for heart disease progression. Despite promising outcomes, the study had limitations. These include employing a single dataset, only considering particular clinical and demographic features, and not comparing results to other test datasets. More research is needed to overcome these restrictions, compare clustering algorithms, test the model on new data, and improves interpretability. Machine learning—particularly clustering algorithms—can effectively predict cardiac illness and guide focused treatment and diagnostic measures.
In 2023, Abood Kadhim et al. (19) examines the growing use of artificial intelligence—specifically machine learning—in cardiac disease diagnosis and prediction. Support vector machines, random forests, and logistic regression are tested on Cleveland Clinic data. Research on artificial intelligence in cardiac care is also examined. The study found that support vector machines are the most accurate heart disease diagnosis tools at 96%. It also presents a 95.4% accurate random forest model for cardiac attacks. The findings demonstrate the importance of AI in healthcare decision-making and early cardiac problem intervention.
Recent researches have stressed the need for global cardiovascular disease diagnosis and identification. Several papers in 2020 and 2021 studied Naïve Bayes, decision tree, K-nearest neighbor (KNN), and random forest algorithms using data mining and machine learning methods. The primary findings are that K-Nearest Neighbors (KNN) may predict heart disease, that supervised machine learning may make healthcare decisions, and that logistic regression, KNN, and naive Bayes are comparable. These findings show the usefulness of predictive models in addressing the rising number of cardiac ailments, leading to healthcare technology advances for early identification and better patient treatment (Figure 2).
2.1 Motivation
Due to the global the amount of cardiovascular diseases, data mining and machine learnnng research on heart disease prediction is escalating. Heart disease is the most common cause of mortality worldwide. To reduce mortality rates, these medical conditions must be accurately and quickly detected. Researchers are studying machine learning to improve diagnostic skills since conventional methods frequently make inaccurate predictions. These studies aim to enhance early diagnosis and treatment. Medical data is complex and risk variables change, making machine learning an intriguing method for finding meaningful patterns and improving heart disease prediction.
2.2 Research gap
Despite the wealth of knowledge in machine learning approaches to heart disease prediction, additional research is needed. Shah et al. (13), Katarya et al. (14), Jindal et al. (15), Gonsalves et al. (16), Nashif et al. (17), Bhatt et al. (18), and Abood Kadhim et al. (19) all emphasize the importance of accurate and early heart disease detection. These researches have examined how K-nearest neighbor (KNN), Support Vector Machine (SVM), Random Forest, and logistic regression can increase predicted accuracy. These attempts are intriguing, but they also highlight limits like dataset dependence, feature selection optimization issues, and the need for more unsupervised learning research. Address data imbalance and real-time patient monitoring equipment concerns. Thus, even though machine learning could change cardiac illness prediction, more research is needed to improve algorithms, overcome data constraints, and improve cardiovascular health care outcomes. The current study lacks detailed algorithm assessments, leaving the best technique for exact predictions unknown. There is also insufficient research into using advanced data mining methods like time series analysis and evolutionary algorithms to better forecast heart illness. Overcome these gaps to increase prediction model robustness and precision in this critical healthcare sector.
The research’s scope is to create trustworthy and effective cardiovascular disease diagnostic tools. Our goal is to reduce heart disease deaths and improve heart disease predictions using powerful machine learning.
• SMOTE, IQR outlier identification, and MICE are used to solve data difficulties in this work. We also introduce Hybrid GOL2-2 T, a hybrid feature selection approach.
• It uses L2 regularization and the Grasshopper Optimization Algorithm.
• A babysitter algorithm and Adaptive Boosted Decision Fusion (ABDF) ensemble learning increase predictive modeling accuracy.
• Our model will be assessed by accuracy, recall, and AUC score.
The main goal of this project is to develop reliable diagnostic tools for early diagnosis and treatment of cardiovascular diseases. This can help doctors improve patient outcomes and reduce illness.
In the subsequent sections, Section 2 provides a comprehensive literature analysis of the corpus of recent publications. The suggested methodology is then presented in Section 3. Section 4 offers a thorough summary of the results and the discussion that follows. In Section 5, prospective avenues for further research are explored and the article is summarized with a conclusion.
3 Proposed methodology
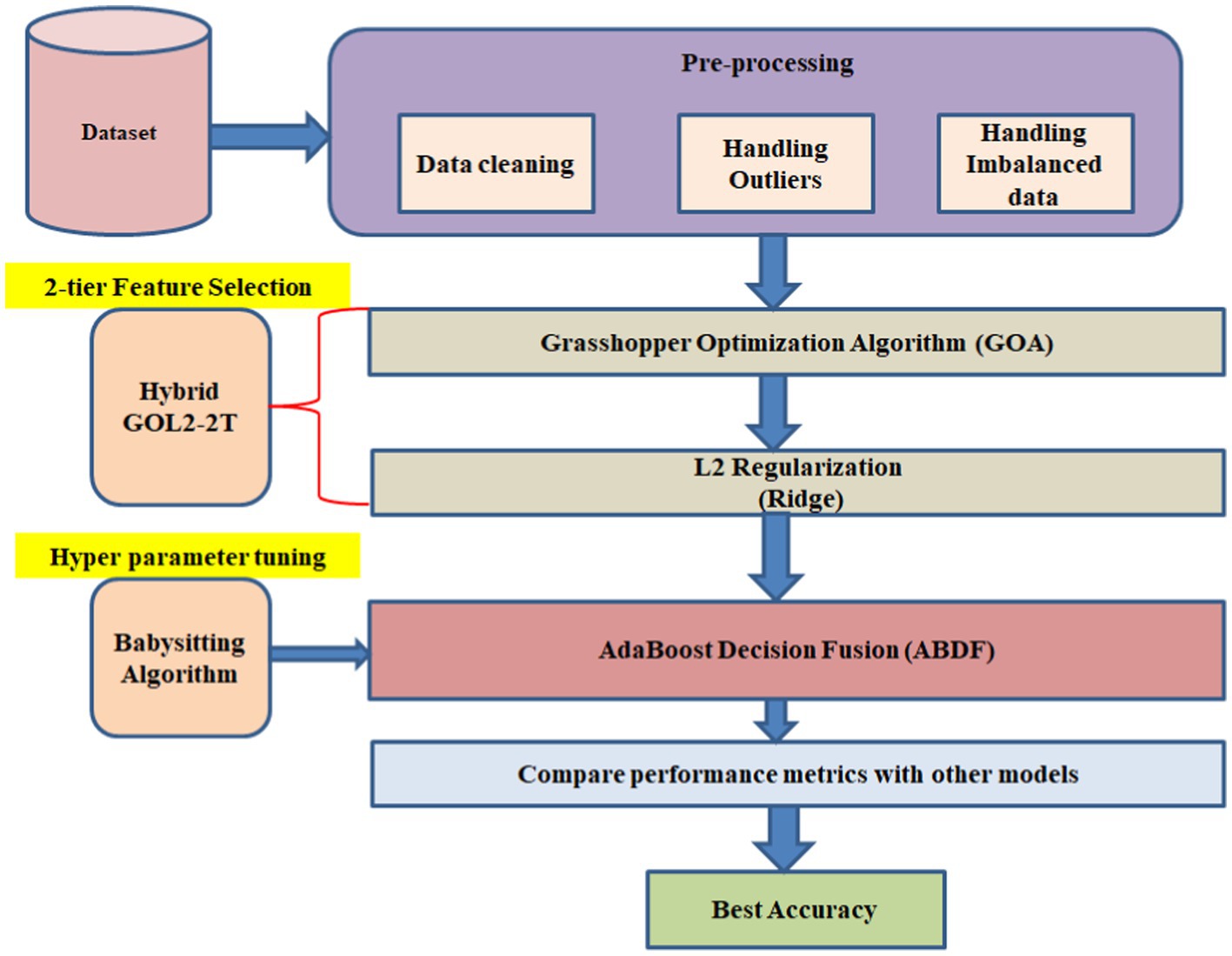
For the two-tier Feature Selection Hybrid GOL2-2 T, starting from the data pre-processing stage among the partitions, 70% of the data partition is allotted for the training set and 30% for the testing set. An objective under this category makes it easy to evaluate the performance of the models in question based on it deeply. The second to the last step is the missing data estimate, which makes use of the Multivariate Imputation by Chained Equations (MICE) approach. This, in return, ensures the completeness of information from one or many variables. In this case, the following techniques were corrected with a deficiency of training the model and have high interoperability with the techniques of machine learning; Imputation, Data scaling, and Label encoding. Inside the method, it has the Inter Quartile Range (IQR) to identify and deal with an outlier in an effort to enhance the resilience of the model through a reduction in influence that emanates from abnormal data points. The major maxim is SMOTE, which a synthetic minority is over-sampling technique aimed at the problem of class imbalance. The technique established a fair representation through the development of synthetic minorities, toward the reduction of biases that may associate with the general over-representation of the dominant class.
2-tier Feature Selection is based on the L2 Regularization (Ridge) (20) along with the Grasshopper Optimization (GOA) method; therefore, the proposed Hybrid GOL2-2 T model is going to form a 2-level model for Feature Selection. It also employs ABDF hyperparameters, which have been babysitting algorithm to be fine-tuned after proper pre-processing of the dataset. Therefore, AdaBoost Decision Fusion (ABDF) maximizes the predictive modeling tasks’ accuracies by pulling the performance measures out with respect to other models for comparison (Figure 3).
3.1 Data collection
The 1988 heart disease dataset (21) is an excellent resource for studying and forecasting cardiovascular disease prevalence. Age, gender, type of chest pain, blood pressure, cholesterol levels, and the presence of numerous cardiovascular diseases are among the 14 important factors. There is a large variety of ages represented in the dataset, with the majority falling between 40 and 60. Of those, 207 are male and 96 are female. With a value of 1 for males and 0 for females, the variable “sex” is included in the data for each issue as an essential health indicator. While we display resting blood pressure (trestbps) and serum cholesterol levels (chol) as whole numbers, we categorize chest discomfort as 1, 2, 3, or 0. Exang, exercise-induced angina, exercise-induced ST depression compared to rest, the slope of the peak exercise ST segment, the number of major vessels colored by fluoroscopy, and thalassemia type are some other factors that improve the dataset. In order to promote a thorough study of cardiovascular health and facilitate the development of reliable prediction systems, the “target” property shows whether heart disease is present (1) or absent (0) (Figure 4).
