
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 03 July 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1398024
This article is part of the Research TopicInfections in the Intensive Care Unit, volume IIView all 17 articles
Sepsis is a intricate pathological process characterized by life-threatening organ dysfunction resulting from a dysregulated host response to infection. It stands as a prominent cause of mortality among critically ill patients globally. The pivotal focus in sepsis management lies in the early identification and prompt administration of antimicrobial agents. Owing to the constraints of current diagnostic methodologies, marked by insufficient sensitivity and delayed outcomes, extensive research has been undertaken to ascertain novel biomarkers for sepsis. In this review, we provide an overview discussing the latest advancements in the study of PTX-3 as a biomarker for sepsis. We acknowledge pivotal discoveries from preceding research and engage in discourse regarding the challenges and limitations confronted by PTX-3 as a sepsis biomarker.
Sepsis is characterized by life-threatening organ dysfunction resulting from a dysregulated host response to infection (1). Recent research estimates globally recorded sepsis cases at 489 million, with reported sepsis-related deaths reaching 11 million, accounting for 19.7% of global mortality (2). In China, a study indicated a higher burden of sepsis in hospitalized patients compared to estimates (3). Therefore, early diagnosis of sepsis is crucial, as it allows for the prompt initiation of supportive and antibiotic therapy to reduce sepsis mortality. However, due to the complexity and heterogeneity of sepsis, accurate early diagnosis and prognosis assessment are challenging. Traditional diagnostic methods are both time-consuming and expensive, and require operation by professionally medically trained personnel (4). Blood culture is the gold standard for diagnosing sepsis; however, it has a high false-negative rate and often results in delayed outcomes (5, 6). Over the past few decades, extensive research has explored various biomarkers, utilizing them to diagnose and predict outcomes in sepsis patients, playing crucial roles in the pathophysiology of sepsis.
Pentraxin-3 (PTX-3), also known as human serum penetratin-3, is a representative member of the long-chain pentraxin subfamily in the pentraxin family (7). PTX-3 is a new type of acute-phase inflammatory factor and plays an important role in infectious diseases. In recent years, it has been found that the PTX-3 level increases sharply in the blood of sepsis patients, and may be superior to traditional biomarkers in judging the severity of their condition and prognosis assessment. The purpose of this review is to summarize the current literature on one of the sepsis biomarkers, PTX-3, to provide a comprehensive overview of the research progress on PTX-3 as a sepsis biomarker. This aims to offer a brief outlook for further research on the sepsis biomarker PTX-3.
Pentraxins is an evolutionarily conserved protein superfamily characterized by a structural motif, that is, the pentraxin domain (8). Pentraxin-3 is a 45-kilodalton protein that forms high-molecular-weight oligomers through interchain disulfide bonds (9). The C-terminal domain (203 amino acids) of PTX-3 has homology with classic short pentameric proteins such as C-reactive protein (CRP) and serum amyloid protein P (SAP), while its N-terminal domain (178 amino acids) has no significant homology with other known proteins.
Unlike classic short pentameric proteins such as CRP and SAP that are systemically produced by hepatocytes, PTX-3 is produced by various cell types and shows differences in genomic organization, cellular source, and ligand-binding characteristics (8). Due to significant differences in sequence and regulation, CRP (which is not an acute-phase protein in mice) cannot be used in genetic methods to study its in vivo functions, while PTX-3 remains highly conserved throughout the evolutionary process. Notably, PTX-3 plays a complex and irreplaceable role in vivo, being able to recognize various pathogens, regulate complement activity by binding to C1q, and promote the recognition of pathogens by macrophages and dendritic cells. PTX-3, as a liquid-phase effector molecule of the innate immune system, its production is stimulated by cytokines and bacterial endotoxins. PTX-3 can bind to specific pathogens, activate complement, promote cell recognition and clearance, and can also act as a matrix component (8).
