- 1Institute of Outcomes Research, Medical University of Vienna, Vienna, Austria
- 2Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Vienna, Austria
- 3Academic Department of Rehabilitation Medicine, University of Leeds, Leeds, United Kingdom
- 4Department for Primary Care Medicine, Center for Public Health, Medical University of Vienna, Vienna, Austria
- 5Institute of Occupational Therapy, School of Health Sciences, ZHAW Zurich University of Applied Sciences, Winterthur, Switzerland
- 6Department of Rheumatology and Immunology, University Hospital, Inselspital, University of Bern, Bern, Switzerland
Background: Experts estimate that in up to 10% of the infected, SARS-CoV-2 would cause persistent symptoms, activity limitations and reduced quality of life. Referred to as long COVID, these conditions might, in the future, specifically impact German-speaking countries due to their higher rates of unvaccinated people compared to other Western countries. Accurate measurement of symptom burden and its consequences is needed to manage conditions such as long COVID, and several tools have been developed to do so. However, no patient-reported instrument existed in the German language at the time of writing.
Objective: This study, therefore, aimed to develop a German version of the COVID-19 Yorkshire Rehabilitation Scale (C19-YRS).
Methods: We conducted a translation and qualitative evaluation, including cultural adaptation, of the C19-YRS and assessed its face validity. After creating a preliminary version, 26 individuals (14 women [53%]) participated in cognitive interviews (January 2022 to March 2022). Using cognitive debriefing interviews, we ensured the content’s comprehensibility. The matrix-framework method guided the qualitative data analysis.
Results: Compared to the original English version, adaptations were necessary, resulting in changes to the introductory text, while the items for recording persistent symptoms were hardly changed.
Conclusion: The German version of the C19-YRS is expected to support standardized long COVID care.
1 Introduction
Experts estimate that in up to 10% of the infected, the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) causes persistent symptoms, activity limitations and reduced quality of life (1, 2). Literature refers to these conditions as long COVID and defines them as a combination of manifestations such as fatigue, exhaustion, decreased physical endurance, post-exertional malaise, breathing difficulties, anxiety, depression, posttraumatic stress disorder or pain, lasting for more than 4 weeks after the infection (3–9). Long COVID can affect people of all ages, regardless of whether they had a severe or mild course of disease (5, 9, 10). Vaccination tends to lead to a risk reduction regarding long COVID symptoms (1, 11–13), whereas reinfections seem to cumulatively increase the risk (14).
Long COVID could particularly impact German-speaking countries in Europe, where the number of unvaccinated people is high (15) as well as reinfection rate due to the lack of primary prevention since spring 2023. Accurate measurement of symptom burden and its impact on people’s daily lives is needed to manage this condition effectively, and rapid assessment is necessary to fully assess patients’ problems and enable targeted multidisciplinary intervention (16). To date, the C19-YRS is the only tool that provides exemplary ideas for patient-centered management and interventions based on the severity of problems reported in the screening tool (17). Completing the C19-YRS over time provides a comprehensive overview of a patient’s progress, whether their condition is improving, worsening or fluctuating, which then also supports goal setting and the planning of patient-centered therapeutic interventions. Translating and adapting a multi-professional, COVID-19-specific assessment tool such as the C19-YRS can facilitate comprehensive assessment and intervention counseling, potentially improving standardized care for people with persistent symptoms after acute COVID-19 infection (17). No patient-reported instrument existed in the German language at the time of writing. Therefore, this study aimed to develop a German version of the COVID-19 Yorkshire Rehabilitation Scale (C19-YRS).
2 Materials and methods
We conducted a translation and qualitative evaluation, including cultural adaptation, of the C19-YRS into German and assessed its face validity.
2.1 COVID-19 Yorkshire Rehabilitation Scale (C19-YRS)
The COVID-19 Yorkshire Rehabilitation Scale is a 22-item patient-reported outcome measure for assessing and monitoring long COVID symptoms and was the first long COVID-specific scale reported in the literature (18). Considering psychometric properties, the English version of the C19-YRS showed good internal consistency, and scaling and targeting assumptions were satisfied (19).
Information collected includes 16 symptoms (including shortness of breath, persistent cough, fatigue, pain or discomfort, cognitive problems, anxiety, depression, symptoms of posttraumatic stress disorder [PTSD], palpitations, dizziness, weakness, and sleep problems) as well as their impact on five areas of activities and participation (including communication, mobility, personal care, activities of daily living, and social life) (see Supplementary Appendix 1). The patient is asked to rate each symptom or functional ability on a scale from 0 to 10 (0 being not present and ten being most severe and life-disturbing) (18). The C19-YRS is recommended for initial assessment, at 6 weeks, and at 6 months for follow-up and includes a self-report version. In a self-reported screening tool, outcomes are reported directly without interference from clinicians or health professionals (20), which supports people’s active participation in the decision-making process regarding their health care (21). For this reason, it was decided to first translate the self-reported version of the C19-YRS within the scope of this paper.