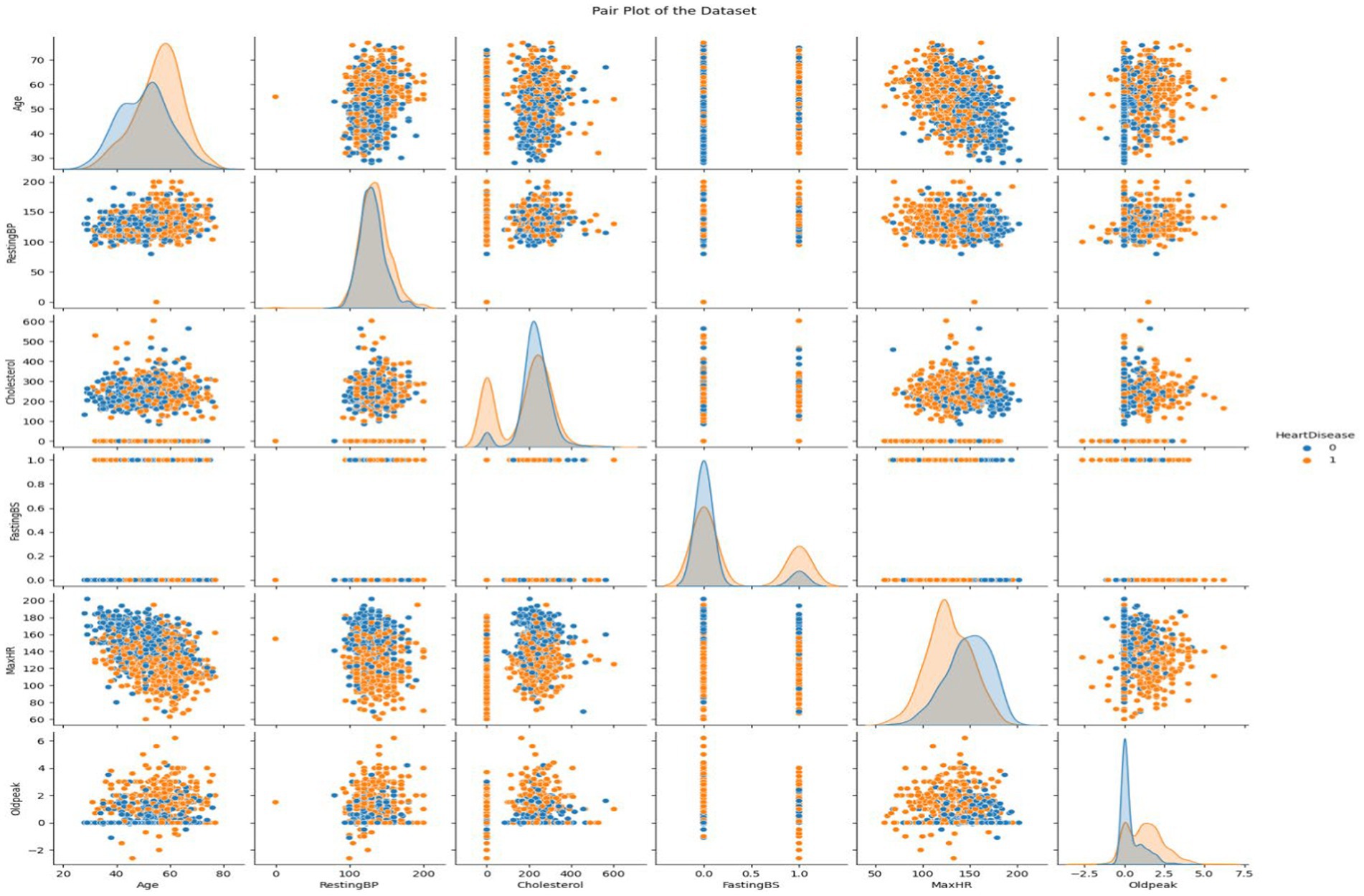
3.1.1 Visualizing the attributes of heart disease dataset using pair plot
This dataset encompasses six numerical variables: RestingBP, Cholesterol, FastingBS, MaxHR, Oldpeak. Two variables are distributed in each grid subplot. Variable correlations in the Heart Disease dataset are shown in the pair plot. The correlation between two variables is displayed in every matrix scatterplot. The level of heart disease dictates the color of the dots. Early detection of data patterns and trends can be aided by this. It can reveal whether there are commonalities between those who have cardiac disease and those who do not.
Histograms show variable distribution, while scatter plots show the connection between paired variables. In the upper left subplot, RestingBP distribution is presented. The y-axis shows data point frequency, and the x-axis shows RestingBP levels. The bottom right subplot displays the association between MaxHR and Oldpeak, an off-diagonal plot. This subplot shows Oldpeak on the y-axis and MaxHR on the x-axis. Examining the pair plot can reveal patterns and linkages, such as cholesterol-resting blood pressure correlations. This graphical tool simplifies dataset analysis, especially for outliers and linear correlations. We consider non-diagonal scatter plots while examining linear relationships. Straight lines between scatter plot dots indicate the variables’ direction and strength. Outliers are scatter plot data points far from the main cluster. If we want to use machine learning to forecast cardiac disease from patient data, we need to understand these tendencies. It might be necessary to make adjustments and do further research on visual representations in order to have a better understanding of the dataset (Figure 5).
3.2 Pre-processing
3.2.1 Data cleaning with MICE
Data pretreatment requirements include cleaning the data to ensure dataset correctness and completeness and that it is analysis or model training ready. Absent data often hurts machine learning models. MICE (22) handle missing data thoroughly and statistically through Multiple Imputation by Chained Equations shown in equation (1). In an iterative process, MICE calculate conditional distributions for all variables with missing data using observed data and other variable imputations. As iterations continue until convergence, the process creates various entire datasets. To accommodate for missing value uncertainty, each dataset has its own imputations. Multiple Imputation by Chained Equations (MICE) works well for non-random missing data patterns in real-world datasets where observed values may affect missing. It evaluates variables and predicts data distributions. The MICE technique provides imputations, updates models, and combines findings to provide credible imputed datasets. Finalized datasets can be used to train machine-learning models. MICE address missing data to improve model performance and assure unbiased parameter estimates.
• shows the value that has been ascribed to the absent item.
• f: The missing value is estimated by the function. The data type of variable j might affect this function.
• : With the exception of variable j, all observed values of the variables are represented by the vector in the ith observation.
• Error term
The observed values of all the variables in this context, with the exception of variable j in observation i, are stored in the vector . By using these observed values, the function f is used to estimate the missing value. The assumed value’s error word denotes any inexplicable volatility or unpredictability.
3.2.2 Scaling with label encoder
There are two essential methods for preparing machine learning data: label encoding and scaling. To transform categorical data into a numerical form, Label Encoding assigns unique integer labels to each category. One method for giving numerical values to categorical variables is Label Encoding (23). With Label Encoding, “Male” and “Female” would be represented as 0 and 1, respectively, in a “Gender” column. For algorithms that can only take numerical input, this simplifies the usage of categorical variables. On the flip side, numerical features can be scaled to be uniform in size so that no one characteristic can have an outsized impact due to size disparities. Model convergence and performance are both enhanced by methods Standard Scaling, [shown in equation (2)] which ensure that all features contribute equally. A typical preprocessing step involves converting categorical characteristics using Label Encoding and then scaling numerical features to make their magnitudes consistent. Label Encoding and Scaling, when used together; make it easy to get datasets ready to be used in machine learning algorithms.
• The initial feature value was X.
• The feature values mean is represented by μ.
• The feature values’ standard deviation is represented by σ.
3.2.3 Handling outliers with IQR
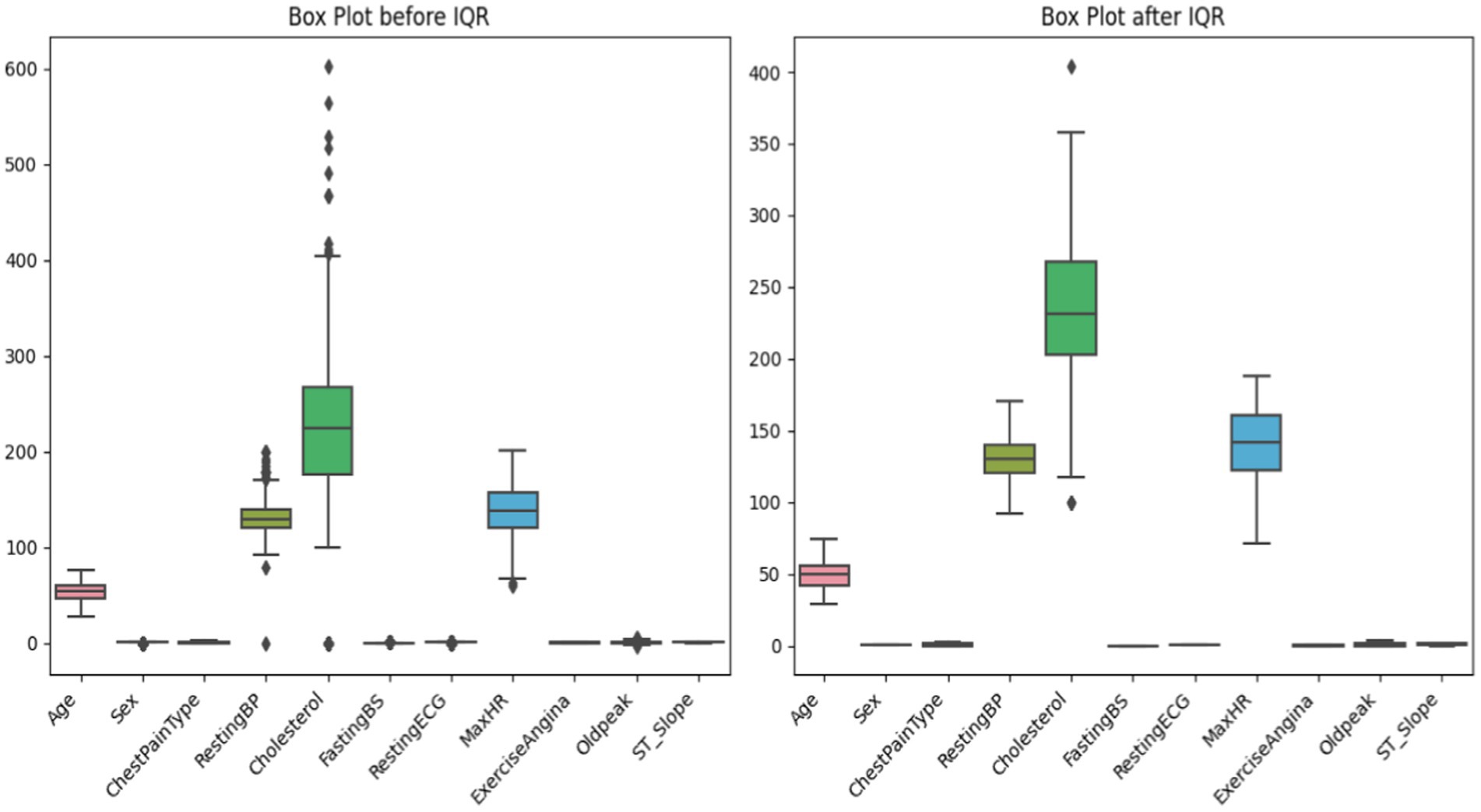
Careful data preparation, including outlier removal, improves machine learning model durability. Interquartile Range (IQR) is a prominent method for finding and treating dataset outliers. Interquartile range (IQR) is the difference between a distribution’s third and first quartiles, or 75th and 25th percentiles [shown in equation (3)]. Abnormal data points fall below or above the lower and higher limits (Q1–1.5 * IQR and Q3 + 1.5 * IQR, respectively) [shown in equations (4, 5)]. Outliers might hurt the model’s performance, but the IQR-based technique would find and fix them. To minimize outliers’ impact on learning, alter them. This reduces model sensitivity to unexpected data sets. This is crucial for algorithms that respond fast to data distribution changes (Table 1).
