- 1Department of Oncology, Liaocheng People's Hospital, Liaocheng, Shandong, China
- 2Department of Central Laboratory, Liaocheng People's Hospital, Liaocheng, Shandong, China
- 3Department of Radiotherapy, Liaocheng People's Hospital, Liaocheng, Shandong, China
Background: The status of the sentinel lymph nodes (SLNs) is an important prognostic factor for many different types of cancer. The one-step nucleic acid amplification (OSNA) assay has emerged as a rapid intraoperative molecular diagnostic tool for LN metastasis detection. We aimed to evaluate and summarize the value of the OSNA assay for the diagnosis of SLN metastasis in cytokeratin 19 (CK19)-positive breast cancer.
Methods: To evaluate the diagnostic value, the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) were pooled. The threshold effect, followed by subgroup analysis, was performed to explore the source of heterogeneity. A sensitivity analysis was performed to assess the stability of this meta-analysis model. Fagan plots and likelihood ratio scattergrams were used to explore the potential clinical significance.
Results: A total of 29 eligible studies, which consisted of 5,331 patients with 10,343 SLNs, were included in this meta-analysis. The pooled sensitivity, specificity, PLR, NLR, and DOR were 0.86 (95% CI: 0.85–0.88), 0.94 (95% CI, 0.94–0.95), 18.00 (95% CI, 13.54–23.92), 0.13 (95% CI, 0.10–0.17), and 138.99 (95% CI, 86.66–222.92), respectively. The AUC was 0.97 (95% CI, 0.95–0.98). Sensitivity analysis showed that four studies had an impact on the pooled results and mainly contributed to the heterogeneity. Fagan's nomogram revealed that the prior probability was 50%, the post-probability positive was 95%, and the post-probability negative was 11%.
Discussion: Our results suggested that OSNA can predict the occurrence of SLN metastasis in CK19-positive breast cancer. However, more well-designed and multicenter diagnostic tests are needed to validate our results.
1 Introduction
Breast cancer is the most common malignancy in women worldwide and is a leading cause of death for women in their 40's (1). Approximately one-third of patients with primary breast cancer eventually develop distant metastases and succumb to the disease (2). The spread of tumor cells to SLNs is still an important prognostic factor for patients with breast cancer and a key criterion for determining individual treatment plans. The nodal status quantifies the number, locations, or size of involved LNs with metastases in clinical tumor node metastasis (TNM) staging of breast cancer patients (2, 3). A SLN biopsy is the standard procedure used to accurately stage axillary nodal involvement in early-stage breast cancer patients without clinical evidence of node metastasis (4). During SLN biopsy, a reliable intraoperative examination of SLN plays an important role in the decision-making process, which may help in selecting a surgical procedure or conservative treatment of the axilla in a single procedure (5). Intraoperative hematoxylin and eosin (H&E) pathological examinations of the frozen section (FS) or imprint cytology are recommended (6). However, all these methods have some drawbacks, such as inaccuracy and long required time, and do not offer timely intraoperative SLN evaluation. Therefore, there is an urgent need for a more efficient method for intraoperative detection of SLN metastasis in CK19-positive breast cancer.
The OSNA assay has emerged as a rapid intraoperative molecular diagnostic tool for lymph node metastasis (LNM) detection (7). It is a standardized and observer-independent molecular technique that can detect tumor-specific CK19 mRNA and is widely used in hospitals (8). CK19, a member of the keratin family, is widely used as an epithelial marker in clinical applications and has been used as a useful tool in the diagnosis, treatment, and prognosis of tumors (9). CK19 is one of the main cytoskeleton proteins in epithelial cells, which is released as a full-length protein by viable epithelial tumor cells and is associated with metastatic progression in cancer patients (10). To date, numerous preliminary and multicenter clinical studies have confirmed that the OSNA assay can be applied to evaluate LNM in various cancers expressing CK19, such as breast cancer, cervical and endometrial cancer, lung cancer, gastric cancer, and colorectal cancer (8, 11–13). The comparative studies between OSNA and pathological evaluation for detecting LNM in breast cancer showed that OSNA has high specificity (94.8%), a high concordant rate (93.8%), and a negative predictive value (97.6%) in a pooled assessment (13). Similar results have been found in multicenter studies for gastric cancer, colorectal cancer, and lung cancer (13). The OSNA assay is a quick and semiautomatic procedure and can be completed in ~40 min, making it suitable as an intraoperative procedure for the detection of LNM (14). Importantly, the OSNA assay is more objective, sensitive, and accurate compared with routine histopathological examination (15, 16). To date, many studies have focused on the diagnostic value of the OSNA assay for SLN detection in breast cancer, but the results are still uncertain. The expected drawbacks of the OSNA method are the false-negative results caused by unstable CK19 expression.
To the best of our knowledge, although some studies have performed intraoperative evaluation for SLN metastases using the OSNA assay in invasive breast cancer patients, there is no systematic review or meta-analysis about the value of the OSNA assay in breast cancer diagnosis. Therefore, we aimed to conduct a meta-analysis to evaluate and summarize the value of the OSNA assay for the diagnosis of SLN metastasis in CK19-positive breast cancer patients.