2.2 Translation and cross-cultural adaptation
First, the authors contacted the team of developers of the original C19-YRS, who granted permission to translate the self-report version of the C19-YRS into German (Austria). The translation process followed five steps according to the guideline established by Beaton et al. (22) namely (1) initial translation, (2) synthesis of translations, (3) back translation, (4) expert committee, and (5) review of preliminary version. In the first step (1), two translators each produced a German version of the C19-YRS (T1&T2). In a second step (2), a third person, who also has expertise in research and translation processes, helped synthesis these translations (T1&T2) into a new version (T12). At this point, the translation team added a step to Beaton’s guideline, as a person who is not a native German speaker reviewed the synthesized version to ensure that it was easy to understand. Then, in the third step (3), two English first language translators who were not familiar with the screening tool worked from the T12 translation and produced two back translations (BT1&BT2). In the fourth step (4), an expert committee (methodologist [TS], linguist [LD], translators) reviewed all reports to reach a consensus and jointly produced a preliminary version. Beaton et al. (22) suggest that individuals from the target group should subsequently complete the preliminary version to test understanding of the items. For this purpose, the authors then decided to apply cognitive interviewing methods in the last step of the translation and adaptation process (5) (23). In addition, the cross-cultural adaptation of a health status screening instrument usually involves assessing validity and reliability (24). In the context of this study, an initial aspect of content, validity face validity, which assesses the extent to which a measurement instrument adequately reflects the construct being measured (25), was chosen.
2.3 Participants and sampling
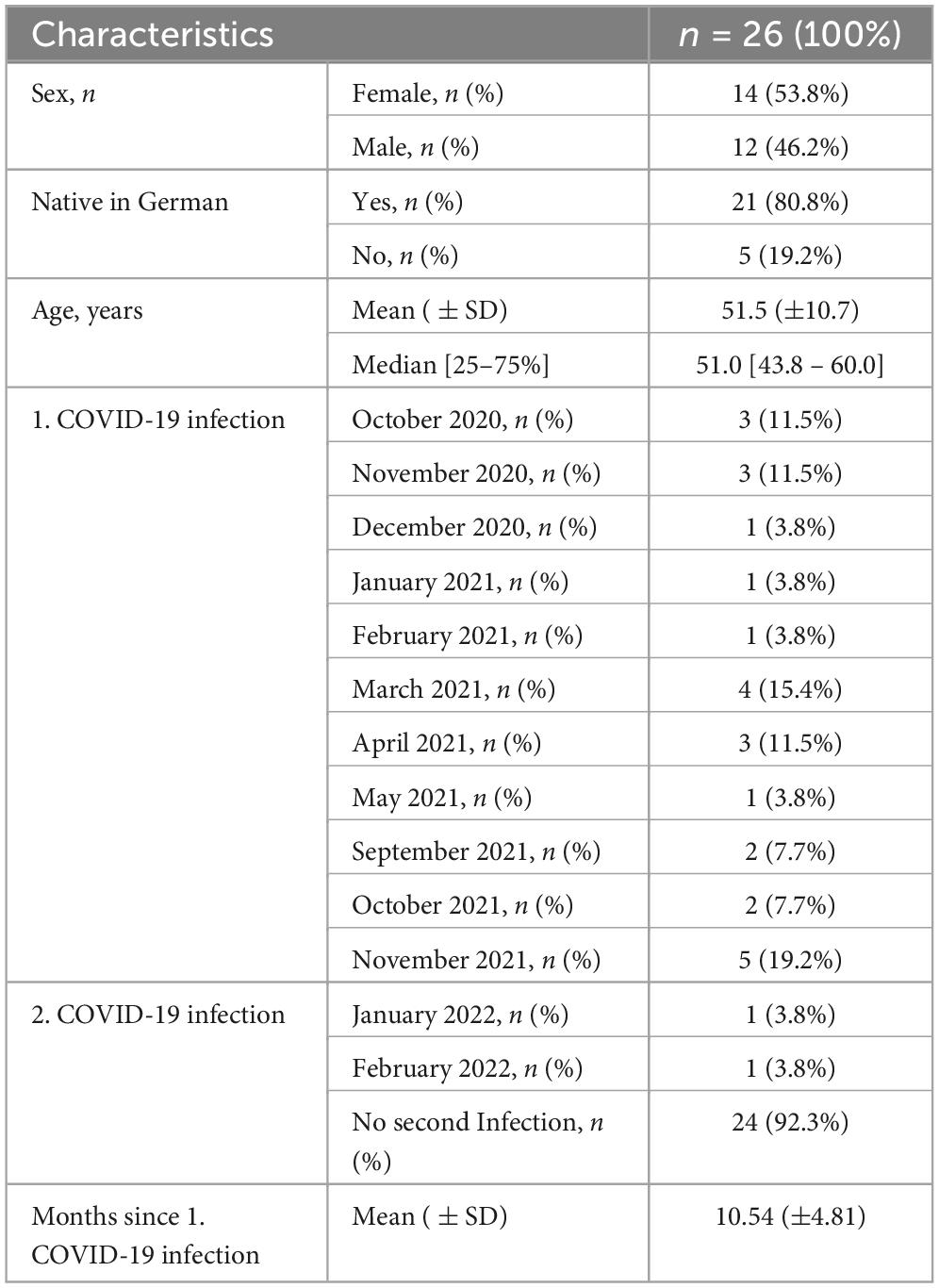
For the cognitive interviews, the first author purposively recruited patients from Austrian rehabilitation centers participating in the Austrian long COVID registry. Participation was open to people who had been diagnosed with COVID-19 infection, who were experiencing long-term symptoms following a COVID-19 infection, who were at least 18 years old, who understood and spoke sufficient German and who agreed to participate. This study involving human participants was reviewed and approved by the ethics committee of the Medical University Vienna as part of the Austrian Long COVID registry Project (EK 1591/2021). Written informed consent to participate in this study was provided by participants.
