- 1Department of Critical Care Medicine, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 2Department of Emergency Medicine, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3Department of Emergency and Critical Care Medicine, Henan Engineering Research Center for Critical Care Medicine, Henan Key Laboratory of Critical Care Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 4Critical Care Medicine, The Second Xiangya Hospital, Changsha, Hunan, China
- 5Department of Critical Care Medicine, Guiqian International General Hospital, Guiyang, Guizhou, China
Background: Some cohort studies have explored the effects and safety of polymyxin B (PMB) in comparison to other antibiotics for the treatment of nosocomial infections, yielding inconsistent results. This systematic review aims to explore the effectiveness and safety of PMB and compared it with other antibiotics.
Methods: A systematic literature search was conducted in PubMed, Embase, the Cochrane Library, and Web of Science, searching specific terms to identify quantitative cohort studies or RCTs that compared the effects of PMB with other antibiotics in terms of their efficacy and safety. The Newcastle–Ottawa Scale (NOS) was conducted to evaluate the risk of bias of observational studies. Odds ratios with 95% confidence intervals were used for outcome assessment. We evaluated heterogeneity using the I2 test.
Results: A total of 22 observational trials were included in the analysis. The PMB group had a higher mortality rate compared to the control group (odds ratio: 1.84, 95% CI: 1.36–2.50, p<0.00001, I2 = 73%). while, the ceftazidime-avibactam group demonstrated a distinct advantage with lower mortality rates, despite still exhibiting high heterogeneity (odds ratio 2.73, 95% confidence interval 1.59–4.69; p = 0.0003; I2 = 53%). Additionally, the PMB group had a lower nephrotoxicity rate compared to the colistin group but exhibited high heterogeneity in the results (odds ratio 0.58, 95% CI 0.36–0.93; p = 0.02; I2 = 73%).
Conclusion: In patients with nosocomial infections, PMB is not superior to other antibiotics in terms of mortality, specifically when compared to ceftazidime-avibactam. However, PMB demonstrated an advantage in terms of nephrotoxicity compared to colistin.
Background
In the past decade, nosocomial infections caused by multidrug-resistant (MDR) bacteria have emerged as a significant cause of mortality and morbidity worldwide, particularly among critically ill patients (1–8). Notably, limitation in antibiotics options against MDR bacterial infections became an extreme challenge in nosocomial settings in recent decades. Estimates suggest that in the United States alone between 5 and 10% of hospitalized patients admitted to acute-care hospitals may acquire nosocomial infections (3, 4).
Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae are the most common MDR bacteria causing fatal nosocomial infections, especially the strains producing extended-spectrum β-lactamases (ESBLs), New Delhi Metallo-beta-lactamase (NDM) and Carbapenemases as well. With a growing threat of MDR bacteria, however, there have been only a few new drugs developed in recent decades, such as tigecycline, ceftazidime-avibactam and eravacycline. As instead, some old antibiotics were reassessed for their potential effectiveness against MDR Gram-negative bacteria. Of them, colistin and polymyxin B (PMB) have gained a great of attention in the last decade (9, 10). Significantly, they were reintroduced as salvage therapy for infections caused by MDR Gram-negative bacteria that did not respond to other treatments (11). Meanwhile, a controversy remains in physician’s options for their clinical uses owing to the adverse events, especially with regard to the nephrotoxicity and the neurotoxicity. For instance, PMB demonstrated a lower renal toxicity than the comparable dose of colistin in several trials (12–16). Additionally, pharmacologically experimental data demonstrated that PMB achieved effective plasma concentrations more rapidly than colistin while administrating intravenously (17, 18). Moreover, previous researches suggested that PMB showed a better efficacy in treatment of nosocomial infections caused by MDR Gram-negative bacteria including Acinetobacter baumannii, Pseudomonas aeruginosa, and CRO (12, 19). On the other hand, a few cohort studies showed that Colistin exhibited a lower mortality rate compared to PMB (12). In this systematic review and meta-analysis, therefore, we sought to study the effectiveness and safety of PMB in treatment of nosocomial infection caused by MDR bacteria comparing with colistin and other antibiotics as well.
Method
We conducted our systematic review and meta-analysis by using predefined protocol according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) reporting guideline (20). The review protocol was registered in PROSPERO (CRD42023446418).
Data sources and search strategy
A systematic literature search was conducted in PubMed, Embase, the Cochrane Library, and Web of Science from the beginning to August 1, 2023, to include clinical trials in patients of nosocomial infections associated with polymyxin B (PMB) and other antibiotics. The search only included English publications. The search terms were “Polymyxin B,” “Drug Resistance, Multiple, Bacterial,” “Aerosporin,” “mortality,” “Hospital-Acquired Pneumonia.” In addition, the reference lists of relevant studies, systematic reviews, and meta-analysis were manually examined to identify any additional publications that could contribute to our analysis. For studies that did not report complete data on our dichotomous or continuous outcomes but were eligible for inclusion in the meta-analysis, we made efforts to contact the original investigators via email in order to acquire Supplementary Data. The search strategy was presented on Supplementary Table S1 (including PubMed database, Embase, Cochrane library and Web of science).
Study selection
Two of us (LP and XL) screened the titles and abstracts of all initially selected articles independently in accordance with the eligibility criteria. Then, the above two reviewers independently assessed full text of the selected articles from first step. Disagreements were resolved by reaching a consensus or seeking help from a third author (PM). We considered overall mortality as the primary outcome of our meta-analysis. Secondary outcomes were nephrotoxicity and microbiological eradiation.
Inclusion criteria
We considered studies to be following criteria:
1. Enrolled were adults (≥18 years old) with nosocomial infections.
2. Treated by PMB compared with other antibiotics.
3. Outcomes (mortality, nephrotoxicity or microbiological eradiation) were available.
4. Randomized controlled trials or cohort studies.
Data extraction and study quality assessment
Data extraction was conducted by 2 reviewers independently (YH and XL) using a standardized form and discussed with another reviewer (PM). And the following date were extracted: the study characteristics (setting, sample size), participant characteristics (sex, age, duration of medication), specification of interventions (types of pathogenic bacteria, the control group).
Quality assessment
Two reviewers (LP and ZZ) independently conducted risk-of-bias assessments using the Newcastle-Ottawa Scale (NOS) to evaluate the observational studies included in the meta-analysis. The NOS tool assesses the risk of bias across three domains: selection, comparability, and outcome. The NOS criteria yield a quality score ranging from 0 to 9, with higher scores indicating better study quality (21, 22). Disagreements between the two investigators were resolved through discussions involving the third investigator (LC).
Subgroup analysis
We performed subgroup analysis based on the risk of bias in the trials to optimize heterogeneity and obtain more reliable results. We also performed subgroup analysis to compare the effectiveness of PMB with different antibiotics, based on the type of antibiotic used in the control group.
Heterogeneity
Heterogeneity was assessed visually using forest plots and quantified with I2 statistics, categorized as low (0–25%), moderate (26–50%), and high (>50%). If substantial heterogeneity was present and an adequate number of publications were available (n = 10), we aimed to investigate potential sources of heterogeneity through prespecified subgroup analysis outlined in the study protocol, such as restricting the analysis to high-quality studies. Additionally, a post hoc sensitivity analysis was conducted to assess heterogeneity after excluding studies that demonstrated a statistically significant benefit from placebo treatment.
Publication bias
We intended to assess publication bias by utilizing funnel plots for outcomes with data available from 10 or more studies.
Statistical analysis
All statistical analysis were performed with the use of RevMan software (version 5.3.3; The Cochrane Collaboration). The odds ratios with associated 95% confidence intervals were used to assess outcomes. Heterogeneity was assessed by the I2 test. Random effects models are used for all analysis. We considered p values <0.05 statistically significant. Comparison adjusted funnel plots are used to evaluate the possibility of study.