2 Materials and methods
2.1 Literature search strategy
We conducted this meta-analysis according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A systematic search was conducted in PubMed, Cochrane Library, and Web of Science to identify all potential literature, with a search period up to 1 September 2023. In addition, we supplemented the manual search to find relevant literature. The search strategy was performed by two investigators independently. Quality studies were needed to provide information on the diagnostic accuracy of OSNA for SLN metastasis in patients with breast cancer. Subject terms used for the literature search included “molecular intraoperative” or “intraoperative molecular analysis” or “intraoperative nucleic acid amplification” or “one step nucleic acid amplification” or “one-step nucleic acid amplification” or “OSNA” combined with “breast cancer” or “breast neoplasm” or “breast carcinoma” or “breast tumor.” No further ethical approval is required since the program does not require the recruitment of patients or the collection of personal information. The review of this meta-analysis has not been registered with PROSPERO or INPLASY.
2.2 Literature selection criteria
All articles were screened according to the inclusion and exclusion criteria by two independent reviewers (Meirong Liu and Weihua Wang). Disagreements were adjudicated by the third reviewer (Yufang Wang). The inclusion criteria were as follows: (1) patients who were diagnosed with breast cancer; (2) the specimens collected were fresh SLNs; (3) the study's purpose was to investigate the performance of the OSNA assay for detecting SLN metastasis in breast cancer patients; (4) the reference method for detecting SLN metastasis was postoperative pathology; (5) the study adopted identical machines and thresholds recommended by the OSNA manufacturer, Sysmex company; (6) the method of pathological examination was described in detail; (7) the study analysis was based on per node; and (8) extracted data were available for obtaining true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) values. The exclusion criteria were as follows: (1) non-English articles; (2) non-clinical research literature, including basic experiments, reviews, conference abstracts, and letters to journal editors; and (3) intraoperative pathologies, such as FS or touch imprint cytology (TIC) (17).
2.3 Data extraction and quality assessment
Two researchers extracted the information and assessed the quality of the included studies. The data extracted included the details on the first author, year of publication, country, type of study design, number of patients, number of LNs, number of study centers, section interval, reference standard method, and type of samples. Diagnostic accuracy estimates included TP, TN, FPs, and FN. The quality of the diagnostic studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) (18). Different opinions will be settled through group consultation.
2.4 Statistical analyses
All analyses were performed with STATA 16.0, RevMan 5.2, and Meta-Disc 1.4 software (19). Heterogeneity was assessed using Higgin's I2 and Cochran's Q tests. I2 > 50% was considered a significant heterogeneity (20). Subgroup analyses were performed to uncover the source of heterogeneity. Sensitivity analysis was used to assess the stability of the model. The diagnostic accuracy of OSNA for SLN metastasis was quantified by the area under the summary receiver operating characteristic curve (AUC), summary DOR, summary sensitivity, specificity, PLR, NLR, and their 95% confidence interval (CI). Deeks' funnel plot was conducted to assess publication bias (21, 22). To explore the potential clinical significance, the Fagan plot was drawn to reveal the relevance between pre-test probability, post-test probability, and likelihood ratio. Moreover, we generated a likelihood ratio scattergram, which showed the different diagnostic values of OSNA for SLN metastasis in CK19-positive breast cancer patients. All statistical tests were two-sided, and a P-value of < 0.05 was considered significant unless otherwise indicated.
3 Results
3.1 Literature search
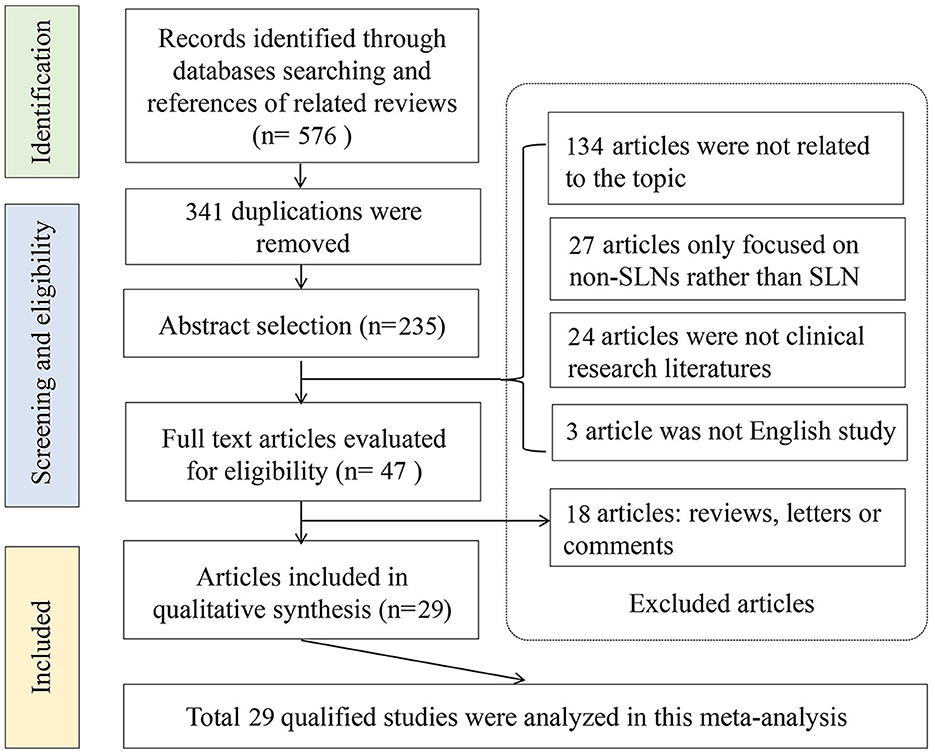
An initial literature search yielded 576 potential articles from three databases. After excluding duplicate publications, 235 studies remained. After browsing the titles and abstracts, studies were also excluded because 134 articles were not related to the topic, 27 articles only focused on the non-SLNs, and 24 articles were not clinical studies. Then, the 47 remaining studies were further evaluated through full-text reading. A total of 18 articles were further excluded due to insufficient data for diagnostic testing. Finally, 29 articles involving 5,331 patients were included in the meta-analysis (5, 14, 15, 23–48). The flow chart of the literature screening process is shown in Figure 1.