The Austrian long COVID registry is a nationwide registry, supported by Gesundheit Österreich GmbH, the Ministry of Health, Medical University of Vienna, Austria Health Insurance Fund, Danube University Krems and two Ludwig Boltzmann Institutes. The overall aim of the registry is to assess the disease course of post/long-COVID-19, evaluate its impact on quality of life on functional capacity, and, e.g., assess the interventions offered (26). Individuals who are referred to one of the participating centers - from primary care centers to rehabilitation centers - and who meet the above inclusion criteria will be asked to complete an online questionnaire covering the above objectives in self-report form and will then be registered in this way.
2.4 Data collection
The authors chose the two most common cognitive interviewing techniques for data collection, thinking aloud and probing (27). The interviews were conducted by the first author and took place either in person at the rehabilitation center or by telephone. Participants were instructed to express what came to their mind (“thinking aloud”) while concurrently completing the C19-YRS (23), and in addition they answered probes retrospectively (28) (see Supplementary Appendix 3).
Typically, 10 to 50 interviews are conducted and analyzed in cognitive interviewing studies (29). Since no further new aspects emerged after 26 interviews, the author ended the data collection at this point as she assumed that thematic saturation was reached. Thematic saturation is achieved when further observations and analyzes do not yield any newer topics (30).
2.5 Data analysis
The authors used a method of analysis called framework, a matrix-based approach to manage qualitative data (31, 32). They entered summaries of individual interviews into a series of grids. Domains of inquiry, in this case, each question and text of the C19-YRS were in the same order as in the test questionnaire. This approach allowed the authors to review the data collected systematically (33).
2.5.1 Descriptive analysis
Next, the authors conducted a descriptive analysis to understand how participants interpreted the test questions and identified factors that influenced interpretation and responses. Familiarity with the data was ensured by reading the matrices and taking notes at each step to record ideas that emerged and were considered helpful for moving forward. The range of responses for each question was noted and then categorized in the next step to identify similarities and differences as participants may have interpreted to test questions differently (34).
2.5.2 Explanatory analysis
Then, the authors conducted an explanatory analysis to understand how these potential problems arose. First, they identified patterns in the data, and then these patterns were linked to, for example, participant understanding or response, which helped identify mechanisms by which problems emerged. The next step was to develop explanations for the patterns, which relied on participants’ accounts captured in the cognitive interviews, whether through individual utterances or observations (34). The authors assessed whether the problem could potentially affect the quality of the data extracted from the Screening tool and made the necessary changes to the preliminary version of the C19-YRS (34).
During translation and cultural adaptation, translators wrote a report for each step they were involved. The expert committee then produced a preliminary version for the first author to use for pre-testing in the target population. All translators tried to stay close to the original version in this translation and adaptation process, but some changes were also necessary due to cultural differences. After completing the descriptive and explanatory analysis, the preliminary version was revised and sent to two health professionals experienced in working with people suffering from persistent symptoms after acute COVID-19 infection. Similar to peer-reviewing, their comments were then incorporated when creating the final Austrian-German version of the C19-YRS.
3 Results
First, the translators decided to expand the subtitle to “Self Report Version” to make the objective of the screening tool sufficiently clear from the outset, although the original English title of the C19-YRS, “COVID-19 Yorkshire Rehabilitation Scale (C19-YRS)”, was retained. Therefore, they decided to expand the subtitle to “Self-Assessment Questionnaire for the Assessment of Persistent COVID-19 Symptoms”. Second, the original introductory text stated, “Your answers will be recorded in your clinical record”. The translators recognized they could not generally claim that the data collected would be recorded in clinical records, so this was deleted. Third it states, “If you can’t remember, just indicate “don’t know” Since there was no box for “don’t know” where this could have been written, this was erased because the translators felt it could be misleading and confusing.
The first author then used this preliminary version to conduct the cognitive interviews. Twenty-six participants agreed to take part in the cognitive interviews. All of these participants were still suffering from persistent symptoms at the time of recruitment and thus constituted the target population of the C19-YRS. Characteristics of the study population are presented in Table 1.