Results
Search results
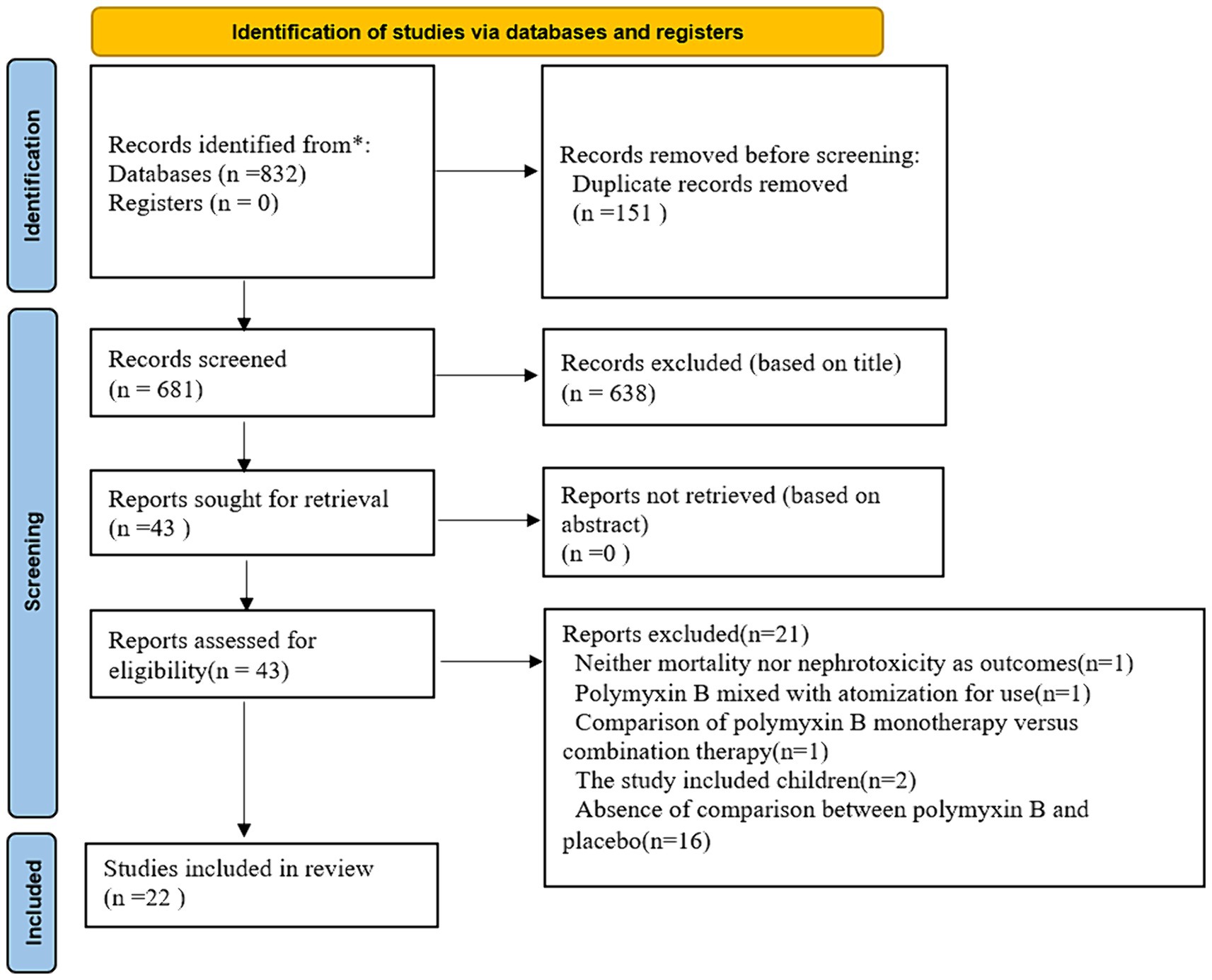
We identified 832 citations and finally 22 studies met the inclusion criteria (Figure 1). The searcher strategies were shown in Supplementary Table S1. Nineteen studies reported data on the mortality, while 16 studies described nephrotoxicity.
Study characteristics
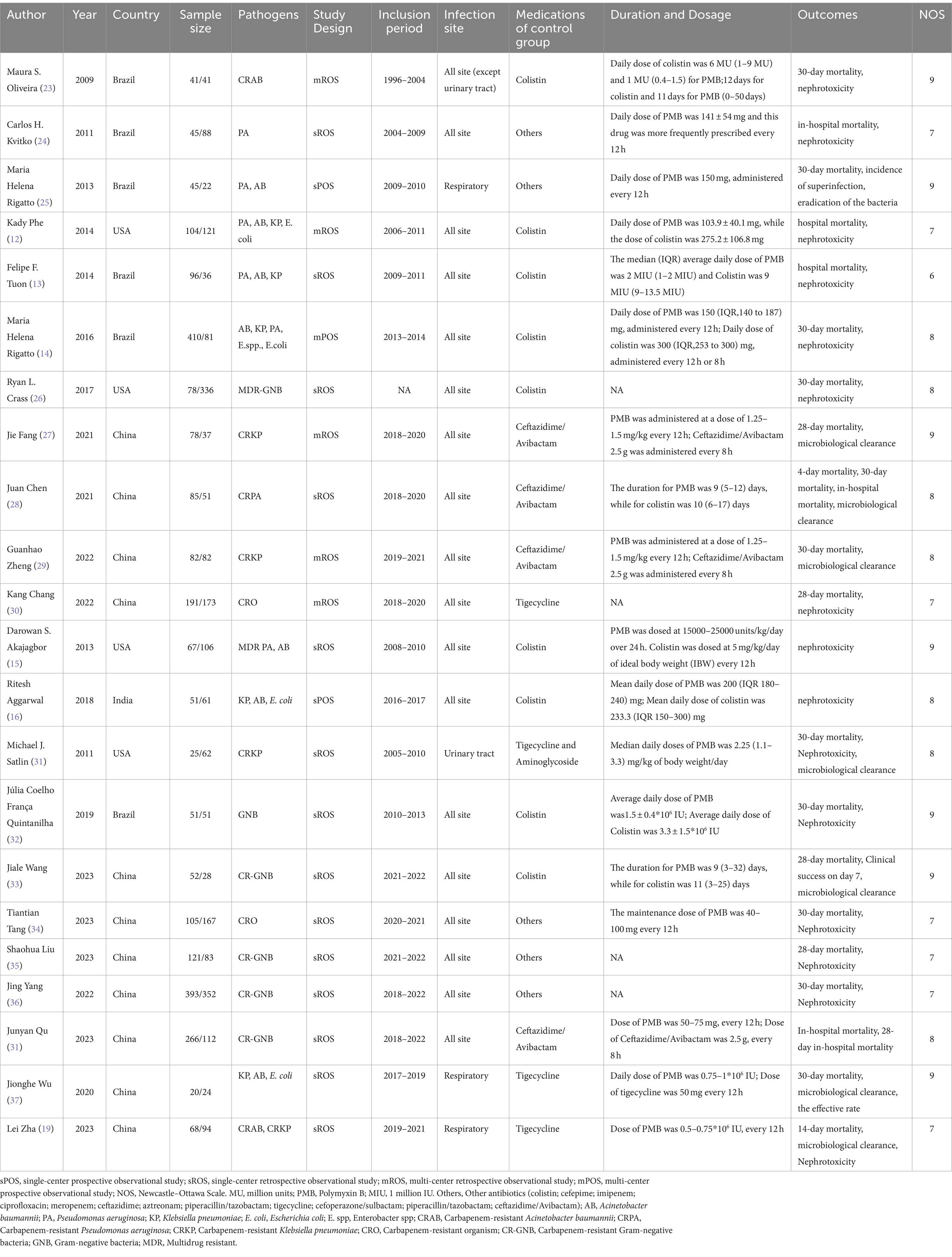
The study characteristics are displayed in Table 1. Colistin was reported as the control group antibiotic in 9 studies, tigecycline was reported in 7 studies, and ceftazidime-avibactam was reported in 7 studies. The pathogen species, the control group and the dosage and duration of antibiotic use among all studies were shown in Table 1.