PTX-3 is an acute-phase reactant with relatively low blood levels under normal circumstances (about 25 ng/mL in mice and < 2 ng/mL in humans). It has been reported that human plasma PTX-3 levels are related to gender, significantly lower in men than in women, and increase significantly with age. In infectious shock, sepsis, and other inflammatory and infectious states, plasma PTX-3 levels increase sharply, reaching a peak level within 6–8 h (10), and its concentration can increase up to 100 ng/mL during sepsis (11). When injury occurs, the PTX-3 level in the blood is extremely high, which is related to the release of pre-formed PTX-3 in neutrophils. Maugeri et al. (12) clearly described this, reporting that neutrophils led to an increase in plasma PTX-3 concentration within 6 h after the appearance of clinical symptoms of acute myocardial infarction and returned to normal within 48 h. Studies using in vivo neutrophil depletion and multiple vascular proteomics have concluded that PTX-3 may be stored and released in a polymeric form. Proteomic analysis of the aorta of mice injected with lipopolysaccharide (LPS) showed that PTX-3 was the most upregulated protein, and polymeric PTX-3 was deposited along with other neutrophil-derived proteins as early as 2 h after LPS injection. In healthy volunteers, a rapid degranulation reaction was observed 6 h after LPS injection, followed by an acute-phase response (13). In addition, studies have shown that plasma PTX-3 levels increase as the glomerular filtration rate (GFR) decreases (14), and the increase in liver PTX-3 levels in liver necrosis may be a marker of acute histological liver injury (15).
PTX-3 has a tendency to rise faster in the pathogenesis of sepsis than the previously used biomarkers and is superior to traditional biomarkers, which may be due to the fact that it is locally produced at the site of infection or tissue damage rather than relying on other molecules to trigger the synthesis of body organs. In contrast, CRP is systemically generated by liver cells in response to IL-6 stimulation, and the process takes longer and only begins to change significantly after 24–30 h (16). The comparison of PTX-3 with other common biomarkers is shown in Table 1.
The overexpression of PTX-3 will exacerbate the inflammatory response and reduce the survival rate of mice subjected to intestinal ischemia–reperfusion injury (17). The data collected from various pathological processes indicate that there is a correlation between plasma PTX-3 level and disease severity, suggesting the potential role of PTX-3 as a pathological biomarker. Whether the significant correlation between the result and the severity reflects its role in the pathogenesis of the injury mechanism, such as amplifying the complement and coagulation cascade reactions, remains to be elucidated (18). Elevated PTX-3 levels have been observed in various infectious diseases including sepsis, septic shock, aspergillus infection, tuberculosis and dengue fever (19, 20). PTX-3 is also expressed in aseptic inflammation. It has been reported that the PTX-3 level of patients with acute coronary syndrome increases by about 6–7 ng/mL (21, 22), the PTX-3 level of patients with congestive heart failure increases (about 3–4 ng/mL) (23), the PTX-3 level in renal failure increases by about 5–6 ng/mL (14, 24), and the PTX-3 level in acute respiratory distress syndrome increases by about 70 ng/mL (25). In addition, in patients with AMI with ST-segment elevation, PTX-3 can predict the 3-month mortality rate after adjusting important risk factors and other acute phase prognostic indicators (26). Early detection of PTX-3 level is an independent indicator for predicting multiple organ dysfunction syndrome (MODS) and premature death in patients with cardiac arrest (27, 28). In autoimmune diseases, PTX-3 mediates the complement regulatory mechanism, leading to inflammation and tissue damage in RA (29–31). PTX-3 may be a new non-invasive biomarker indicating the clinical arthritis activity of RA patients (32). In addition, PTX-3 is significantly correlated with the activity degree of SLE (33, 34). PTX-3 may participate in the pathogenesis of psoriasis and can indicate the disease activity of psoriasis (35, 36). The increase of PTX-3 level during the acute attack of acute rheumatic fever may help predict the clinical course (37). PTX-3 can also be used for the early severity assessment and prediction of acute pancreatitis (AP) (38). However, PTX-3 is not as good as CRP and APACHE II score in predicting the mortality of AP, and the combination of PTX-3 and CRP cannot improve the predictive value of CRP (39).
In conclusion, PTX-3 is a multifunctional protein at the intersection of immunity, inflammation, extracellular matrix construction, and female reproduction (8, 40). In all these cases, the PTX-3 level is correlated with the clinical activity of the disease, making it a candidate biomarker for disease monitoring (41).
As an acute-phase reactant protein, PTX-3 has been widely studied as a biomarker to distinguish common bacterial infections from sepsis or septic shock. The results of one study showed that the area under the curve (AUC) for the discrimination between the healthy control group and the SIRS group was 0.922 (cut-off value 16.0 ng/mL, sensitivity 89.1%, specificity 85%), and the difference was statistically significant (42). At the same time, among suspected infection patients visiting the emergency department, the AUC of PTX-3 in predicting severe sepsis from day 0 to day 28 was 0.73 (cut-off value 14.1 ng/mL, sensitivity 63%, specificity 80%) (43). Another study showed that in adult febrile patients in the emergency department, the AUC for predicting bloodstream infection was 0.71, the critical value was 16.1 ng/mL, and the sensitivity was 76% and the specificity was 61% (44). Hamed et al. (45) studied the PTX-3 level on the 1st, 3rd, and 8th days of treatment in ICU sepsis patients and found that at the cut-off value of 5 μg/L, the lowest sensitivity and specificity were 92% and 64%, respectively. Moreover, this study defined a unified diagnostic boundary, diagnosing sepsis at least (≥5.0 ng/mL) and septic shock (≥9.0 ng/mL) (45).