Cognitive interviews lasted from 20 to 30 min, whether the interviewer conducted the interviews via telephone (n = 10) or on site (n = 16), while participants were filling out the pre-final version of the C19-YRS. The results of the interviews were primarily based on statements made by participants during the think aloud process, supported by the probes administered, as well as observations made from the interviewer. Observations were limited in telephone interviews, however, think aloud was more sufficient on the telephone as the participants appeared to talk the interviewer through each question more than those on site. The sample size of 26 seems appropriate, as no new topics emerged.
Questions 1-3
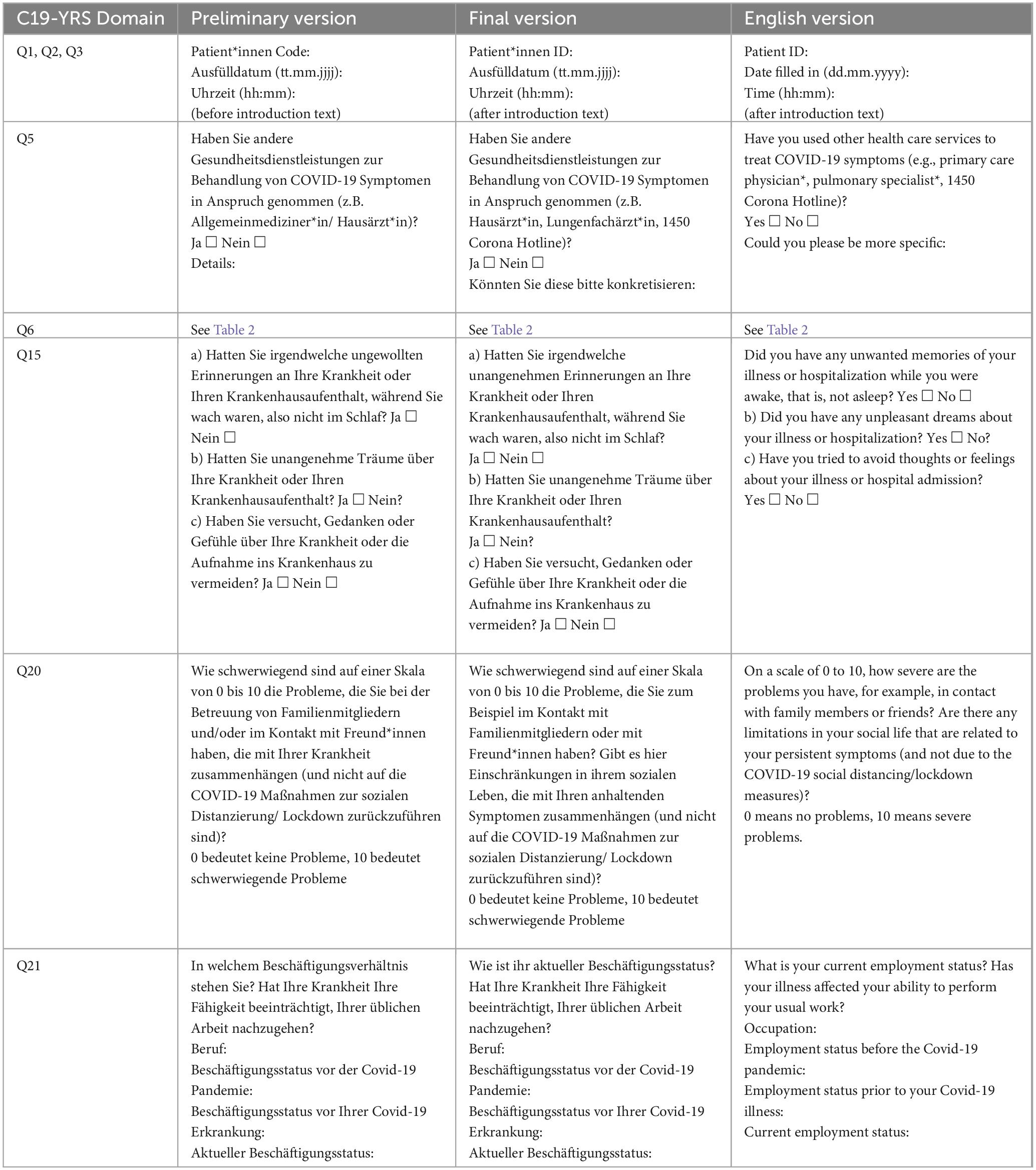
Q1 to 3 asked for the patient code, date and time, first under the heading. Fourteen of twenty-six participants skipped completing Q1 to 3 without further comment. The author observed that respondents were more likely to start with reading the introduction and therefore overlook these short questions. She suggested that these questions be listed after the introduction, and before the opening questions, and changed the location of Q1 to 3 accordingly.