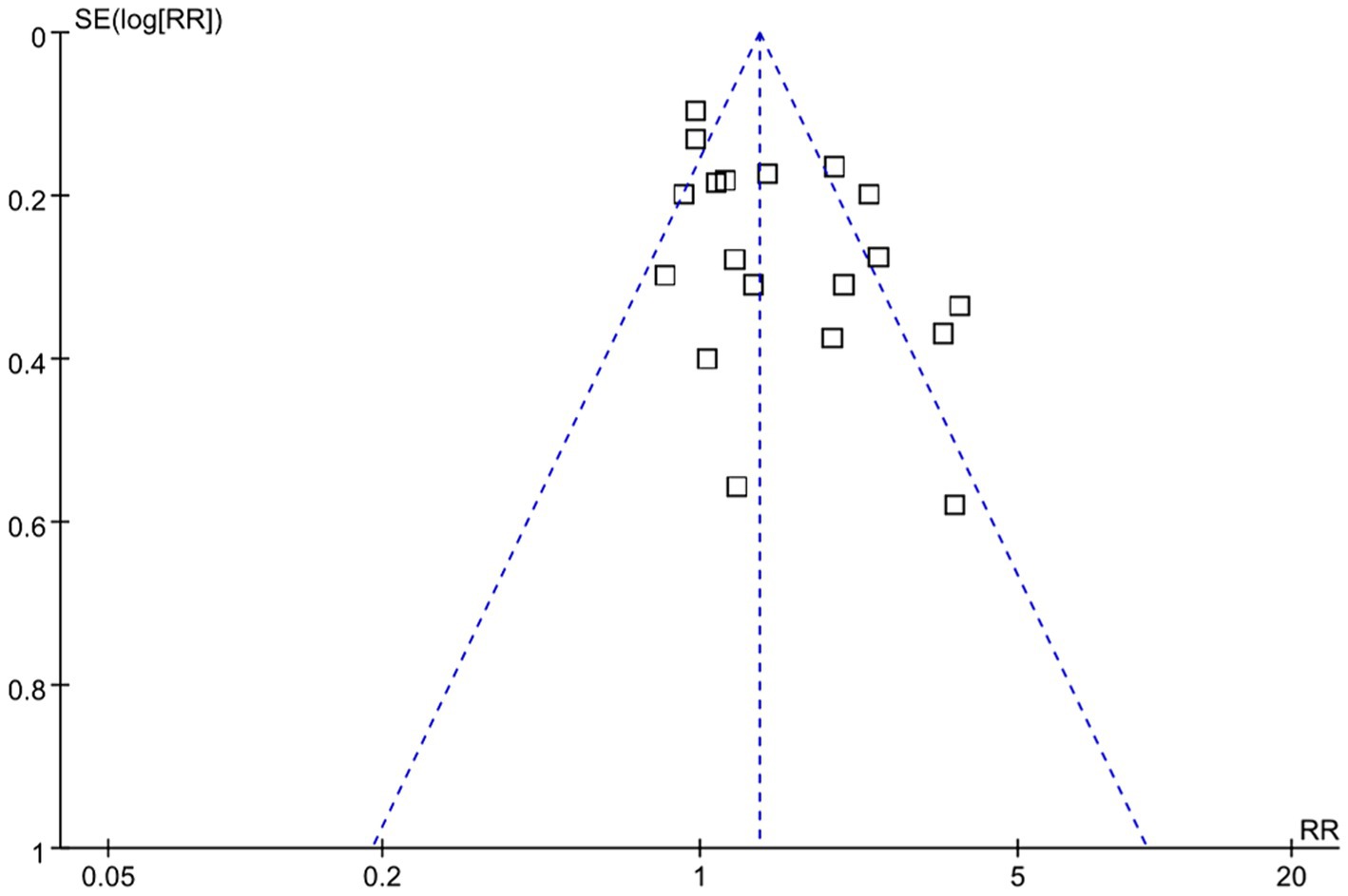
According to NOS analysis, quality scores ranged from 6 to 9, and 7 trials were rated as low risk (equal 9 score), 7 trials were rated as moderate risk (equal 8 score), and 8 trials were rated as high risk (equal 7 score or less than 7 score). The risk bias of included studies was shown in Table 1 and Supplementary Table S2. Visual analysis of the funnel plots suggested no publication bias (Figure 2).
Mortality
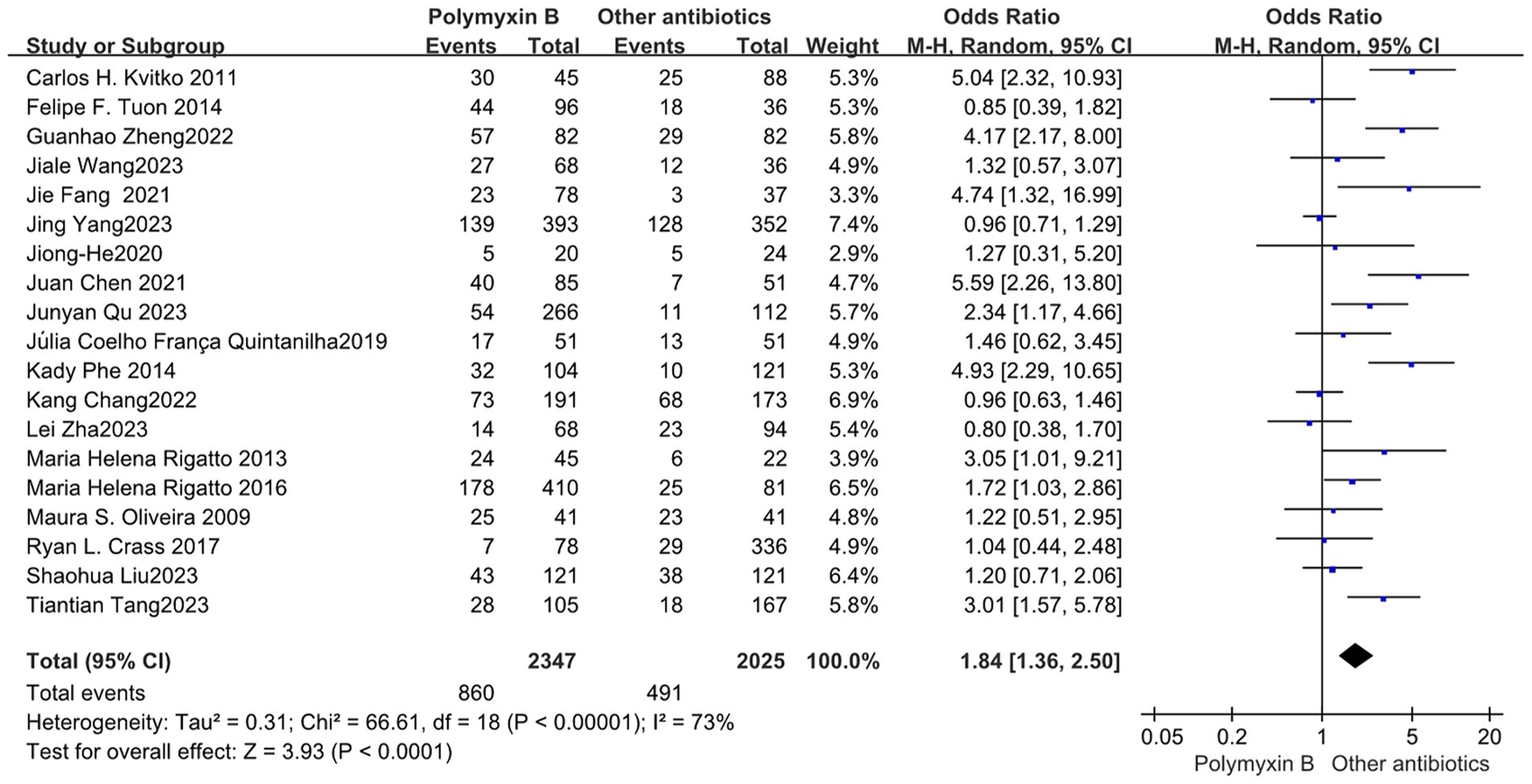
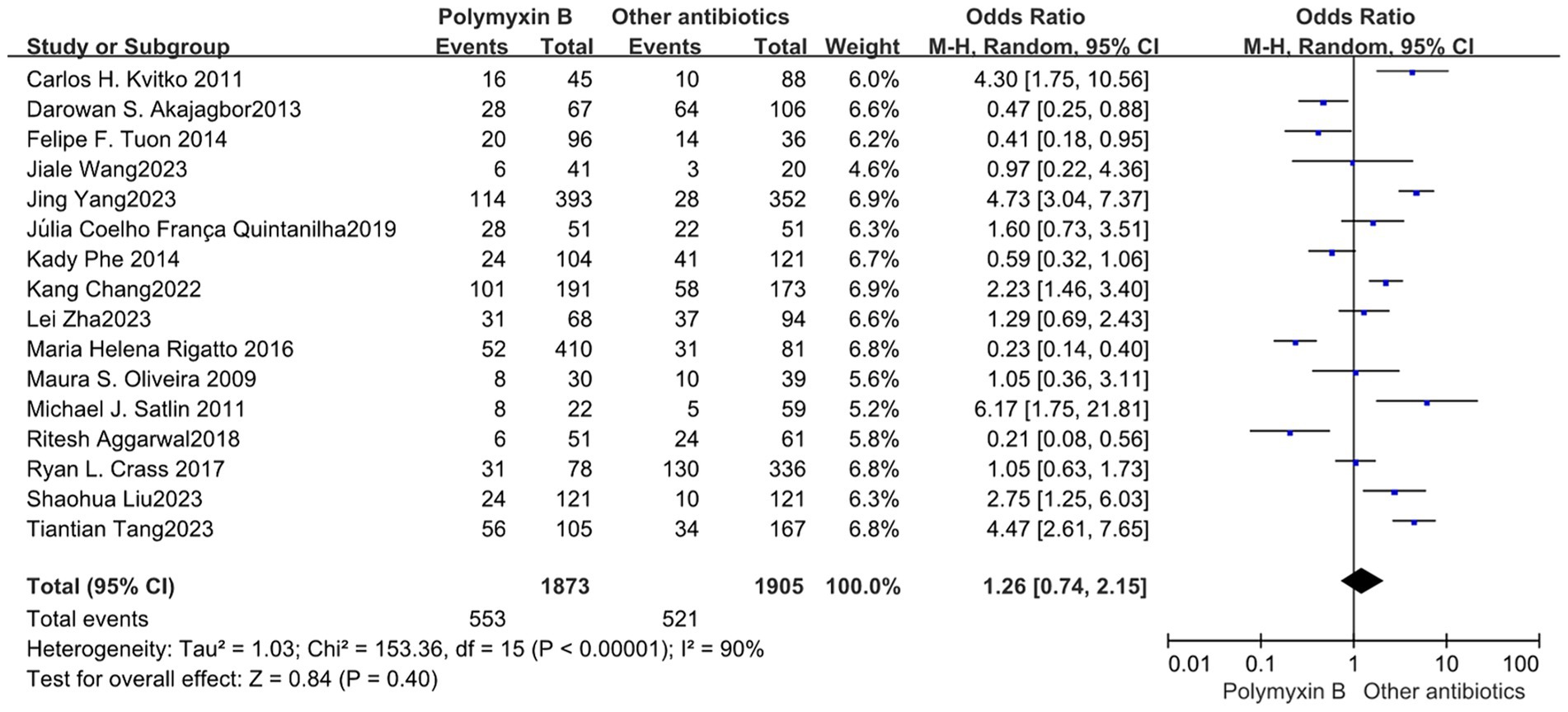