Similar to adults, PTX-3 can be used as a reliable biomarker for neonatal sepsis with high sensitivity and specificity. One study showed that when using the PTX-3 cut-off value of 5.6 μg/L to diagnose sepsis, the sensitivity was 98.3%, the specificity was 96.7%, the positive predictive value (PPV) was 98%, and the negative predictive value (NPV) was 96%. While the critical value of CRP was 8 mg/dL, the sensitivity was 96.7%, the specificity was 96.7%, the positive predictive value (PPV) = 98.3%, and the negative predictive value (NPV) = 93.5%, and the accuracy (area under the curve) = 0.989 (46). Although this study may have some limitations, for the diagnosis of neonatal sepsis, the sensitivity of biomarkers is more important than the specificity, so PTX-3 seems to be superior to CRP as a diagnostic biomarker for neonatal sepsis.
Abnormally elevated plasma PTX-3 levels are closely related to the mortality rate of sepsis and have important significance in predicting the risk of death for sepsis patients. Multiple studies have shown that the systemic PTX-3 level of critically ill patients with a fatal outcome is significantly higher than that of surviving critically ill patients (25, 42, 47–50). Among sepsis patients, the maximum PTX-3 value of non-surviving patients in the first to fourth days is significantly higher than that of surviving patients (44.8 vs. 6.4 ng/mL, 𝑃<0.001), and the AUC for predicting the case fatality rate is 0.82 (cut-off value 15 ng/mL, sensitivity 72%, specificity 81%) (51). Similarly, when predicting the mortality rate on the 28th day, the AUC of suspected infected emergency patients is 0.69 (95% confidence interval 0.58 ~ 0.79, p < 0.001), and the critical value is 7.7 ng/mL, (sensitivity 70%, specificity 63%) (43). Wang et al.’s (52) meta-analysis included 17 studies with 3,658 sepsis patients, and the results showed that the PTX-3 level of sepsis patients who died was significantly higher than that of surviving patients, indicating that a high level of PTX-3 is significantly related to the risk of death in sepsis and can predict the patient mortality rate. Lee et al.’s (53) meta-analysis found that an increase in PTX-3 doubles the risk of death in sepsis patients. Another trial conducted a prospective study on 160 sepsis patients and showed that when predicting the 28-day mortality rate of sepsis, at the cut-off value of 26.90 ng/mL, its sensitivity is 88.9% and specificity is 49.5%, and the AUC is 0.734 (95% CI 0.656 ~ 0.811) (54). A prospective cohort study by Kim et al. found that compared with PCT, neutrophil count and CRP, PTX-3 has a larger AUC value (0.819, 95% CI 0.677 ~ 0.961) in predicting the 28-day all-cause mortality rate of severe sepsis patients. At the same time, by establishing a Cox proportional hazards model, it was found that the plasma PTX-3 level measured at admission is an independent predictor of the 28-day all-cause mortality rate of severe sepsis patients (HR = 7.16, 95% CI 2.46 ~ 15.85) (55).
The above research findings suggest that PTX-3 is an early biomarker for predicting the mortality rate of sepsis. Timely detection of PTX-3 can provide guidance for subsequent treatment and management, which has significant clinical importance. Additionally, a single-center prospective study reveals that in comparison to PCT, IL-6, CRP, lactic acid, and platelet count, PTX-3 demonstrates a higher diagnostic value in differentiating between patients with infectious shock and those without (p < 0.001) (56). However, when compared to the traditional Simplified Acute Physiological Score II (SAPS II), the ability of PTX-3 to predict mortality is poorer (49).
In different studies, the optimal cut-off value of PTX-3 for predicting bacterial infection, sepsis, septic shock and mortality varies, and the reported sensitivity and specificity are also discrepant. The comparison with other indicators is presented in Table 2.
