- 1Faculty of Medicine, University “Fehmi Agani” in Gjakova, Gjakovë, Kosovo
- 2Faculty of Pharmacy, AMECC Rezonanca, Prishtinë, Kosovo
1 Introduction
Sarcopenia is a condition characterized by loss of muscle mass and function (1) occurring as a natural part of aging process. Even though initially suggested as a concept to embody the “poverty of flesh” (2), sarcopenia underwent certain transformations throughout its formation, particularly when shifting from muscle mass to muscle strength for the key diagnostic component as suggested by the revised consensus criteria by the European Working Group in Sarcopenia for Older People—EWGSOP2 (1). In its pathway sarcopenia managed to receive an ICD-10-CM code (M62.84) in 2016 (3), thus making a significant step ahead on its establishment and distinction. To date, it's certain and undeniable that sarcopenia presents a topic of ever-growing interest amongst researchers and clinicians, while being relatively unknown amongst the general population. Nonetheless, the continuous increase of expected life expectancy together with aging of worldwide population has inevitably raised the need to include the policy makers and end-users in the matter.
Global prevalence of sarcopenia has been shown to vary widely in between different studies and countries, ranging from 10% using the EWGSOP2 algorithm and diagnostic criteria to 27% using the overall muscle mass definition in a systematic review and meta-analysis involving adults aged ≥ 18 years (4). Another review involving studies with elderly participants while following the most commonly used sarcopenia definitions (EWGSOP, EWGSOP2, Asian Working Group in Sarcopenia—AWGS, International Working Group in Sarcopenia—IWGS and Foundations of National Institutes of Health—FNIH) reported a sarcopenia prevalence ranging between 10 and 16% (5). However, a very comprehensive review including all the available sarcopenia diagnostic approaches that was conducted by Petermann-Rocha et al. (4) showed ranges in outcomes from as low as 0.2% to as high as 86.5% (0.3–91.2% in biological women and 0.4–87.7% in biological men).
It has been a couple of years since Haase et al. (6) addressed the potential implications of sarcopenia diagnosis, particularly arguing about the lack of differentiation of current treatments from the general health recommendations. Elsewhere, Tagliafico et al. (7) even suggested for sarcopenia to be clearly underdiagnosed in clinical practice while arguing for the necessity to involve radiologists in the muscle mass assessment process.
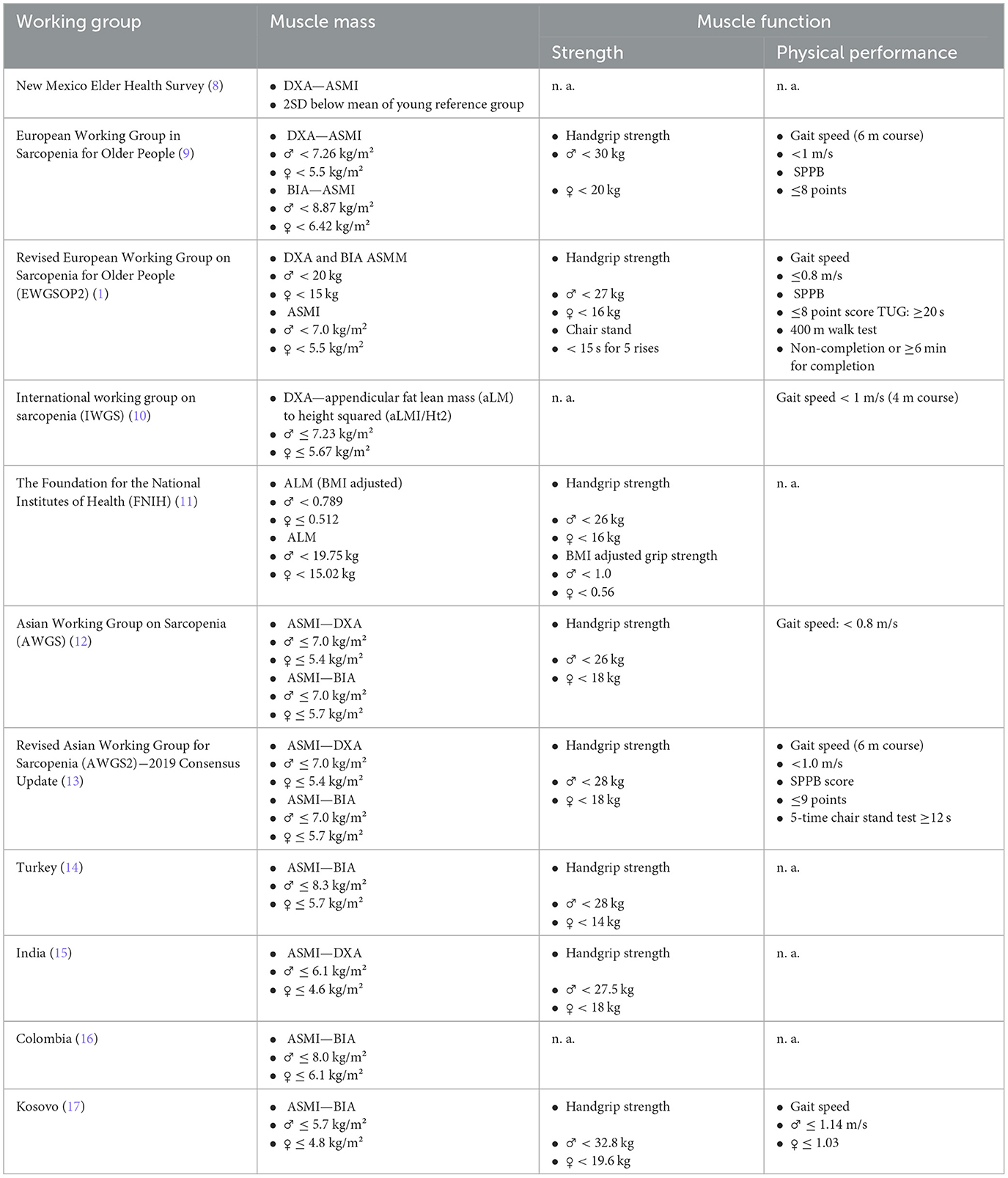
