- 1School of Stomatology, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2Changsha Hospital for Maternal and Child Health Care, Changsha, Hunan, China
- 3Department of Stomatology, The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
Background: Oral lichen planus (OLP) is a relatively common chronic T cell-mediated disease characterized by pain and inflammation. Clobetasol propionate (CLO) is the first-line drug in the treatment of OLP. The meta-analysis aimed to evaluate the efficacy and safety of CLO for treating patients with OLP.
Methods: PubMed, Embase and Web of Science were systematically searched from the database inception date up to August 2023. There were no restrictions on language or date of publication. The outcomes of our interest were as follows: improvement of clinical signs and/or symptoms, total lesion size, relapse and adverse events.
Results: A total of 17 RCTs evaluating the effects of CLO were included in this study. The results revealed no significant difference in the clinical score (WMD = 0.14, 95% CI: −0.39, 0.66; p = 0.609) and pain score (WMD = 0.17, 95% CI: −0.44, 0.79; p = 0.582) between CLO and other treatments. However, clinical resolution (RR = 1.61, 95% CI: 1.17, 2.22; p = 0.003) and symptoms improvement (RR = 1.80, 95% CI: 1.17, 2.77; p = 0.008) were significantly different between CLO and other treatments. Moreover, there was a significant reduction in the total lesion size with CLO treatment (WMD = -0.58, 95% CI: −1.03, −0.13; p = 0.011). In addition, CLO showed no statistical incidence of adverse events (RR = 1.46, 95% CI: 0.86, 2.50; p = 0.161) and relapse (RR = 1.56, 95% CI: 0.66, 3.71; p = 0.314) than other therapies.
Conclusion: This systematic review and meta-analysis of 17 randomized clinical trials supported the long-term application of CLO as an effective regimen in OLP patients.
Introduction
Oral lichen planus (OLP) is a common T-cell-mediated autoimmune inflammatory (1). The global prevalence of OLP is 1–2%, typically appearing in the fourth decade of life, and more commonly affects women than men (2–4). The feature of OLP lesions in the buccal mucosa are normally white reticular, which are frequently asymptomatic (5). Conversely, the symptoms of ulcerative or erosive lesions vary from mild discomfort to severe pain that may negatively affect quality of patient’s life (6, 7). OLP is clinically unpredictable, with worsening over time (8). In fact, due to the cases of relapse are not uncommon, long-term symptoms relief is a great challenge in treating OLP (9). More importantly, an analysis of the existing literature suggests that patients diagnosed with OLP possess an increased risk of developing oral squamous cell carcinoma (OSCC) (10, 11). Consequently, OLP has been classified as a potentially malignant oral disorder (12).
While significant strides have been made in OLP research, a definitive cure and effective treatment for OLP is yet to be discovered (13). Most therapeutic strategies, given the present scenario, are focused on relieving symptomatic pain and inflammation. In the management of non-erosive OLP, the use of topical corticosteroids, such as 0.05% clobetasol propionate (CLO), is commonly employed. For erosive OLP, localized treatment via triamcinolone acetonide injections is often utilized (14). However, in cases of severe erosive or refractory OLP, alternative therapeutic approaches have been explored. Systemic therapies such as systemic corticosteroids, apremilast, hydroxychloroquine, and systemic retinoids have been proposed for their immunomodulatory effects (3). Additionally, biologics targeting specific inflammatory pathways, such as anti-IL-13, anti-IL-12/IL-23, and anti-IL-23 monoclonal antibodies, have shown promising results in refractory cases of erosive OLP, suggesting a potential role in personalized treatment strategies (14, 15). Emerging non-pharmacological treatments, including CO2 laser therapy and photodynamic therapy, have also demonstrated varying degrees of efficacy, offering alternative options for patients with contraindications or poor responsiveness to conventional therapies (16, 17). Other novel therapies, such as low-level laser therapy (LLLT) and ozone therapy, have shown promising results in reducing pain and inflammation in OLP patients (18). However, it is important to note that their effectiveness may vary among different patients.
As it stands, CLO, a corticosteroid, is considered an effective anti-inflammatory and pain relief for managing mild symptoms of OLP (14). Alternative therapies are only recommended in some cases, such as those characterized by poor responsiveness, intolerance, or existing contraindications to corticosteroids (19). Recent studies have also indicated that the topical application of CLO demonstrated efficacy in treating erosive, refractory, or recurrent forms of OLP (20, 21).
To elucidate the clinical application scenarios of CLO in OLP treatment, this study conducted a comprehensive comparison of the efficacy and safety between CLO therapy and alternative treatments. Although current meta-analyses do not robustly support the superiority of CLO in treating OLP, the increasing number of randomized controlled trials (RCTs) centered on CLO has prompted an update of our review. This update aims to provide a more accurate assessment of the effectiveness and safety of CLO in treating OLP, thereby offering a more informed basis for clinical decision-making.
Materials and methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and the revised intervention assessment methodology from the Cochrane Handbook (22, 23).
Search strategy
We systematically searched PubMed, EMBASE and Web of Science, encompassing all relevant studies ranging from the database inception date up to August 2023. References of the selected articles were also manually searched to identify additional relevant studies. To avoid missing pertinent literature as much as possible, there were no restrictions on language, publication date, or type. Literature management was done using EndNote (X9), and two reviewers independently assessed all research. Discrepancies were negotiated to reach a consensus.
Inclusion and exclusion criteria
The following inclusion criteria were employed: (1) RCTs involved patients diagnosed with OLP either clinically or through histopathology. (2) Original studies investigating the application of CLO in the treatment of OLP. (3) Placebo or alternative treatment as the control. (4) At least one outcome regarding efficacy, safety, and stability. We excluded the following studies: (1) Reviews, case reports, in vitro research, letters, book chapters, and conference papers. (2) Studies with insufficient data and inability to establish contact with authors.
The primary efficacy outcomes comprised clinical response (score and resolution) and symptoms response (pain score and symptoms improvement). Clinical response was evaluated through the Thongprasom scale and signs improved, categorized into complete resolution and partial or no resolution. The secondary outcomes incorporated total lesion size, relapse rate post-treatment, and safety as determined by the proportion of patients with adverse reactions at any study phase.
Data extraction and management
Two investigators (Z.T. and W.Y.T) independently screened articles for relevance. Essential data from full texts, including the first author’s name, publication year, geographical location, sample size, interventions, and outcomes, were extracted. Any differences between reviewers were consulted by the third reviewer (L.C.Y) to ensure consistency.
Quality assessment
The risk of bias of all the included RCTs was evaluated by two independent investigators (Z.T. and W.Y.T.) with Cochrane ROB_2 (24). Disagreements were resolved by discussions with the third reviewer (L.C.Y) until a consensus was reached. These trials were examined across seven different RCT domains (25), including random sequence generation (selection bias), allocation concealment (selection bias), the blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. For each domain, the risk of bias was assessed as low, high, or unclear based on the Cochrane Collaboration guidelines (25). If all domains showcased low risk, then the study was deemed to have an overall low risk of bias. If the study was high risk in any one domain, then it was classified as having an overall high risk of bias. Otherwise, the study was considered to be unclear risk.
Statistical analysis
We employed the Stata 12.0 software to quantify treatment effects. For dichotomous data, risk ratios (RR) with 95% confidence intervals (CIs) were calculated, while continuous outcomes were represented by weighted mean differences (WMD) with 95% CIs. Heterogeneity among the included studies was assessed using the Q and I2statistics, in which p < 0.1 or I2 > 50% indicated significance. In the presence of significant heterogeneity, a random-effects model was adopted (26); otherwise, a fixed-effect model was chosen (27). For potential publication bias evaluation, Begg (28) and Egger’s (29) tests were applied when 10 or more studies were included in the analysis. Subgroup analyses were stratified by intervention treatment, control treatment and treatment duration. A sensitivity analysis was conducted by excluding one study at a time. All statistical tests were two-sided, with p < 0.05 being indicative of statistical significance.
