- 1Binzhou Medical University Hospital, Binzhou, China
- 2Shandong University of Aeronautics, Binzhou, Shandong, China
Introduction: Adult diffuse hepatic hemangiomatosis (DHH) is an extremely rare disease. Consequently, its characteristics are poorly understood. Herein, we report a case of adult DHH involving both liver lobes but without extrahepatic involvement. To the best of our knowledge, this the largest reported adult DHH to date.
Case presentation: A 51-year-old man was admitted due to abdominal distension and dyspnea. Physical examination revealed marked liver enlargement. Color Doppler, plain and contrast-enhanced computed tomography, and contrast-enhanced magnetic resonance imaging revealed a hepatic lesion sized 35.1 × 32.1 × 14.1 cm occupying nearly the entire abdominal and pelvic cavities. Diagnosis was established by liver puncture biopsy. The patient exhibited clinical signs of portal hypertension and hypersplenism, but remains free of serious DHH-related complications. He is followed up regularly, with proactive evaluation for future liver transplantation.
Conclusion: This case will contribute to the current knowledge on the clinical and imaging features of this rare entity.
1 Introduction
Hepatic hemangiomas are the most common benign tumors of the liver, with an incidence of 5–20% (1). In most cases, they are isolated, asymptomatic, and incidentally detected. Contrarily, diffuse hepatic hemangiomatosis (DHH) is a rare disease that usually develops in newborns and is characterized by extensive replacement of the liver parenchyma with hemangiomatous lesions (2). The difference between DHH and multiple or giant hepatic hemangiomas is that, in the former, the lesions have poorly defined margins with diffuse replacement of hepatic tissue, whereas in the latter, hemangiomas are surrounded by a fibrous capsule with well-defined margins. Adult DHH, particularly without extrahepatic involvement, is extremely rare.
Herein, we present a case of adult DHH without extrahepatic involvement presenting with extremely enlarged liver occupying the entire abdominal and pelvic cavities, and describe its imaging features as determined by multimodality evaluation.
2 Case presentation
A 51-year-old man was hospitalized on May 31, 2023 due to abdominal distension lasting for 3 years and chest tightness lasting for 1 week. Three years previously, the patient was diagnosed with venous thrombosis of the right lower extremity and had been taking rivaroxaban for an extended period. He had no history of hepatitis, denied a history of alcoholism, and had diabetes and hypertension. He did not have telangiectasia of the lips or mouth and denied episodes of epistaxis.
Physical examination revealed markedly enlarged liver occupying nearly the entire abdominal and pelvic cavities, with the lower margin reaching the superior margin of the pubic symphysis.
Complete blood count analysis revealed leukopenia and thrombocytopenia, with a white blood cell count of 2.9 × 109/L (reference range: 4–10 × 109/L) and platelet count of 80 × 109/L (reference range: 100–300 × 109/L). Liver function and coagulation function test results were normal. Tests for hepatitis A, B, C, D, and E, Epstein–Barr virus, cytomegalovirus, liver immunological markers, and ceruloplasmin yielded negative results. Parasitic infection was ruled out based on epidemiological history. Serum tumor marker levels (cancer antigen 125, alpha fetoprotein, carcinoembryonic antigen, cancer antigen 199, total prostate-specific antigen, and squamous cell carcinoma antigen) were within normal ranges.