The initial stage in IQR-based outlier treatment is splitting the sample into quartiles and determining the IQR (24). Outliers can be deleted or altered by comparing them against boundaries. This technique emphasizes creating more extensive and reliable datasets to improve ML model generalizability and prediction accuracy. The IQR outlier control approach must be used to prepare data for future machine learning experiments to ensure reliability and efficiency (Figure 6).
3.2.4 Handling imbalanced dataset with SMOTE
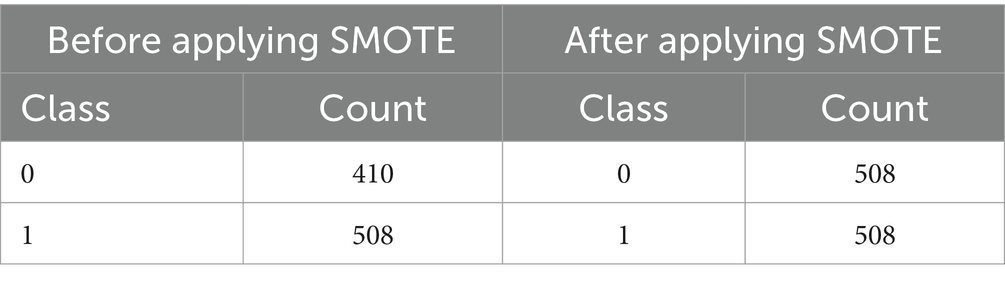
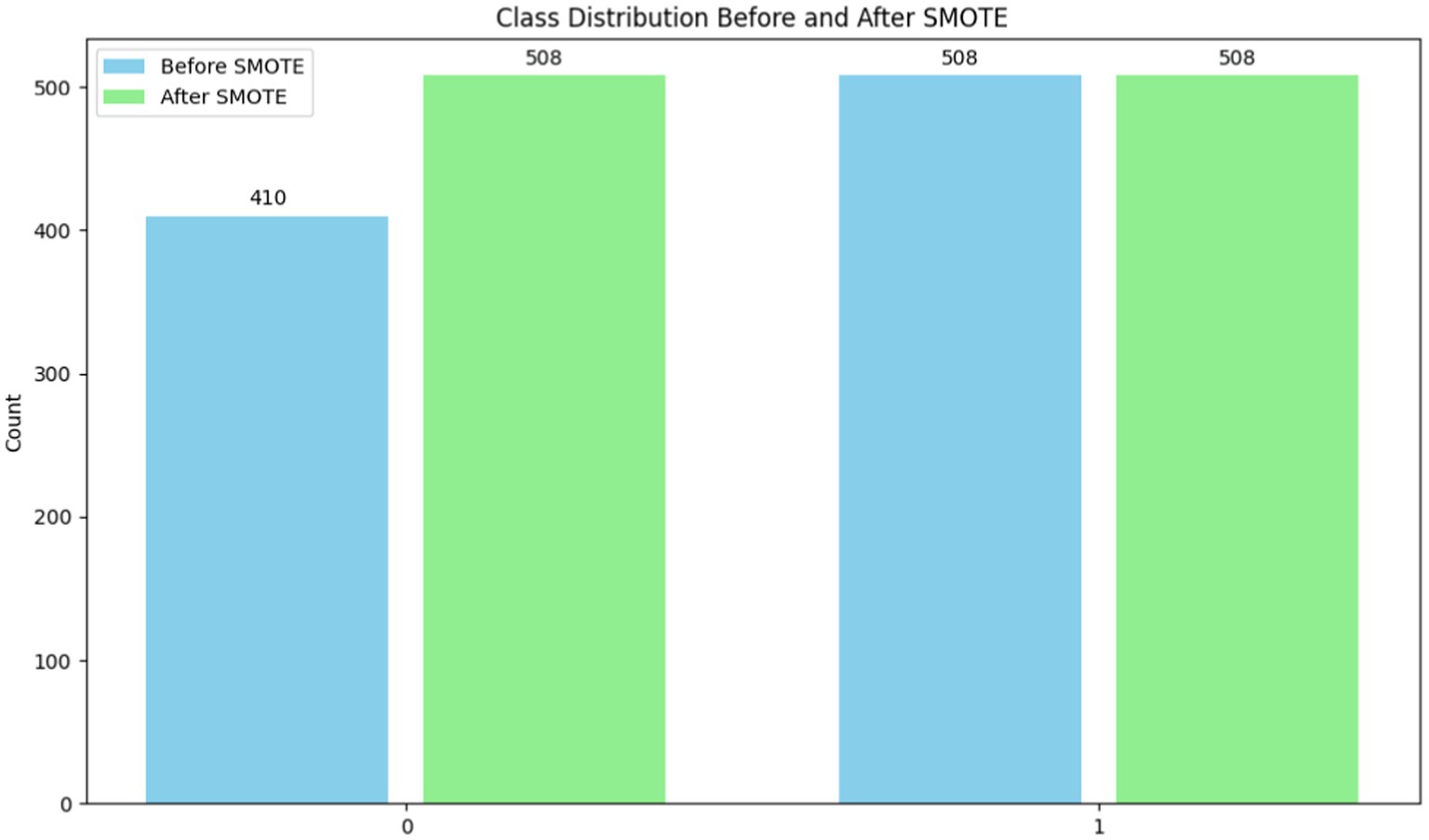
To ensure that machine learning algorithms are not biased toward the dominant class and hence reduce prediction accuracy, imbalanced datasets must be handled. In order to rectify class imbalance, particularly in cases when minority occurrences are underrepresented, this system applies the Synthetic Minority Over-sampling Technique (SMOTE) (25) [shown in equation (6)]. Class distribution has an imbalance with 508 class 1 instances and 410 class 0 instances (shown in Table 2 and Figure 7). It would indicate that the 0.8071 imbalance ratio is less than the 1 - imbalance_threshold threshold. SMOTE manipulates the underrepresented class’s dataset presence by creating false instances of it. This is accomplished by building artificial instances along line segments that connect instances of minority classes. With a more evenly distributed dataset, the model may learn from more examples and, perhaps, make better predictions with new data.
Model prediction is improved with SMOTE (26) because it decreases class imbalance. When data from minority groups is limited, this strategy really shines in terms of model performance. To aid in the management of unbalanced datasets, SMOTE encourages correct and equitable predictions across all classes.
3.2.5 Feature selection using hybrid GOL2-2 T
A new hybrid feature selection approach called the Hybrid GOL2-2 T, in which L2 regularization is fused with the Grasshopper Optimization Algorithm (GOA) (27), is discussed. This solution of the metaheuristic attracts a promising subset of the feature set through the application of an objective function and global search. We then applied L2 regularization to the selected feature set. Majorly, the objective of L2 regularization is to penalize too many coefficients, promote sparsity, and preserve only the most useful features. Hybrid GOL-2 T combining fine tuning powers from L2 regularizations with the muscular strength of GOA combined gives a dependable feature selection technique. In this respect, models that provide predictive classification via two-level approaches should have higher classification accuracy and dependability since they help in selecting the most relevant characteristics and reducing overfitting. As has been correctly pointed out, for these reasons, this approach has gained significant acceptance and has become an indispensable tool for many machine learning applications, like regression and classification tasks.
3.2.6 Grasshopper optimization algorithm
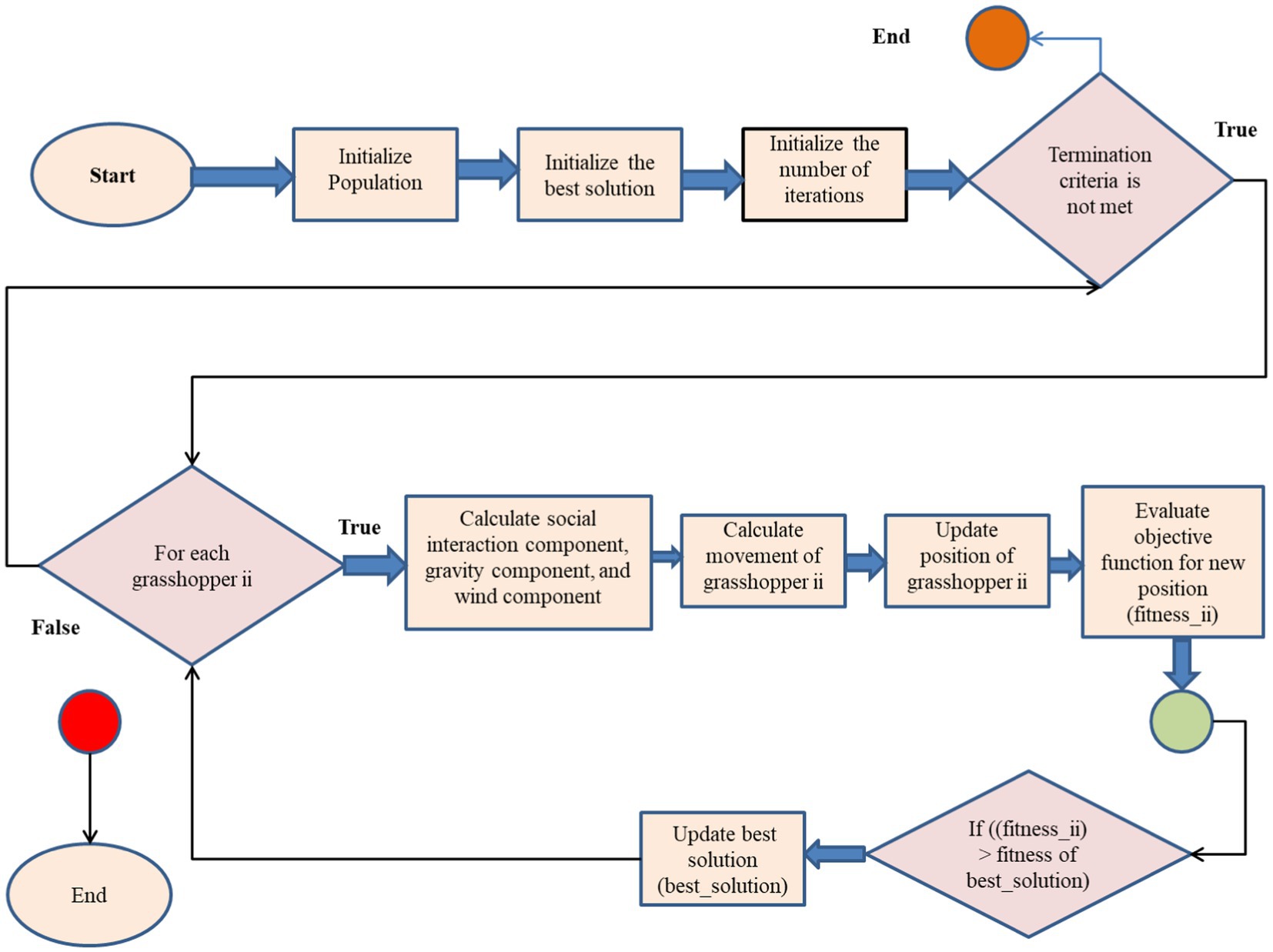
Developed in 2017 by Saremi et al. (32), the Grasshopper Optimization Technique (GOA) is a metaheuristic optimization technique inspired by nature. The idea originated from the way grasshoppers behaved in unison. GOA has been used to solve a variety of optimization problems, including feature selection in the context of machine learning. Here is a brief description of how GOA works shown in Algorithm 1, complete with formulas and the algorithm itself:
Algorithm 1: Grasshopper Optimization Algorithm (GOA)
Initialize population of grasshoppers (solutions)
Initialize best solution (best_solution)
Initialize number of iterations (iterations)
While (termination criterion is not met)
For each grasshopper ii
Calculate social interaction component Sii shown in equation (7)
Calculate gravity component Gii shown in equation (8)
Calculate wind component Aii shown in equation (9)
Calculate movement of grasshopper ii (xii)
Update position of grasshopper ii (xii)
Evaluate objective function for new position (fitnessii)
If ((fitnessii) > fitness of best_solution)
Update best solution (best_solution)
End If
End For
Update number of iterations (iterations)
End While
Return best solution
Where,
• c is a decreasing coefficient that balances the processes of exploration and exploitation.