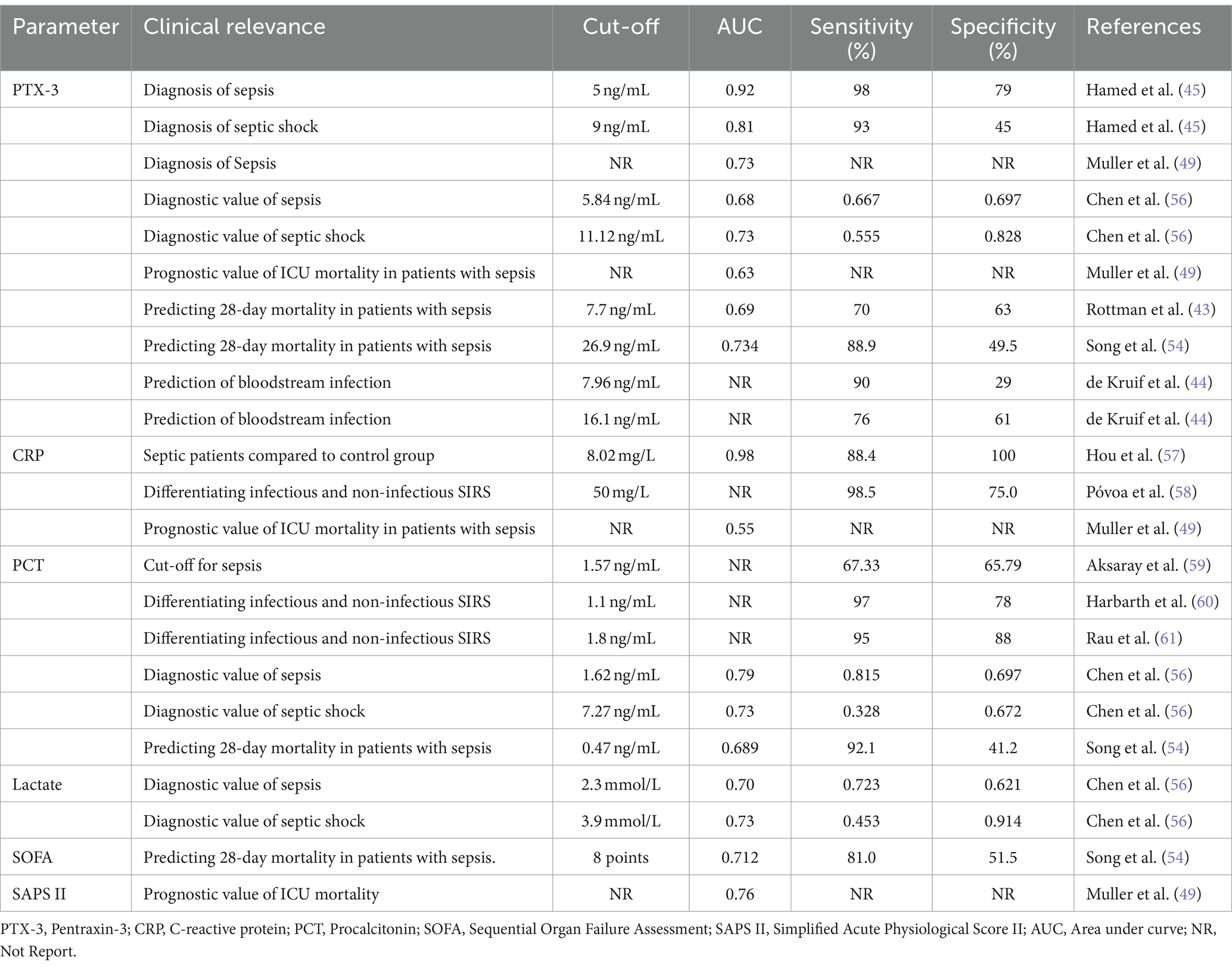
Table 2. The cut-off values of PTX-3 and other commonly used indicators in differencing infection, sepsis and predicting mortality.
The correlation between the PTX-3 level and the disease severity and organ dysfunction exceeds that of other measured mediators such as tumor necrosis factor-α, interleukin-6, and C-reactive protein (62). Although PTX-3 alone may not perform as well as the Simplified Acute Physiology Score II (SAPS II), this does not necessarily rule out its potential application in prognosis. In recent decades, scoring systems such as the Acute Physiology and Chronic Health Evaluation System (APACHE II), the Simplified Acute Physiology Score (SAPS), and the Sequential Organ Failure Assessment (SOFA) have become increasingly popular in assessing the risk of death in critically ill patients. However, these scoring systems have significant limitations in clinical practice. Data collection requires multiple laboratory measurements and the calculation involving numerous variables, making it both laborious and costly (63, 64).