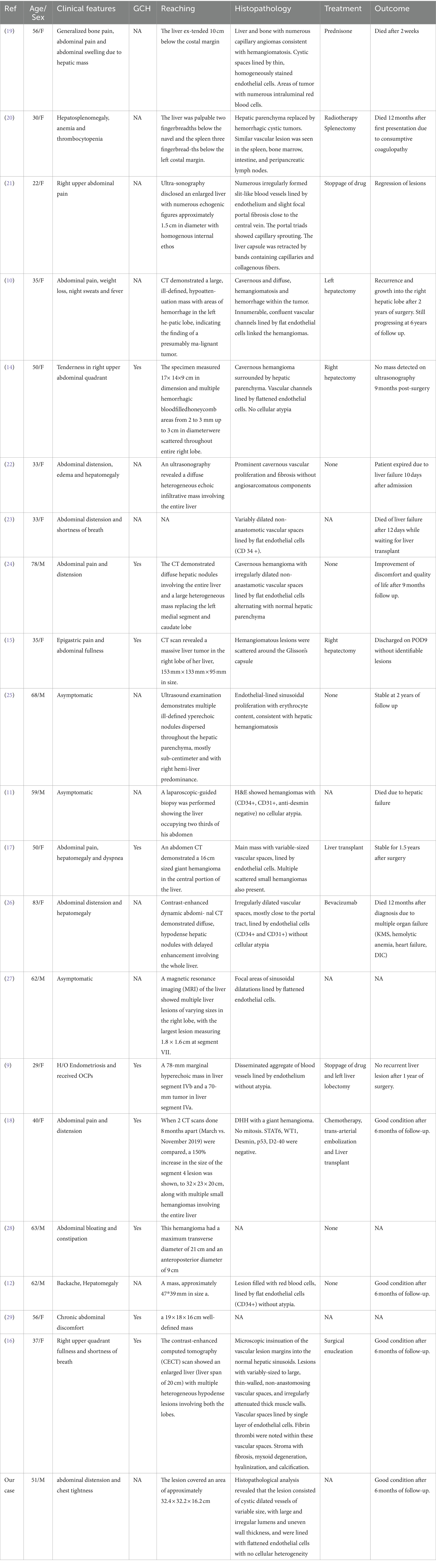
Abdominal Doppler ultrasound examination suggested massive liver enlargement. The liver capsule was smooth, and a giant space-occupying lesion was detected. The lesion had irregular morphology and protruded from the liver, extending to the xiphoid process superiorly, 8.0 cm below the umbilicus inferiorly, and the posterior axillary line on the left side. The liver showed inhomogeneous echogenicity, with only a small amount of normal liver tissue remaining in the right lobe (Figure 1).
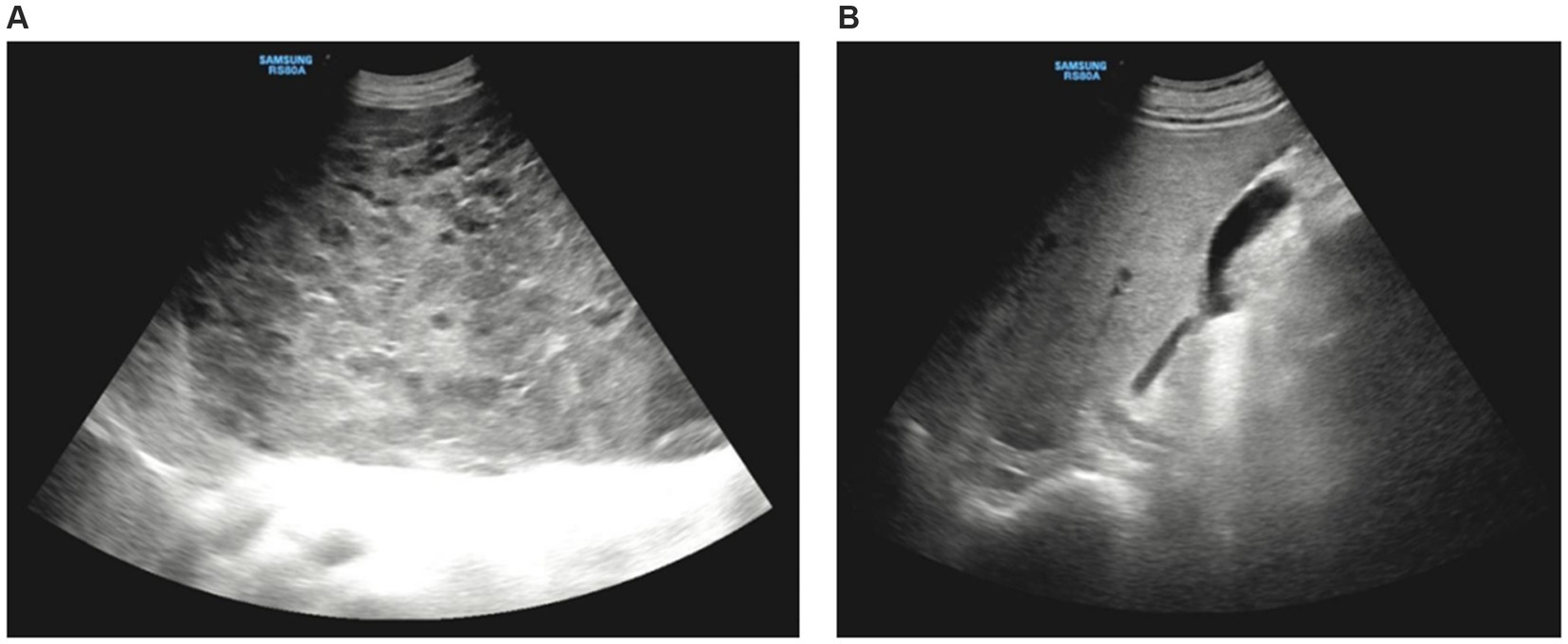
Figure 1. Doppler ultrasound imaging. (A) Ultrasound showed huge occupation in the liver, with uneven internal echoes, and morphological under-rule. (B) The normal liver tissue in the right lobe of liver.