3.2 Characteristics of the included studies
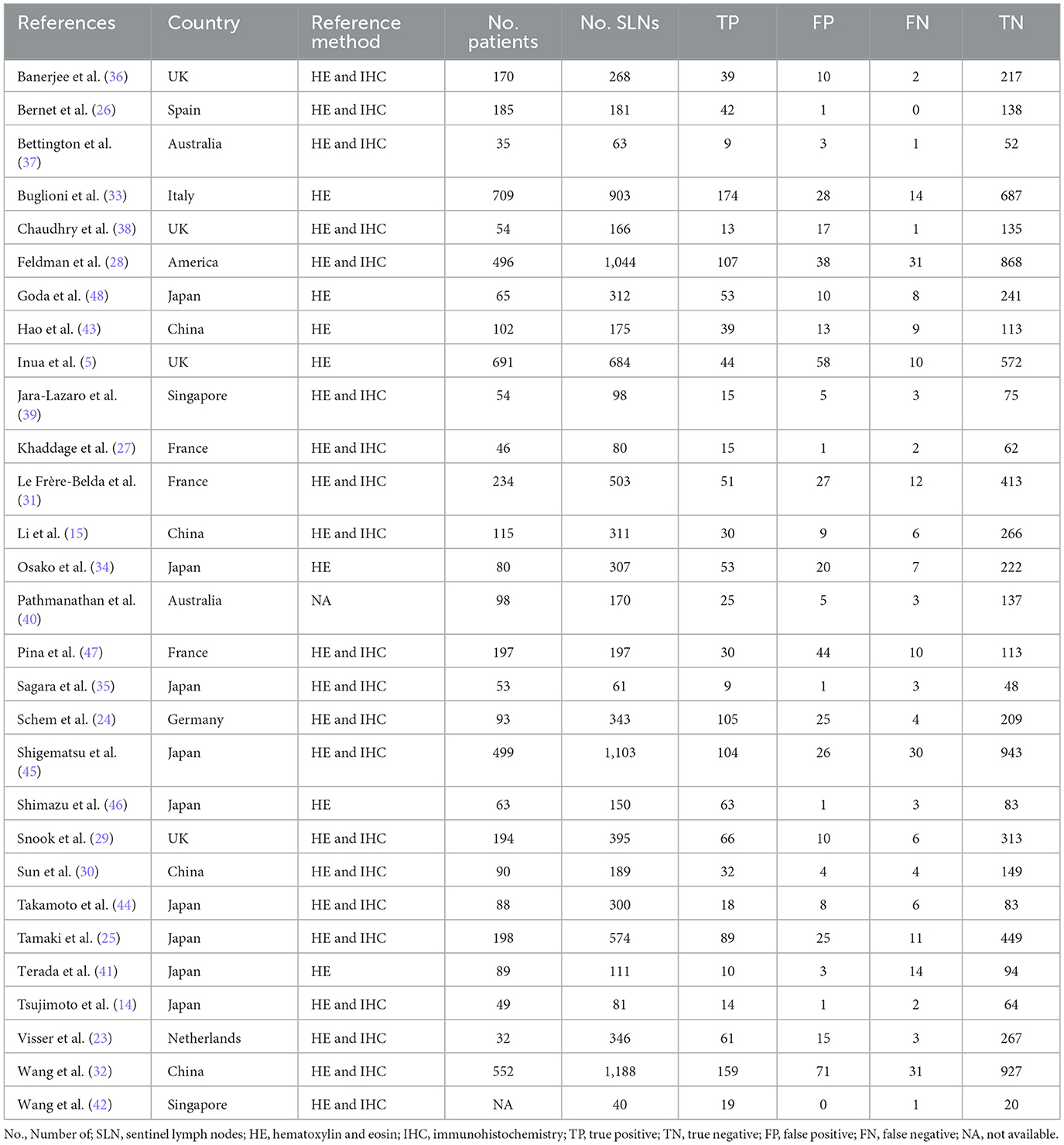
The basic characteristics of the 29 included studies (5, 14, 15, 23–47) are shown in Table 1. Our study consisted of 5,331 patients with 10,343 SLNs. The literature was published from 2008 to 2023; 15 literature articles (7, 11, 21, 26, 28, 30, 31, 35, 37–42, 44) were conducted in Asian countries, 11 trials (5, 19, 20, 22, 23, 25, 27, 29, 32, 34, 43) were conducted in Europe, two trials (33, 36) were conducted in Oceania, and one study (24) was conducted in America. The reference standards of all studies were assessed by postoperative pathology, but the detailed approaches were different because 21 studies (7, 11, 19–28, 31–36, 38, 40, 41, 43) were taken with serial sections with HE staining and IHC and seven studies (5, 29, 30, 37, 39, 42, 44) were taken with serial sections with HE staining only.