However, despite the fact that the prevalence and diagnosis of sarcopenia remains a topic of increasing interest, the question of how to appropriately diagnose it in clinical practice remains a gray area. Many international working groups have been trying to address this concern, though almost each one ends up bringing novelties to the matter with still no universal consensus guideline. This way, notwithstanding the scientific progress, the greatest issue surrounding sarcopenia remains the lack of its applicability in clinical practices. The reasons remain vague, with the lack of unified diagnostic criteria being above all. Table 1 describes some of the major recommended diagnostic guideline criteria and certain population specific diagnostic cut-off points.
2 Diagnostic approaches of sarcopenia
In principle, there are two common approaches to diagnose sarcopenia: international working group consensus guideline cutoff points vs. population-specific cutoff points. The international working groups cutoff points are based on certain consensus resulting from the agreement between field experts on a specific criterion for the diagnosis of sarcopenia. These specifically-defined criterions are mainly set based on the state-of-the-art research evidence from patterns deriving from one or more populations with similar characteristics, or from big and comprehensive studies were performed in the similar populations. However, the population-specific ones are tailored-based diagnostic criteria deriving from the population specifics, generated on the distribution of muscle mass, strength and physical performance amongst the specific population. They are calculated by determining the standard deviation from the respective mean of a given parameter within a younger population group, and then subtracting that standard deviation (usually from 2 to 2.5 times) from a respective mean population, thus defining a cut point that represents a certain number of standard deviations below the mean (8).
As observed in Table 2, both approaches have their own advantages and disadvantages of (and when) being followed. Unfortunately, certain limitations that come in the expense of an extra benefit are undeniably worrisome, like the case of standardization in between populations that the international guidelines endure in expense to the demography accuracy. A similar case is as well-observed with clinical relevancy and research accuracy that the population-specific cutoff points provide, though in the expense of practicality and consistency.
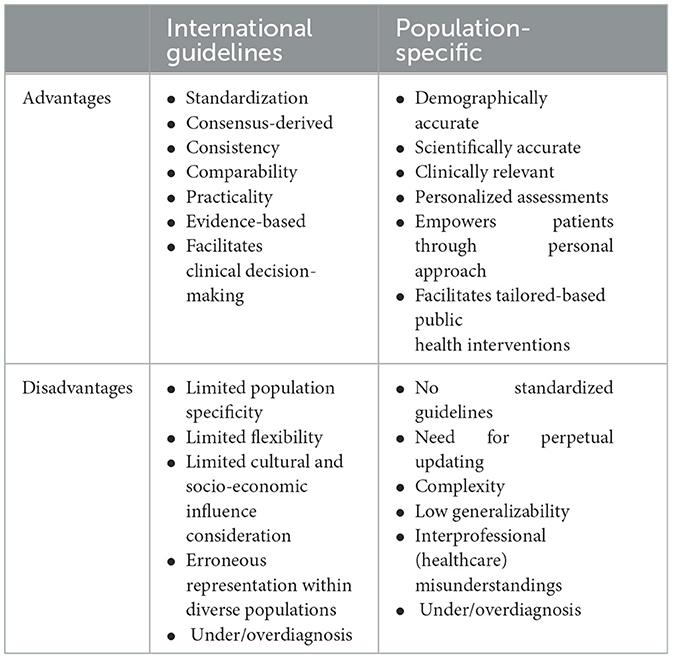
Table 2. Advantages and disadvantages of international consensus vs. population-specific diagnostic criteria for sarcopenia.