Contrast-enhanced abdominal computed tomography (CT) revealed a massive area of abnormal enhancement in the liver parenchyma partially protruding from the contour of the liver and growing toward the abdominal cavity. The lesion covered an area of approximately 32.4 × 32.2 × 16.2 cm, with only a small amount of normal liver tissue remaining in the right lobe. In the arterial phase, the lesion contained multiple patchy foci of significant enhancement. In the portal venous and delayed phases, the extent of enhancement increased with increased scanning time. Delayed scanning after about 30 min showed uniform enhancement of most of the lesion and patchy low-density shadows in the lateral side of the lesion. The portal vein was slightly narrowed by compression, there was no significant dilation of the splenic vein, and no significant splenomegaly (Figure 2).
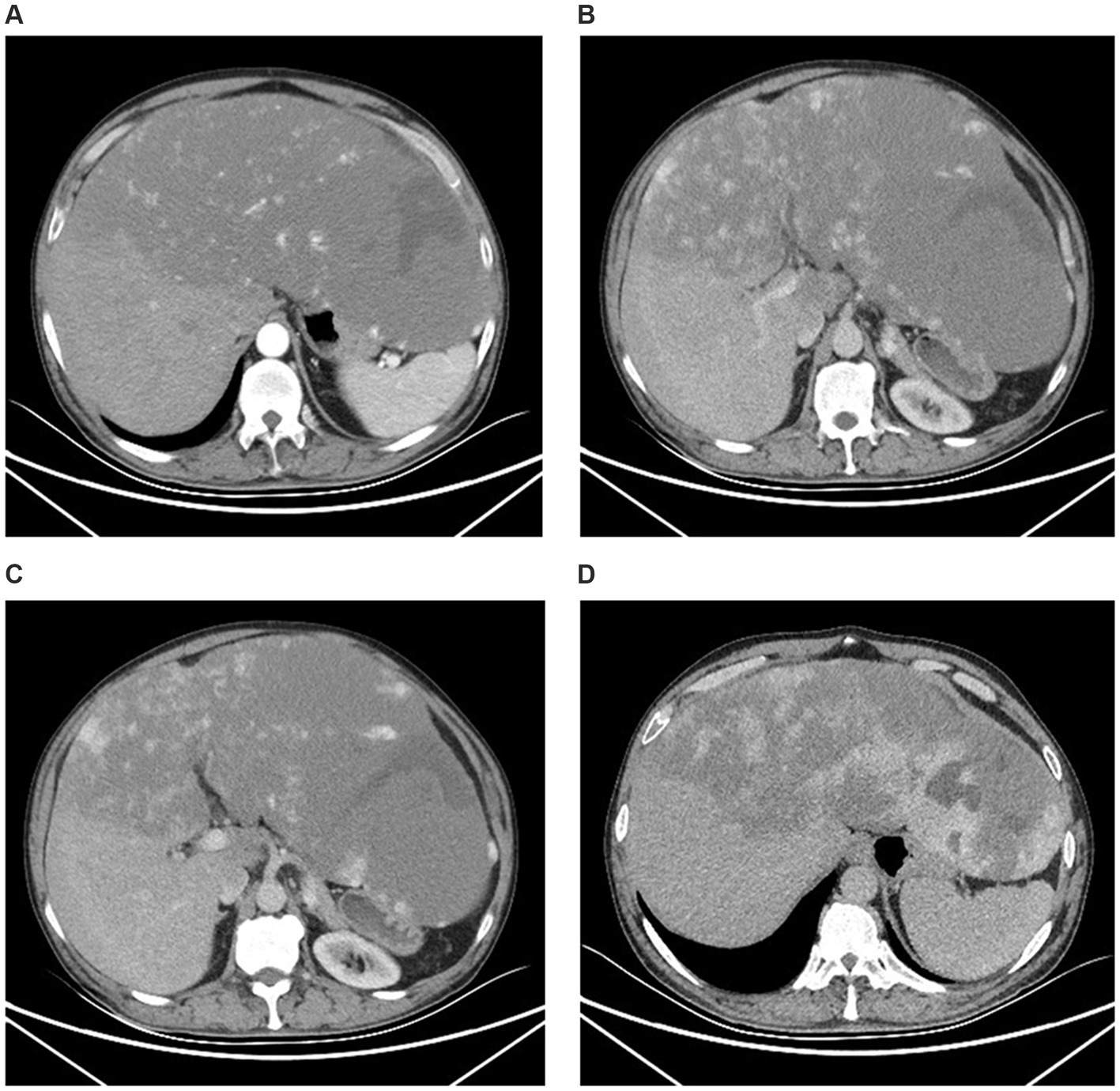
Figure 2. Computed tomography imaging. (A) The arterial phase showed the irregular patchy foci of enhancement. (B,C) The portal venous phase expressed the range of enhancing gradually increases. (B) The left portal vein branch was extremely thin and the right portal vein branch become thinner. (C) The main portal vein in hepatic hilar region was slightly narrowed. (D) The delayed phases showed uniform enhancement of most of the lesion and patchy low-density shadows in the lateral side of the left lobe.
Contrast-enhanced abdominal magnetic resonance imaging (MRI) showed marked liver enlargement with a mass-like abnormal signal in the left lobe and most of the right lobe. The lesion was slightly hypointense on T1-weighted images, slightly hyperintense on T2-weighted and diffuse-weighed images, and locally hypointense on the apparent diffusion coefficient map. The left lobe exhibited local streaks with even lower T1 and even longer T2 signals, with streaks of hypointense segments within. The lesion had clear margins, measured 35.1 × 32.1 × 14.1 cm, and partially protruded from the liver contour, compressing the surrounding structures (Figure 3).