3.3 Quality assessment
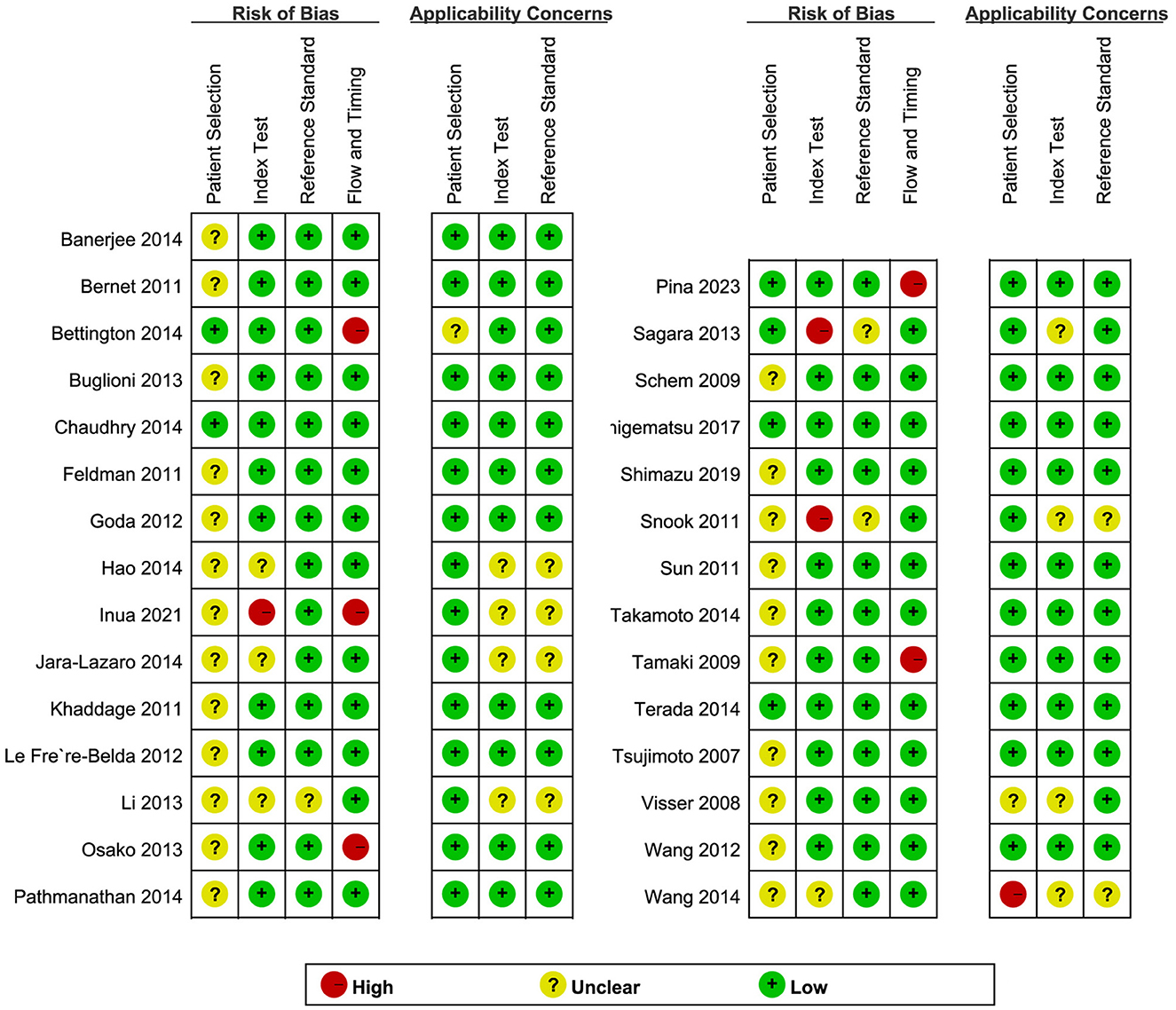
QUADAS-2 was used to assess the quality of the included literature (Figure 2). In the assessment of the index test, three studies (5, 25, 31) were found to have a high risk of bias, and the remainder had an unclear or low risk of bias. With regard to the flow and timing domain, five studies (5, 21, 30, 33, 43) were high risk and the rest were unclear or low risk. This is mainly related to the unclear implementation of literature blinding and reporting of thresholds and loss to follow-up. In the remaining two QUADAS-2 domains, namely, patient selection and reference standard, most studies were found to be of unclear or low risk. Regarding applicability concerns, only one study (38) showed high risk in “patient selection” and no high risk in the “index test” or the “reference standard.” Overall, the quality of the studies included in this review was indicated to be acceptable.
3.4 Diagnostic accuracy of OSNA for SLNs in breast cancer
3.4.1 Sensitivity and specificity
The pooled sensitivity and specificity of OSNA to diagnose SLN metastasis in CK19-positive breast cancer were 0.86 (95% CI from 0.85 to 0.88) and 0.94 (95% CI from 0.94 to 0.95), respectively (Figures 3A, B). Cochran Q and I2-tests were conducted to assess heterogeneity, which indicated significant heterogeneity (I2 = 73.6%, P < 0.001) and specificity (I2 = 85.2%, P < 0.001). Thus, a random effect model was used.

Figure 3. Forest plot of the sensitivity (A), specificity (B), and SROC curve (C) of OSNA for SLN metastasis in breast cancer. SROC, summary receiver operating characteristic; AUC, area under the curve; OSNA, one-step nucleic acid amplification; SLNs, sentinel lymph nodes.