Question 5 (Q5)
When completing Q5, which asked for health care services used for treatment of COVID-19 symptoms, eight participants wondered whether this question also meant specific health services such as rehabilitation centers and pulmonary physicians. They stated that the term “other health services” was not specific enough, therefore, the author decided to add other examples besides general practitioner (GP) that were mentioned by participants when thinking aloud (e.g., pulmonary physicians, 1450 Corona hotline in Austria). In addition, two participants asked whether Q5 meant the time of the acute infection or the time after, which could also have an influence on the answers, as the responses may vary depending on the requested time. It was clear from the outset of the original questionnaire that this question asked about treatment during the acute infection, so the author adjusted the translation accordingly.
Question 6 (Q6)
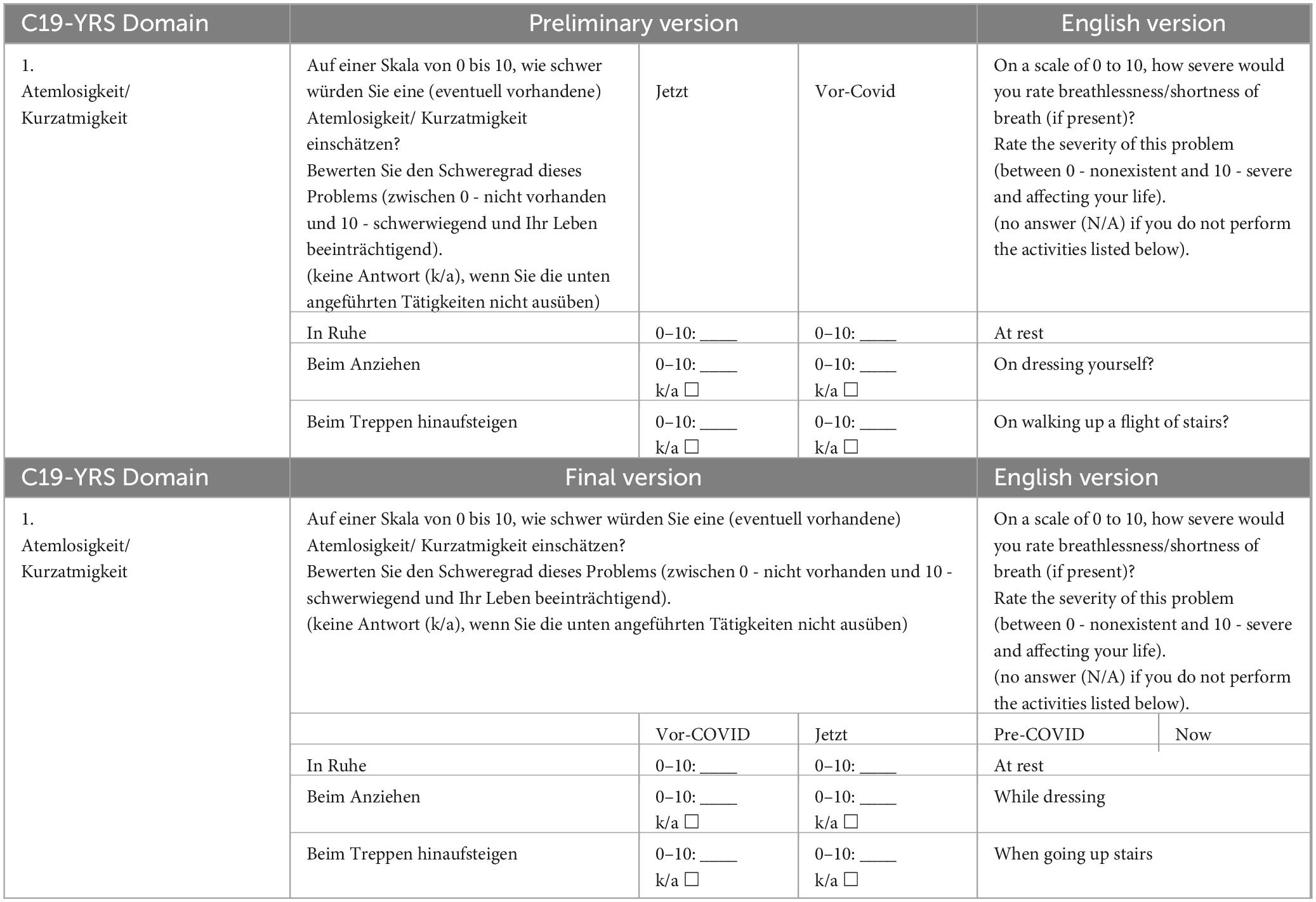
Eight out of twenty-six respondents were observed to complete Q6, which measures the extent of breathlessness, incorrectly because the answer to the question was misplaced. Three respondents additionally asked for help in completing it. The author observed that the visual representation of the question was misleading in this part of the questionnaire, as was the case with Q1 to 3, and therefore adapted this question to the visual appearance of the others (see Table 2).
Question 15 (Q15)
All participants agreed that Q15, which was screening post-traumatic stress disorder (PTSD), had to be read repeatedly to be understood while some also asked the interviewer for a verbal explanation. Four other participants interpreted the question as referring only to people who were in the hospital. The author concluded that the wording of the question was flawed and changed the question according to the verbal explanations she gave to the participants during the interviews, which were well understood.