Figure 3. Magnetic resonance imaging. (A) T1-weighted image. (B) T2-weighted image. (C) Apparent diffusion coefficient image. (D) The coronal MRI revealed enlarged liver occupying nearly the entire abdominal and pelvic cavities.
To confirm the diagnosis, ultrasound-guided biopsy of the right liver lobe was performed. Histopathological analysis revealed that the lesion consisted of cystic dilated vessels of variable size, with large and irregular lumens and uneven wall thickness, and were lined with flattened endothelial cells with no cellular heterogeneity (Figure 4).
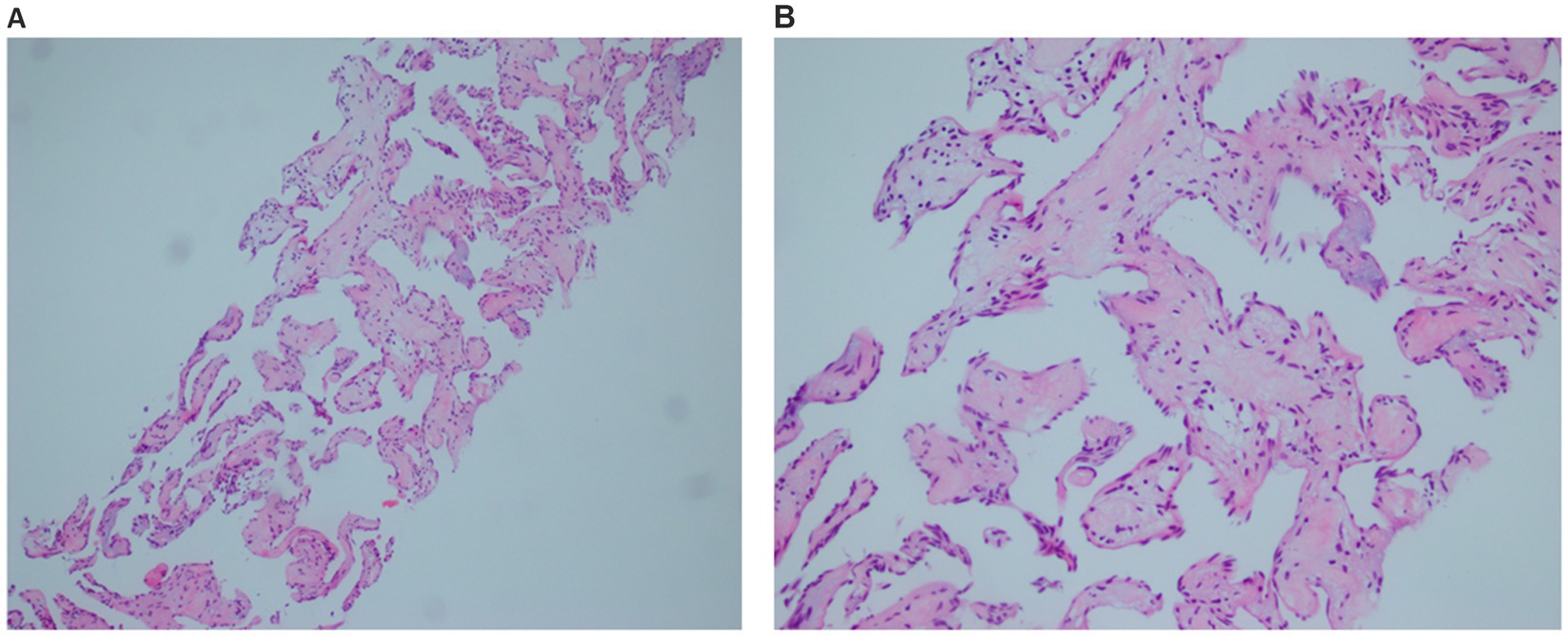
Figure 4. Pathological image. Histological section showing that the lesion consisted of cystic dilated vessels of variable size, with large and irregular lumens and uneven wall thickness, and were lined with flattened endothelial cells with no cellular heterogeneity. (A) The low magnification. (B) The higher magnification.
Our differential diagnoses included diffuse hamartoma, multiple metastases, polycystic iver, diffuse hepatocellular carcinoma, angiosarcoma, and hemangioendothelioma.
Based on the histopathological and imaging findings, the patient was diagnosed with adult DHH. At present, no effective treatment is available for DHH with near-total liver involvement. The patient is free of significant complications, such as liver failure, high-output heart failure, Kasabach-Merritt syndrome, or disseminated intravascular coagulation. Therefore, regular follow-up is performed with consideration for future liver transplantation. At nearly 6 months of follow-up, the patient remains stable with intermittent epigastric pain.
3 Discussion