All included 19 trials (12–14, 19, 23–30, 32–38) with 4,372 patients totally mentioned the overall mortality, and our meta-analysis indicated that the mortality was higher in the PMB group than the control group (odds ratio 1.84, 95% confidence interval 1.36–2.50; p<0.00001; I2 = 73%, Figure 3). Subgroup analysis stratified by risk level indicates that the control group shows lower mortality rates in the low-risk level, along with reduced heterogeneity (odds ratio 1.69, 95% confidence interval 1.12–2.55; p = 0.01; I2 = 0%, Figure 4).
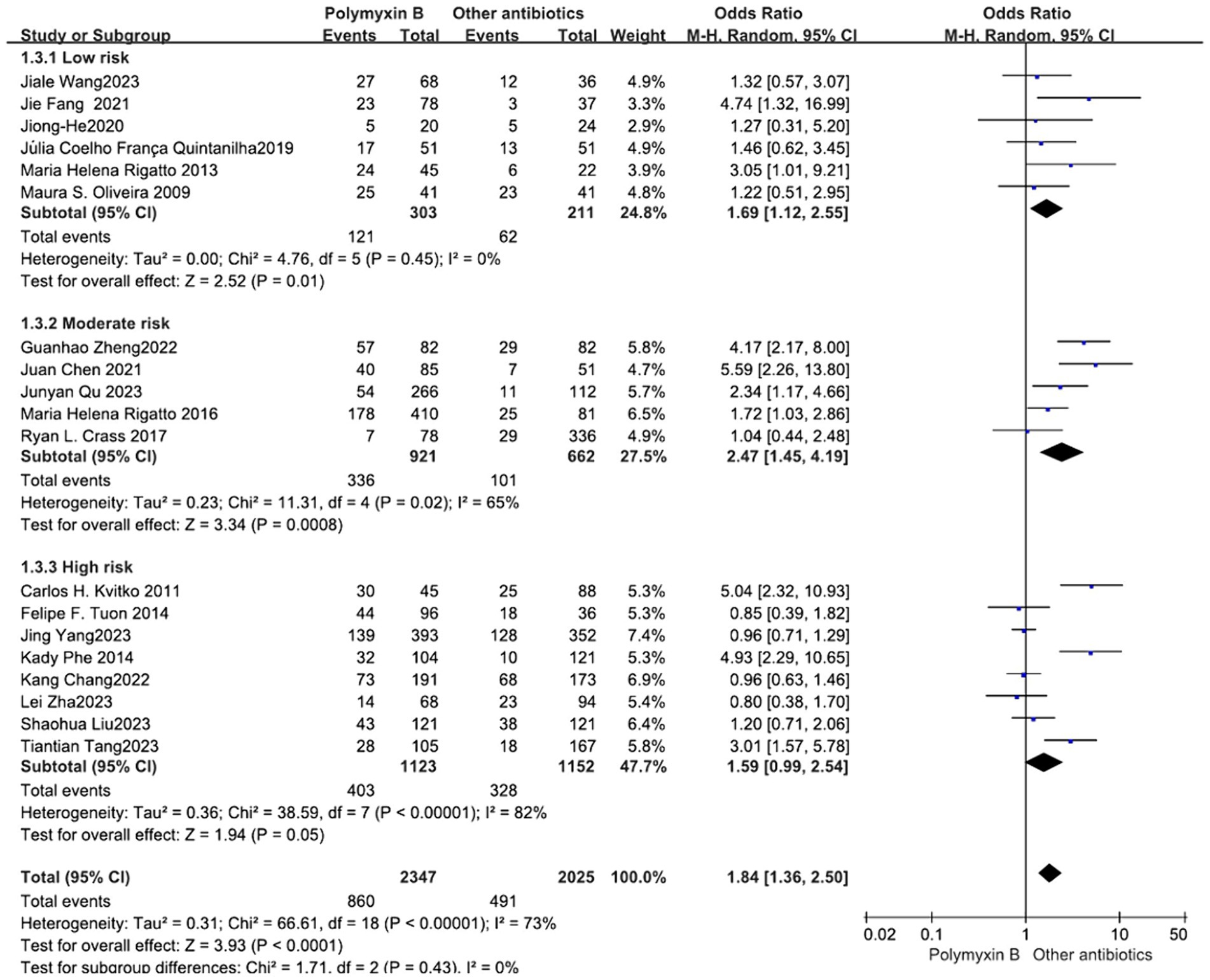
Figure 4. The mortality was compared between PMB and other antibiotics (subgroup analysis based on the risk of bias).