Question 20 (Q20)
Nine participants indicated that Q20, evaluating the social role, was not easy to understand and required a verbal explanation from the author. Participants suggested that the question be reworded and Q20, like Q15 above, was rephrased according to the author’s verbal explanation.
Question 21 (Q21)
Eight participants mentioned that Q21, requesting information on employment, was not clear, and they said that the answer choices provided did not correspond to the question asked. Therefore, the author tried to rephrase the original question to connect to the answers more appropriately.
According to every participant, the screening instrument has generally worked well for capturing their long COVID condition. Participants indicated that the C19-YRS was clear, feasible, user-friendly, and appropriate. Therefore, the authors would like to state that it appears that the face validity in the C19-YRS is good.
Following the results of the cognitive interview data analysis, the authors made changes to the preliminary version of the C19-YRS. The changes to the preliminary version were then proofread by two health professionals who work with patients with persistent symptoms after acute COVID-19 infection. After the authors incorporated their comments, which were mainly wording recommendations, a final version was prepared (see Supplementary Appendix 2). Table 3 illustrates the changes made from the preliminary to the final version, including feedback from both participants and health professionals.
4 Discussion
The present work is the first German version of the C19-YRS, a screening tool for long COVID. To date, this appears to be the only translation of a COVID-19-specific assessment or screening tool into German. Based on a rigorous translation and cross-cultural adaptation process that included 26 cognitive interviews with patients and feedback from health professionals working in the field of long COVID, as well as the evaluation of its face validity, a German version was created. Overall, this study emphasizes that the translated version of the C19-YRS appears suitable to assess persistent symptoms and to support establishment of standardized care in German speaking countries.
The final German C19-YRS has been carefully reviewed and compared with the original, as equivalence between the original and the translated version is considered important (35). No relevant differences were found that could affect the use of the C19-YRS in any way. Adjustments were necessary to adequately reflect the context in which the screening instrument is embedded. These inconsistencies primarily led to changes in the introductory text of the C19-YRS and the items recording the persistent symptoms were hardly affected.
However, instrument translation is usually accompanied by a change in context (25, 36). But compared to previous studies in which researchers reported that translation and cultural adaptation of assessments often lead to alteration in meaning and even deletion of items (37, 38), changes in the case of this study are marginal. Although the current work could be seen as an unproblematic adaptation process with only few changes, other explanations should also be discussed. As such, perhaps the premature state of current research on long COVID and the resulting limited knowledge of individuals affected and health professionals involved in their care (39) has actually restricted more critical examination of the C19-YRS. Widely varying descriptions and definitions of long COVID continue to appear in the literature, as well as in everyday language. With primary studies and reviews appearing at a rapid pace, current findings are inevitably associated with methodological differences and limitations. To improve our knowledge of long COVID, well-designed prospective studies are needed to establish long COVID definitions, accurate differentiation of symptoms, and appropriate treatment of this emerging condition (39). The increase in knowledge could then lead to a more critical review and adaptation process with the C19-YRS at a later date, possibly involving even greater significant changes.
The evaluation of the face validity was also rather superficial compared to other cross-sectional studies focusing on translation and cross-cultural adaptation (40, 41). Further investigation of psychometric properties, including internal consistency, already demonstrated in the original version (19), should be considered in future studies of the Austrian-German version of the C19-YRS.
5 Methodological considerations
The combination of different data sources and methods, as well as the involvement of numerous researchers in the translation process, in the sense of triangulation, enriched the data and reduced bias (42, 43). According to Collins (23), a sufficient number of participants were employed in this study, aiming to verify that the participants’ understanding of the questionnaire items was in line with the intended meaning. The sample was balanced, such that the participants represented both genders, a variety of age groups, and both native speakers and non-native speakers. Peer review by health professionals was also beneficial, as their expertise was particularly helpful in adapting this comprehensive assessment.
Nevertheless, this is a report of a small-scale study. In comparison, Beaton et al. (22) suggests 30–40 participants for the pre-test. This study should be replicated in a bigger sample, including a population characterized by different treatment experiences. In this case, only individuals who were already assigned to a rehabilitation facility, that can currently be considered an essential part of treatment for long COVID in Austria, participated in this study. Also, the fact that the interviews were not recorded and transcribed could lead to a lower credibility of the results. The interviews were conducted by the first author and predominantly evaluated by her. As a control, interviews could have been conducted and evaluated by other researches as well. A high agreement would have been a sign for trustworthiness (44), as in the study of Friedli and Gantschnig (37). Content validity and internal consistency should be considered for further investigations (26), as face validity, often giving a first impression without going into too much detail, is overall a very subjective assessment (45).