Adult DHH is a rare disease, with only 20 reported cases as of August 2023 (Table 1), over half of which were associated with giant cavernous hemangioma (GCH) (3). In the present case, DHH was not accompanied by GCH, but was diffusely distributed throughout the liver, manifesting as poorly defined spongy vascular tissue occupying nearly the entire liver. The involvement of the left lobe was particularly pronounced. It was completely occupied by hemangiomatous tissue, resulting in a marked increase in its size, reaching the superior margin of the pubic symphysis inferiorly and the posterior axillary line on the left side. The enlarged liver occupied nearly the entire abdominal and pelvic cavities, reaching a size of 35.1 × 32.1 × 14.1 cm. To the best of our knowledge, this is the largest DHH reported to date.
DHH usually presents with abdominal pain and distension. Some patients may also develop dyspnea and jaundice. Dyspnea is primarily due to the space occupation of the abdominal cavity by the liver associated with restricted respiration due to diaphragmatic elevation, as in this case. Jaundice may be due to liver dysfunction caused by vascular disease, mass effect, and ischemia. Heart failure, liver failure, disseminated intravascular coagulation, and Kasabach-Merritt syndrome are serious complications of DHH (4) and the leading causes of patient death. Portal hypertension can develop in cases of diffuse DHH progression with portal vein compression (5). These tumors may cause a mass effect when they attain substantial dimensions or have a modified internal component, such as a thrombosis or hemorrhage, transforming the lesion into a firm solid mass (24). It may cause the intrahepatic portal vein obstruction. Mild portal vein compression was also observed in the present case. Although no significant splenomegaly was found, we considered that the leukopenia and thrombocytopenia that could not be explained by other disorders were due to portal hypertension-induced hypersplenism.