Although PTX-3 has good prospects as a biomarker for sepsis, challenges and limitations still need to be addressed. Due to the heterogeneity of research and selection criteria, an accurate cut-off value has not been determined yet. Therefore, laboratory test results should be interpreted in combination with the clinical situation and used in combination with other laboratory findings to ensure accurate diagnosis and guide appropriate management. Previous studies have included studies involving both adults and children, as well as individuals with different severities in infectious diseases. Some scholars strongly recommend subgroup analysis according to age and different disease manifestations (65). Future research should focus on refining the role of PTX-3 in different etiologies of sepsis and evaluating its potential as a therapeutic target, such as whether the PTX-3 level can guide the use of antibiotics, etc., and its molecular biological mechanism still needs to be further clarified.
PTX-3 appears to be a promising prognostic biomarker for critically ill patients. Currently, research is limited to observational designs estimating the predictive potential for mortality risk. Further investigation is needed to determine whether monitoring PTX-3 levels during treatment can be used to guide therapeutic decisions. In conclusion, PTX-3 emerges as a valuable candidate as a biomarker for sepsis, providing insights into its structural characteristics, physiological functions, and potential diagnostic applications. The progress in understanding the role of PTX-3 in sepsis paves the way for improving diagnostic accuracy, prognostic assessment, and targeted therapeutic interventions.
YZ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Funding acquisition. XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. XiaoZ: Conceptualization, Investigation, Methodology, Software, Writing – review & editing. TW: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. XianZ: Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arina, P, and Singer, M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. (2021) 34:77–84. doi: 10.1097/ACO.0000000000000963
2. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Weng, L, Xu, Y, Yin, P, Wang, Y, Chen, Y, Liu, W, et al. National incidence and mortality of hospitalized sepsis in China. Crit Care. (2023) 27:84. doi: 10.1186/s13054-023-04385-x
4. Kumar, S, Tripathy, S, Jyoti, A, and Singh, SG. Recent advances in biosensors for diagnosis and detection of sepsis: a comprehensive review. Biosens Bioelectron. (2019) 124-125:205–15. doi: 10.1016/j.bios.2018.10.034
5. Bellos, I, Fitrou, G, Pergialiotis, V, Thomakos, N, Perrea, DN, and Daskalakis, G. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. (2018) 177:625–32. doi: 10.1007/s00431-018-3114-1
6. Shah, BA, and Padbury, JF. Neonatal sepsis. Virulence. (2013) 5:170–8. doi: 10.1016/S0140-6736(17)31002-4
7. Porte, R, Davoudian, S, Asgari, F, Parente, R, Mantovani, A, Garlanda, C, et al. The long Pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and Sepsis. Front Immunol. (2019) 10:794. doi: 10.3389/fimmu.2019.00794
8. Garlanda, C, Bottazzi, B, Bastone, A, and Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. (2005) 23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756
9. Bottazzi, B, Vouret-Craviari, V, Bastone, A, De Gioia, L, Matteucci, C, Peri, G, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. (1997) 272:32817–23. doi: 10.1074/jbc.272.52.32817
10. Yamasaki, K, Kurimura, M, Kasai, T, Sagara, M, Kodama, T, and Inoue, K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. (2009) 47:471–7. doi: 10.1515/CCLM.2009.110
11. Daigo, K, and Hamakubo, T. Host-protective effect of circulating pentraxin 3 (PTX3) and complex formation with neutrophil extracellular traps. Front Immunol. (2012) 3:378. doi: 10.3389/fimmu.2012.00378
12. Maugeri, N, Rovere-Querini, P, Slavich, M, Coppi, G, Doni, A, Bottazzi, B, et al. Early and transient release of leukocyte Pentraxin 3 during acute myocardial infarction. J Immunol. (2011) 187:970–9. doi: 10.4049/jimmunol.1100261