Results
Literature selection and included studies
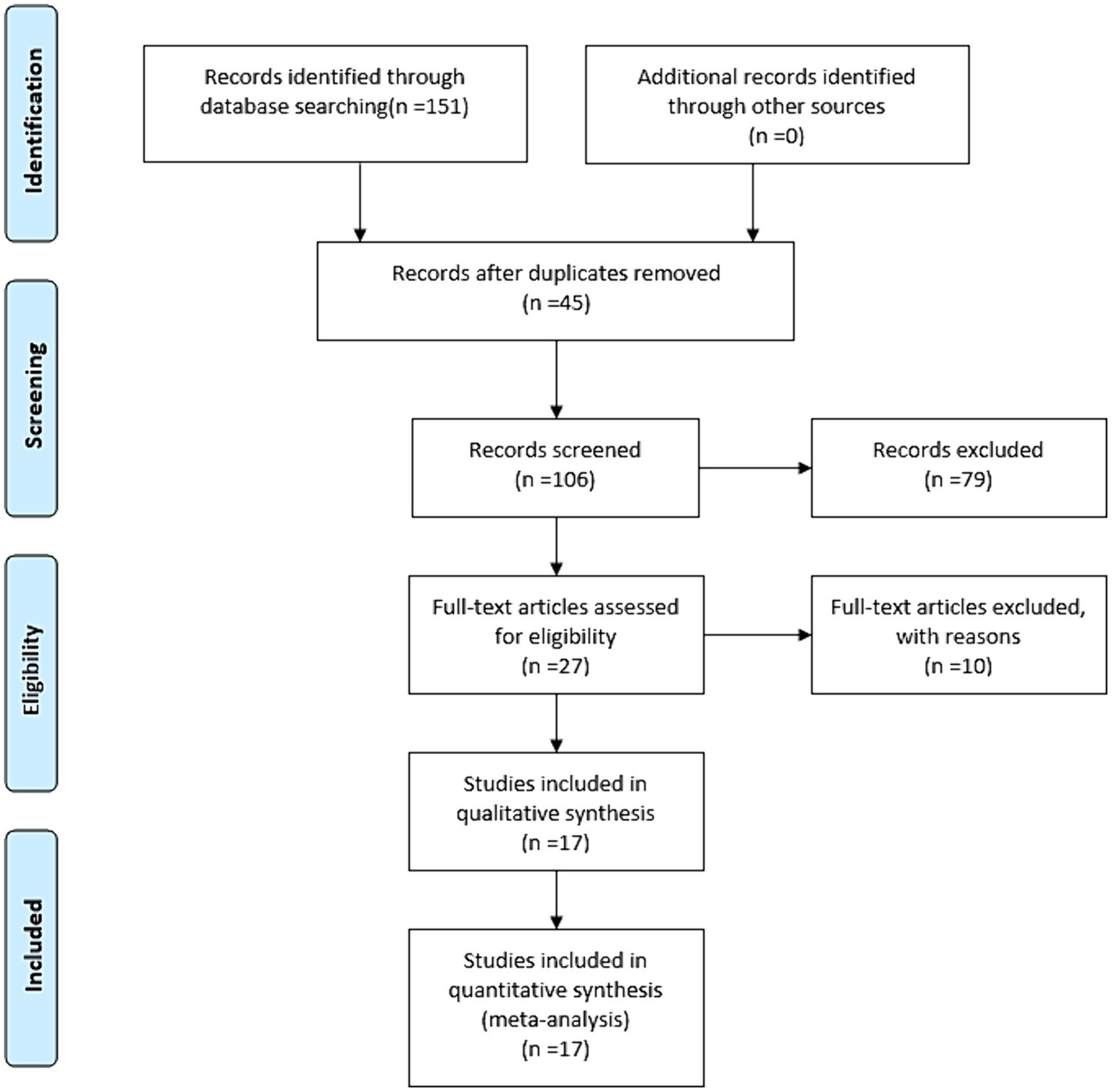
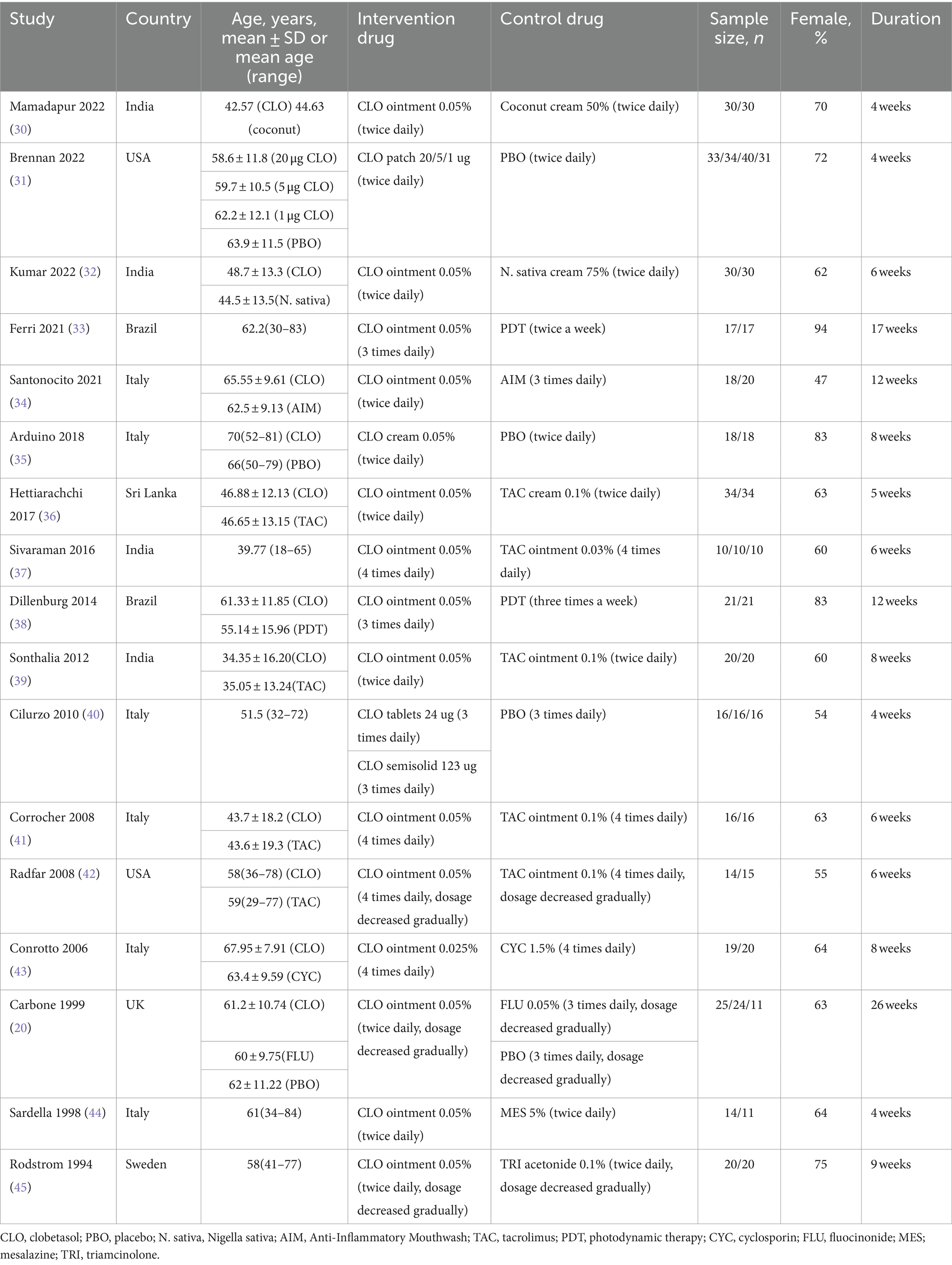
The flow diagram of the literature search and study selection process is depicted in Figure 1. Based on our established search strategy, a total of 151 relevant studies were identified. From these, 45 were excluded due to duplication, and an additional 79 were eliminated after evaluating their titles and abstracts. This left 27 articles for a full-text review. Following this review, 17 studies were chosen for the meta-analysis. The details and characteristics of these studies are summarized in Table 1.
Quality of the included studies
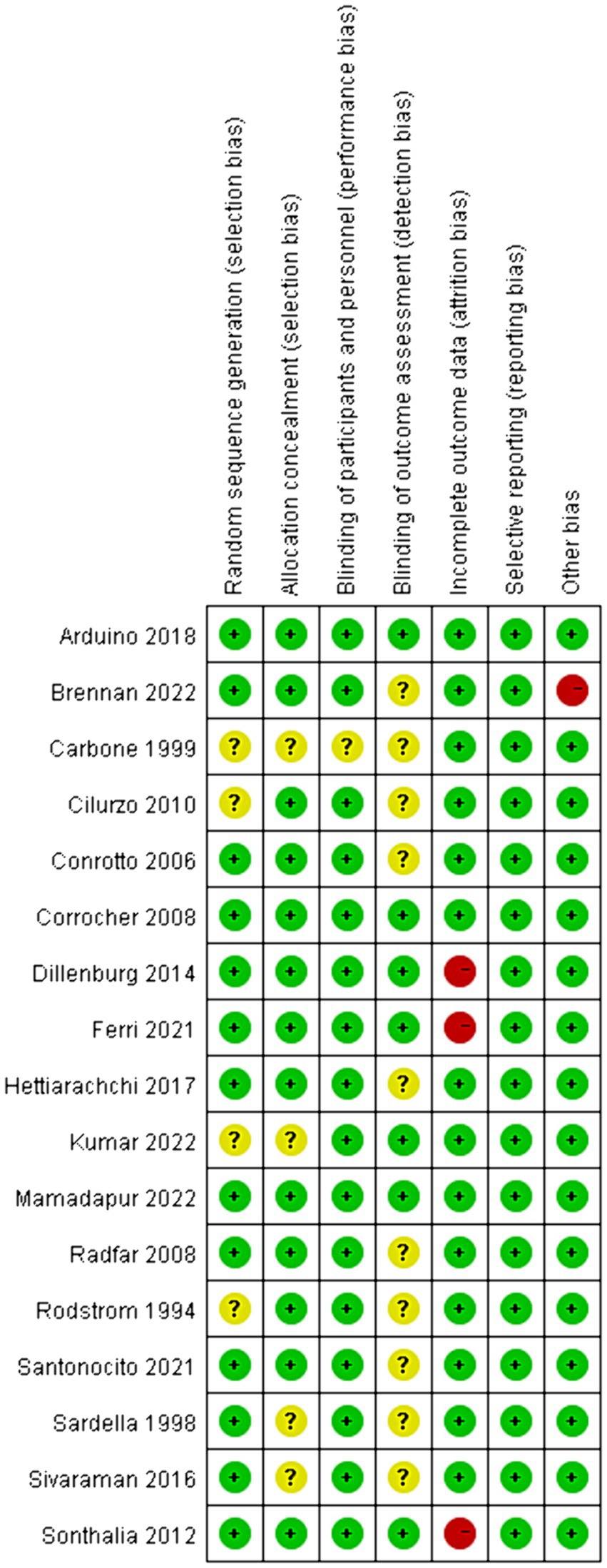
The risk of bias assessments in the included studies was presented in Figure 2. Concerning random sequence generation, 4 out of the 17 studies failed to furnish a clear methodological description, resulting in an “unclear” categorization in their risk of bias assessment (20, 32, 40, 45). A similar ambiguity was observed in the domain of allocation concealment (20, 32, 37, 44). Additionally, in 16 trials, both participants and personnel adopted blinding procedures, leading us to deduce that these trials exhibited a low risk of bias. However, one trial remained ambiguous in this respect (20). In terms of outcome assessment blinding, we observed that 59% of the authors did not specify whether medical staff remained blind to the type of intervention when evaluating patient conditions (20, 31, 34, 36, 37, 40, 42–45). Yet, in 7 trials, we identified a low risk of bias due to their blinding in outcome evaluations. With regard to incomplete outcome data, the majority of studies were evaluated as low risk, but three studies were rated as high risk owing to an excessive number of dropouts (33, 38, 39). Notably, the risk of bias related to selective reporting was generally low across all reviewed studies. Upon considering other biases, we noted that one study, funded by a pharmaceutical company, was assessed as high risk (31). In summary, 4 trials were evaluated with a high risk of bias (31, 33, 38, 39), 3 with a low risk (30, 35, 41), and 10 remained unclear (20, 31, 34, 36, 37, 40, 42–45).