• g is a constant that determines the strength of the gravity component is the center of the search space.
• U is a constant that determines the strength of the wind component.
• is the position of the best solution found so far.
• N is the number of grasshoppers.
• and are the positions of the grasshoppers.
The algorithm generates grasshoppers, each representing a possible solution. The first grasshopper in the population gets the best answer. The algorithm then loops through each grasshopper in the population. The application calculates grasshopper social interaction, gravity, and wind components. These components steer the grasshopper toward the best alternative.
The components calculated in the previous stage are used to modify the grasshopper’s movement. The objective function measures grasshopper positioning and solution efficacy. A new site becomes the ideal option if it outperforms the old one. After reaching grasshopper population termination criteria, the technique continues iteratively. A maximum number of iterations, a minimum fitness value, or any other suitable stopping condition may be used for the job. After optimization, the technique returns the ideal answer (Figure 8).
3.2.7 L2 regularization
L2, sometimes called ridge regression (28), is a machine learning technique used to reduce a model’s complexity by adding a penalty term to the loss function. The penalty term is directly correlated with the square of the magnitudes of the coefficients, encouraging the model to have smaller coefficients and reducing the likelihood of overfitting shown in Algorithm 2.
The L2 regularization term is added to the loss function as shown in equation (10).
Where:
MSE is the mean squared error between the predicted and actual values shown in equation (11).
alpha is the regularization parameter (a hyperparameter).
Coefficient is the coefficient of the feature in the model.
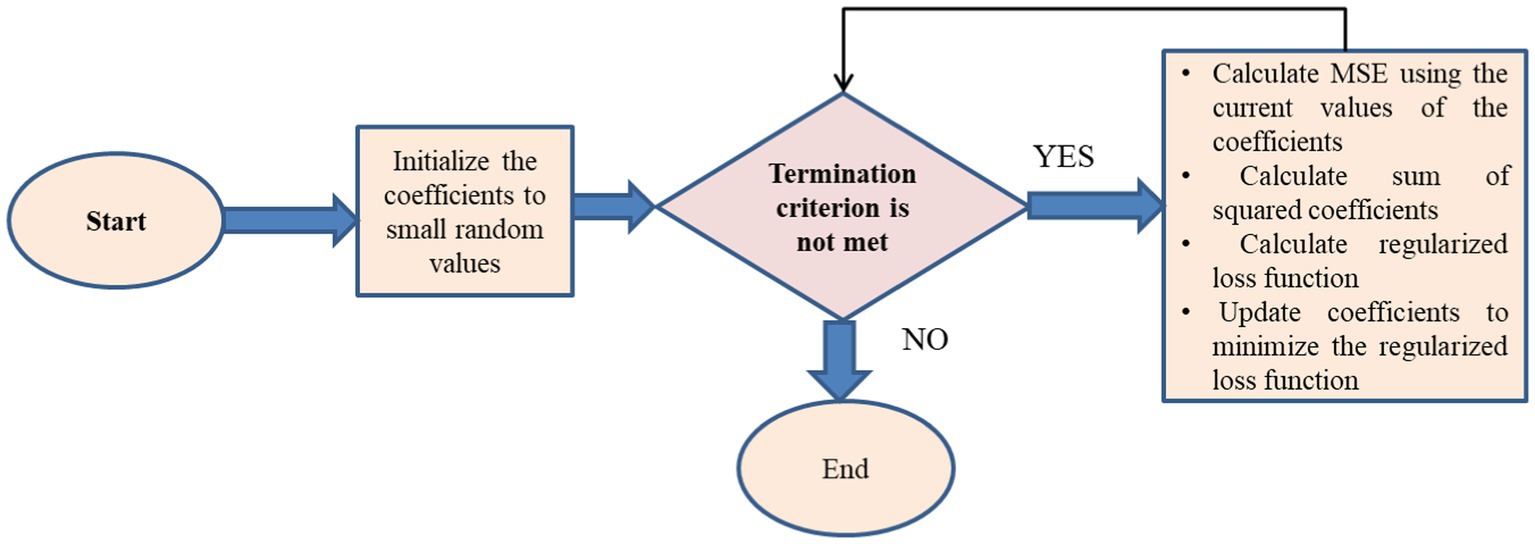
The algorithm for L2 regularization can be described as follows:
Algorithm 2: L2 regularization
Initialize coefficients to small random values
While (termination criterion is not met)
Calculate MSE using the current values of the coefficients by using equation (11)
Calculate sum of squared coefficients by using equation (12)
Calculate regularized loss function as the sum of the MSE and the regularization term (alpha * sum of squared coefficients)
Update coefficients to minimize the regularized loss function
End While
Return the optimized coefficients
Where,
• is the number of coefficients.
• as the data point’s observed value ii
• as the anticipated value for data point ii.
The L2 regularization approach may be used to a wide range of models due to its computational efficiency. To achieve the optimal balance between bias and variance, the regularization hyperparameter alpha has to be changed. Features that are more effective at lowering the Mean Squared Error (MSE) are chosen when L2 regularization reduces the size of the model’s coefficients. L2 regularization may be used as a feature selection method by selecting only those features in the model that have coefficients greater than zero (Figure 9).
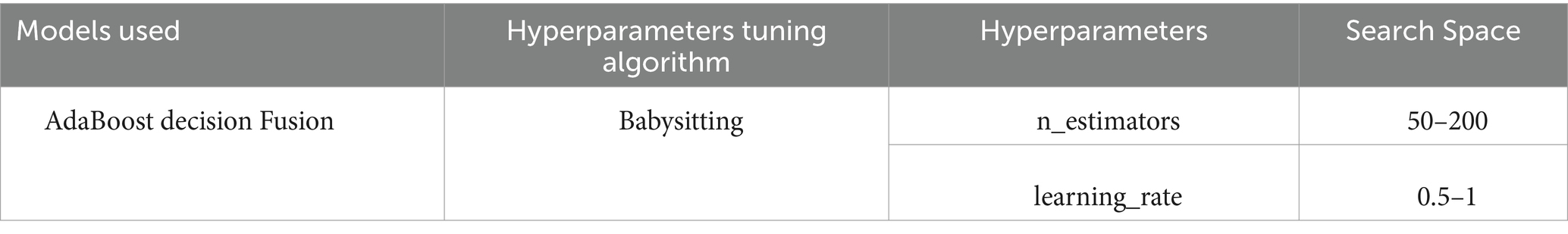
3.3 Hyperparameter tuning using babysitting algorithm
The babysitting Algorithm (BA) (29) in AdaBoost (30) decision fusion manually evaluates the model’s performance after iteratively modifying the hyperparameters. Setting hyperparameters, constructing a table, separating the dataset into training, validation, and testing sets, and progressively experimenting with different combinations are the steps. For each combination, an AdaBoost classifier is trained on the training set and assessed on the validation set using a performance metric. The hyperparameter table is updated when the trial number, hyperparameters, and performance measure change. Select the hyperparameters with the best validation set outcomes after all trials. The training and validation sets are utilized to train a new AdaBoost classifier using the optimum hyperparameters. For an impartial evaluation, the finished model is tested on the testing set shown in Algorithm 3.
Algorithm 3: Hyperparameter Tuning Babysitting on AdaBoost Decision Fusion
// Initialize hyperparameters and performance metric
InitializeHyperparameters()
// Initialize the hyperparameter table
InitializeHyperparameterTable()
// Main loop for hyperparameter tuning
while (stopping criterion not met) do
// Iterate through hyperparameter combinations
for each hyperparameter combination do
// Train AdaBoost classifier with current hyperparameters
model = TrainAdaBoostClassifier(current_hyperparameters)
// Evaluate the model's performance on the validation set
performance_metric = EvaluateModelPerformance(model, validation_set)
// Update hyperparameter table with current hyperparameters and performance metric
UpdateHyperparameterTable(current_hyperparameters, performance_metric)
end for
// Select best hyperparameters based on the highest performance metric
best_hyperparameters = SelectBestHyperparameters()
// Train AdaBoost classifier with the best hyperparameters on the combined training and validation sets
best_model = TrainAdaBoostClassifier(best_hyperparameters, combined_training_validation_set)
// Evaluate the final model on the testing set
final_performance_metric = EvaluateModelPerformance(best_model, testing_set)
// Update stopping criterion based on convergence or maximum iterations
UpdateStoppingCriterion()
end while
3.4 Model building for heart failure prediction
3.4.1 Ensemble technique with adaptive boosted decision fusion
“Adaptive Boosted Decision Fusion (31) is an advanced ensemble learning algorithm that effectively combines the principles of Adaptive Boosting (AdaBoost) and Decision Fusion.” To prioritize instances that are harder to classify, this innovative approach has the algorithm adaptively changing the weights [shown in equation (13)] given to less effective learners. When combined with decision fusion, ABDF sequential training method for weak models allows for the efficient integration of results from many decision-makers [shown in equations (14–18)]. The ultimate result is a very accurate and reliable prediction model that is both adaptable and resilient. One way to make the ensemble better is via adaptive boosted decision fusion, which uses iterative refinement and smartly gives different learners different weights depending on how well they do. When it’s critical to combine multiple decision-making viewpoints to get superior predicted outcomes, this method shines.