13. Burnap, SA, Mayr, U, Shankar-Hari, M, Cuello, F, Thomas, MR, Shah, AM, et al. A proteomics-based assessment of inflammation signatures in Endotoxemia. Mol Cell Proteomics. (2021) 20:100021. doi: 10.1074/mcp.RA120.002305
14. Tong, M, Carrero, JJ, Qureshi, AR, Anderstam, B, Heimburger, O, Barany, P, et al. Plasma Pentraxin 3 in patients with chronic kidney disease. Clin J Am Soc Nephrol. (2007) 2:889–97. doi: 10.2215/CJN.00870207
15. Yaman, H, Cakir, E, Akgul, EO, Aydin, I, Onguru, O, Cayci, T, et al. Pentraxin 3 as a potential biomarker of acetaminophen-induced liver injury. Exp Toxicol Pathol. (2013) 65:147–51. doi: 10.1016/j.etp.2011.07.003
16. Milas, GP, Issaris, V, and Niotis, G. Pentraxin-3 and neonatal sepsis: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2023) 36:2205986. doi: 10.1080/14767058.2023.2205986
17. Souza, DG, Soares, AC, Pinho, V, Torloni, H, Reis, LFL, Martins, MT, et al. Increased mortality and inflammation in tumor necrosis factor-stimulated Gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. (2002) 160:1755–65. doi: 10.1016/S0002-9440(10)61122-4
18. Mantovani, A, Garlanda, C, Doni, A, and Bottazzi, B. Pentraxins in innate immunity: from C-reactive protein to the long Pentraxin PTX3. J Clin Immunol. (2007) 28:1–13. doi: 10.1007/s10875-007-9126-7
19. Azzurri, A, Sow, OY, Amedei, A, Bah, B, Diallo, S, Peri, G, et al. IFN-γ-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. (2005) 7:1–8. doi: 10.1016/j.micinf.2004.09.004
20. Mairuhu, ATA, Peri, G, Setiati, TE, Hack, CE, Koraka, P, Soemantri, A, et al. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. (2005) 76:547–52. doi: 10.1002/jmv.20397
21. Inoue, K, Sugiyama, A, Reid, PC, Ito, Y, Miyauchi, K, Mukai, S, et al. Establishment of a high sensitivity plasma assay for human Pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. (2007) 27:161–7. doi: 10.1161/01.ATV.0000252126.48375.d5
22. Peri, G, Introna, M, Corradi, D, Iacuitti, G, Signorini, S, Avanzini, F, et al. PTX3, a prototypical long Pentraxin, is an early Indicator of acute myocardial infarction in humans. Circulation. (2000) 102:636–41. doi: 10.1161/01.CIR.102.6.636
23. Suzuki, S, Takeishi, Y, Niizeki, T, Koyama, Y, Kitahara, T, Sasaki, T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. (2008) 155:75–81. doi: 10.1016/j.ahj.2007.08.013
24. Suliman, ME, Yilmaz, MI, Carrero, JJ, Qureshi, AR, Saglam, M, Ipcioglu, OM, et al. Novel links between the long Pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol. (2008) 3:976–85. doi: 10.2215/CJN.03960907
25. Mauri, T, Coppadoro, A, Bellani, G, Bombino, M, Patroniti, N, Peri, G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity*. Crit Care Med. (2008) 36:2302–8. doi: 10.1097/CCM.0b013e3181809aaf
26. Latini, R, Maggioni, AP, Peri, G, Gonzini, L, Lucci, D, Mocarelli, P, et al. Prognostic significance of the long Pentraxin PTX3 in acute myocardial infarction. Circulation. (2004) 110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E
27. Ristagno, G, Fumagalli, F, Bottazzi, B, Mantovani, A, Olivari, D, Novelli, D, et al. Pentraxin 3 in cardiovascular disease. Front Immunol. (2019) 10:823. doi: 10.3389/fimmu.2019.00823
28. Ristagno, G, Varpula, T, Masson, S, Greco, M, Bottazzi, B, Milani, V, et al. Elevations of inflammatory markers PTX3 and sST2 after resuscitation from cardiac arrest are associated with multiple organ dysfunction syndrome and early death. Clin Chem Lab Med. (2015) 53:1847–57. doi: 10.1515/cclm-2014-1271
29. Breitner, S, Störkel, S, Reichel, W, and Loos, M. Complement components C1q, C1r/C1s, and C1INH in rheumatoid arthritis. Correlation of in situ hybridization and northern blot results with function and protein concentration in synovium and primary cell cultures. Arthritis Rheum. (1995) 38:492–8. doi: 10.1002/art.1780380406
30. Jose, PJ, Moss, IK, Maini, RN, and Williams, TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. (1990) 49:747–52. doi: 10.1136/ard.49.10.747
31. Katona, IM, Ohura, K, Allen, JB, Wahl, LM, Chenoweth, DE, and Wahl, SM. Modulation of monocyte chemotactic function in inflammatory lesions. Role of inflammatory mediators. J Immunol. (1991) 146:708–14.