Clinical score
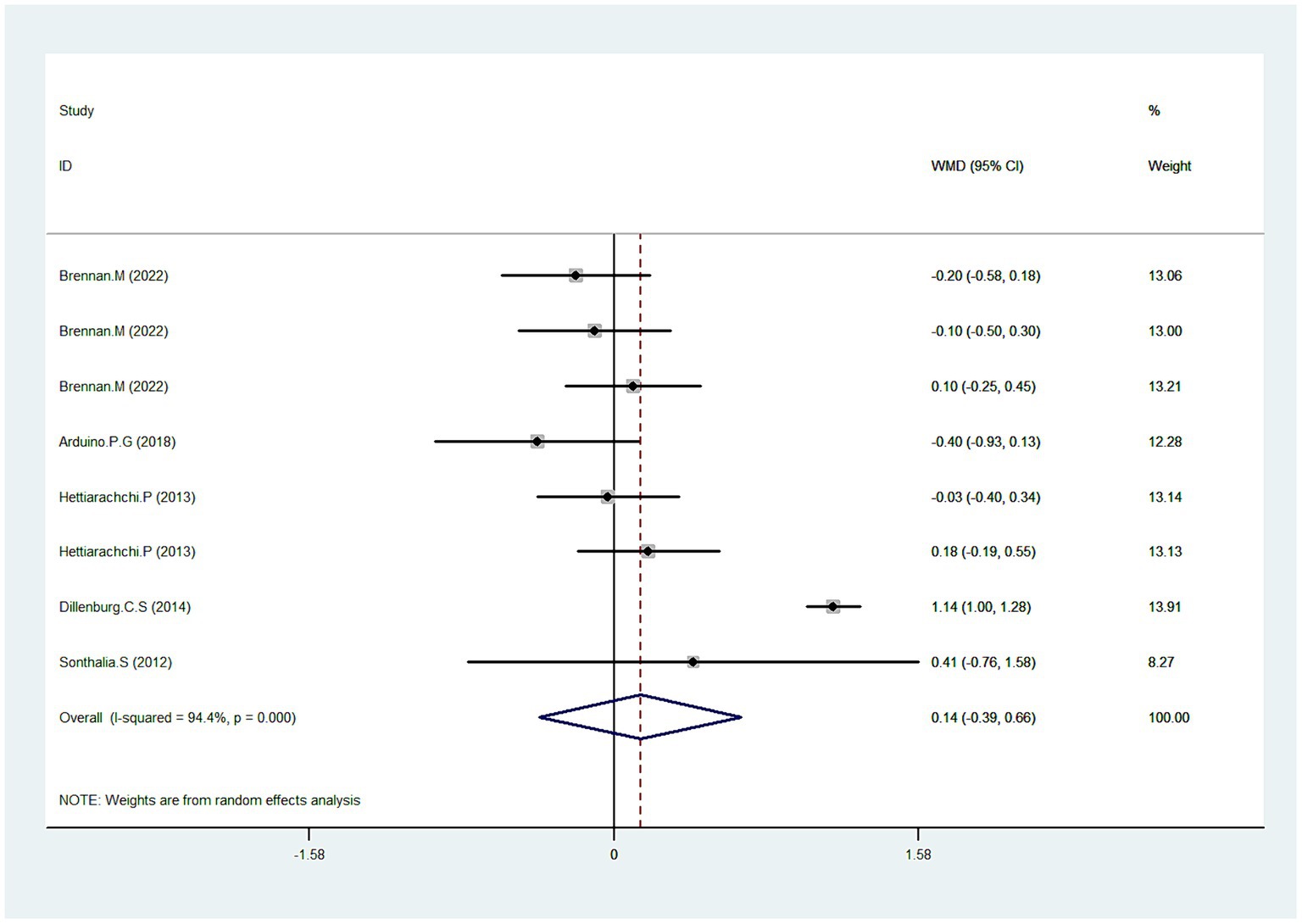
Five studies reported data pertaining to clinical score (31, 35, 36, 38, 39). As depicted in Figure 3, Pooled data demonstrated that CLO exhibited no significant improvement in the clinical score (WMD = 0.1495% CI: −0.39, 0.66; p = 0.609). However, a significant test for heterogeneity was observed (I2 = 94.4%, P < 0.001). To elucidate this inconsistency, we conducted a sensitivity analysis. Results suggested that after excluding the trial identified as an outlier (38), the heterogeneity was resolved (I2 = 0.0%), with the overall estimate essentially unchanged (WMD = −0.03, 95% CI: −0.19, 0.13; p = 0.710). This indicated that the outlier trial was the primary contributor to the observed heterogeneity.
Clinical resolution
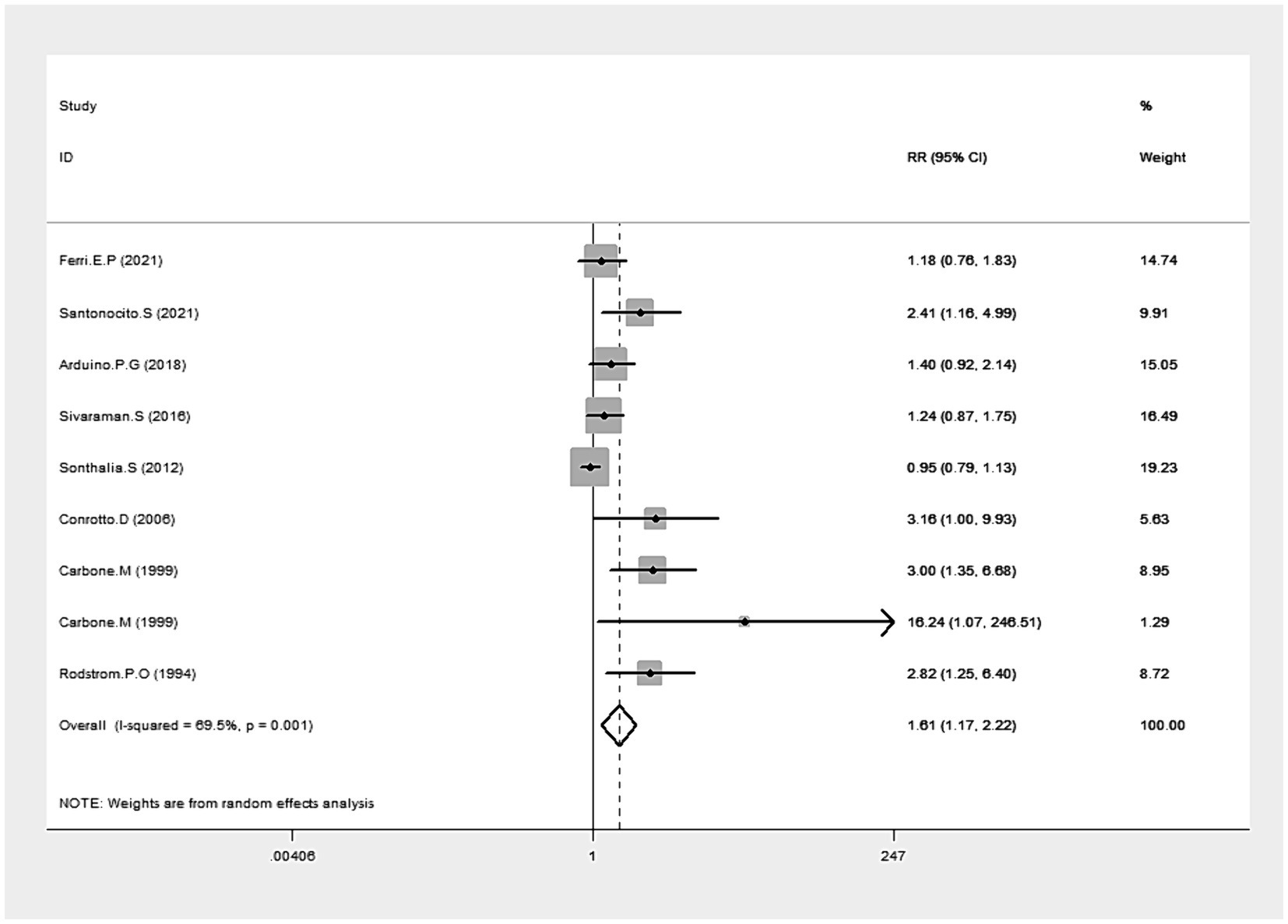
Data from eight studies provided insights into clinical resolution (20, 33–35, 37, 39, 43, 45). The collected data indicated that CLO significantly bolstered clinical resolution (RR = 1.61, 95% CI: 1.17, 2.22; p = 0.003), as illustrated in Figure 4. Notably, there was significant heterogeneity among these studies (I2 = 69.5%, p = 0.001). The robustness of our results was confirmed by a sensitivity analysis performed after excluding the outlier study (39): no single study substantially dominated the results (RR = 1.53, 95% CI: 1.26, 1.87; P < 0.001), and the heterogeneity becomes insignificant (I2 = 49.1%). Subgroup analyses revealed superior clinical resolution with both 0.05 and 0.025% concentrations of CLO compared to alternative treatments like CYC, FLU, TRI, and AIM. Of those, the therapeutic effects of CLO observed at 9 weeks and 26 weeks were more effective than those with shorter treatment durations (Table 2).