Input:
Training dataset:
Where the feature is vector and is the corresponding label.
Number of weak learners: UT
Initialization:
2. Initialize an empty ensemble of weak learners.
3. Train a weak learner using the current instance weights.
where ꟾ(.) is the indicator function.
iv Add the weak learner to the ensemble with weight .
Output:
Predictions:
This method combines the best features of AdaBoost and Decision Fusion in a way that strengthens the ensemble (26), making it better at handling misclassifications and making accurate predictions. A long-lasting ensemble model that frequently outperforms individual models is produced by ABDF iterative method of correcting errors of weak models [shown in equation (19)]. Classification problems, such as the prediction [shown in equation (20)] of cardiac illness, frequently use ABDF. It finds usage in a variety of domains due to its flexibility in accommodating varied poor learners (Tables 3, 4).
4 Result and discussion
4.1 Performance assessments
4.1.1 Feature selection outcome using GOL2-2 T
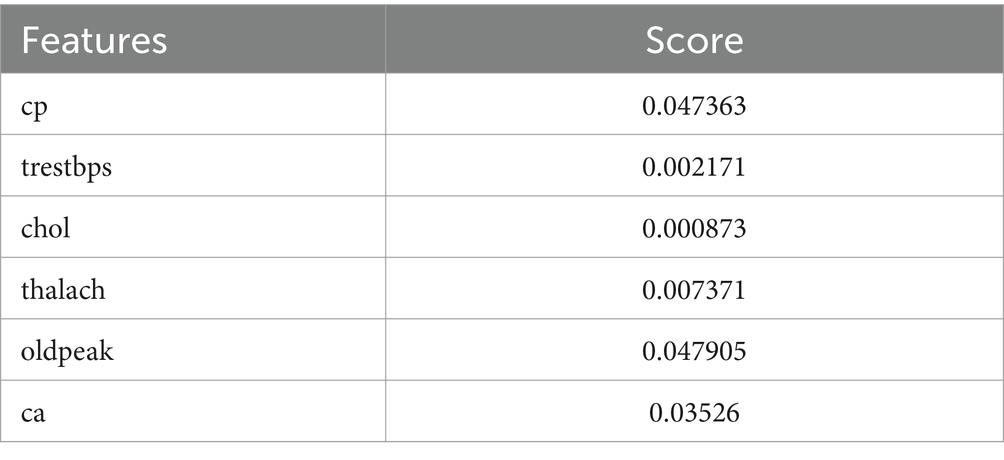
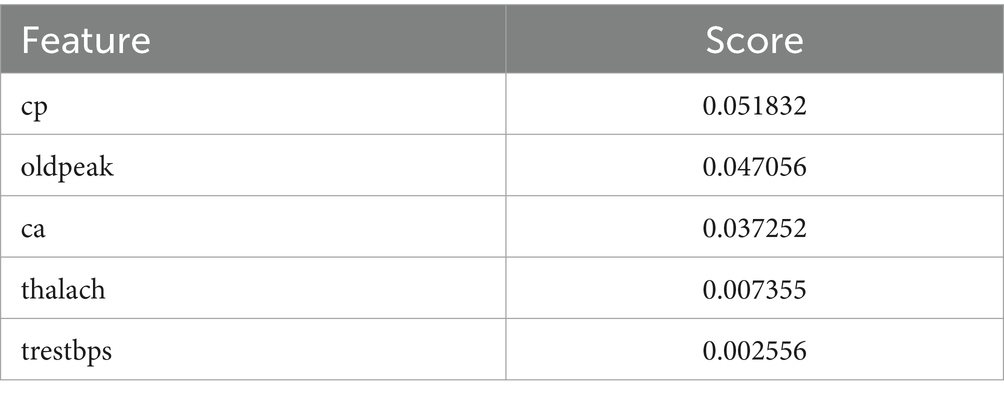
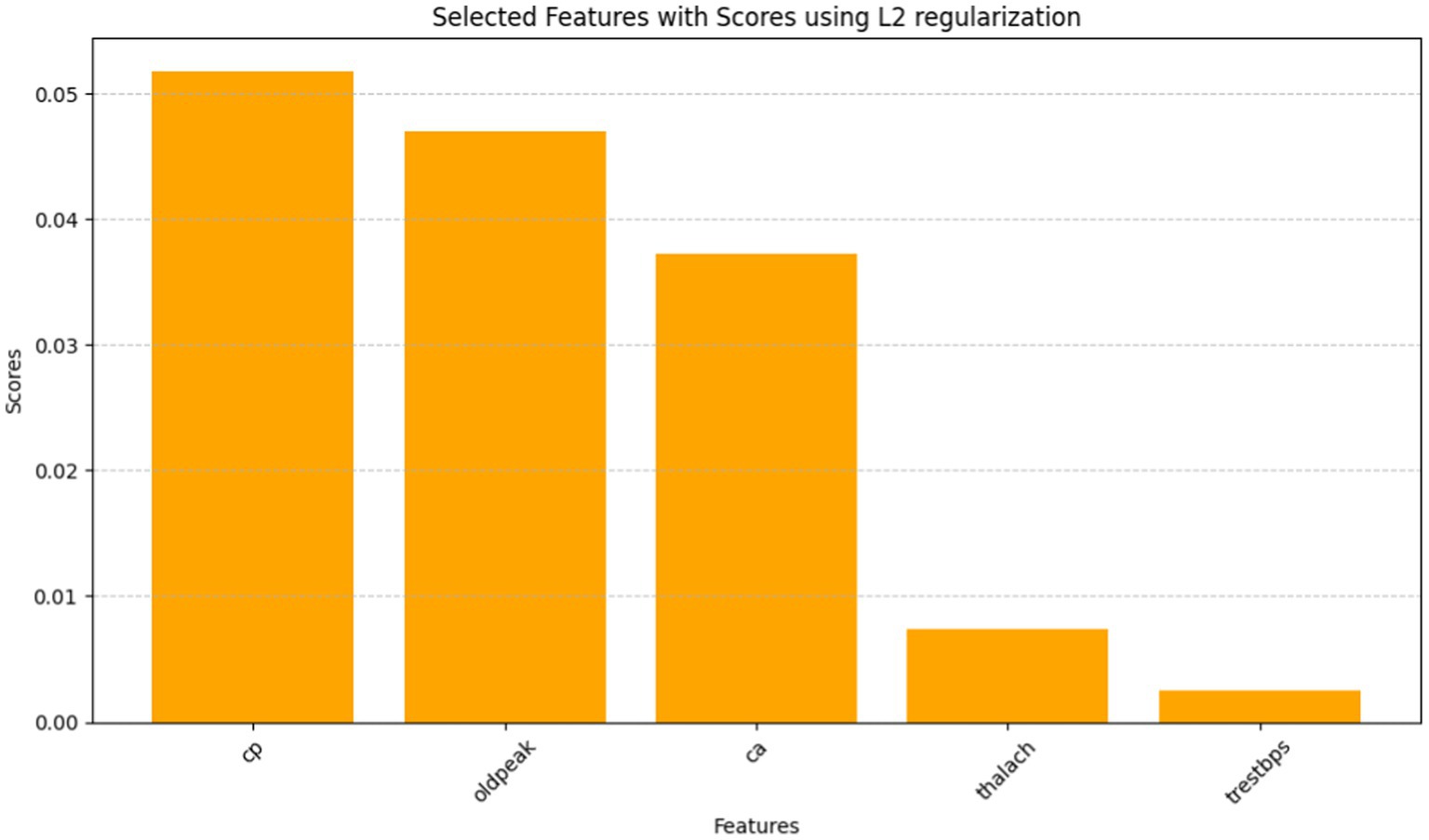
The Grasshopper Optimization Algorithm (GOA) (32) identified heart disease predictors. This method found critical characteristics like chest pain type (cp), resting blood pressure (trestbps), serum cholesterol (chol), maximum heart rate (thalach), ST depression caused by exercise compared to rest (oldpeak), and the number of main vessels colored by fluoroscopy (ca). High scores showed relevancy. The prediction model ranked attributes by score. Next, we used ridge regression, also known as L2 regularization, to enhance feature selection. Revised features included oldpeak, thalach, ca, trestbps, and cp. Revaluating characteristics using L2 regularization yielded scores that accurately represent their value in heart disease prediction. Comparing the two feature selection approaches shows convergence in the selected qualities, suggesting they may be essential for heart disease identification. However, slight discrepancies in feature significance showed that GOA and L2 regularization use different techniques and criteria. We need more study to evaluate the predictive modeling of the upgraded features and the implications for heart disease diagnostics (Figures 10, 11).