32. Sharma, A, Khan, R, Gupta, N, Sharma, A, Zaheer, MS, Abbas, M, et al. Acute phase reactant, Pentraxin 3, as a novel marker for the diagnosis of rheumatoid arthritis. Clin Chim Acta. (2018) 480:65–70. doi: 10.1016/j.cca.2018.01.035
33. Sahin, S, Adrovic, A, Barut, K, Durmus, S, Gelisgen, R, Uzun, H, et al. Pentraxin-3 levels are associated with vasculitis and disease activity in childhood-onset systemic lupus erythematosus. Lupus. (2017) 26:1089–94. doi: 10.1177/0961203317699286
34. Shimada, Y, Asanuma, YF, Yokota, K, Yoshida, Y, Kajiyama, H, Sato, K, et al. Pentraxin 3 is associated with disease activity but not atherosclerosis in patients with systemic lupus erythematosus. Mod Rheumatol. (2013) 24:78–85. doi: 10.3109/14397595.2013.852837
35. Bevelacqua, V, Libra, M, Mazzarino, MC, Gangemi, P, Nicotra, G, Curatolo, S, et al. Long pentraxin 3: a marker of inflammation in untreated psoriatic patients. Int J Mol Med. (2006) 18:415–23. doi: 10.3892/ijmm.18.3.415
36. Uysal, S, Yılmaz, FM, Karatoprak, K, Artüz, F, and Cumbul, NU. The levels of serum pentraxin3, CRP, fetuin-a, and insulin in patients with psoriasis. Eur Rev Med Pharmacol Sci. (2014) 18:3453–8.
37. Gürses, D, Oğuz, M, Yilmaz, M, Aybek, H, and Akpinar, F. Pentraxin 3 levels and correlation with disease severity in patients with acute rheumatic fever. Archives of. Rheumatology. (2021) 36:233–43. doi: 10.46497/ArchRheumatol.2021.8232
38. Kusnierz-Cabala, B, Gurda-Duda, A, Dumnicka, P, Panek, J, Pawlica-Gosiewska, D, Kulig, J, et al. Plasma pentraxin 3 concentrations in patients with acute pancreatitis. Clin Lab. (2013) 59:1003–8. doi: 10.7754/Clin.Lab.2012.120821
39. Staubli, SM, Schäfer, J, Rosenthal, R, Zeindler, J, Oertli, D, and Nebiker, CA. The role of CRP and Pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci Rep. (2019) 9:18340. doi: 10.1038/s41598-019-54910-8
40. Bottazzi, B, Garlanda, C, Salvatori, G, Jeannin, P, Manfredi, A, and Mantovani, A. Pentraxins as a key component of innate immunity. Curr Opin Immunol. (2006) 18:10–5. doi: 10.1016/j.coi.2005.11.009
41. Fazzini, F, Peri, G, Doni, A, Dell'Antonio, G, Dal Cin, E, Bozzolo, E, et al. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. (2001) 44:2841–50. doi: 10.1002/1529-0131(200112)44:12<2841::AID-ART472>3.0.CO;2-6
42. Caldwell, CC, Bastrup-Birk, S, Skjoedt, M-O, Munthe-Fog, L, Strom, JJ, Ma, YJ, et al. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. (2013) 8:e73119. doi: 10.1371/journal.pone.0073119
43. Rottman, M, Uusitalo-Seppälä, R, Huttunen, R, Aittoniemi, J, Koskinen, P, Leino, A, et al. Pentraxin 3 (PTX3) is associated with severe Sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort Study. PLoS One. (2013) 8:e53661. doi: 10.1371/journal.pone.0053661
44. de Kruif, MD, Limper, M, Sierhuis, K, Wagenaar, JFP, Spek, CA, Garlanda, C, et al. PTX3 predicts severe disease in febrile patients at the emergency department. J Infect. (2010) 60:122–7. doi: 10.1016/j.jinf.2009.11.010
45. Hamed, S, Behnes, M, Pauly, D, Lepiorz, D, Barre, M, Becher, T, et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. (2017) 17:554. doi: 10.1186/s12879-017-2606-3
46. Fahmey, SS, and Mostafa, N. Pentraxin 3 as a novel diagnostic marker in neonatal sepsis. J Neonatal-Perinatal Med. (2020) 12:437–42. doi: 10.3233/NPM-190261
47. Lin, Q, Fu, F, Shen, L, and Zhu, B. Pentraxin 3 in the assessment of ventilator-associated pneumonia: an early marker of severity. Heart Lung. (2013) 42:139–45. doi: 10.1016/j.hrtlng.2012.11.005
48. Mauri, T, Bellani, G, Patroniti, N, Coppadoro, A, Peri, G, Cuccovillo, I, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. (2010) 36:621–9. doi: 10.1007/s00134-010-1752-5
49. Muller, B, Peri, G, Doni, A, Torri, V, Landmann, R, Bottazzi, B, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. (2001) 29:1404–7. doi: 10.1097/00003246-200107000-00017