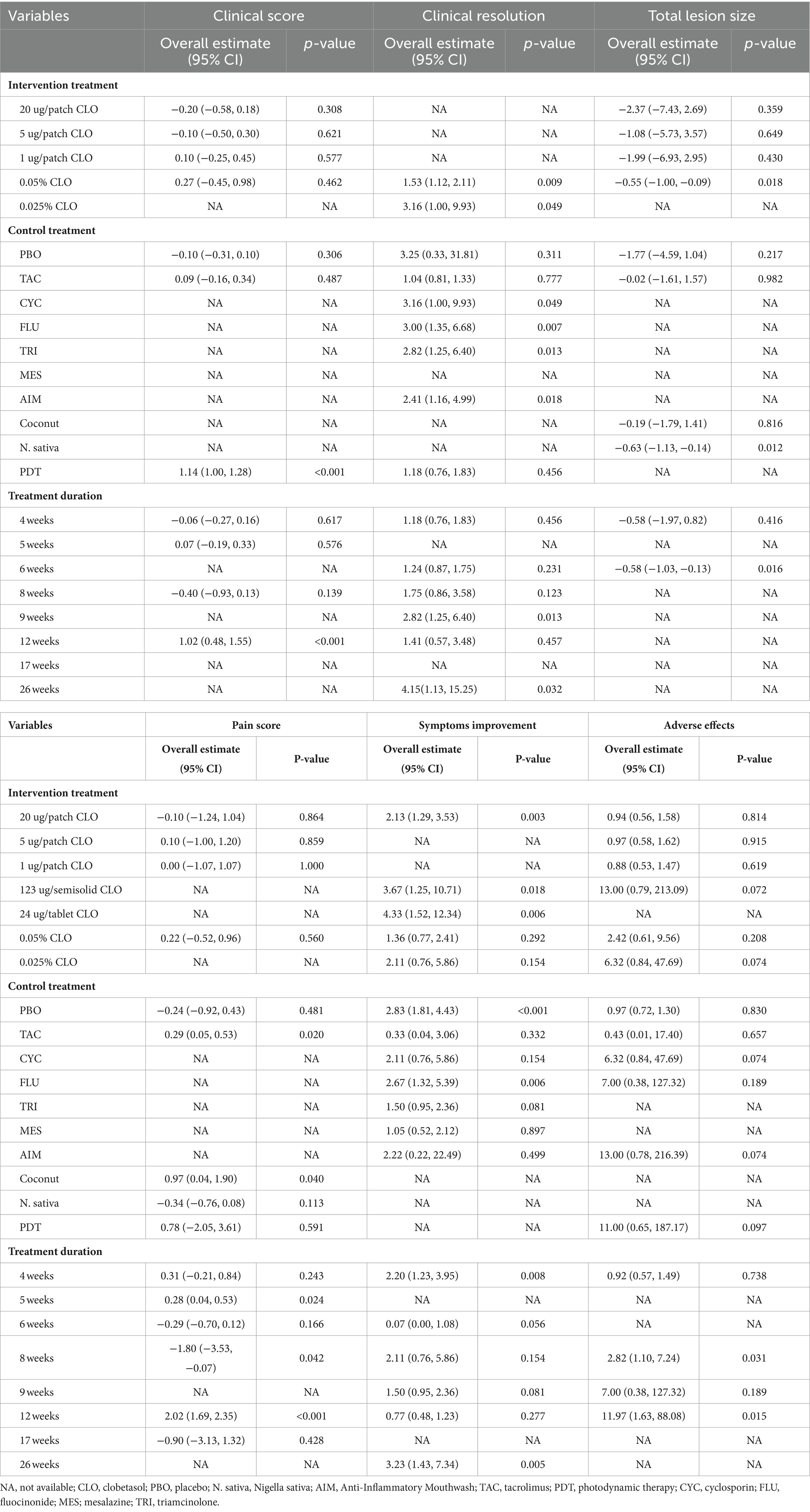
Table 2. Based on intervention treatment, control treatment and treatment duration of subgroup analysis.
Total lesion size
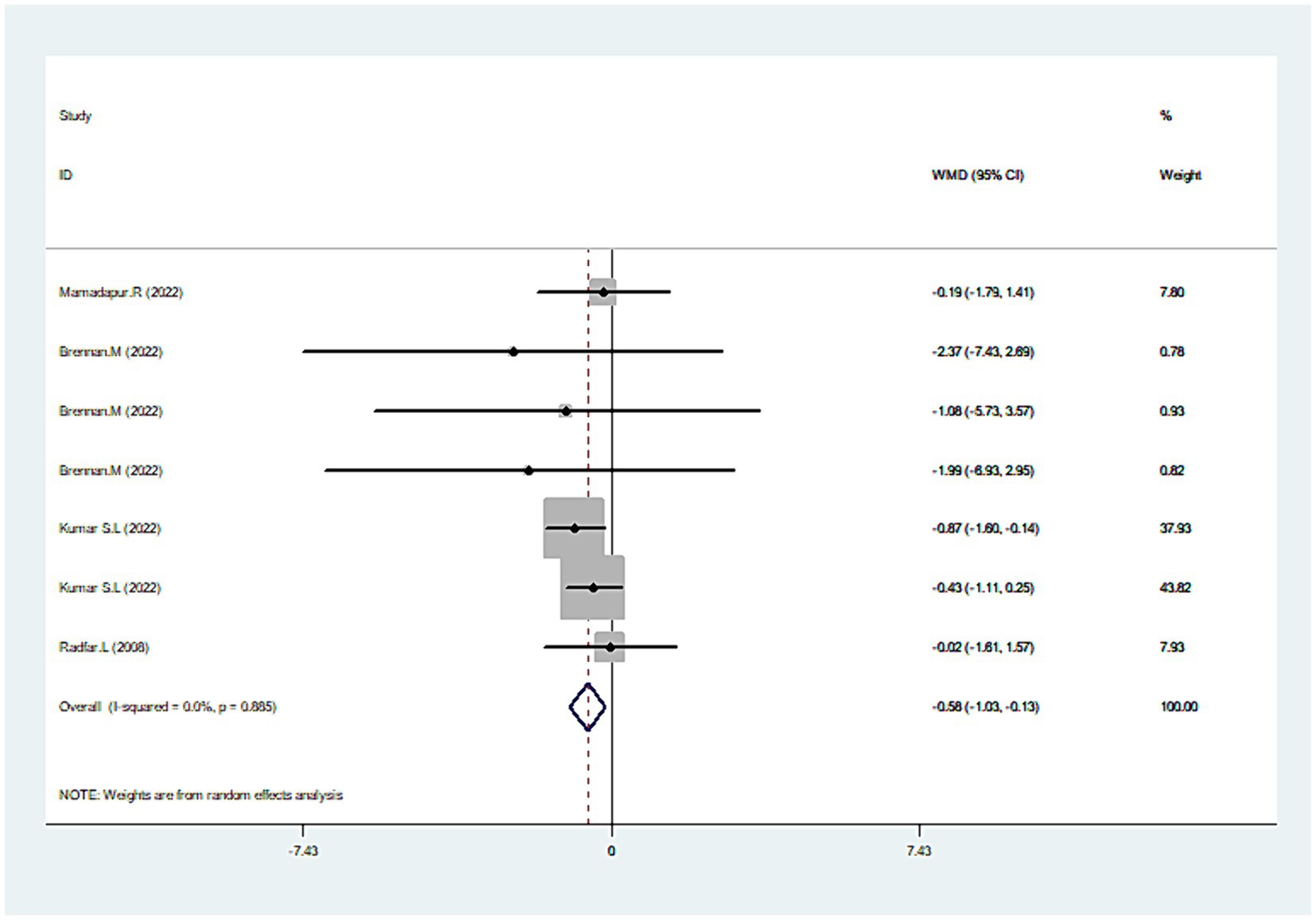
Combining the results from four studies (30–32, 42), there was a significant reduction in the total lesion size with CLO treatment (WMD = −0.5895% CI: −1.03, −0.13; p = 0.011), as seen in Figure 5. No significant heterogeneity was revealed (I2 = 0.0%, p = 0.885), which indicated the consistency of results among these studies. An in-depth subgroup analysis was performed that the common concentration of 0.05% CLO was particularly effective in diminishing the total lesion size (Table 2).