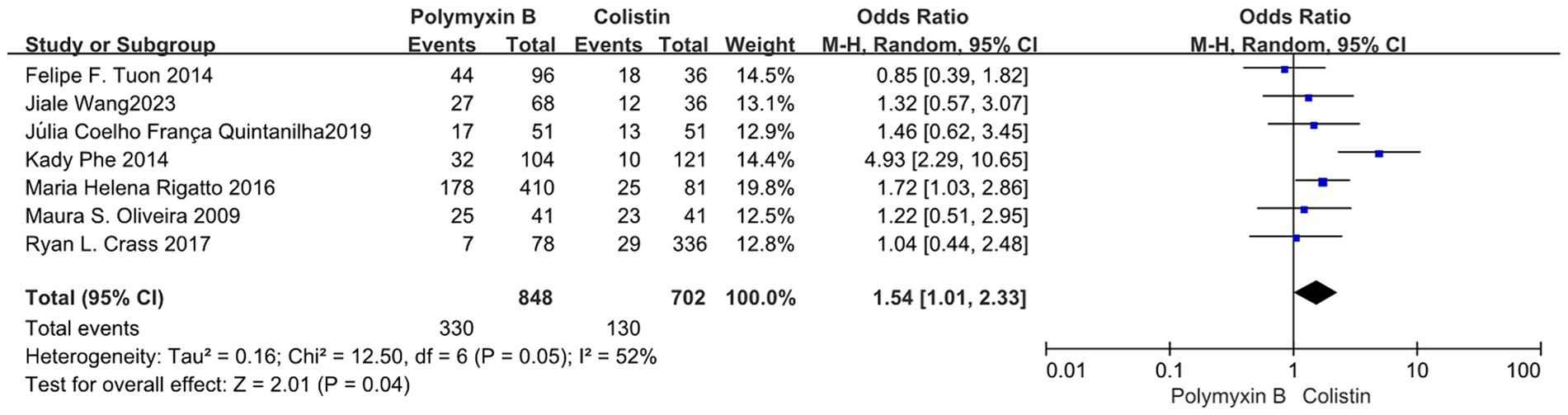
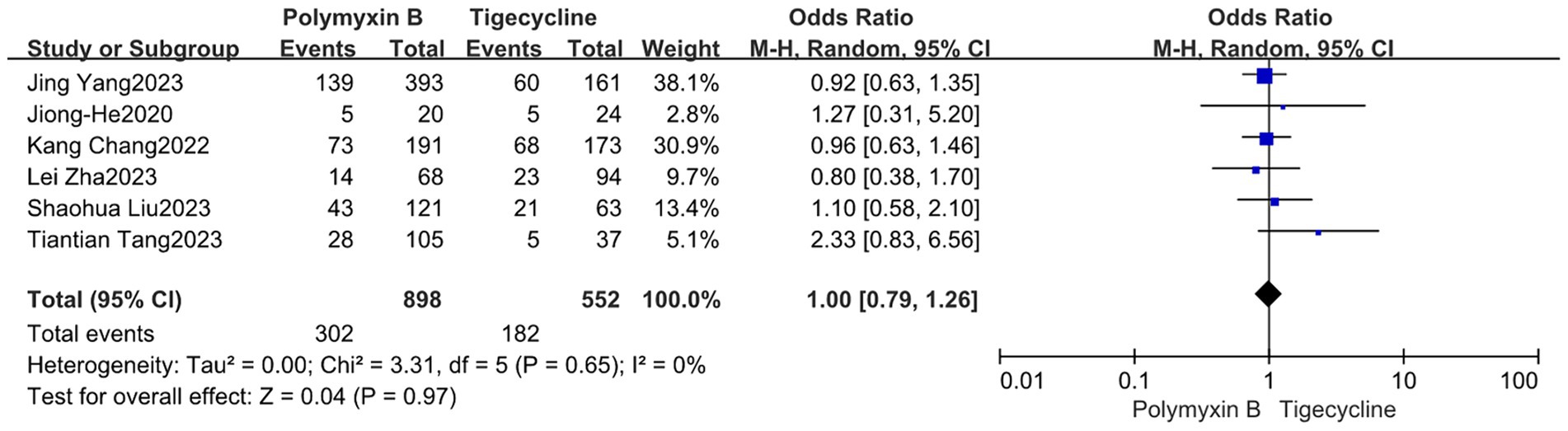
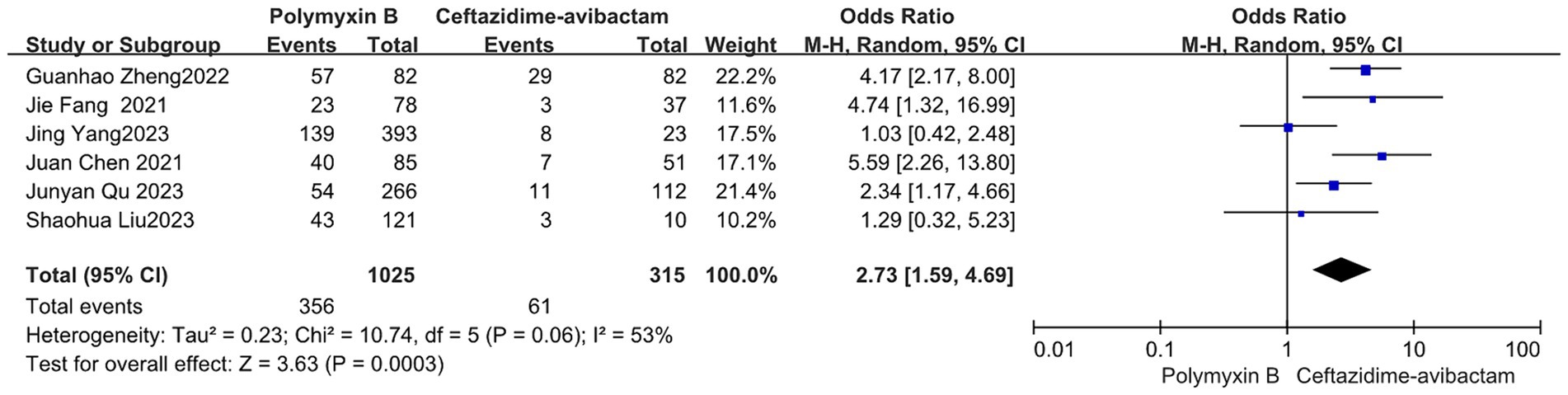
Our findings revealed that the colistin group exhibited a slightly lower mortality rate compared to the PMB group, but with high heterogeneity (odds ratio 1.54, 95% confidence interval 1.01–2.33; p = 0.04; I2 = 52%, Figure 5). Meanwhile, no significant difference in mortality was observed between the PMB group and the tigecycline group (odds ratio 1.00, 95% confidence interval 0.79–1.26; p = 0.65; I2 = 0%, Figure 6). However, the ceftazidime-avibactam group demonstrated a distinct advantage with lower mortality rates, despite still exhibiting high heterogeneity (odds ratio 2.73, 95% confidence interval 1.59–4.69; p = 0.0003; I2 = 53%, Figure 7). Notably, after excluding high-risk studies, we obtained consistent stronger results with improved heterogeneity (odds ratio 3.68, 95% confidence interval 2.47–5.49; p<0.00001; I2 = 0%, Figure 8).
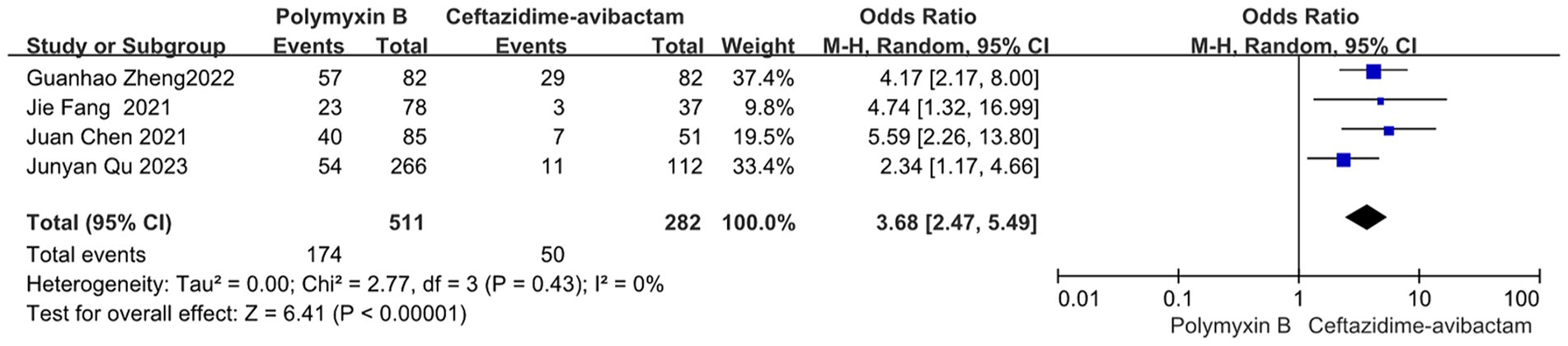
Figure 8. The mortality was compared between PMB and ceftazidime-avibactam (low and moderate risk of studies).
Nephrotoxicity
Our meta-analysis included a total of 16 trials (12–16, 19, 23, 24, 26, 30–36) involving 3,778 patients, all of which reported nephrotoxicity. The results revealed no significant difference in nephrotoxicity between the PMB group and the control group with high heterogeneity (odds ratio: 1.26, 95% confidence interval: 0.74–2.15; p<0.40; I2 = 90%, Figure 9). Stratifying the subgroup analysis by risk level yielded similar results but did not alleviate heterogeneity (Figure 10).