4.1.2 Hyperparameter tuning outcome using babysitting algorithm on ABDF
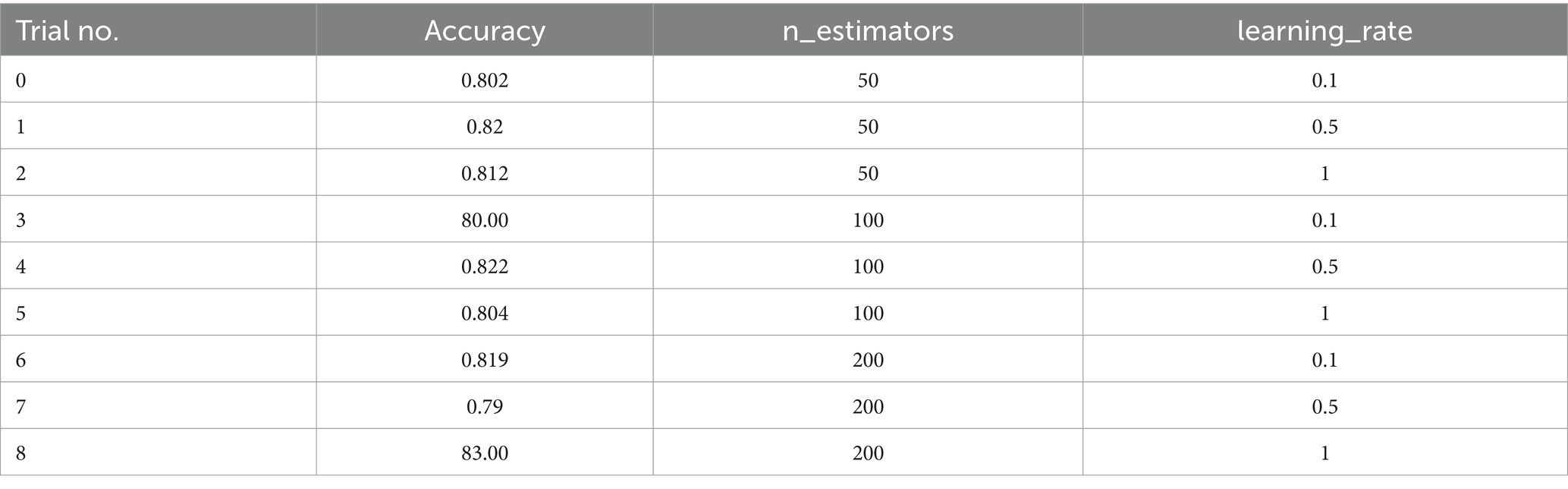
The AdaBoost Decision Fusion model’s hyperparameters were optimized by a two-pronged approach involving tuning the n_estimators and learning rate with the help of the Babysitting Algorithm (see in Table 5). A narrow range of the search space for n_estimators, which was from 50 to 200, and a more broad range of the learning rate, which was from 0.5 to 1, was seen. The hyperparameter optimization was made through a number of runs by substituting various combinations of parameters for n_estimators and learning_rate (see in Table 6 and Figure 12). The data obtained from the ABDF model showed deviation across the many attempts conducted in the experiment; Trial No. 8 gave 8 as the most accurate results, their accuracy being 83.00%. The crucial aspiration of this process was the attainment of an optimal accuracy and robustness model for the ABDF model, specifically as it concerned the given task.
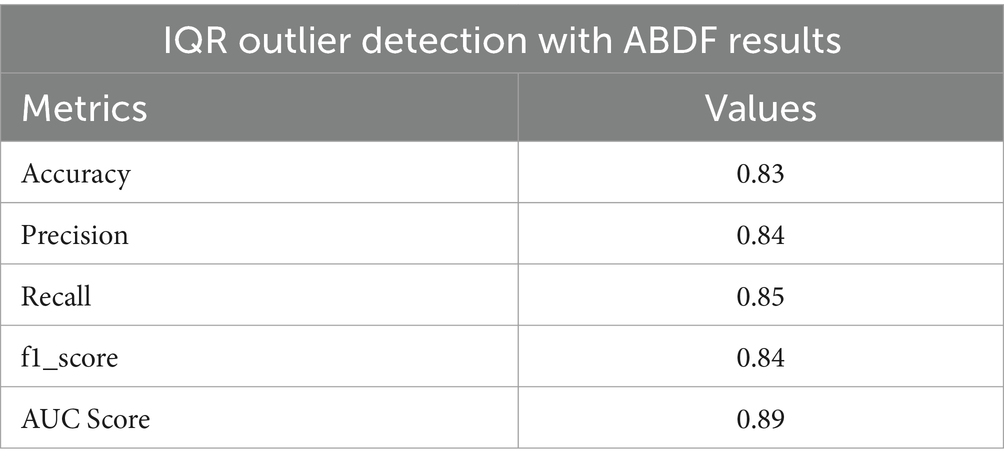
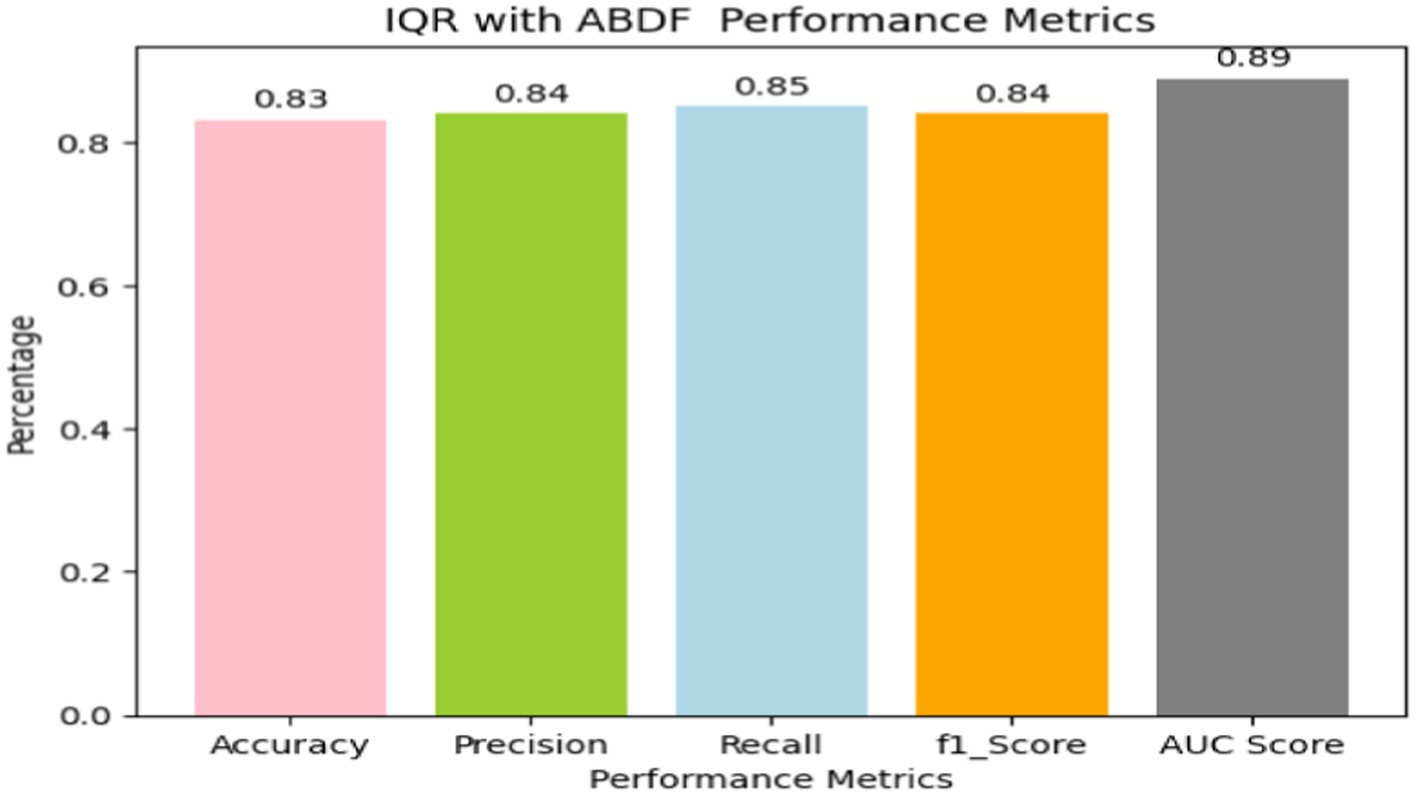
4.2 IQR outlier detection with ABDF
Heart disease may be reliably predicted using the ABDF method and the IQR outlier preprocessing strategy. The model achieves an 83% accuracy rate in instance categorization and an 84% success rate in accurately anticipating predicted positives (see in Table 7 and Figure 13). The model correctly identifies a large number of positive examples, as evidenced by its impressive recall score of 85%. An F1 Score of 84% (a measure of both recall and accuracy) indicates that the model is performing well. With an Area Under the Curve (AUC) score of 89% (see in Figure 14), the model clearly can differentiate between positive and negative occurrences. Based on these metrics, it appears that preprocessing using ABDF and IQR improves the accuracy, precision, recall, and overall predictive performance of models used to forecast cardiac diseases. According to its reliable performance, the model may be relied on by healthcare providers to aid in the rapid identification and treatment for people at risk of heart disease.