Pain score
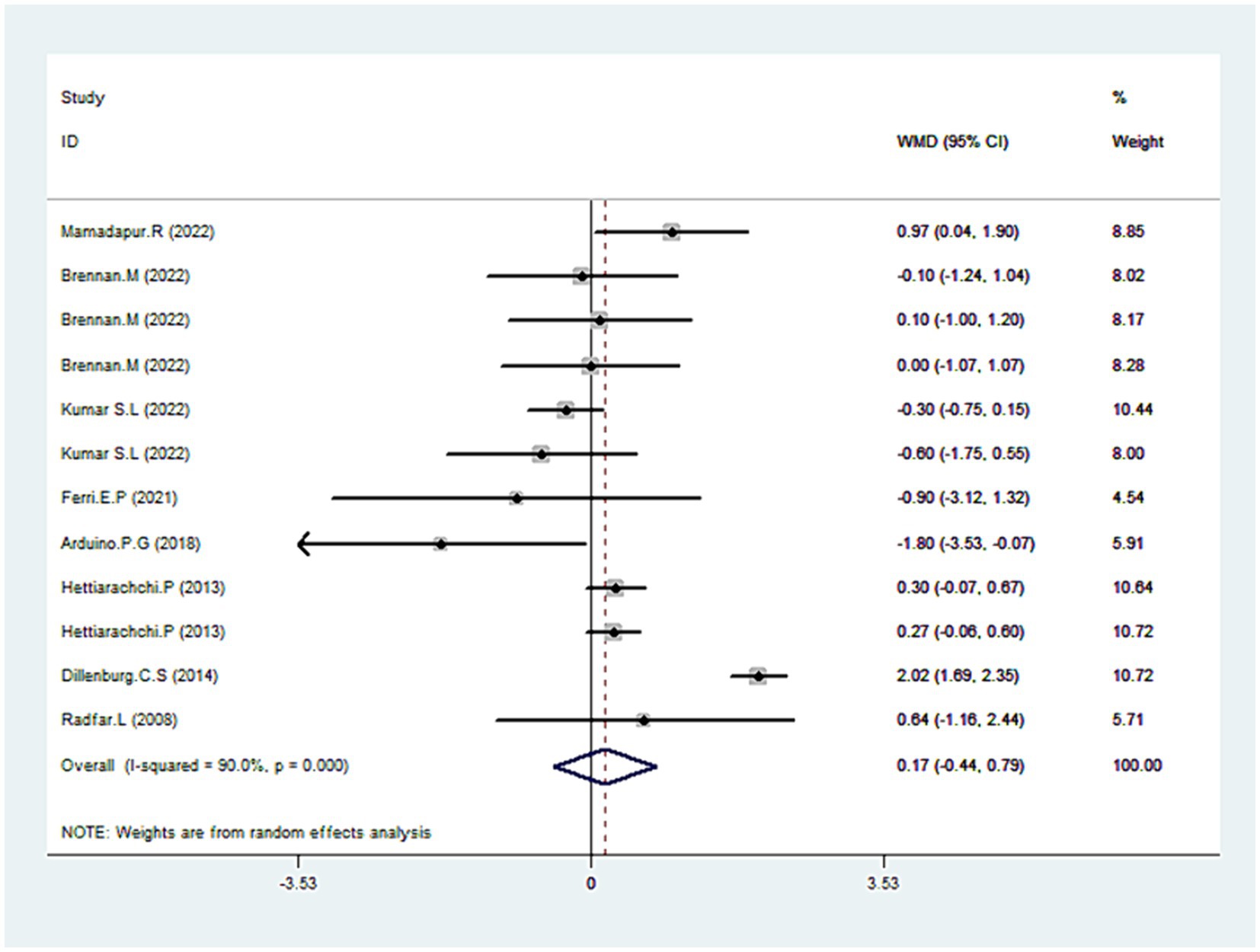
Eight trials reported data on pain scores (30–33, 35, 36, 38, 42). As illustrated in Figure 6, CLO therapy did not produce significant variations in pain score (WMD = 0.1795% CI: −0.44, 0.79; p = 0.582). However, the test for heterogeneity was significant (I2 = 90.0%, p < 0.001). Sensitivity analyses were conducted to determine the robustness of the pooled results. On exclusion of the extreme outlier (38), the heterogeneity was substantially reduced (I2 = 36.7%), yet the overall estimated remained statistically non-significant (WMD = 0.06, 95% CI: −0.23, 0.36; p = 0.672). Further subgroup analysis revealed that other treatments like TAC or Coconut demonstrated superior efficacy over CLO in pain mitigation (Table 2).
Symptoms improvement
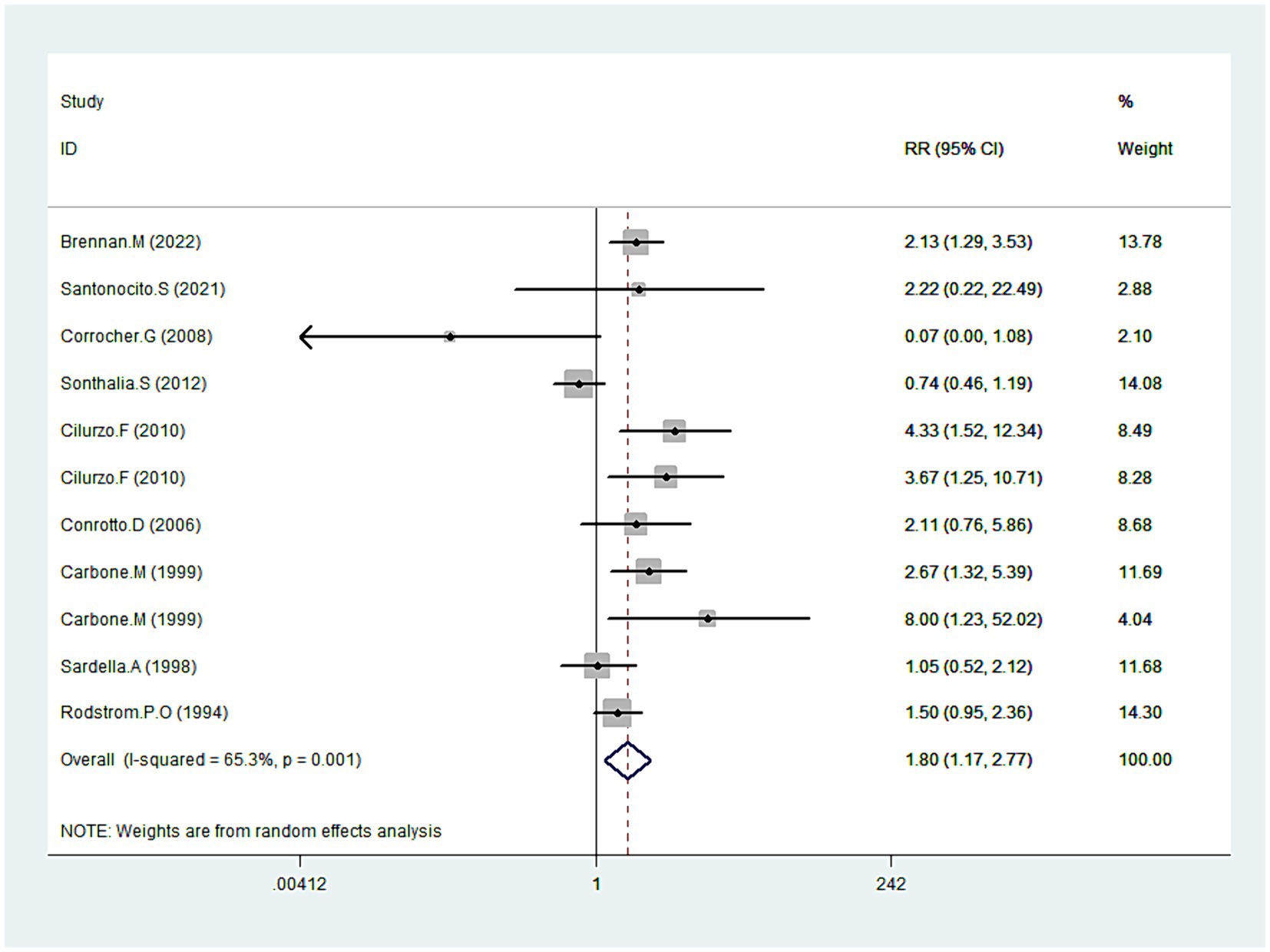
Data on symptom improvement was offered by nine studies (20, 31, 34, 39–41, 43–45). Analysis revealed that symptoms improved notably during CLO treatment (RR = 1.8095% CI: 1.17, 2.77; p = 0.008), detailed in Figure 7. But still, pronounced heterogeneity was detected with this analysis (I2 = 65.3%, p = 0.001). In sensitivity analysis, heterogeneity was reduced to 46.1% by excluding the outlier trial (39), with the overall estimate essentially unchanged (RR = 2.00, 95% CI: 1.55, 2.58; p < 0.001). Additional subgroup analyses underscored that different dosage forms of CLO, including patch, semisolid, and tablet, demonstrated more pronounced effects on symptom improvement. Moreover, these therapeutic effects also showed significant differences when compared with PBO and FLU (Table 2).
Relapse
Only three studies presented relapse data (20, 38, 43). The aggregated results did not indicate that CLO treatment led to a significant relapse increase (RR = 1.56, 95% CI: 0.66, 3.71; p = 0.314).