3.4.2 Summary receiver operating characteristic (SROC) curve analysis
An SROC curve analysis was performed, and the AUC of the SROC curve was calculated to be 0.9708 (95% CI from 0.95 to 0.98; Figure 3C). This indicates the high diagnostic value of SLN metastasis for CK19-positive breast cancer.
3.4.3 Positive and negative likelihood ratios (PLR and NLR)
Owing to significant heterogeneity in PLR (I2 = 85.30%, 95% CI from 85.30 to 91.89, P < 0.001) and NLR (I2 = 81.07%, 95% CI from 74.75 to 87.38, P < 0.001), meta-analyses were performed using a random-effects model. The pooled PLR and NLR of the studies were 18.00 (95% CI from 13.54 to 23.92) and 0.13 (95% CI from 0.10 to 0.17), respectively (Figures 4A, B).

Figure 4. Forest plot of the PLR (A), NLR (B), DS (C), and DOR (D) of OSNA for SLN metastasis in breast cancer. PLR, positive likelihood ratio; NLR, negative likelihood ratio; DS, diagnostic score; DOR, summary diagnostic odds ratio; OSNA, one-step nucleic acid amplification; SLNs, sentinel lymph nodes.
3.4.4 Diagnostic score (DS) and odds ratio (DOR)
As there was significant heterogeneity in DS (I2 = 77.13%, P < 0.001) and DOR (I2 = 100.00%, P < 0.001), a meta-analysis of DS and DOR was conducted using a random-effects model. The overall pooled DS and DOR of the studies were 4.93 (95% CI: 4.46–5.41) and 138.99 (95% CI: 86.66–222.92), respectively (Figures 4C, D).
3.4.5 Sensitivity analysis
Sensitivity analysis showed a good fit for goodness of fit and binary normality (Figures 5A, B). There were four articles weighted (Figure 5C), which may be a source of heterogeneity shown by outlier detection (Figure 5D). After the exclusion of abnormal studies, the pooled specificity varied from 0.94 to 0.95; the sensitivity, AUC value, and NLR remained unchanged; the DLR decreased slightly from 18.26 to 18.00, and the DS and DOR decreased from 4.93 to 4.91 and 138.99 to 135.57, respectively. These data suggested that the re-analysis was slightly different compared with the combined results before exclusion which indicates that the conclusions of this study are robust.
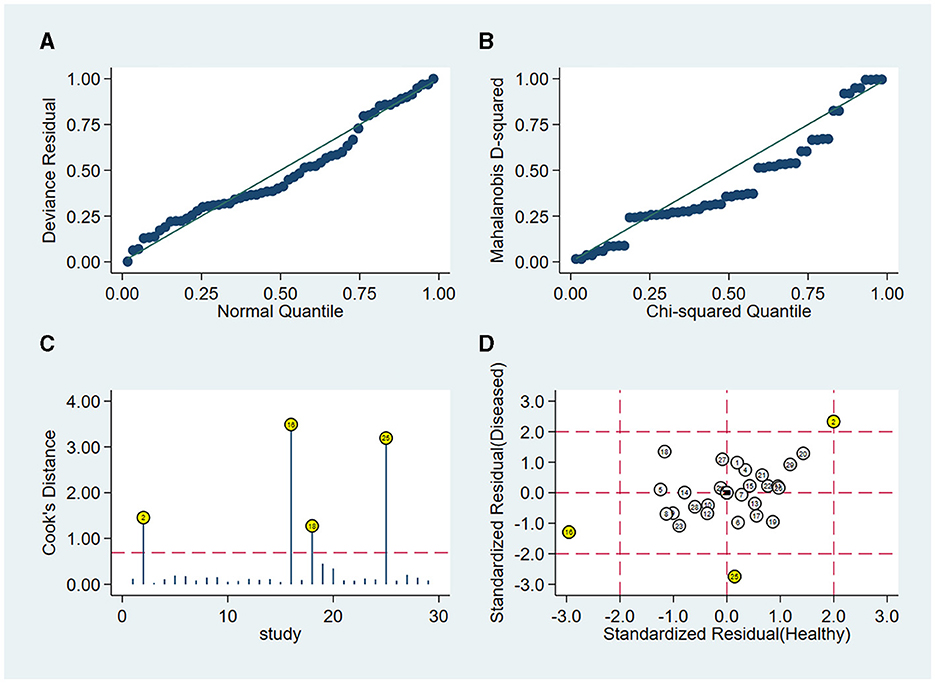
Figure 5. Sensitivity analysis results of the included studies. (A) Goodness of fit, (B) bivariate normality, (C) influence analysis, and (D) outlier detection.