6 Conclusion
In conclusion, this study resulted in a German version of the C19-YRS. It is expected that the provision of this multi-professional screening tool will support the initial assessment of persistent symptoms, and the establishment of standardized care pathways in Austria. First, however, the psychometric properties should be further explored. Then, the efforts of the broader multi-professional rehabilitation team will be essential to ensure that this screening tool is successfully used in practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study involving human participants was reviewed and approved by the Ethics Committee of the Medical University Vienna as part of the Austrian Long COVID registry. Participation was open to people who had been diagnosed with COVID-19 infection, who were experiencing long-term symptoms following a COVID-19 infection, who were at least 18 years old, who understood and spoke sufficient German and who agreed to participate.
Author contributions
LS: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review and editing. TS: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review and editing. EM: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review and editing. VR: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review and editing. MS: Resources, Supervision, Writing – review and editing. KH: Supervision, Writing – review and editing. BG: Methodology, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank everyone involved for their help with the translations and also thank all the participants who took the time to participate in the cognitive interview process during their rehabilitation.
Conflict of interest
TS has received grant/research support from AbbVie and Roche, has been a consultant for AbbVie and Sanofi Genzyme, and has been a paid speaker for AbbVie, Novartis, Roche, Sanofi, and Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1401491/full#supplementary-material
References
1. Davis H, McCorkell L, Vogel J, Topol E. Long COVID: Major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-22
3. Baricich A, Borg M, Cuneo D, Cadario E, Azzolina D, Balbo P, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur J Phys Rehabil Med. (2021) 57:199–207. doi: 10.23736/S1973-9087.21.06699-5
4. Halpin S, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. (2021) 93:1013–22. doi: 10.1002/jmv.26368
5. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-673632656-8
6. Mandal S, Barnett J, Brill S, Brown J, Denneny E, Hare S, et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. (2021) 76:396–8. doi: 10.1136/thoraxjnl-2020-215818
7. Méndez R, Balanzá-Martínez V, Luperdi S, Estrada I, Latorre A, González-Jiménez P, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. (2021) 290:621–31. doi: 10.1111/joim.13262
8. Miskowiak K, Johnsen S, Sattler S, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol. (2021) 46:39–48. doi: 10.1016/j.euroneuro.2021.03.019
9. van den Borst B, Peters J, Brink M, Schoon Y, Bleeker-Rovers C, Schers H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis. (2020) 73:e1089–98. doi: 10.1093/cid/ciaa1750
10. Nalbandian A, Sehgal K, Gupta A, Madhavan M, McGroder C, Stevens J, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
11. Ayoubkhani D, Bermingham C, Pouwels K, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ. (2022) 377:e069676. doi: 10.1136/bmj-2021-069676
12. Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. (2022) 328:676–8. doi: 10.1001/jama.2022.11691
13. Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. (2023) 2:e000385. doi: 10.1136/bmjmed-2022-000385
14. Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. (2022) 28:2398–405. doi: 10.1038/s41591-022-02051-3
15. Stamm T, Partheymüller J, Mosor E, Ritschl V, Kritzinger S, Eberl J. Coronavirus vaccine hesitancy among unvaccinated Austrians: Assessing underlying motivations and the effectiveness of interventions based on a cross-sectional survey with two embedded conjoint experiments. Lancet Reg Health Eur. (2022) 17:100389. doi: 10.1016/j.lanepe.2022.100389
16. Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Developing services for long COVID: Lessons from a study of wounded healers. Clin Med (Lond). (2021) 21:59–65. doi: 10.7861/clinmed.2020-0962
17. Parkin A, Davison J, Tarrant R, Ross D, Halpin S, Simms A, et al. A multidisciplinary NHS COVID-19 service to manage post-COVID-19 syndrome in the community. J Prim Care Community Health. (2021) 12:21501327211010994. doi: 10.1177/21501327211010994
18. Sivan M. The self-report version and digital format of the COVID-19 Yorkshire rehabilitation scale (C19-YRS) for long COVID or Post-COVID syndrome assessment and monitoring. Adv Clin Neurosci Rehabil. (2021) 20:9348.
19. O’Connor R, Preston N, Parkin A, Makower S, Ross D, Gee J, et al. The COVID-19 Yorkshire rehabilitation scale (C19-YRS): Application and psychometric analysis in a post-COVID-19 syndrome cohort. J Med Virol. (2022) 94:1027–34. doi: 10.1002/jmv.27415
20. Calvert M, Blazeby J, Altman D, Revicki D, Moher D, Brundage M, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. (2013) 309:814–22. doi: 10.1001/jama.2013.879
21. Bingham C, Noonan V, Auger C, Feldman D, Ahmed S, Bartlett S. Montreal accord on patient-reported outcomes (PROs) use series - Paper 4: Patient-reported outcomes can inform clinical decision making in chronic care. J Clin Epidemiol. (2017) 89:136–41. doi: 10.1016/j.jclinepi.2017.04.014
22. Beaton D, Bombardier C, Guillemin F, Ferraz M. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. (1976) 2000:3186–91. doi: 10.1097/00007632-200012150-00014
23. Collins D. Cognitive interviewing: Origin, purpose and limitations. In: Collins D editor. Cognitive interviewing practice. Thousand Oaks, CA: SAGE (2015).