According to the imaging features, DHH can manifest as a multinodular type, consisting of multiple discrete or coalescing nodules, and diffuse type, consisting of numerous poorly demarcated lesions with a tendency to converge to the point of replacing the entire liver (6–8). Unlike in cases of multiple hepatic hemangiomas, the lesion margins in DHH are usually indistinct and not clearly demarcated from the normal liver tissue. On Doppler ultrasound, the lesions appear as homogeneously hyperechoic areas with poorly defined margins or multiple small hypoechoic nodules on a hyperechoic background, varying significantly between patients. The lesions lack typical peripheral enhancement on contrast-enhanced CT and tend to present as patchy or crescent-shaped enhancements. On dynamic contrast-enhanced MRI, the lesions are hypointense on T1-weighted and hyperintense on T2-weighted images, with restricted diffusion on diffusion-weighted images, and exhibit discontinuous centripetal fill-in on axial dynamic images. In the present case, the hemangiomatous tissue occupied nearly the entire liver, with a diffuse and continuous distribution, and CT and MRI results were consistent with the typical presentation described above. The lesion exhibited diffuse and slightly hypointense areas with homogeneous density on plain CT, which was not easily distinguished from normal liver tissue and differed from the more common diffuse DHH or GCH with DHH manifestations, leading to possible misdiagnosis without contrast-enhanced CT.
Due to the rarity of adult DHH, little is known about its etiology or natural history. DHH is among the hepatic manifestations of Rendu-Osler-Weber disease and skeletal hemangiomatosis (9). Prior studies have reported possible etiologic associations between DHH and medication use (e.g., metoclopramide, estrogens, and Chinese patent medicines) (9–12). The correlation between hypothyroidism and DHH has been described in infants but not in adults (13). Jhaveri et al. (3) reported that GCH can be accompanied by DHH. In the present case, there was no extrahepatic involvement, no history of medication use, including metoclopramide, estrogens, or Chinese patent medicines, and no GCH; thus, a clear etiology could not be established.
If the tumor borderline is clear and confined to one lobe, surgical resection can be performed. In the literature, five patients underwent surgery with right/left hepatectomy (9, 10, 14–16). Radiation and Anti-VEGF (anti-vascular endothelial growth fac-tor) treatment has also been reported. The liver transplantation is the last resort. Two patients underwent living-donor liver transplantation and was in good condition at the follow-up (17, 18).
4 Conclusion
The present case provides description of the clinical and imaging characteristic of adult DHH. We conducted multimodality evaluation, including abdominal color Doppler ultrasound, CT, MRI, and liver puncture biopsy and pathology. We believe that the described findings can help improve our understanding of this rare disease. Since the probability of serious complications is significantly higher in cases of near-total liver involvement and severe liver enlargement, continuous monitoring, regular reexamination, and proactive evaluation for liver transplantation are necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Binzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual (s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-NG: Writing – original draft, Writing – review & editing. YS: Formal analysis, Writing – review & editing. S-CD: Methodology, Writing – review & editing. X-BM: Formal analysis, Writing – review & editing. WW: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present case study was supported by the Key Clinical Specialty of Shandong Province.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing. Thanks Ying Wang for the pictures revision and Thanks Liang Chen for his help in iconography.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weimann, A, Ringe, B, Klempnauer, J, Lamesch, P, Gratz, KF, Prokop, M, et al. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg. (1997) 21:983–91. doi: 10.1007/s002689900337