50. Vanska, M, Koivula, I, Hamalainen, S, Pulkki, K, Nousiainen, T, Jantunen, E, et al. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica. (2011) 96:1385–9. doi: 10.3324/haematol.2011.044925
51. Neyrolles, O, Huttunen, R, Hurme, M, Aittoniemi, J, Huhtala, H, Vuento, R, et al. High plasma level of long Pentraxin 3 (PTX3) is associated with fatal disease in Bacteremic patients: a prospective cohort Study. PLoS One. (2011) 6:e17653. doi: 10.1371/journal.pone.0017653
52. Wang, G, Jiang, C, Fang, J, Li, Z, and Cai, H. Pentraxin-3 as a predictive marker of mortality in sepsis: an updated systematic review and meta-analysis. Crit Care. (2022) 26:167. doi: 10.1186/s13054-022-04032-x
53. Lee, YT, Gong, M, Chau, A, Wong, WT, Bazoukis, G, Wong, SH, et al. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J Infect. (2018) 76:1–10. doi: 10.1016/j.jinf.2017.10.016
54. Song, J, Moon, S, Park, DW, Cho, H-J, Kim, JY, Park, J, et al. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock. Medicine. (2020) 99:e20495. doi: 10.1097/MD.0000000000020495
55. Kim, SB, Lee, KH, Lee, JU, Ann, HW, Ahn, JY, Jeon, YD, et al. Long Pentraxin 3 as a predictive marker of mortality in severe septic patients who received successful early goal-directed therapy. Yonsei Med J. (2017) 58:370–9. doi: 10.3349/ymj.2017.58.2.370
56. Chen, H, Li, T, Yan, S, Liu, M, Liu, K, Zhang, H, et al. Pentraxin-3 is a strong biomarker of Sepsis severity identification and predictor of 90-day mortality in intensive care units via Sepsis 3.0 definitions. Diagnostics. (2021) 11:1906. doi: 10.3390/diagnostics11101906
57. Hou, X, Liu, C, Lian, H, Xu, Z, Ma, L, Zang, X, et al. The value of neutrophil gelatinase-associated lipocalin and citrullinated alpha enolase peptide-1 antibody in diagnosis, classification, and prognosis for patients with sepsis. Medicine. (2020) 99:e21893. doi: 10.1097/MD.0000000000021893
58. Póvoa, P, Almeida, E, Moreira, P, Fernandes, A, Mealha, R, Aragão, A, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. (1998) 24:1052–6. doi: 10.1007/s001340050715
59. Aksaray, S, Alagoz, P, Inan, A, Cevan, S, and Ozgultekin, A. Diagnostic value of sTREM-1 and procalcitonin levels in the early diagnosis of sepsis. Northern Clin Istanb. (2016) 3:175–82. doi: 10.14744/nci.2016.26023
60. Harbarth, S, Holeckova, K, Froidevaux, C, Pittet, D, Ricou, B, Grau, GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. (2001) 164:396–402. doi: 10.1164/ajrccm.164.3.2009052
61. Rau, B, Steinbach, G, Baumgart, K, Gansauge, F, Grünert, A, and Beger, HG. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med. (2000) 26:S159–64. doi: 10.1007/s001340051136
62. Zhang, H, Damas, P, and Preiser, JC. The long way of biomarkers: from bench to bedside. Intensive Care Med. (2010) 36:565–6. doi: 10.1007/s00134-010-1758-z
63. Le Gall, JR, Lemeshow, S, and Saulnier, F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.1993.03510240069035
64. Litton, E, Ho, KM, and Webb, SAR. Comparison of physician prediction with 2 prognostic scoring systems in predicting 2-year mortality after intensive care admission: a linked-data cohort study. J Crit Care. (2012) 27:423.e9–e15. doi: 10.1016/j.jcrc.2011.11.013
Keywords: sepsis, pentraxin-3 (PTX-3), biomarker, diagnosis, prognosis
Citation: Zhang Y, Li X, Zhang X, Wang T and Zhang X (2024) Progress in the study of pentraxin-3(PTX-3) as a biomarker for sepsis. Front. Med. 11:1398024. doi: 10.3389/fmed.2024.1398024
Received: 08 March 2024; Accepted: 18 June 2024;
Published: 03 July 2024.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Martin Helán, St. Anne’s University Hospital, CzechiaCopyright © 2024 Zhang, Li, Zhang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangcheng Zhang, Wmh4YzAzMThAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.