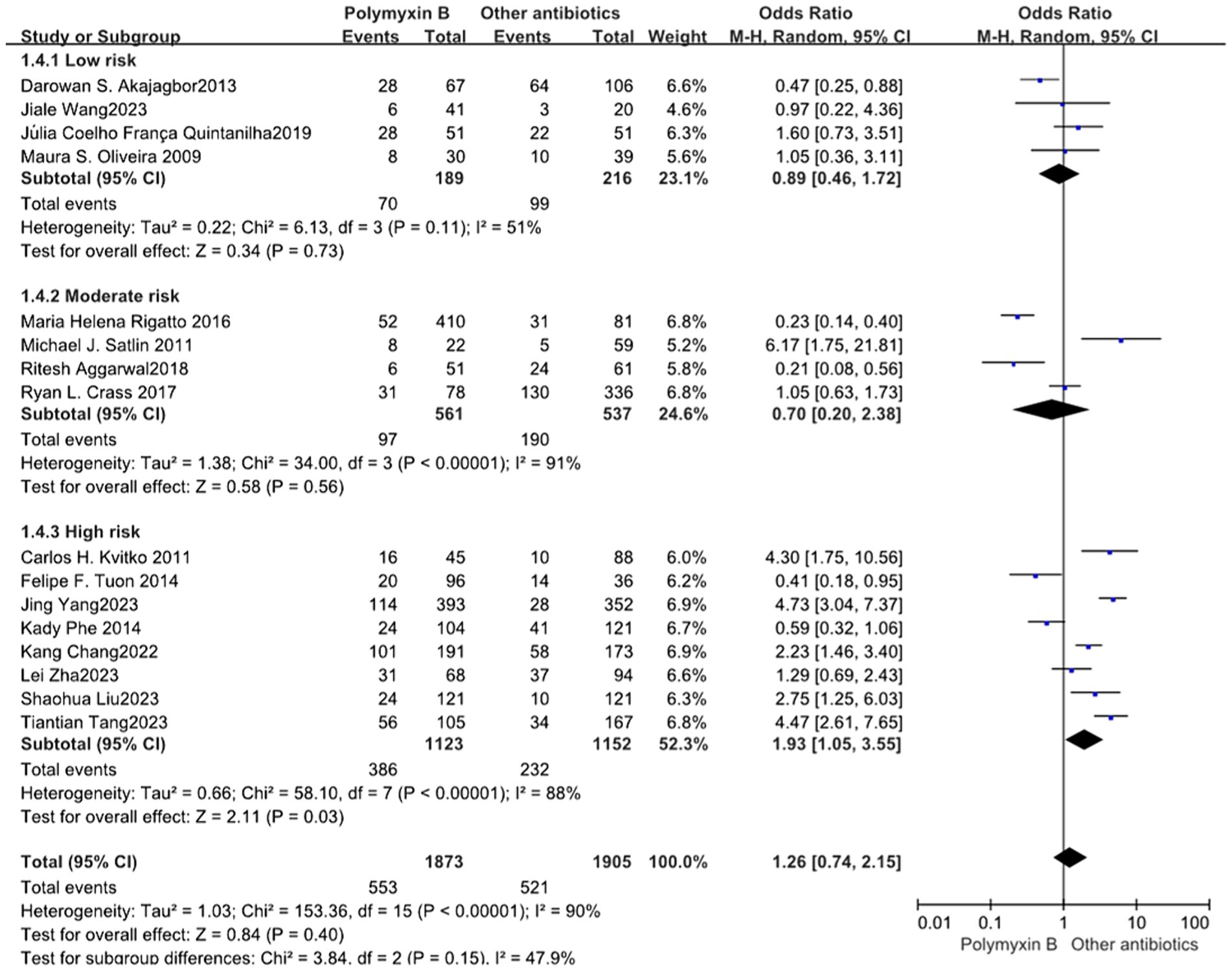
Figure 10. The nephrotoxicity was compared between PMB and other antibiotics (subgroup analysis based on the risk of bias).
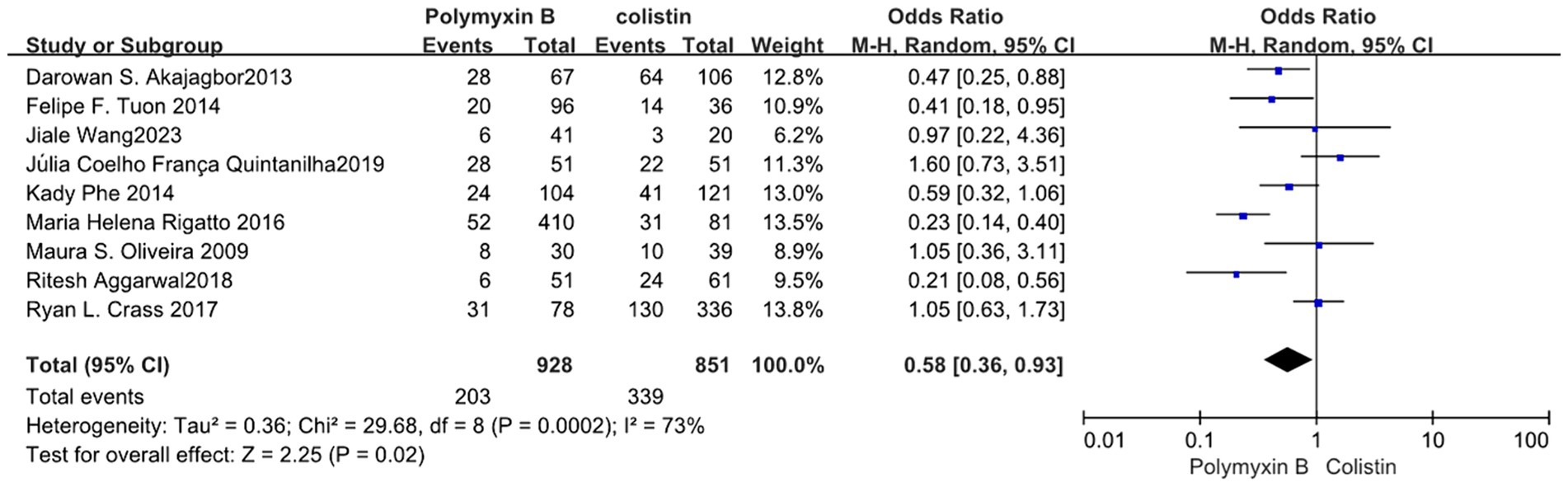
We found that the PMB group had a lower nephrotoxicity rate compared to the colistin group but exhibited high heterogeneity in the results (odds ratio 0.58, 95% CI 0.36–0.93; p = 0.02; I2 = 73%, Figure 11). Conversely, the tigecycline group had a lower nephrotoxicity rate, despite similarly displaying high heterogeneity (odds ratio 3.08, 95% CI 1.71–5.55; p = 0.0002; I2 = 70%, Figure 12).
Bacterial clearance
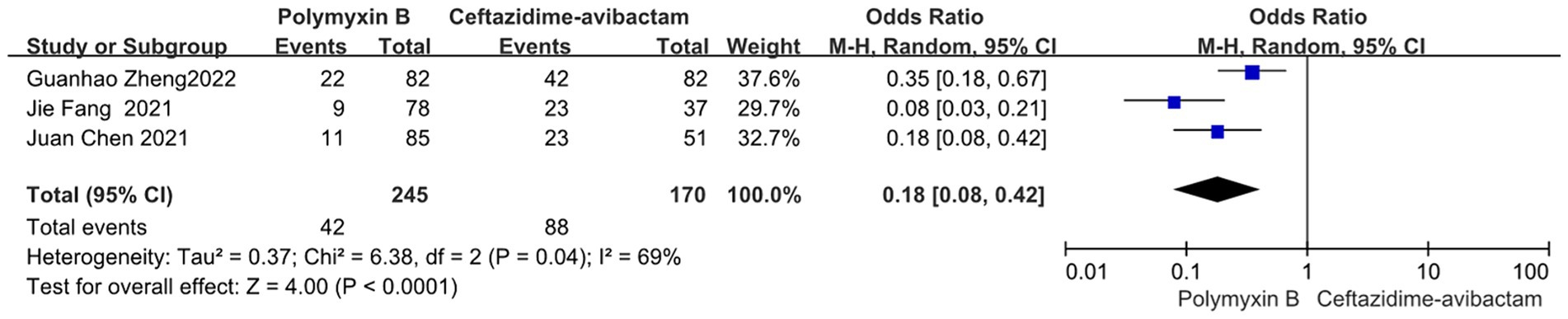
Compared to the PMB group, the ceftazidime-avibactam group demonstrated better bacterial clearance (odds ratio 0.18, 95% CI 0.08–0.42; p<0.0001; I2 = 69%, Figure 13).
Discussion
This is a systematic review and meta-analysis of 22 clinical trials to evaluate the effectiveness and safety of PMB in patients with nosocomial infections. Our meta-analysis revealed that PMB is not superior to other antibiotics in terms of mortality, specifically when compared to ceftazidime-avibactam. However, PMB demonstrated an advantage in terms of nephrotoxicity compared to colistin.