2. Ohnishi, S, Miyagishima, T, Nakagawa, M, Kamata, T, Kishimoto, A, Choi, GH, et al. Diffuse neonatal hemangiomatosis without cutaneous lesions in an adult—a case report. Angiology. (2002) 53:235–7. doi: 10.1177/000331970205300217
3. Jhaveri, KS, Vlachou, PA, Guindi, M, Fischer, S, Khalili, K, Cleary, SP, et al. Association of hepatic hemangiomatosis with giant cavernous hemangioma in the adult population: prevalence, imaging appearance, and relevance. Am J Roentgenol. (2011) 196:809–15. doi: 10.2214/AJR.09.4143
4. Aslan, A, Meyer Zu Vilsendorf, A, Kleine, M, Bredt, M, and Bektas, H. Adult Kasabach-Merritt syndrome due to hepatic giant hemangioma. Case Rep Gastroenterol. (2009) 3:306–12. doi: 10.1159/000242420
5. Romero, AH, Domínguez, EG, Gómez, RM, Codoceo, CM, and Vázquez, IF. Portal hypertension secondary to multiple hepatic hemangiomatosis. Gastroenterol Hepatol. (2018) 41:323–4. doi: 10.1016/j.gastrohep.2017.06.007
6. Vilgrain, V, Boulos, L, Vullierme, MP, Denys, A, Terris, B, and Menu, Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. (2000) 20:379–97. doi: 10.1148/radiographics.20.2.g00mc01379
7. Liu, MC, and Little, EC. Isolated hepatic hemangiomatosis in 2 septuagenarians. Radiol Case Rep. (2018) 13:1097–103. doi: 10.1016/j.radcr.2018.08.018
8. Guerra, A, Infante, A, Rinninella, E, Spinelli, I, Mazziotti, MA, De Gaetano, AM, et al. A peculiar case of diffuse hemangiomatosis of the left hepatic lobe in an asymptomatic adult patient: case report and literature review. Eur Rev Med Pharmacol Sci. (2017) 21:1593–7.
9. Ota, T, Kamiyama, T, Kato, T, Hanamoto, T, Hirose, K, Otsuka, N, et al. A rare case of cavernous hemangioma accompanied with diffuse hepatic hemangiomatosis. Surg Case Rep. (2020) 6:251. doi: 10.1186/s40792-020-01023-4
10. Lehmann, FS, Beglinger, C, Schnabel, K, and Terracciano, L. Progressive development of diffuse liver hemangiomatosis. J Hepatol. (1999) 30:951–4. doi: 10.1016/S0168-8278(99)80152-4
11. Militz, M, Carlotto, JR, Schmitz, LD, and Dal Vesco, JA. Diffuse hepatic hemangiomatosis of rapid growth after bariatric surgery. Dig Liver Dis. (2016) 48:1513. doi: 10.1016/j.dld.2016.06.012
12. He, S, Chen, W, Yang, Y, Tang, X, Zhou, G, Zhou, J, et al. Adult diffuse hepatic hemangiomatosis: a case report and review of the literature. Clin Res Hepatol Gastroenterol. (2022) 46:101789. doi: 10.1016/j.clinre.2021.101789
13. He, X, Guo, ZW, and Niu, XM. Case report: CTC1 mutations in a patient with diffuse hepatic and splenic hemangiomatosis complicated by Kasabach-Merritt syndrome. Front Oncol. (2023) 13:1087790. doi: 10.3389/fonc.2023.1087790
14. Moon, WS, Yu, HC, Lee, JM, and Kang, MJ. Diffuse hepatic hemangiomatosis in an adult. J Korean Med Sci. (2000) 15:471–4. doi: 10.3346/jkms.2000.15.4.471
15. Hkura, Y, Hashimoto, M, Lee, S, Sasaki, K, Matsuda, M, and Watanabe, G. Right hepatectomy for giant cavernous hemangioma with diffuse hemangiomatosis around Glisson’s capsule. World J Gastroenterol. (2014) 20:8312–6. doi: 10.3748/wjg.v20.i25.8312
16. Bhardwaj, N, Parkhi, M, Kumar, M, Kaman, L, and Mitra, S. Adult diffuse hepatic hemangiomatosis. Autops Case Rep. (2022) 12:e2021401. doi: 10.4322/acr.2021.401
17. Lee, JH, Yoon, CJ, Kim, YH, Han, HS, Cho, JY, Kim, H, et al. Living-donor liver transplantation for giant hepatic hemangioma with diffuse hemangiomatosis in an adult: a case report. Clin Mol Hepatol. (2018) 24:163–8. doi: 10.3350/cmh.2017.0002
18. Shankar, S, Rammohan, A, Kulaseharan, VH, Kanagavelu, R, Reddy, MS, and Rela, M. Liver transplantation for rapidly progressive giant hepatic hemangioma with diffuse hemangiomatosis. Exp Clin Transplant. (2021) 19:1106. doi: 10.6002/ect.2020.0330
19. Kane, RC, and Newman, AB. Case reports. Diffuse skeletal and hepatic hemangiomatosis. Calif Med. (1973) 118:41–4. doi: 10.1111/j.1440-1827.1981.tb00992.x
20. Maeda, H, Matsuo, T, Nagaishi, T, Ikeda, T, Tomonaua, Y, and Mori, H. Diffuse hemangiomatosis, coagulopathy and microangiopathic hemolytic anemia. Acta Pathol Jpn. (1981) 31:135–42.