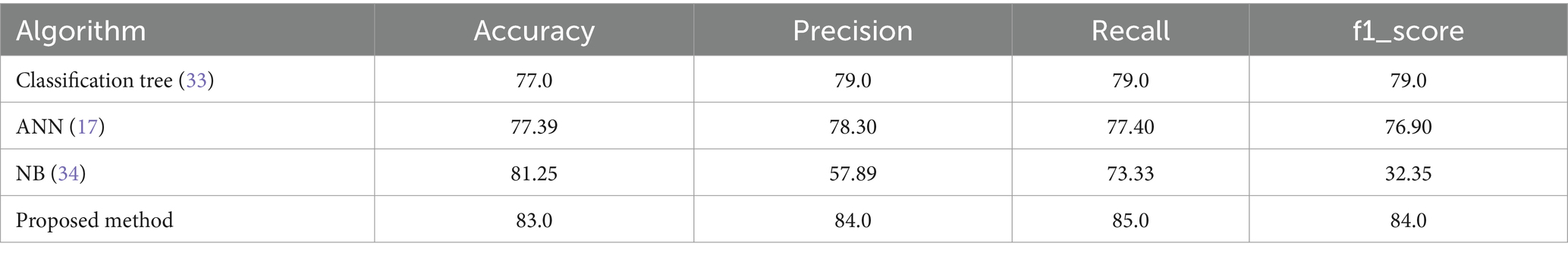
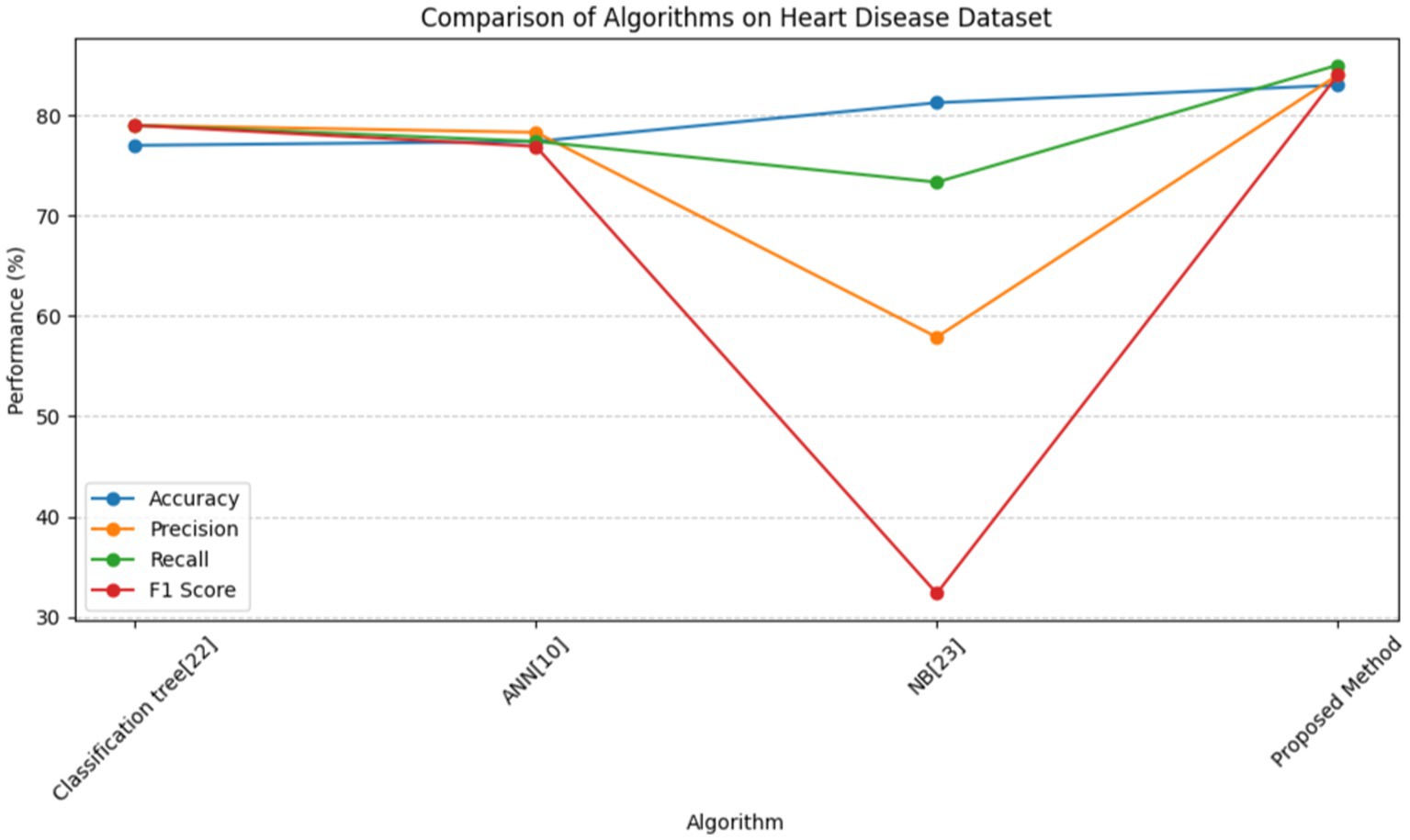
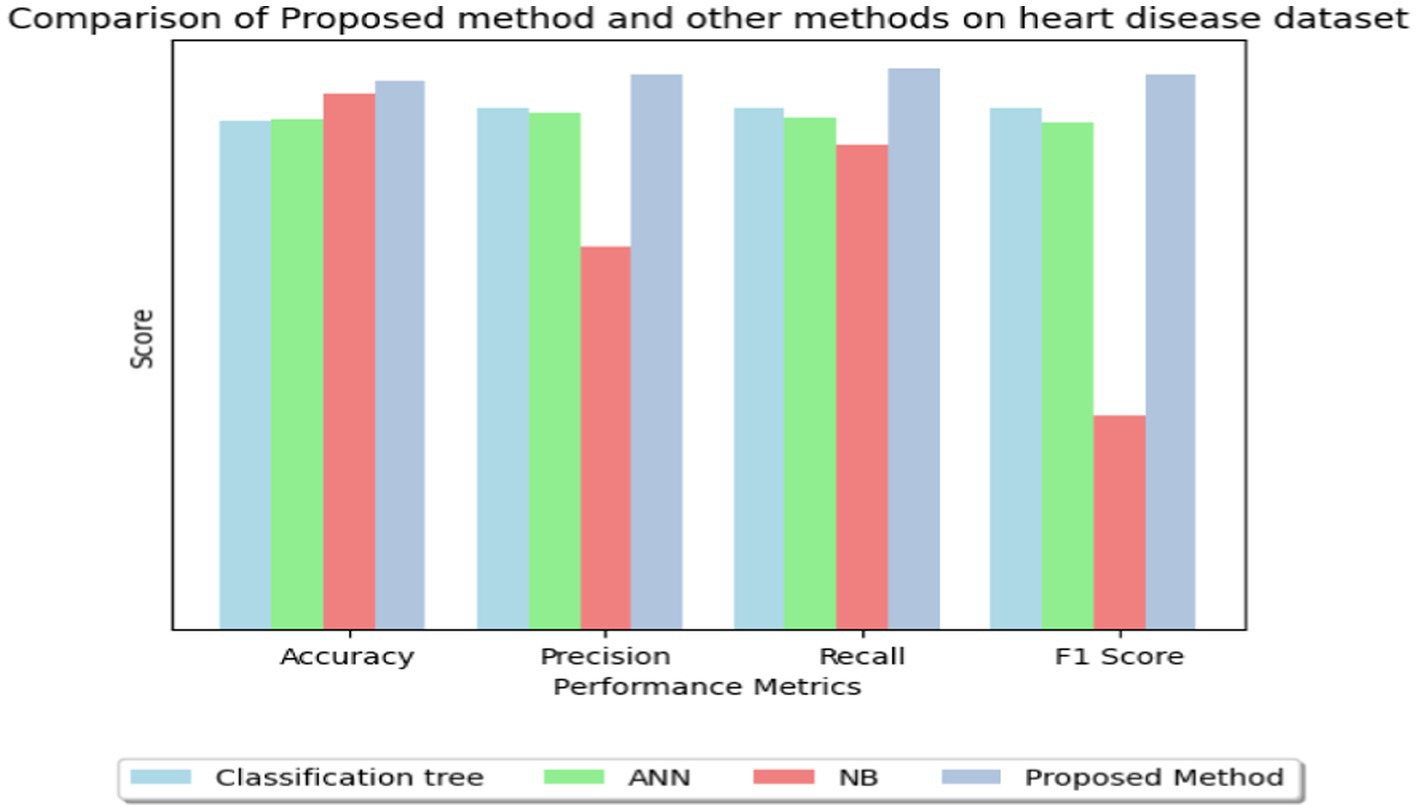
4.2.1 Comparison of proposed method and other methods on heart disease dataset
In Table 8, multiple approaches are used to a heart disease dataset to assess accuracy, precision, recall, and F1-score. The suggested technique outperforms the others with 83.0% accuracy. This shows that it locates dataset instances properly. This method outperforms the Classification Tree and Artificial Neural Network (ANN) methods in classification testing. The new approach outperforms previous methods in accuracy, recall, and F1-score. Its great overall performance is due to its balanced trade-off between precisely recognizing positive examples (precision) and capturing all positive occurrences (recall).
The Naive Bayes (NB) technique exceeds the suggested method in accuracy (81.25%) but much worse in precision, recall, and F1-score. More particular, the NB technique has poorer precision and F1-score than the suggested strategy, suggesting more false positives and a worse accuracy-recall trade-off. The findings suggest that the proposed technique balances accuracy and precision-recall, making it suitable for heart illness classification (see in Figures 15, 16). The comparison research also emphasizes the need of choosing the right technique for favorable performance indicators. This scenario shows that the recommended strategy is better than the present options.
5 Discussions
Our work presents an 83% reliable machine learning heart disease prediction approach. We used cutting-edge methods like SMOTE, IQR outlier detection, MICE, and GOL2-2 T, a hybrid feature selection technique, to improve predictive accuracy and robustness. Combining these techniques improved feature selection and model performance, according to our findings. Our heart disease patient identification approach is very accurate. These results demonstrate the need of using cutting-edge machine learning algorithms in medicine to identify and cure diseases early.
Our findings may help doctors predict cardiac disease, improving patient care and intervention. Our accurate diagnostic equipment may enhance patient outcomes and minimize cardiovascular disease mortality. However, our research has some drawbacks. Our hopeful results are limited to a dataset and may not apply to other patient populations or healthcare situations. Data quality and feature selection criteria may also affect our model’s performance.
We urge additional research to corroborate our results across a variety of datasets and populations. Using additional machine learning methods (35–40) and domain-specific information may improve the model’s interpretability and prediction accuracy. To evaluate the long-term effects of early cardiac disease identification on patient outcomes, longitudinal studies are needed. In conclusion, our results emphasize the necessity for ongoing study to develop cardiovascular prediction analytics.
6 Conclusion and future scope
In conclusion, our study met the urgent demand for precise and effective cardiovascular disease prognostic diagnostic tools. MICE, IQR outlier detection, SMOTE, and Adaptive Boosted Decision Fusion (ABDF) were used to improve heart disease prediction models’ precision and reliability. The Hybrid GOL2-2 T feature selection technique has enhanced our process by discovering important features and decreasing overfitting.
We solved class imbalance, missing data, and outlier identification to create a model that outperforms previous methods. The accuracy rate of 83.0% and balanced F1 score of 84.0% of our heart disease prediction method were impressive. The accuracy, recall, and AUC score demonstrate the validity and applicability of our methods. Our findings show that powerful machine learning techniques must be used in healthcare to produce reliable cardiovascular disease diagnosis tools. The study gives doctors tools for early diagnosis and effective treatment of cardiovascular disease risk.
Future study may improve prediction models and examine additional factors to improve diagnostic precision.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SP: Visualization, Data curation, Investigation, Software, Validation, Writing – original draft. MH: Data curation, Investigation, Funding acquisition, Project administration, Supervision, Writing – review & editing. SA: Writing – review & editing. US: Conceptualization, Software, Validation, Writing – original draft. NT: Conceptualization, Formal analysis, Investigation, Writing – review & editing. SI: Investigation, Methodology, Resources, Visualization, Writing – review & editing. FA: Resources, Software, Validation, Writing – review & editing. TA: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AN: Data curation, Investigation, Writing – review & editing. GS: Writing – review & editing. CY: Funding acquisition, Writing – review & editing, Conceptualization, Formal analysis, Resources, Visualization. TG: Formal analysis, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barik, S, Mohanty, S, Rout, D, Mohanty, S, Patra, AK, and Mishra, AK. Heart disease prediction using machine learning techniques. Adv Electr Control Signal Syst. (2020) 665:879–88. doi: 10.1007/978-981-15-5262-5_67
2. Riyaz, L, Butt, MA, Zaman, M, and Ayob, O. Heart disease prediction using machine learning techniques: a quantitative review. Adv Intell Syst Comput. (2021)1394:81–94. doi: 10.1007/978-981-16-3071-2_8
3. Fu, Q, Chen, R, Ding, Y, Xu, S, Huang, C, He, B, et al. Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front Nutr. (2023) 10:509. doi: 10.3389/fnut.2023.1250509
4. Huang, L, Wu, J, Lian, B, Zhang, D, Zhai, Y, and Cao, L. Successful robot-assisted laparoscopic resection of pheochromocytoma in a patient with dilated cardiomyopathy: a case report on extremely high-risk anesthesia management. Medicine. (2023) 102:e35467. doi: 10.1097/md.0000000000035467
5. Wang, Y, Zhai, W, Zhang, H, Cheng, S, and Li, J. Injectable Polyzwitterionic lubricant for complete prevention of cardiac adhesion. Macromol Biosci. (2023) 23:e2200554. doi: 10.1002/mabi.202200554
6. Zhou, Y, Sun, X, Yang, G, Ding, N, Pan, X, Zhong, A, et al. Sex-specific differences in the association between steps per day and all-cause mortality among a cohort of adult patients from the United States with congestive heart failure. Heart Lung. (2023) 62:175–9. doi: 10.1016/j.hrtlng.2023.07.009
7. Liu, Z, Fan, Y, Zhang, Z, Fang, Y, Cheng, X, Yang, Q, et al. mTOR in the mechanisms of atherosclerosis and cardiovascular disease. Discov Med. (2021) 31:129–40.