3.4.6 Risk of publication bias
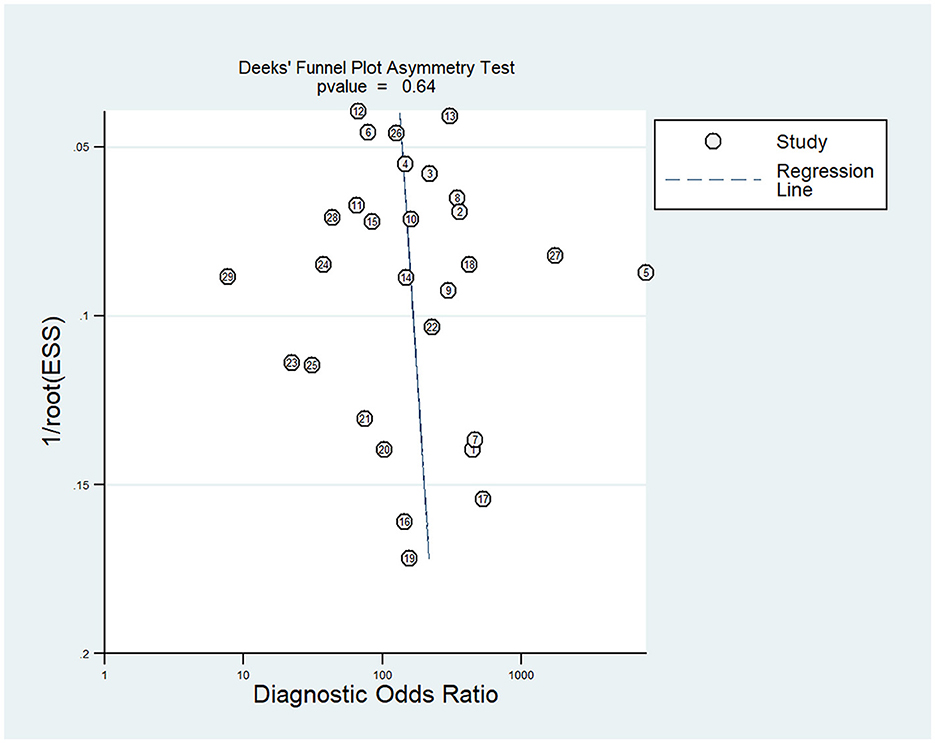
Deek's funnel chart was used to analyze any potential publication bias. As shown in Figure 6, the funnel chart was symmetric, and P = 0.64 suggested that no significant publication bias existed in this meta-analysis (Figure 6).
3.5 Subgroup analysis
We also conducted subgroup analysis to explore the sources of heterogeneity among different populations, different numbers of included patients, and whether it was conducted in multiple centers. As shown in Table 2, our analysis showed that these variables did not have a significant impact on the pooled analysis.
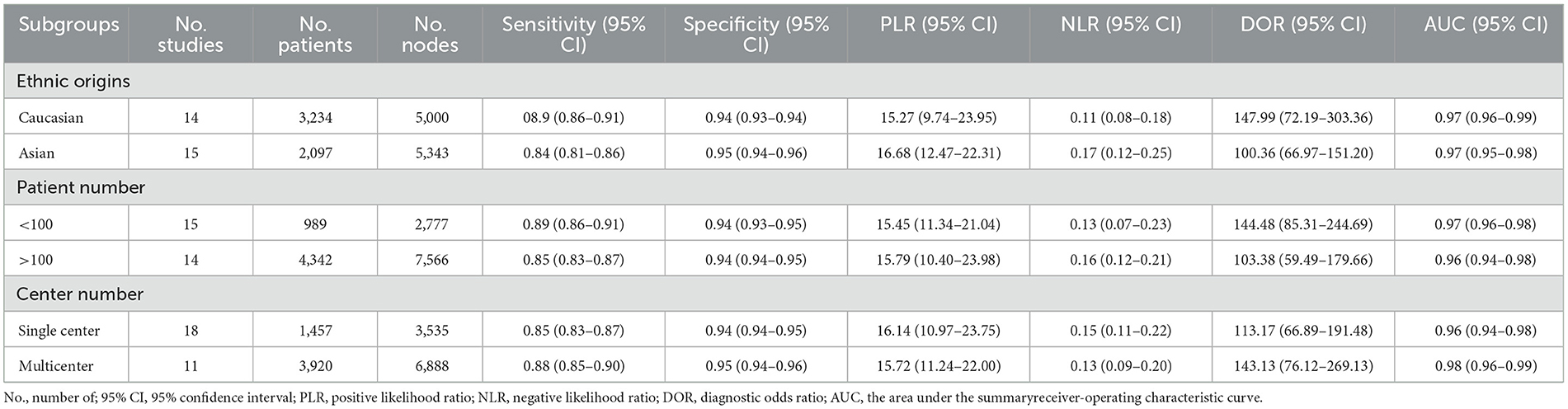
Table 2. Subgroup analysis of diagnostic accuracies of the OSNA assay in breast cancer based on a per-node analysis.
3.6 Clinical diagnostic value
Fagan's nomogram (Figure 7A) showed a prior probability of 50%. The post-probability positive and post-probability negative were 95 and 11%, respectively. Furthermore, the likelihood ratio scattergram (Figure 7B) showed the different clinical significances of SLN metastasis in CK19-positive breast cancer. On the upper left quadrant, PLR was >10 and NLR was < 0.1, which indicated that these markers could be used to make an exclusion or confirmation diagnosis. On the upper right quadrant, PLR was >10 and NLR was >0.1, which indicated that these markers could be used to make a confirmation diagnosis only. On the lower right quadrant, PLR was < 10 and NLR was >0.1, which indicated that these markers were not able to be used to make an exclusion or confirmation diagnosis.