Due to the rise of pathogen resistance, there is a need for the development of novel antibiotics and strategies. Despite the availability of new antimicrobial agents, the issue of their high cost persists. It is of paramount importance to reevaluate old antibiotics and investigate their clinical efficacy. Our meta-analysis revealed that PMB is not superior to other antibiotics in terms of mortality, particularly in comparison to ceftazidime-avibactam. Therefore, ceftazidime-avibactam could be a favorable option for nosocomial infections. However, the cost-effectiveness of the comparing antibiotics is not addressed in the present study. Moreover, PMB serves as an invaluable salvage therapy and should be contemplated for combination therapy. Naturally, additional evidence and further qualified studies are necessary to ascertain the cost-efficacy of PMB usage.
Both PMB and colistin are commonly administered in combination with other antibiotics to enhance effectiveness and reduce resistance development. In Maura S. Oliveira’s study, both the PMB and colistin groups received combined antibiotics, including amphotericin, ampicillin, carbapenem, cephalosporin, ciprofloxacin, metronidazole, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, and vancomycin, with no statistically significant differences observed (23). Similar results were also seen in Ryan L. Crass’s study (26). All the studies included were retrospective. The extensive range of antibiotics used in both groups limited further statistical analysis. The variety of antibiotics used in combination may potentially impact outcomes, underscoring the need for further research to investigate the efficacy of antibiotic combination therapy.
Our meta-analysis indicated that PMB demonstrated an advantage in terms of nephrotoxicity compared to colistin. Therefore, PMB may be a more favorable choice than colistin for treating nosocomial infections in patients with renal insufficiency. Additionally, the nebulized administration of both PMB and colistin has been investigated (11, 39–44). While we acknowledge the association between drug dosage and nephrotoxicity, the PMB dosages in different studies vary in presentation. Some articles specify dosages based on unit weight, while others mention total daily dosages. This inconsistency in reporting methods hinders the feasibility of performing regression analysis to explore the relationship between PMB dosage and nephrotoxicity. To explore new potential applications for old antibiotics, such as PMB and colistin, further research is warranted to investigate appropriate selection criteria, administration methods, and dosage recommendations.
The majority of the included articles are from Brazil and China. Subgroup analysis was also performed (Supplementary Figures S1, S2), revealing that there was no significant difference in nephrotoxicity between the PMB group and the control group in Brazil and the USA. However, the PMB group exhibited a higher risk of developing nephrotoxicity when compared to the control group. The possible reason for this observation could be that many studies included from China in recent years predominantly used tigecycline and tigecycline as comparator groups. PMB shows stronger nephrotoxicity compared to these two drugs. In earlier studies, colistin was the primary comparator, and there was minimal difference in nephrotoxicity.
A few limitations were still present in our study. First, all the studies included in this analysis were cohort studies, and the level of evidence was very low. Second, there is variability in the timing of mortality among different studies. Most studies assessed 30-day mortality, while others focused on in-hospital mortality, 28-day mortality, and 14-day mortality. Such heterogeneity may potentially impair the quality of the synthesized evidence. Third, nosocomial infections often require the combined application of antibiotics. However, our study did not account for the concurrent use of medications in each group. Finally, there was significant heterogeneity in the dosage and duration of antibiotic between studies for many of our outcomes. Hence, our current findings were weakened, and our study was downgraded one level.
Conclusion
Our meta-analysis showed that PMB is not superior to other antibiotics in terms of mortality, specifically when compared to ceftazidime-avibactam. However, PMB demonstrated an advantage in terms of nephrotoxicity compared to colistin. Additionally, there is a need for further high-quality studies to elucidate the appropriate antibiotic selection for nosocomial infections.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LP: Writing – original draft, Software, Methodology, Formal analysis, Data curation. ZZ: Writing – original draft, Funding acquisition. XQ: Writing – original draft, Data curation. YZ: Writing – original draft, Project administration, Data curation. TS: Writing – review & editing, Resources, Investigation. LC: Writing – original draft, Validation, Investigation. JZ: Writing – original draft, Resources, Methodology, Conceptualization. XL: Writing – original draft, Validation, Methodology, Data curation. PM: Writing – review & editing, Visualization, Supervision, Resources, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ZZ received funding from China National Key Research and Development Program (no. 2022YFC2504500), the National natural science foundation of China (82272180) and China National Key Research and Development Program (no. 2023YFC3603104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1400757/full#supplementary-material
Supplementary FIGURE S1 | The mortality was compared between PMB and other antibiotics (subgroup analysis based on different countries).
Supplementary FIGURE S2 | The nephrotoxicity was compared between PMB and other antibiotics (subgroup analysis based on different countries).
Supplementary Table S1 | Search strategy.
Supplementary Table S2 | Quality evaluation of the eligible studies with Newcastle-Ottawa scale.
References
1. Gastmeier, P. Nosocomial infection surveillance and control policies. Curr Opin Infect Dis. (2004) 17:295–301. doi: 10.1097/01.qco.0000136929.75543.8a
2. Liu, JY, and Dickter, JK. Nosocomial infections: a history of hospital-acquired infections. Gastrointest Endosc Clin N Am. (2020) 30:637–52. doi: 10.1016/j.giec.2020.06.001
3. Sydnor, ER, and Perl, TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. (2011) 24:141–73. doi: 10.1128/CMR.00027-10
4. Trivic, I, and Hojsak, I. Use of probiotics in the prevention of nosocomial infections. J Clin Gastroenterol. (2017) 52:S62–5. doi: 10.1097/MCG.0000000000001070
5. Kollef, M, Torres, A, Shorr, A, Martin-Loeches, I, and Micek, S. Nosocomial infection. Crit Care Med. (2021) 49:169–87. doi: 10.1097/ccm.0000000000004783
6. Jay, S. Nosocomial infections. Med Clin North Am. (1983) 67:1251–77. doi: 10.1016/s0025-7125(16)31152-x
7. Eickhoff, T. Nosocomial infections. N Engl J Med. (1982) 306:1545–6. doi: 10.1056/nejm198206243062508
8. Vincent, J. Nosocomial infections in adult intensive-care units. Lancet. (2003) 361:2068–77. doi: 10.1016/s0140-6736(03)13644-6
9. Vardakas, KZ, and Falagas, ME. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents. (2017) 49:233–8. doi: 10.1016/j.ijantimicag.2016.07.023
10. Medeiros, GS, Rigatto, MH, Falci, DR, and Zavascki, AP. Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. (2019) 53:152–7. doi: 10.1016/j.ijantimicag.2018.10.010
11. Zhang, X, Cui, X, Jiang, M, Huang, S, and Yang, M. Nebulized colistin as the adjunctive treatment for ventilator-associated pneumonia: a systematic review and meta-analysis. J Crit Care. (2023) 77:154315. doi: 10.1016/j.jcrc.2023.154315
12. Phe, K, Lee, Y, McDaneld, PM, Prasad, N, Yin, T, Figueroa, DA, et al. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother. (2014) 58:2740–6. doi: 10.1128/AAC.02476-13
13. Tuon, FF, Rigatto, MH, Lopes, CK, Kamei, LK, Rocha, JL, and Zavascki, AP. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents. (2014) 43:349–52. doi: 10.1016/j.ijantimicag.2013.12.002