24. Anthoine E, Moret L, Regnault A, Sébille V, Hardouin J. Sample size used to validate a scale: A review of publications on newly-developed patient reported outcomes measures. Health Qual Life Outcomes. (2014) 12:176. doi: 10.1186/s12955-014-0176-2
25. Mokkink L, Terwee C, Patrick D, Alonso J, Stratford P, Knol D, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. (2010) 63:737–45. doi: 10.1016/j.jclinepi.2010.02.006
26. Stamm T. Study protocol for the registry for post/long COVID-19 conditions in Austria. Vienna: Ethikkommission Medizinische Universität Wien (2021).
27. Gray M. Conductive cognitive interviews. In: Collins D editor. Cognitive interviewing practice. Thousand Oaks, CA: SAGE (2015).
28. D’ardenne J. Developing interview protocol. In: Collins D editor. Cognitive interviewing practice. Thousand Oaks, CA: SAGE (2015).
29. Collins D, Gray M. Sampling and recruitment. In: Collins D editor. Cognitive interviewing practice. Thousand Oaks, CA: SAGE (2015). 4 p.
30. Green J, Thorogood N. Qualitative methods for health research. 2nd ed. Thousand Oaks, CA: Sage Publications (2004).
31. Miles M, Huberman A. Qualitative data analysis: An expanded sourcebook. Thousand Oaks, CA: Sage Publications (1994).
32. Spencer L, Ritchie J, O’Connor W. Analysis: Practices, principles and processes. 1st ed. In: Ritchie J, Lewis J editors. Qualitative research practice. Thousand Oaks, CA: Sage Publications (2003). p. 199–218.
33. D’ardenne J, Collins D. Data management. In: Collins D editor. Cognitve interviewing practice. Thousand Oaks, CA: SAGE (2015).
34. Collins D. Analysis and interpretation. In: Collins D editor. Cognitive interviewing practice. Thousand Oaks, CA: SAGE (2015).
35. Streiner D, Norman G, Cairney J. Health measurement scales. A practical guide to their development and use. 5th ed. Oxford: Oxford University Press (2015).
36. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: Report of the ISPOR task force for translation and cultural adaptation. Value Health. (2005) 8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x
37. Friedli T, Gantschnig B. Testing the iMTA productivity costs questionnaie (iPCQ) for the use with chronic disease patients in Switzerland. Int J Health Profess. (2022) 9:25–38.
38. Schulze C, Page J, Lilja M, Kottorp A. Cross-cultural validity of the German version of the pediatric evaluation of disability inventory (PEDI-G)-a Rasch model application. Child Care Health Dev. (2017) 43:48–58. doi: 10.1111/cch.12401
39. Nittas V, Gao M, West E, Ballouz T, Menges D, Wulf Hanson S, et al. Long COVID through a public health lens: An umbrella review. Public Health Rev. (2022) 43:1604501. doi: 10.3389/phrs.2022.1604501
40. Axelsson M, Kottorp A, Carlson E, Gudmundsson P, Kumlien C, Jakobsson J. Translation and validation of the Swedish version of the IPECC-SET 9 item version. J Interprof Care. (2022) 36:900–7. doi: 10.1080/13561820.2022.2034762
41. Kamwesiga J, von Koch L, Kottorp A, Guidetti S. Cultural adaptation and validation of stroke impact scale 3.0 version in Uganda: A small-scale study. SAGE Open Med. (2016) 4:2050312116671859. doi: 10.1177/2050312116671859
43. Guba E, Lincoln Y. Competing paradigms in qualitative research. In: Denzin N, Lincoln Y editors. Handbook of qualitative research. Thousand Oaks, CA: Sage Publications (1994). p. 105–17.
Keywords: long-COVID, long-term consequences, post-acute sequelae of SARS-CoV-2 (PASC), rehabilitation, patient-reported outcomes
Citation: Sperl L, Stamm T, Mosor E, Ritschl V, Sivan M, Hoffmann K and Gantschnig B (2024) Translation and cultural adaptation of the COVID-19 Yorkshire Rehabilitation Scale into German. Front. Med. 11:1401491. doi: 10.3389/fmed.2024.1401491
Received: 15 March 2024; Accepted: 16 August 2024;
Published: 30 August 2024.
Edited by:
Arch Mainous, University of Florida, United StatesReviewed by:
Stefan Tino Kulnik, Ludwig Boltzmann Institute for Digital Health and Prevention, AustriaRoland Axmann, Gesundheitszentrum Peterhof ÖGK, Austria
Copyright © 2024 Sperl, Stamm, Mosor, Ritschl, Sivan, Hoffmann and Gantschnig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanja Stamm, dGFuamEuc3RhbW1AbWVkdW5pd2llbi5hYy5hdA==
 Lisa Sperl1,2
Lisa Sperl1,2 Tanja Stamm
Tanja Stamm Erika Mosor
Erika Mosor Valentin Ritschl
Valentin Ritschl Manoj Sivan
Manoj Sivan