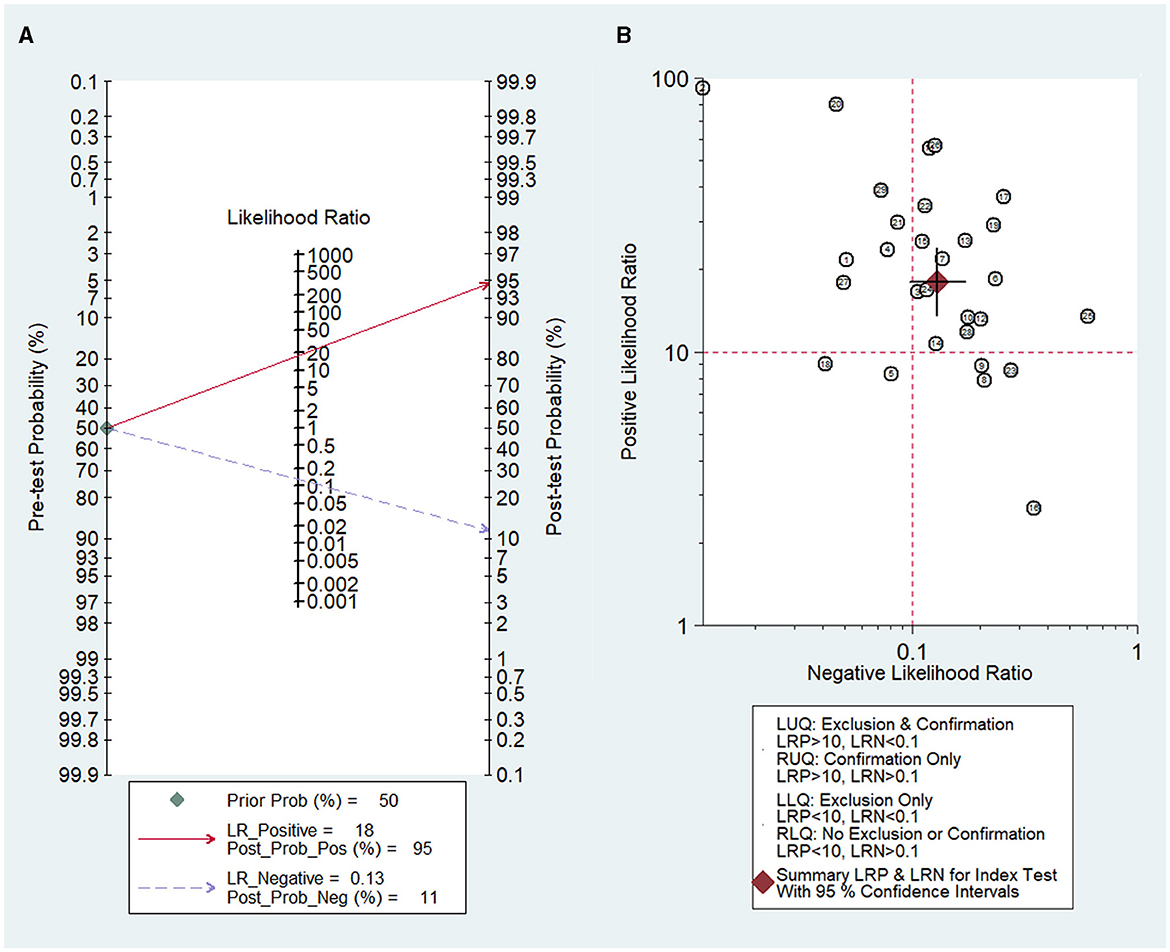
Figure 7. Diagnostic value of OSNA for SLN metastasis in breast cancer. (A) Fagan's nomogram evaluates the clinical diagnostic value of OSNA for SLN metastasis in breast cancer. (B) The likelihood ratio scattergram shows the different clinical significances of OSNA for SLN metastasis in breast cancer. OSNA, one-step nucleic acid amplification; SLNs, sentinel lymph nodes.
4 Discussion
The formation of distant LNM is the most lethal step in cancer progression and affects surgical decision-making, which is an important prognostic indicator in various cancer types (49). Traditional intraoperative SLN detection methods, including FS and TIC, are limited due to their low sensitivity and lack of standardized methods. An SLN biopsy combined with an intraoperative molecular-based detection of SLN metastasis using the OSNA assay provides a more standardized, objective, and reproducible whole-node assessment than the traditional pathological examination methods in breast cancer patients (16, 50). The OSNA assay can more effectively detect micrometastasis, reducing the number of false-negative histological examinations caused by the small size of micrometastases, which may not be included in any microscopical section (12). It can also avoid sampling errors and second operations due to false-negative results, thereby expediting the progression to adjuvant treatment (38). In particular, it succeeded in significantly reducing the surgical time from a mean operation length of 70.1 ± 10.5 min in the case of the FS to a mean operation length of 42.1 ± 5.1 min using OSNA (12). Therefore, it can reduce breast cancer patient healthcare costs and, correspondingly, ease the economic burden on patients. These advantages contribute to OSNA detection becoming a routine intraoperative SLN detection method during breast cancer surgery.
Although the sensitivity and specificity of the OSNA assay have been described, no pooled analysis has been conducted to evaluate the diagnostic performance of SLN metastases in breast cancer patients. In this study, we attempted to conduct a meta-analysis to quantify the diagnostic accuracy of the OSNA assay in detecting SLN metastasis in CK19-positive breast cancer patients.