14. Rigatto, MH, Oliveira, MS, Perdigão-Neto, LV, Levin, AS, Carrilho, CM, Tanita, MT, et al. Multicenter prospective cohort study of renal failure in patients treated with Colistin versus Polymyxin B. Antimicrob Agents Chemother. (2016) 60:2443–9. doi: 10.1128/AAC.02634-15
15. Akajagbor, DS, Wilson, SL, Shere-Wolfe, KD, Dakum, P, Charurat, ME, and Gilliam, BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis. (2013) 57:1300–3. doi: 10.1093/cid/cit453
16. Aggarwal, R, and Dewan, A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: a prospective study. Ann Clin Microbiol Antimicrob. (2018) 17:15. doi: 10.1186/s12941-018-0262-0
17. Cai, Y, Lee, W, and Kwa, AL. Polymyxin B versus colistin: an update. Expert Rev Anti-Infect Ther. (2015) 13:1481–97. doi: 10.1586/14787210.2015.1093933
18. Tran, TB, Velkov, T, Nation, RL, Forrest, A, Tsuji, BT, Bergen, PJ, et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int J Antimicrob Agents. (2016) 48:592–7. doi: 10.1016/j.ijantimicag.2016.09.010
19. Zha, L, Zhang, X, Cheng, Y, Xu, Q, Liu, L, Chen, S, et al. Intravenous Polymyxin B as adjunctive therapy to high-dose Tigecycline for the treatment of nosocomial pneumonia due to Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae: a propensity score-matched cohort study. Antibiotics. (2023) 12:273. doi: 10.3390/antibiotics12020273
20. Liberati, A, Altman, D, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
21. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
22. Min, B, Grant-Orser, A, and Johannson, KA. Peripheral blood monocyte count and outcomes in patients with interstitial lung disease: a systematic review and meta-analysis. Eur Respir Rev. (2023) 32:230072. doi: 10.1183/16000617.0072-2023
23. Oliveira, MS, Prado, GV, Costa, SF, Grinbaum, RS, and Levin, AS. Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagn Microbiol Infect Dis. (2009) 65:431–4. doi: 10.1016/j.diagmicrobio.2009.07.018
24. Kvitko, CH, Rigatto, MH, Moro, AL, and Zavascki, AP. Polymyxin B versus other antimicrobials for the treatment of pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother. (2011) 66:175–9. doi: 10.1093/jac/dkq390
25. Rigatto, MH, Ribeiro, VB, Konzen, D, and Zavascki, AP. Comparison of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia and tracheobronchitis caused by Pseudomonas aeruginosa or Acinetobacter baumannii. Infection. (2013) 41:321–8. doi: 10.1007/s15010-012-0349-z
26. Crass, RL, Rutter, WC, Burgess, DR, Martin, CA, and Burgess, DS. Nephrotoxicity in patients with or without cystic fibrosis treated with Polymyxin B compared to Colistin. Antimicrob Agents Chemother. (2017) 61:e02329-16. doi: 10.1128/AAC.02329-16
27. Fang, J, Li, H, Zhang, M, Shi, G, Liu, M, Wang, Y, et al. Efficacy of ceftazidime-avibactam versus Polymyxin B and risk factors affecting clinical outcomes in patients with Carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. (2021) 12:780940. doi: 10.3389/fphar.2021.780940
28. Chen, J, Liang, Q, Chen, X, Wu, J, Wu, Y, Teng, G, et al. Ceftazidime/avibactam versus Polymyxin B in the challenge of Carbapenem-resistant Pseudomonas aeruginosa infection. Infect Drug Resist. (2022) 15:655–67. doi: 10.2147/IDR.S350976
29. Zheng, G, Cai, J, Zhang, L, Chen, D, Wang, L, Qiu, Y, et al. Ceftazidime/avibactam-based versus Polymyxin B-based therapeutic regimens for the treatment of Carbapenem-resistant Klebsiella pneumoniae infection in critically ill patients: a retrospective cohort study. Infect Dis Ther. (2022) 11:1917–34. doi: 10.1007/s40121-022-00682-0
30. Chang, K, Wang, H, Zhao, J, Yang, X, Wu, B, Sun, W, et al. Polymyxin B/Tigecycline combination vs. Polymyxin B or Tigecycline alone for the treatment of hospital-acquired pneumonia caused by Carbapenem-resistant Enterobacteriaceae or Carbapenem-resistant Acinetobacter baumannii. Front Med. (2022) 9:772372. doi: 10.3389/fmed.2022.772372
31. Satlin, MJ, Kubin, CJ, Blumenthal, JS, Cohen, AB, Furuya, EY, Wilson, SJ, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother. (2011) 55:5893–9. doi: 10.1128/AAC.00387-11
32. Quintanilha, JCF, Duarte, NDC, Lloret, GR, Visacri, MB, Mattos, KPH, Dragosavac, D, et al. Colistin and polymyxin B for treatment of nosocomial infections in intensive care unit patients: pharmacoeconomic analysis. Int J Clin Pharm. (2019) 41:74–80. doi: 10.1007/s11096-018-0766-x
33. Wang, J, Shah, BK, Zhao, J, Xiong, J, Wang, C, and Xie, S. Comparative study of polymyxin B and colistin sulfate in the treatment of severe comorbid patients infected with CR-GNB. BMC Infect Dis. (2023) 23:351. doi: 10.1186/s12879-023-08339-0
34. Tang, T, Li, Y, Xu, P, Zhong, Y, Yang, M, Ma, W, et al. Optimization of polymyxin B regimens for the treatment of carbapenem-resistant organism nosocomial pneumonia: a real-world prospective study. Crit Care. (2023) 27:164. doi: 10.1186/s13054-023-04448-z
35. Liu, S, Wu, Y, Qi, S, Shao, H, Feng, M, Xing, L, et al. Polymyxin B therapy based on therapeutic drug monitoring in carbapenem-resistant organisms sepsis: the PMB-CROS randomized clinical trial. Crit Care. (2023) 27:232. doi: 10.1186/s13054-023-04522-6
36. Yang, J, Liu, S, Lu, J, Sun, T, Wang, P, and Zhang, X. An area under the concentration-time curve threshold as a predictor of efficacy and nephrotoxicity for individualizing polymyxin B dosing in patients with carbapenem-resistant gram-negative bacteria. Crit Care. (2022) 26:320. doi: 10.1186/s13054-022-04195-7
37. Wu, J, Liu, T, Yuan, Y, Wang, C, Fang, X, and Li, H. The evaluation of clinical efficacy and safety of polymyxin B in treatment of senile patients with hospital-acquired pneumonia caused by carbapenem-resistant bacteria. Med J Chin People's Lib Army. (2020) 12:1277–81. doi: 10.11855/j.issn.0577-7402.2020.12.11
38. Junyan, Q, Jian, X, Liu, Y, Chenggong, H, Zhong, C, and Lv, X. Real-world effectiveness of ceftazidime/avibactam versus polymyxin B in treating patients with carbapenem-resistant gram-negative bacterial infections. Int J Antimicrob Agents. (2023) 62:106872. doi: 10.1016/j.ijantimicag.2023.106872
39. Boisson, M, Grégoire, N, Cormier, M, Gobin, P, Marchand, S, Couet, W, et al. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J Antimicrob Chemother. (2017) 72:2607–12. doi: 10.1093/jac/dkx167
40. Boisson, M, Jacobs, M, Grégoire, N, Gobin, P, Marchand, S, Couet, W, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother. (2014) 58:7331–9. doi: 10.1128/AAC.03510-14
41. Cao, B, and Cao, L. Case report: a case of spinal muscular atrophy with extensively drug-resistant Acinetobacter baumannii pneumonia treated with nebulization combined with intravenous polymyxin B: experience and a literature review. Front Cell Infect Microbiol. (2023) 13:1163341. doi: 10.3389/fcimb.2023.1163341
42. Shi, R, Fu, Y, Gan, Y, Wu, D, Zhou, S, and Huang, M. Use of polymyxin B with different administration methods in the critically ill patients with ventilation associated pneumonia: a single-center experience. Front Pharmacol. (2023) 14:1222044. doi: 10.3389/fphar.2023.1222044
43. Wu, Z, Zhang, S, Cao, Y, Wang, Q, Sun, K, and Zheng, X. Comparison of the clinical efficacy and toxicity of nebulized polymyxin monotherapy and combined intravenous and nebulized polymyxin for the treatment of ventilator-associated pneumonia caused by carbapenem-resistant gram-negative bacteria: a retrospective cohort study. Front Pharmacol. (2023) 14:1209063. doi: 10.3389/fphar.2023.1209063
Keywords: nosocomial infections, Polymyxin B, colistin, tigecycline, ceftazidime-avibactam, meta-analysis
Citation: Peng L, Zhang Z, Qi X, Zhong Y, Sun T, Chen L, Zhu J, Lv X and Ma P (2024) Efficiency of polymyxin B treatment against nosocomial infection: a systematic review and meta-analysis. Front. Med. 11:1400757. doi: 10.3389/fmed.2024.1400757
Edited by:
Jingwei Li, University of New South Wales, AustraliaReviewed by:
Muhammad Usman, University of Veterinary and Animal Sciences, PakistanShixing Zhu, Ocean University of China, China
Copyright © 2024 Peng, Zhang, Qi, Zhong, Sun, Chen, Zhu, Lv and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Penglin Ma, bWFwZW5nbGluMUAxNjMuY29t
†These authors have contributed equally to this work
 Liyuan Peng
Liyuan Peng Zhongheng Zhang
Zhongheng Zhang Xueyan Qi
Xueyan Qi Yanjun Zhong
Yanjun Zhong Tongwen Sun
Tongwen Sun Lvlin Chen1
Lvlin Chen1 Xiangui Lv
Xiangui Lv Penglin Ma
Penglin Ma