21. Feurle, GE. Arteriovenous shunting and cholestasis in hepatic hemangiomatosis associated with metoclopramide. Gastroenterology. (1990) 99:258–62. doi: 10.1016/0016-5085(90)91256-6
22. Kim, EH, Park, SY, Ihn, YK, and Hwang, SS. Diffuse hepatic hemangiomatosis without extrahepatic involvement in an adult patient. Korean J Radiol. (2008) 9:559–62. doi: 10.3348/kjr.2008.9.6.559
23. Kim, JD, Chang, UI, and Yang, JM. Clinical challenges and images in GI: diffuse hepatic hemangiomatosis involving the entire liver. Gastroenterology. (2008) 134:1830–2197. doi: 10.1053/j.gastro.2008.05.006
24. Yoo, BR, Han, HY, Choi, SY, and Kim, JH. Giant cavernous hemangioma coexistent with diffuse hepatic hemangiomatosis presenting as portal vein thrombosis and hepatic lobar atrophy. Ultrasonography. (2014) 33:65–70. doi: 10.14366/usg.13003
25. Batista, A, Matos, AP, Neta, JO, and Ramalho, M. Diffuse hepatic hemangiomatosis in the adult without extrahepatic involvement: an extremely rare occurrence. J Clin Imaging Sci. (2014) 4:43. doi: 10.4103/2156-7514.139733
26. Shimizu, Y, Komura, T, Seike, T, Omura, H, Kumai, T, Kagaya, T, et al. A case of an elderly female with diffuse hepatic hemangiomatosis complicated with multiple organic dysfunction and Kasabach-Merritt syndrome. Clin J Gastroenterol. (2018) 11:411–6. doi: 10.1007/s12328-018-0871-3
27. Rao, R, Naidu, J, Muhammad Nawawi, KN, Wong, ZQ, Ngiu, CS, Mohammed, F, et al. Diffuse hepatic haemangiomatosis: a case report and review of literature. Med J Malaysia. (2018) 73:436–8.
28. González-Nieto, MI, and Escobar Hoyos, LA. A case of diffuse hepatic hemangiomatosis coexistent with giant hemangioma: case report and literature review. Radiol Case Rep. (2021) 16:1518–23. doi: 10.1016/j.radcr.2021.03.058
Keywords: diffuse hepatic hemangiomatosis, giant cavernous hemangioma, imaging features, Kasabach-Merritt syndrome, multimodality evaluation
Citation: Ge Y-N, Shao Y, Dong S-C, Ma X-B and Wang W (2024) Adult diffuse hepatic hemangiomatosis lesion occupying the entire abdominal and pelvic cavities: a case report. Front. Med. 11:1399913. doi: 10.3389/fmed.2024.1399913
Edited by:
Michele Di Stefano, IRCCS S.Matteo Hospital Foundation, ItalyReviewed by:
Lovenish Bains, University of Delhi, IndiaTakamichi Ishikawa, Hamamatsu University School of Medicine, Japan
Copyright © 2024 Ge, Shao, Dong, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, eGhrb25naG1AMTI2LmNvbQ==
†ORCID: Ya-Nan Ge, orcid.org/0009-0006-8306-9346
Yan Shao, orcid.org/0000-0002-9770-8114
Shu-Chen Dong, orcid.org/0009-0008-1771-5666
Xing-Bin Ma, orcid.org/0000-0002-6854-0420
Wei Wang, orcid.org/0000-0001-7561-1015
 Ya-Nan Ge1†
Ya-Nan Ge1† Wei Wang
Wei Wang