Notwithstanding the applicability of both approaches, the internationally-suggested criterions are generally considered as more reliable and valid when dealing with prevalence/epidemiological studies, or studies within big populations with similar characteristics. To some degree this is understandable based on the standardization they provide, resulting in a more practical applicability (while not having to keep developing population-specific diagnostic criteria), as well as a generalizability (encompassing different populations) and consistency (by following the same pathway). On the contrary to that, population-specific criteria might raise concerns on their reliability, mainly due to their high reliance on the distribution of muscle mass and strength within a specific population, as something that can vary widely across different geographic regions (latitude) and demography (ethnicity, biological gender, age). Though if population specific diagnostic criteria are derived from a well-planed and high-end customization study conducted within a particular population, the certainty of diagnostic pathway could be very high. In certain cases, even the international guidelines do suggest to use regional normative populations (when available), particularly when dealing with variables prone to stature variations like strength and gait speed (1). Unfortunately, this is often not the case in scientific practice, particularly amongst developing countries where age-related diseases like sarcopenia are rather neglected in comparison to others like emergency medicine and acute medical interventions that often take the spotlight (18). Such situations seriously limit researchers and/or practitioners' available possibilities to diagnose and perform in their practice. This way the comparability between different populations (in particular) becomes obsolete due to the specific circumstances around them. Nonetheless, if there is an outcome deriving from the current state-of-the-art, it seems that both approaches do provide the ground for either under or over diagnosis of sarcopenia and its conceptual stages.
3 Which approach should we follow?
Both, mostly depending on the context. It is a fact that we cannot develop population-specific diagnostic criteria for every disease out there! Though in clinical practice, the most important factor is to find cases with potential to develop disease. If the population-specific diagnostic cut-off points result from a comprehensive epidemiological study that considers other inter-related covariates like the individual health, environment and lifestyle behaviors, they most definitely provide the more accurate diagnostic pathway. It is particularly true for the un-/under—explored populations (developing countries) or for the small populations (e.g., small countries). Therefore, population specific diagnostic criteria should be the frontline to be used in clinical practices, for as long as they would have a strong “backbone” criterion-derived study from which they would be based (17, 19). Then, sarcopenia diagnosis should be followed by other means of diagnostic approach including qualitative muscle assessments (echogenicity analysis) through diagnostic ultrasound to provide insights within the pathophysiology of sarcopenia (20), as a reliable and valid diagnostic method for quantitative assessment of age-related changes in appendicular muscle mass (21). And this is the momentum which we would need to catch since the skeletal muscle provides the qualitative context besides the quantitative one. It is particularly important to visualize and better understand the qualitative muscle changes that accompany the sarcopenia diagnosis (and/or its conceptual stages).
The revised EWGSOP2 consensus definition and diagnostic algorithm (1) has been one of the more frequent and novel diagnostic pathways followed in many recent studies (though predominantly coming from European populations since being developed for these populations). The standard diagnostic flowchart suggests to start with the sarcopenia screening questionnaire (SARC-F) for potential case findings, and proceed with muscle strength assessment by either isometric grip strength (assessed by dynamometry) or lower body strength (assessed by chair stand test). When either of strength parameters is below the threshold the term “sarcopenia probable” is given, which requires to undergo the sarcopenia confirmation process that involves the assessment of appendicular skeletal muscle mass (or index) through one of the recommended techniques (either dual x-ray energy absorptiometry—DXA, or bio electrical impedance analysis—BIA). If the outcomes are below the set thresholds in this phase as well, estimating the severity of sarcopenia through physical performance assessment (gait speed at normal walking pace) remains the final step. In this context, the international consensus guidelines are definitely important for the populations they encompass or that were taken into consideration when being developed, and they should be used in research, for prevalence studies and in addition to the other assessment approach. Their potential population specificity limitations could be overcome with the qualitative approach as well. Though, sarcopenia in clinical practice shouldn't be just about filling or not some certain criteria, but rather as a comprehensive geriatric condition that needs to be detected and tackled accordingly. However, until a unified worldwide diagnostic pathway to follow is brought up, clinicians are most definitely going to hesitate to move forward toward mass diagnosing. Until then, diagnosis of sarcopenia should be made carefully following both approaches (international and population-specific criteria), always evaluated in conjunction with a comprehensive medical evaluation, including a physical exam, medical history, and laboratory tests, as well as followed by muscle echogenicity analysis to estimate muscle quality. This should be important particularly in developing countries where numbers of sarcopenia cases are expected to raise with time going by, whereas normative data remain inconclusive (18). In this context, the inclusion of certain experts from such countries (notwithstanding the size of the representative population) within international working groups might offer other (different) perspectives on the matter. This might allow clinician to make decisions based on gathered evidences from different facts, thus giving some time for the more appropriate sarcopenia-diagnostic approach to settle. After all, if the sarcopenia diagnosis does not start to be applied in clinical practice, it risks becoming an obsolete. It might even take the path of overdiagnosis (6) and regress from the brave steps taken to date.