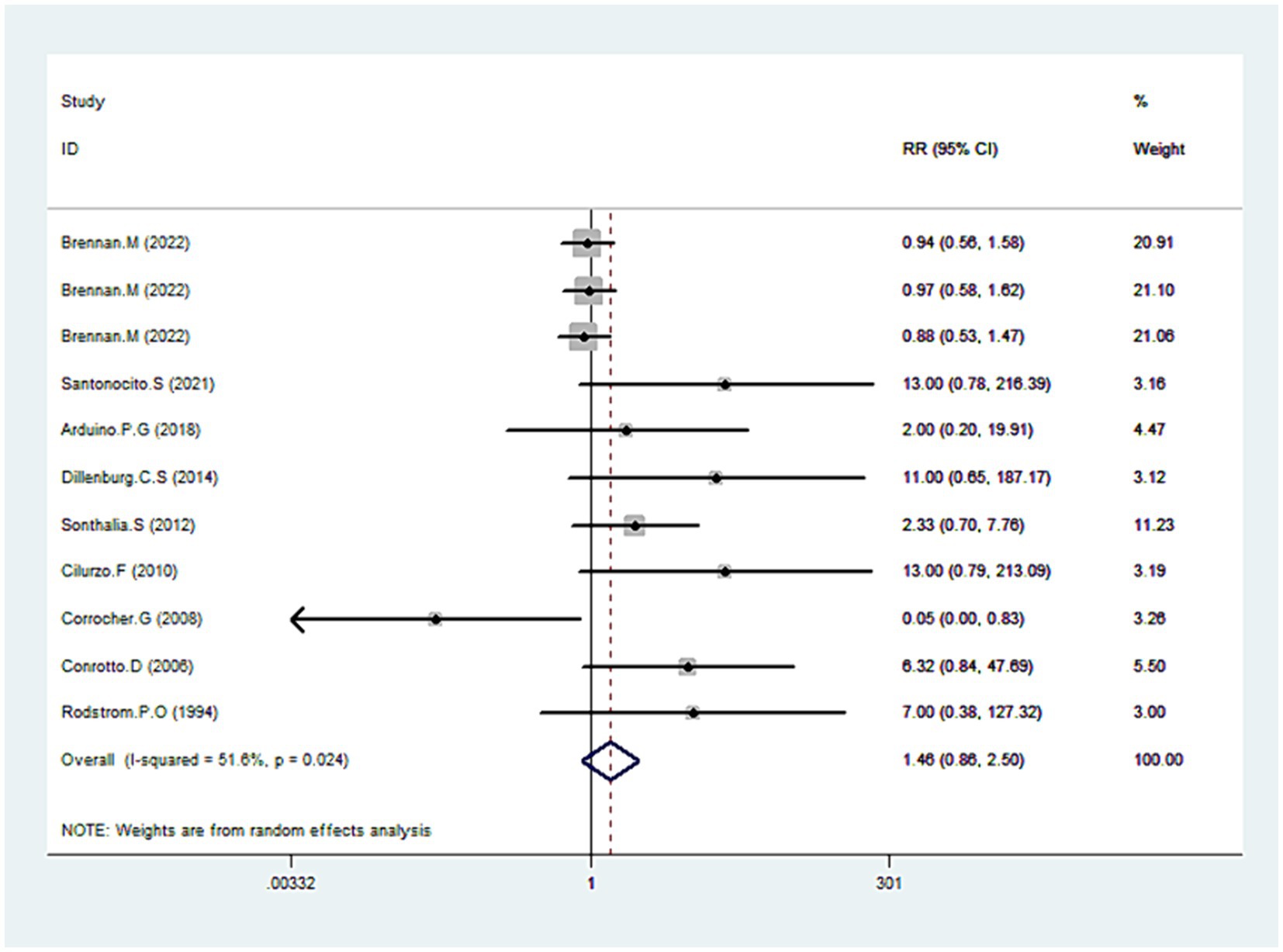
Adverse effects
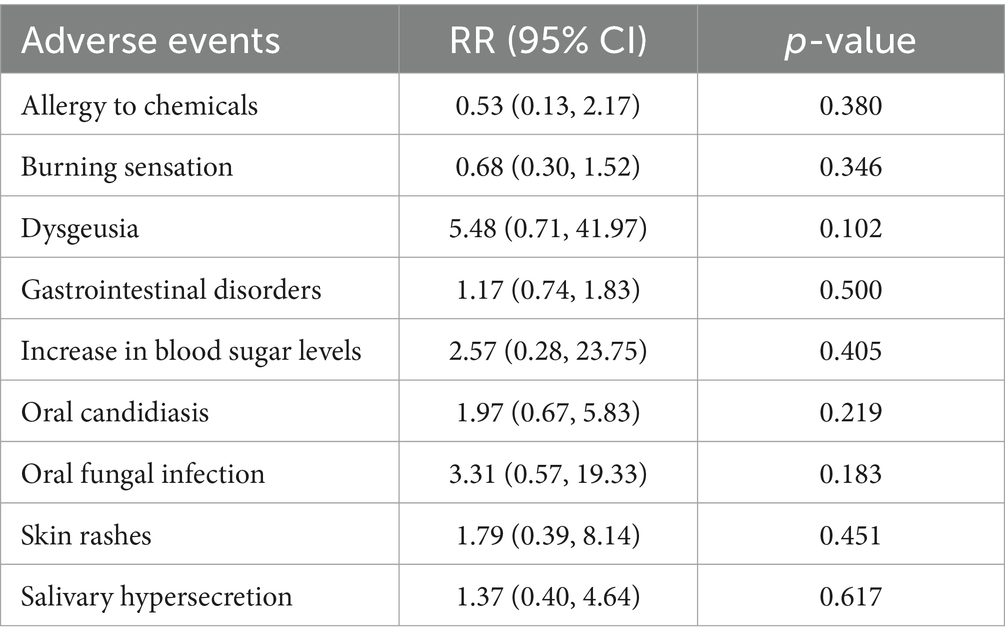
Nine studies’ detailed data provided comprehensive data regarding adverse effects (31, 34, 35, 38–41, 43, 45). Our analysis revealed no statistically significant difference between CLO and other therapies in terms of adverse effects (RR = 1.46, 95% CI: 0.86, 2.50; p = 0.161), shown in Figure 8. However, there was notable heterogeneity among the included studies (I2 = 51.6%, p = 0.024). To address this, we excluded an outlier from the dataset, which resulted in reduced heterogeneity (I2 = 43.7%) without a significant change in the overall risk estimate (RR = 1.12, 95% CI: 0.85, 1.48; p = 0.409). Upon a detailed examination of specific adverse effects, no significant difference was observed between the CLO and other therapy groups (Table 3).
Discussion
Our meta-analysis has evaluated the efficacy and safety of CLO in treating OLP compared to other therapeutic options. The results indicated that OLP patients who underwent CLO treatment experienced notable improvements in both lesion size and symptomatic relief, as well as in overall clinical outcomes. Previous studies have shown that CLO is particularly effective for erosive and atrophic forms of OLP, significantly reducing mucosal inflammation and stabilizing the epithelial barrier (46). Conversely, reticular OLP, characterized by milder symptoms, generally does not require potent topical corticosteroid treatment (47). Although significant heterogeneity was present in this study, potentially due to variations in treatment regimens, duration, and baseline patient conditions, the application of the sensitivity analysis successfully mitigated these potential influences, thereby enhancing the stability and reliability of the results.
In this updated search, we included eight more trials, building upon the previous network meta-analysis (NMA) (48). Unlike the previous NMA that found no clear clinical advantage of CLO over other treatments, our current meta-analysis distinctively underscores CLO’s superiority. It emerges as more effective in clinical resolution than therapies such as CYC, FLU, TRI, and AIM. This disparity can be attributed to the inclusion of newer trials and data with varied follow-up durations (48). Firstly, our study encompassed a larger number of relevant trials. Secondly, the research that was included had a longer treatment duration. Thirdly, some data that had slipped through the previous study were fortunately captured, enriching our analysis (20, 45). Another meta-analysis indicated that CLO’s role in pain management wasn’t as prominent as with PBO. This conclusion, however, mainly came from a single RCT with a small sample size, making its findings inconclusive (47). Conversely, by incorporating three additional RCTs, we found that CLO significantly outperforms placebo in alleviating symptoms (20, 31, 40). Regarding relapse rates and side effects, our findings differed from an earlier study. The prior NMA implied that CLO treatment might elevate relapse risks and adverse events (49). However, a newly included phase II RCT comprehensively evaluated side effects (31). Along with a more substantial sample size, our research offers robust evidence, suggesting a minimal link between CLO and adverse outcomes. Additionally, drawing from three newly added RCTs, ours is the inaugural meta-analysis to probe CLO’s efficiency in reducing overall lesion size (30–32).
In this study, we have observed that the clinical score of OLP patients remained consistent after undergoing CLO treatment, aligning with existing literature (31). Despite this, our subgroup analysis over a 12-week period revealed that the efficacy of PDT significantly surpassed that of CLO (P < 0.001). Supporting this, Dillenburg et al. (38) found that, over a prolonged treatment duration, the group subjected to PDT demonstrated a more pronounced decrease in clinical score compared to the CLO group (P < 0.001). However, it’s important to note two significant concerns. Firstly, the safety of PDT remains under scrutiny. There are studies that suggest that PDT might induce genomic instability, potentially heightening carcinogenesis risks (50). Secondly, the financial strain of PDT treatment is significant. Considering the initial costs of equipment and specialist training, PDT proves to be more expensive than CLO 39). Therefore, balancing economic feasibility with safety considerations positions CLO as a more appealing choice for OLP therapy.
Then, we turned our focus to assessing the ability of CLO to induce clinical resolution in OLP. Across the studies included in our meta-analysis, the mean treatment duration was 11 weeks, with a range of 4–26 weeks. In our meta-analysis, the concentration of CLO primarily used was 0.05%, which is consistent with its common application in treating OLP. A few studies also explored the efficacy of a lower concentration of 0.025%, contributing to the comprehensive evaluation of CLO’s therapeutic potential in our analysis. Although the effectiveness is somewhat modest in clinical score, two CLO concentrations (0.05% and 0.025%) both significantly outperformed other treatments, such as CYC, FLU, TRI, and AIM, in achieving clinical resolution. However, this promising finding does not come without its nuances; some studies painted a different picture. Arduino et al. (35) recognized CLO’s advantageous role in pain alleviation, yet they did not note a similar enhancement in clinical resolution. In contrast, Carbone et al. observed favorable results with CLO in both pain relief and clinical resolution (20). This disparity may rest in methodological nuances: Arduino’s study had a smaller sample size and shorter follow-up interval, leading to missing the optimal therapeutic effects of CLO. Indeed, our subgroup analysis definitively confirmed that a longer follow-up duration is more likely to highlight the benefits of CLO therapy. Hence, for patients seeking long-term treatment, CLO is a preferable choice.