8. Statista. Share of People with Heart Problems India 2020, by Age Group. (2023). Available at: https://www.statista.com/statistics/1123509/india-share-of-respondents-with-heart-issues-by-age-group/.
9. Manikandan, S. “Heart attack prediction system.” (2017). International Conference on Energy, Communication, Data Analytics and Soft Computing (ICECDS).
10. Sirisha, U, Praveen, SP, Srinivasu, PN, Barsocchi, P, and Bhoi, AK. Statistical analysis of design aspects of various YOLO-based deep learning models for object detection. Int J Comput Intell Syst. (2023) 16:126. doi: 10.1007/s44196-023-00302-w
11. Srinivasu, PN, JayaLakshmi, G, Jhaveri, RH, and Praveen, SP. Ambient assistive living for monitoring the physical activity of diabetic adults through body area networks. Mob Inf Syst. (2022) 2022:1–18. doi: 10.1155/2022/3169927
12. Krishna, T, Praveen, SP, Ahmed, S, and Srinivasu, PN. Software-driven secure framework for mobile healthcare applications in IoMT. Intell Decis Technol. (2023) 17:377–93. doi: 10.3233/IDT-220132
13. Shah, D, Patel, S, and Bharti, SK. Heart disease prediction using machine learning techniques. SN Comp Sci. (2020) 1:1–6. doi: 10.1007/s42979-020-00365-y
14. Katarya, R, and Srinivas, P. “Predicting heart disease at early stages using machine learning: a survey.” (2020). International Conference on Electronics and Sustainable Communication Systems (ICESC).
15. Jindal, H, Agrawal, S, Khera, R, Jain, R, and Nagrath, P. Heart disease prediction using machine learning algorithms. IOP Conf Ser Mater Sci Eng. (2021) 1022:012072. doi: 10.1088/1757-899x/1022/1/012072
16. Gonsalves, AH, Thabtah, F, Mohammad, RMA, and Singh, G. “Prediction of coronary heart disease using machine learning.” Proceedings of the 2019 3rd International Conference on Deep Learning Technologies (2019).
17. Nashif, S, Rakib Raihan, M, Rasedul Islam, M, and Imam, MH. Heart disease detection by using machine learning algorithms and a real-time cardiovascular health monitoring system. World J Eng Technol. (2018) 6:854–73. doi: 10.4236/wjet.2018.64057
18. Bhatt, CM, Patel, P, Ghetia, T, and Mazzeo, PL. Effective heart disease prediction using machine learning techniques. Algorithms. (2023) 16:88. doi: 10.3390/a16020088
19. Abood Kadhim, M, and Radhi, AM. Heart disease classification using optimized machine learning algorithms. Iraqi J. Comp. Sci. Math. (2023) 4:31–42. doi: 10.52866/ijcsm.2023.02.02.004
20. Huang, H, Wu, N, Liang, Y, Peng, X, and Shu, J. SLNL: a novel method for gene selection and phenotype classification. Int J Intell Syst. (2022) 37:6283–04. doi: 10.1002/int.22844
21. Heart Disease Dataset. Kaggle. (2019). Available at: https://www.kaggle.com/datasets/johnsmith88/heart-disease-dataset/data.
22. Samad, MD, Abrar, S, and Diawara, N. Missing value estimation using clustering and deep learning within multiple imputation framework. Knowl-Based Syst. (2022) 249:108968. doi: 10.1016/j.knosys.2022.108968
23. Gandla, VR, Mallela, DV, and Chaurasiya, R. “Heart failure prediction using machine learning.” International Conference on Applied Computational Intelligence and Analytics (Acia-2022) (2023).
24. Mamun, A, Paul, BK, Ahmed, K, Bui, FM, Quinn, JM, and Moni, MA. Heart disease prediction using supervised machine learning algorithms: performance analysis and comparison. Comput Biol Med. (2021) 136:104672. doi: 10.1016/j.compbiomed.2021.104672
25. Ishaq, A, Sadiq, S, Umer, M, Ullah, S, Mirjalili, S, Rupapara, V, et al. Improving the prediction of heart failure patients’ survival using SMOTE and effective.
27. Rani, P, Kumar, R, Ahmed, NS, and Jain, A. A decision support system for heart disease prediction based upon machine learning. J Rel Intell Environ. (2021) 7:263–75. doi: 10.1007/s40860-021-00133-6
28. Hasan, MK, Habib, AA, Islam, S, Safie, N, Ghazal, TM, Khan, MA, et al. Federated learning enables 6 G communication technology: requirements, applications, and integrated with intelligence framework. Alex Eng J. (2024) 91:658–68. doi: 10.1016/j.aej.2024.02.044
29. Hasan, MK, Hosain, M, Ahmed, MK, Islam, S, Aledaily, AN, Yasmeen, S, et al. Encrypted images in a V-BLAST assisted SC-FDMA wireless communication system. Trans Emerg Telecommun Technol. (2024) 35:e4882. doi: 10.1002/ett.4882
30. Ahmed, AA, Hasan, MK, Nafi, NS, Aman, AH, Islam, S, and Nahi, MS Optimization technique for deep learning methodology on power Side Channel attacks. 2023 33rd International Telecommunication Networks and Applications Conference IEEE (2023).
31. Tirumanadham, K, Kumar, M, Thaiyalnayaki, S, and Sriram, M. “Evaluating boosting algorithms for academic performance prediction in E-learning environments.” (2024) International Conference on Intelligent and Innovative Technologies in Computing, Electrical and Electronics (IITCEE).
32. Dey, C, Bose, R, Ghosh, KK, Malakar, S, and Sarkar, R. LAGOA: learning automata based grasshopper optimization algorithm for feature selection in disease datasets. J Ambient Intell Human Comp. (2021) 13:3175–94. doi: 10.1007/s12652-021-03155-3
33. Ponti, J, and Moacir, P. Combining classifiers: from the creation of ensembles to the decision fusion. (2011). 24th SIBGRAPI Conference on graphics, patterns, and images tutorials.
34. Dwivedi, AK. Performance evaluation of different machine learning techniques for prediction of heart disease. Neural Comp Appl. (2016) 29:685–93. doi: 10.1007/s00521-016-2604-1
35. Liao, H, Murah, MZ, Hasan, MK, Aman, AHM, Fang, J, Hu, X, et al. A survey of deep learning Technologies for Intrusion Detection in internet of things. IEEE Access. (2024) 12:4745–61. doi: 10.1109/ACCESS.2023.3349287
36. Islam, MM, Hasan, MK, Islam, S, Balfaqih, M, Alzahrani, AI, Alalwan, N, et al. Enabling pandemic-resilient healthcare: narrowband internet of things and edge intelligence for real-time monitoring. CAAI Trans Intell Technol. (2024). doi: 10.1049/cit2.12314
37. Lu, S, Yang, J, Yang, B, Li, X, Yin, Z, Yin, L, et al. Surgical instrument posture estimation and tracking based on LSTM. ICT Express. (2024). doi: 10.1016/j.icte.2024.01.002
38. Kim, S, Kim, M, Kim, J, Jeon, JS, Park, J, and Yi, HG. Bioprinting methods for fabricating in vitro tubular blood vessel models. Cyborg Bionic Syst. (2023) 4:43. doi: 10.34133/cbsystems.0043
39. Bing, P, Liu, Y, Liu, W, Zhou, J, and Zhu, L. Electrocardiogram classification using TSST-based spectrogram and ConViT. Front Cardiovasc Med. (2022) 9:543. doi: 10.3389/fcvm.2022.983543
40. Gao, X, Huang, D, Hu, Y, Chen, Y, Zhang, H, Liu, F, et al. Direct Oral anticoagulants vs. vitamin K antagonists in atrial fibrillation patients at risk of falling: a Meta-analysis. Front Cardiovasc Med. 9:329. doi: 10.3389/fcvm.2022.833329
Keywords: multivariate imputation by chained equations, synthetic minority over-sampling technique, interquartile range, adaptive boosted decision fusion, cardiovascular disease, adaboost decision fusion (ABDF)
Citation: Praveen SP, Hasan MK, Abdullah SNHS, Sirisha U, Tirumanadham NSKMK, Islam S, Ahmed FRA, Ahmed TE, Noboni AA, Sampedro GA, Yeun CY and Ghazal TM (2024) Enhanced feature selection and ensemble learning for cardiovascular disease prediction: hybrid GOL2-2 T and adaptive boosted decision fusion with babysitting refinement. Front. Med. 11:1407376. doi: 10.3389/fmed.2024.1407376
Edited by:
Amin Ul Haq, University of Electronic Science and Technology of China, ChinaReviewed by:
Lakshmeeswari Gondi, Gandhi Institute of Technology and Management (GITAM), IndiaSaeed Mian Qaisar, Effat University, Saudi Arabia
Kishore Konda Chidella, University of Nevada, Las Vegas, United States
J. Mohana Lakshmi, Malnad College of Engineering, India
Copyright © 2024 Praveen, Hasan, Abdullah, Sirisha, Tirumanadham, Islam, Ahmed, Ahmed, Noboni, Sampedro, Yeun and Ghazal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shayla Islam, c2hheWxhQHVjc2l1bml2ZXJzaXR5LmVkdS5teQ==; Chan Yeob Yeun, Y2hhbi55ZXVuQGt1LmFjLmFl
 S. Phani Praveen
S. Phani Praveen Mohammad Kamrul Hasan2
Mohammad Kamrul Hasan2 Uddagiri Sirisha
Uddagiri Sirisha Thowiba E. Ahmed
Thowiba E. Ahmed Gabriel Avelino Sampedro
Gabriel Avelino Sampedro Chan Yeob Yeun
Chan Yeob Yeun Taher M. Ghazal
Taher M. Ghazal