Author contributions
AB: Conceptualization, Methodology, Resources, Visualization, Writing – original draft. EK: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
2. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr (1989) 50:1121–235.
3. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc. (2016) 17:675–7. doi: 10.1016/j.jamda.2016.06.001
4. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcop Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
5. Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
6. Haase CB, Brodersen JB, Bülow J. Sarcopenia: early prevention or overdiagnosis? BMJ. (2022) 376:e052592. doi: 10.1136/bmj-2019-052592
7. Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. (2022) 127:228–37. doi: 10.1007/s11547-022-01450-3
8. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. (1998) 147:755–63. doi: 10.1093/oxfordjournals.aje.a009520
9. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
10. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults current consensus definition: prevalence, etiology, and Consequences International Working Group on Sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
11. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol Ser A. (2014) 69:547–58. doi: 10.1093/gerona/glu010
12. Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
13. Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
14. Ates Bulut E, Soysal P, Dokuzlar O, Kocyigit SE, Aydin AE, Yavuz I, et al. Validation of population-based cutoffs for low muscle mass and strength in a population of Turkish elderly adults. Aging Clin Exp Res. (2020) 32:1749–55. doi: 10.1007/s40520-019-01448-4
15. Pal R, Aggarwal A, Singh T, Sharma S, Khandelwal N, Garg A, et al. Diagnostic cut-offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: The Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES). Eur Geriatr Med. (2020) 11:725–36. doi: 10.1007/s41999-020-00332-z
16. Pineda-Zuluaga MC, González-Correa CH, Sepulveda-Gallego LE. Cut-off points for low skeletal muscle mass in older adults: Colombia versus other populations. F1000Res. (2023) 11:304. doi: 10.12688/f1000research.109195.2
17. Boshnjaku A, Bahtiri A, Feka K, Krasniqi E, Tschan H, Wessner B. Impact of using population-specific cut-points, self-reported health, and socio-economic parameters to predict sarcopenia: a cross-sectional study in community-dwelling Kosovans aged 60 years and older. J Clin Med. (2022) 11:5579. doi: 10.3390/jcm11195579
18. Boshnjaku A. Is age-related sarcopenia a real concern for my developing country? J Cachexia Sarcop Muscle. (2022) 13:2589–92. doi: 10.1002/jcsm.13107
19. Fernandes S, Rodrigues da Silva E, New York B, Macedo P, Gonçalves R, Camara S, et al. Cutoff points for grip strength in screening for sarcopenia in community-dwelling older-adults: a systematic review. J Nutr Health Aging. (2022) 26:452–60. doi: 10.1007/s12603-022-1788-6
20. Stringer HJ, Wilson D. The role of ultrasound as a diagnostic tool for sarcopenia. J Frailty Aging. (2018) 7:258–61. doi: 10.14283/jfa.2018.24
Keywords: diagnostic criteria, aging, older people, overdiagnosis, muscle decline
Citation: Boshnjaku A and Krasniqi E (2024) Diagnosing sarcopenia in clinical practice: international guidelines vs. population-specific cutoff criteria. Front. Med. 11:1405438. doi: 10.3389/fmed.2024.1405438
Received: 22 March 2024; Accepted: 15 July 2024;
Published: 26 July 2024.
Edited by:
Klara Komici, University of Molise, ItalyReviewed by:
Grazia Daniela Femminella, University of Naples Federico II, ItalyCopyright © 2024 Boshnjaku and Krasniqi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ermira Krasniqi, cGguZXJtaXJha3Jhc25pcWlAZ21haWwuY29t
 Arben Boshnjaku
Arben Boshnjaku Ermira Krasniqi
Ermira Krasniqi