In this research, we first evaluated the alteration in total lesion size in patients with OLP after CLO therapy. Echoing the sentiments of previous RCTs (32), CLO effectively shrank the total lesion size. Yet, Brennan et al. (31) contended that CLO did not offer a clear advantage over PBO in terms of lesion size reduction. This difference in findings might stem from Brennan’s decision to use the patch form of CLO, instead of the more commonly used ointment form. This choice could influence CLO’s efficacy. Our subgroup analysis supports this hypothesis, suggesting that for patients with larger lesion areas, the traditional ointment form of CLO is more advantageous.
Pain management emerges as a paramount goal in the treatment of OLP. However, clinical trials have shown that CLO alone has not yielded significant improvements in pain scores. Strikingly, a study by Brennan et al. (31) reported that a 20 μg/patch of CLO outperformed PBO in pain score. The efficacy of patch-based CLO in pain control is likely attributed to its precision, allowing for fine control of dosage and duration to ensure prolonged contact with the oral mucosa. Additionally, Ferri et al. (33) emphasized the sustained pain-relieving efficacy of CLO with extended follow-up periods. Consequently, CLO emerges as a favorable therapeutic option for long-term pain management in OLP. Furthermore, subgroup analysis, based on the choice of control treatment, revealed that both Coconut and TAC exhibited efficacy in alleviating pain. However, the TAC protocol involved intermittent long-term treatments, heightening the risk of adverse effects, notably its potential carcinogenicity (36). Conversely, Coconut faces a significant limitation due to the scarcity of evidence from RCTs, with only one referenced trial (30). As a result, it is a choice that strikes a balance between efficacy and safety to prescribe CLO to patients, given its enhanced safety profile and validation through multiple RCTs.
Regarding symptoms improvement, CLO has demonstrated significant efficacy, as corroborated by multiple studies (20, 31). Conversely, Sardella et al. (44) highlighted that MES outperformed CLO in symptoms improvement. This difference might arise from the adhesive base in MES, enhancing its adhesion to damaged tissues. Such an adhesive property is crucial in shielding the oral mucosa and reducing pain. Our subgroup analysis, based on the intervention treatments, further emphasized the significance of adhesive duration for symptom relief. The tablet, semisolid, and patch forms of CLO were more effective than the commonly used ointment form, primarily due to their extended adhesive durations. Interestingly, the non-traditional CLO formulations provided pain relief in the early phases of OLP treatment (at 4 and 6 weeks), whereas the 0.05% ointment CLO was more beneficial in later stages (at 26 weeks). Hence, for OLP patients struggling with overwhelming pain, an initial burst therapeutic intervention using patch CLO for swift pain management, segueing into a prolonged regimen of 0.05% ointment CLO, seems to be an effective strategy. Nonetheless, this proposed pharmacological approach needs comprehensive exploration and empirical validation before widespread clinical or research application.
Our study offers robust evidence that warrants further investigation into the long-term stability and safety of CLO treatment for OLP patients. Three other RCTs noted no significant difference in patient-reported relapses between CLO and alternative treatments. Yet, Conrotto et al. (43) recommended caution with the 0.025% CLO due to its higher relapse rate, which might be attributed to its reduced stability at milder concentrations compared to more concentrated formulations. In terms of side effects, our research found no major differences between CLO and other treatments. Nonetheless, Einarsdottir et al. (18) highlights that topical application of CLO may induce severe adverse reactions, such as adrenal insufficiency. This finding underscores the importance of regular assessments of adrenal function in patients undergoing long-term treatment with topical clobetasol. Further, some studies have indicated an increased risk of candidal infections with CLO (39, 40), but this link appears inconclusive, mainly due to limited sample sizes. A subsequent, larger RCT also endorsed CLO, asserting it did not result in a heightened risk of side effects (31). However, considering the significance of minimizing side effects for patient adherence, we delved deeper into the manifestation of these effects across several RCTs. Predominant adverse effects included gastrointestinal symptoms, localized burning sensations, and oral candidiasis. In most cases, these symptoms were mild and did not obstruct the treatment (34, 38). Only one instance of medication discontinuation due to abdominal pain was deemed exceptionally rare (35). Prophylactic antifungal drugs or chlorhexidine can effectively combat oral candidiasis, with most patients reacting favorably to such combination therapies (39). Few side effects, like excessive salivation and skin rashes, could be traced back to chlorhexidine (45), emphasizing the preference for antifungals in treating candidiasis. Lastly, albeit rare, CLO might induce hypersensitivity reactions, which are reversible upon discontinuation (34). To conclude, clinicians should prioritize antifungal drugs to preemptively tackle oral candidiasis, advise patients against accidental ingestion during medication use to prevent gastrointestinal issues, and aim to reduce CLO’s contact duration with oral mucosa, minimizing potential side effects.
Our meta-analysis is not without limitations. Firstly, we confined our search to RCTs, excluding other types of studies. Coupled with most of which had a relatively small sample size, this might lead to an overestimation of the therapeutic effect. Secondly, our analysis aggregated various CLO data, including different intervention treatments, control treatments and treatment duration. Furthermore, the clinical manifestations, quantity, and ages of OLP patients in the studies varied, introducing potential heterogeneity and bias into our results. Thirdly, our study did not conduct a cost comparison between CLO and alternative therapies. Fourthly, even though specific data for subgroup analyses based on lesion severity were not provided in the trials, the size or severity of the lesions might exert a potential influence on the outcomes. Lastly, the current research on OLP generally lacks randomized well-controlled blinded Good Clinical Practice (GCP) studies, and there is an inconsistency in the use of relevant, well-developed outcome assessments across studies. This limitation hinders our ability to conduct a higher quality meta-analysis.
In conclusion, our comprehensive meta-analysis affirms that CLO treatment offers significant relief from pain and clinical symptoms in patients, underscoring its therapeutic potential. On the safety front−a paramount aspect in medical research—the post-CLO treatment data indicates that both the relapse rate and incidence of adverse effects are within acceptable bounds. While there have been accounts of occasional adverse effects, most are mild, and the presence of effective countermeasures further bolsters the treatment’s safety profile. Despite the insights gained, this study has its limitations. To solidify our conclusions and delve deeper into the long-term efficacy and safety of CLO, more extensive multicenter, prospective RCTs are imperative.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CL: Data curation, Writing – original draft. YW: Data curation, Methodology, Writing – original draft. RZ: Conceptualization, Formal analysis, Writing – review & editing. DW: Conceptualization, Supervision, Writing – review & editing. JT: Conceptualization, Supervision, Writing – review & editing. KZ: Conceptualization, Data curation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Program of National Natural Science Foundation (NSFC82374530); Research Project supported by Hunan Provincial Administration of Traditional Chinese Medicine (2021240); Research Project supported by Hunan Provincial Department of Education (22C0190); and 712 Talent Project supported by Changsha Maternal and Child Health Hospital (20211159). Research Project supported by Hunan Provincial Administration of Chinese Medicine (Grant No: B2024068), Hunan University of Chinese Medicine Innovation Training Project (Grant No: 220).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El-Howati, A, Thornhill, MH, Colley, HE, and Murdoch, C. Immune mechanisms in oral lichen planus. Oral Dis. (2023) 29:1400–15. doi: 10.1111/odi.14142
2. McCartan, BE, and Healy, CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. (2008) 37:447–53. doi: 10.1111/j.1600-0714.2008.00662.x
3. Alrashdan, MS, Cirillo, N, and McCullough, M. Oral lichen planus: a literature review and update. Arch Dermatol Res. (2016) 308:539–51. doi: 10.1007/s00403-016-1667-2
4. González-Moles, MÁ, Warnakulasuriya, S, González-Ruiz, I, González-Ruiz, L, Ayén, Á, Lenouvel, D, et al. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. (2021) 27:813–28. doi: 10.1111/odi.13323
5. Carrozzo, M, and Gandolfo, S. The management of oral lichen planus. Oral Dis. (1999) 5:196–205. doi: 10.1111/j.1601-0825.1999.tb00301.x
6. Tadakamadla, J, Kumar, S, and Johnson, NW. Quality of life in patients with oral potentially malignant disorders: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. (2015) 119:644–55. doi: 10.1016/j.oooo.2015.01.025
7. López-Jornet, P, and Camacho-Alonso, F. Quality of life in patients with oral lichen planus. J Eval Clin Pract. (2010) 16:111–3. doi: 10.1111/j.1365-2753.2009.01124.x
8. Kaplan, I, Ventura-Sharabi, Y, Gal, G, Calderon, S, and Anavi, Y. The dynamics of oral lichen planus: a retrospective clinicopathological study. Head Neck Pathol. (2012) 6:178–83. doi: 10.1007/s12105-011-0318-3
9. Eisen, D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. (2002) 46:207–14. doi: 10.1067/mjd.2002.120452
10. Ramos-García, P, González-Moles, MÁ, and Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions—3.0 evidence level: A systematic review of systematic reviews. Oral Dis. (2021) 27:1919–35. doi: 10.1111/odi.13812
11. González-Moles, MÁ, Ruiz-Ávila, I, González-Ruiz, L, Ayén, Á, Gil-Montoya, JA, and Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. (2019) 96:121–30. doi: 10.1016/j.oraloncology.2019.07.012
12. Gonzalez-Moles, MA, Scully, C, and Gil-Montoya, JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. (2008) 14:229–43. doi: 10.1111/j.1601-0825.2008.01441.x
13. Lodi, G, Scully, C, Carrozzo, M, Griffiths, M, Sugerman, PB, and Thongprasom, K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2005) 100:164–78. doi: 10.1016/j.tripleo.2004.06.076
14. Didona, D, Caposiena Caro, RD, Sequeira Santos, AM, Solimani, F, and Hertl, M. Therapeutic strategies for oral lichen planus: state of the art and new insights. Front Med. (2022) 9:1790. doi: 10.3389/fmed.2022.997190
15. Zheng, T, Liu, C, Wang, Y, Zhou, H, Zhou, R, Zhu, X, et al. Inflammatory cytokines mediating the effect of oral lichen planus on oral cavity cancer risk: a univariable and multivariable mendelian randomization study. BMC Oral Health. (2024) 24:375. doi: 10.1186/s12903-024-04104-0
16. Al-Hallak, N, Hamadah, O, Mouhamad, M, and Kujan, O. Efficacy of injectable platelet-rich fibrin in the treatment of symptomatic oral lichen planus. Oral Dis. (2023) 29:2256–64. doi: 10.1111/odi.14261
17. Ibrahim, R, Abdul-Hak, M, Kujan, O, and Hamadah, O. CO2 laser vaporisation in treating oral lichen planus: A split-mouth randomised clinical trial. Oral Dis. (2023). doi: 10.1111/odi.14669
18. Einarsdottir, MJ, Bankvall, M, Robledo-Sierra, J, Rödström, P-O, Bergthorsdottir, R, Trimpou, P, et al. Topical clobetasol treatment for oral lichen planus can cause adrenal insufficiency. Oral Dis. (2023) 30:1304–1312. doi: 10.1111/odi.14588
19. Canto, AM, Müller, H, Freitas, RR, Santos, PS, and Müller, H. Líquen plano oral (LPO): diagnóstico clínico e complementar. An Bras Dermatol. (2010) 85:669–75. doi: 10.1590/S0365-05962010000500010
20. Carbone, M, Conrotto, D, Carrozzo, M, Broccoletti, R, Gandolfo, S, and Scully, C. Topical corticosteroids in association with miconazole and chlorhexidine in the long-term management of atrophic-erosive oral lichen planus: a placebo-controlled and comparative study between clobetasol and fluocinonide. Oral Dis. (1999) 5:44–9. doi: 10.1111/j.1601-0825.1999.tb00063.x
21. Carbone, M, Goss, E, Carrozzo, M, Castellano, S, Conrotto, D, Broccoletti, R, et al. Systemic and topical corticosteroid treatment of oral lichen planus: a comparative study with long-term follow-up. J Oral Pathol Med. (2003) 32:323–9. doi: 10.1034/j.1600-0714.2003.00173.x
22. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
23. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
25. Higgins, JPT, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
27. Mantel, N, and Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48.
28. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
29. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
30. Mamadapur, R, Naik, Z, Kumar, SL, and Bagewadi, A. Comparative efficacy of topical coconut cream and clobetasol propionate ointment for the management of oral lichen planus: A double-blinded randomized control trial. Indian J Pharm. (2022) 54:84–9. doi: 10.4103/ijp.ijp_984_20
31. Brennan, MT, Madsen, LS, Saunders, DP, Napenas, JJ, McCreary, C, Riordain, RN, et al. Efficacy and safety of a novel mucoadhesive clobetasol patch for treatment of erosive oral lichen planus: A phase 2 randomized clinical trial. J Oral Pathol Med. (2022) 51:86–97. doi: 10.1111/jop.13270
32. Kumar, SL, Naik, Z, Panwar, A, Keluskar, V, and Kumar, RS. Comparative evaluation of the efficacy of Nigella sativa (75% v/v) cream and clobetasol propionate (0.05% w/w) gel in oral lichen planus-a double-blinded randomized control trial. Oral Maxillofac Surg. (2022) 28:225–34. doi: 10.1007/s10006-022-01130-6
33. Ferri, EP, Cunha, KRL, Abboud, CS, de Barros, GC, de Sousa, SS, de Fatima Teixeira da Silva, D, et al. Photobiomodulation is effective in oral lichen planus: A randomized, controlled, double-blind study. Oral Dis. (2021) 27:1205–16. doi: 10.1111/odi.13662
34. Santonocito, S, Polizzi, A, De Pasquale, R, Ronsivalle, V, Giudice, AL, and Isola, G. Analysis of the efficacy of two treatment protocols for patients with symptomatic oral lichen planus: A randomized clinical trial. Int J Environ Res Public Health. (2021) 18:1–11. doi: 10.3390/ijerph18010056
35. Arduino, PG, Campolongo, MG, Sciannameo, V, Conrotto, D, Gambino, A, Cabras, M, et al. Randomized, placebo-controlled, double-blind trial of clobetasol propionate 0.05% in the treatment of oral lichen planus. Oral Dis. (2018) 24:772–7. doi: 10.1111/odi.12821
36. Hettiarachchi, P, Hettiarachchi, RM, Jayasinghe, RD, and Sitheeque, M. Comparison of topical tacrolimus and clobetasol in the management of symptomatic oral lichen planus: A double-blinded, randomized clinical trial in Sri Lanka. J Investig Clin Dent. (2017) 8:237. doi: 10.1111/jicd.12237
37. Sivaraman, S, Santham, K, Nelson, A, Laliytha, B, Azhalvel, P, and Deepak, J. A randomized triple-blind clinical trial to compare the effectiveness of topical triamcinolone acetonate (0.1%), clobetasol propionate (0.05%), and tacrolimus orabase (0.03%) in the management of oral lichen planus. J Pharm Bioallied Sci. (2016) 8:S86–9. doi: 10.4103/0975-7406.191976
38. Dillenburg, CS, Martins, MA, Munerato, MC, Marques, MM, Carrard, VC, SantAna Filho, M, et al. Efficacy of laser phototherapy in comparison to topical clobetasol for the treatment of oral lichen planus: a randomized controlled trial. J Biomed Opt. (2014) 19:068002. doi: 10.1117/1.Jbo.19.6.068002
39. Sonthalia, S, and Singal, A. Comparative efficacy of tacrolimus 0.1% ointment and clobetasol propionate 0.05% ointment in oral lichen planus: a randomized double-blind trial. Int J Dermatol. (2012) 51:1371–8. doi: 10.1111/j.1365-4632.2012.05459.x
40. Cilurzo, F, Gennari, CGM, Selmin, F, Epstein, JB, Gaeta, GM, Colella, G, et al. A new mucoadhesive dosage form for the management of oral lichen planus: formulation study and clinical study. Eur J Pharm Biopharm. (2010) 76:437–42. doi: 10.1016/j.ejpb.2010.07.014
41. Corrocher, G, Di Lorenzo, G, Martinelli, N, Mansueto, P, Biasi, D, Nocini, PF, et al. Comparative effect of tacrolimus 0.1% ointment and clobetasol 0.05% ointment in patients with oral lichen planus. J Clin Periodontol. (2008) 35:244–9. doi: 10.1111/j.1600-051X.2007.01191.x
42. Radfar, L, Wild, RC, and Suresh, L. A comparative treatment study of topical tacrolimus and clobetasol in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2008) 105:187–93. doi: 10.1016/j.tripleo.2007.07.029
43. Conrotto, D, Carbone, M, Carrozzo, M, Arduino, P, Broccoletti, R, Pentenero, M, et al. Ciclosporin vs. clobetasol in the topical management of atrophic and erosive oral lichen planus: a double-blind, randomized controlled trial. Br J Dermatol. (2006) 154:139–45. doi: 10.1111/j.1365-2133.2005.06920.x
44. Sardella, A, Demarosi, F, Oltolina, A, Rimondini, L, and Carrassi, A. Efficacy of topical mesalazine compared with clobetasol propionate in treatment of symptomatic oral lichen planus. Oral Dis. (1998) 4:255–9. doi: 10.1111/j.1601-0825.1998.tb00289.x
45. Rodstrom, PO, Hakeberg, M, Jontell, M, and Nordin, P. Erosive oral lichen planus treated with clobetasol propionate and triamcinolone acetonide in Orabase: A double-blind clinical trial. J Dermatol Treat. (1994) 5:7–10. doi: 10.3109/09546639409081837
46. Carbone, M, Arduino, PG, Carrozzo, M, Caiazzo, G, Broccoletti, R, Conrotto, D, et al. Topical clobetasol in the treatment of atrophic-erosive oral lichen planus: a randomized controlled trial to compare two preparations with different concentrations. J Oral Pathol Med. (2009) 38:227–33. doi: 10.1111/j.1600-0714.2008.00688.x
47. Lodi, G, Manfredi, M, Mercadante, V, Murphy, R, and Carrozzo, M. Interventions for treating oral lichen planus: corticosteroid therapies. Cochrane Database Syst Rev. (2020) 2020:CD001168. doi: 10.1002/14651858.CD001168.pub3
48. Leong, XY, Gopinath, D, Syeed, SM, Veettil, SK, Shetty, NY, and Menon, RK. Comparative efficacy and safety of interventions for the treatment of Oral lichen planus: A systematic review and network Meta-analysis. J Clin Med. (2023) 12:2763. doi: 10.3390/jcm12082763
49. Yuan, P, Qiu, X, Ye, L, Hou, F, Liang, Y, Jiang, H, et al. Efficacy of topical administration for oral lichen planus: A network meta-analysis. Oral Dis. (2022) 28:670–81. doi: 10.1111/odi.13790
Keywords: oral lichen planus, systematic review, meta-analysis, clobetasol propionate, corticosteroids
Citation: Zheng T, Liu C, Wang Y, Zhou R, Wu D, Tan J and Zhu K (2024) Efficacy and safety of topical clobetasol propionate in comparison with alternative treatments in oral lichen planus: an updated systematic review and meta-analysis. Front. Med. 11:1391754. doi: 10.3389/fmed.2024.1391754
Edited by:
Guya Diletta Marconi, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Dongmei Shi, Jining No. 1 People’s Hospital, ChinaPia Lopez Lopez Jornet, University of Murcia, Spain
Ioanina Parlatescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Zheng, Liu, Wang, Zhou, Wu, Tan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: KeKe Zhu, emh1a2VrZV9obnVjbUAxNjMuY29t
 Tao Zheng
Tao Zheng ChengYong Liu1
ChengYong Liu1 KeKe Zhu
KeKe Zhu