A total of 29 studies, consisting of 5,331 patients with 10,343 SLNs, were included in this study. The pooled sensitivity, specificity, and AUC of the OSNA assay were 0.86, 0.94, and 0.9708, respectively, which indicated a relatively preferable diagnostic value. A previous meta-analysis based on intraoperative FS or TIC for SLNs showed that their pooled sensitivity and specificity were 0.78 and 1.00 (FS) and 0.74 and 0.98 (TIC), respectively (51). These results have also been validated by Yang et al., indicating that compared to traditional pathological examination methods, the OSNA assay seems to have better performance (52). In addition, our analysis results show that the overall diagnostic accuracy of OSNA for all cases was 0.9708. According to previous reports, the overall diagnostic accuracy of the TS and TIC detection methods was 0.9857 and 0.9837, respectively, which indicated no significant difference compared to OSNA (51). Given these potential benefits derived from the OSNA assay, the OSNA assay appears to be a useful tool in assessing SLN metastasis in CK19-positive breast cancer patients.
We also conducted subgroup analysis to explore the sources of heterogeneity among different subgroups, such as ethnic origins, patient numbers, and center numbers. There is no significant difference between different subgroups, indicating that these variables did not have a significant impact on the pooled analysis. Therefore, the OSNA assay might be quite a stable method for diagnosing SLN metastases in breast cancer. A sensitivity analysis was also performed in this study. After removing four weighted articles, the pooled specificity varied from 0.94 to 0.95, and the sensitivity, AUC value, and NLR remained unchanged. The conclusions of this study have been confirmed to be robust.
Despite our efforts to perform a systematic and comprehensive meta-analysis, there are still some limitations to our study. First, some of the included literature did not provide detailed descriptions of information, such as trial randomization, blind design, and quality control, which may affect the quality of this study. Second, the incidence and medical level were different among different countries and regions, which could affect the accuracy of the diagnosis and thus affect the results of this analysis. Third, heterogeneity among included trials is still an essential issue in this study. Moreover, this meta-analysis mainly focused on the diagnostic value of OSNA, and its prognostic value could be evaluated in future studies. Thus, the diagnostic performance and application of the OSNA assay in clinical practice still need a lot of research.
In summary, this meta-analysis provides evidence that the OSNA assay is a convenient, reliable, and standardized method for the intraoperative detection of SLN metastases in CK19-positive breast cancer patients. It provides satisfactory results in a short time, and with an easy procedure, its clinical application can benefit patients by minimizing the need for second surgeries for SLN detection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ML: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. WW: Data curation, Project administration, Resources, Writing – original draft. YW: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Michaels E, Worthington RO, Rusiecki J. Breast cancer. Med Clin N Am. (2023) 107:271–84. doi: 10.1016/j.mcna.2022.10.007
2. Fischer JR, Jackson HW, de Souza N, Varga Z, Schraml P, Moch H, et al. Multiplex imaging of breast cancer lymph node metastases identifies prognostic single-cell populations independent of clinical classifiers. Cell Rep Med. (2023) 4:100977. doi: 10.1016/j.xcrm.2023.100977
3. Cserni G, Chmielik E, Cserni B, Tot T. The new TNM-based staging of breast cancer. Virchows Archiv. (2018) 472:697–703. doi: 10.1007/s00428-018-2301-9
4. Cheng G, Kurita S, Torigian DA, Alavi A. Current status of sentinel lymph-node biopsy in patients with breast cancer. Eur J Nucl Med Mol Imaging. (2010) 38:562–75. doi: 10.1007/s00259-010-1577-z
5. Inua B, Fung V, Al-Shurbasi N, Howells S, Hatsiopoulou O, Somarajan P, et al. Sentinel lymph node biopsy with one-step nucleic acid assay relegates the need for preoperative ultrasound-guided biopsy staging of the axilla in patients with early stage breast cancer. Mol Clin Oncol. (2021) 14:51. doi: 10.3892/mco.2021.2213
6. Ahmed S, Ahmad M. Comparison of diagnostic accuracy of touch imprint cytology and frozen section techniques in detecting breast malignancies. J Pak Med Assoc. (2016) 66:292–5.
7. Namba K, Suzawa K, Shien K, Miura A, Takahashi Y, Miyauchi S, et al. One-step nucleic acid amplification for intraoperative diagnosis of lymph node metastasis in lung cancer patients: a single-center prospective study. Sci Rep. (2022) 12:7297. doi: 10.1038/s41598-022-11064-4
8. Crafa F, Vanella S, Catalano OA, Pomykala KL, Baiamonte M. Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection. World J Gastroenterol. (2022) 28:4019–43. doi: 10.3748/wjg.v28.i30.4019
9. Mehrpouya M, Pourhashem Z, Yardehnavi N, Oladnabi M. Evaluation of cytokeratin 19 as a prognostic tumoral and metastatic marker with focus on improved detection methods. J Cell Physiol. (2019) 234:21425–35. doi: 10.1002/jcp.28768
10. Alix-Panabières C, Vendrell J-P, Slijper M, Pellé O, Barbotte E, Mercier G, et al. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. (2009) 11:R39. doi: 10.1186/bcr2326
11. Togami S, Ushiwaka T, Kitazono I, Yanazume S, Kamio M, Tanimoto A, et al. One-step nucleic acid amplification (OSNA) assay for detecting lymph node metastasis in cervical and endometrial cancer: a preliminary study. J Gynecol Oncol. (2022) 33:e11. doi: 10.3802/jgo.2022.33.e11
12. Bertozzi S, Londero AP, Bulfoni M, Seriau L, Agakiza D, Pasqualucci A, et al. One-step nucleic acid amplification system in comparison to the intraoperative frozen section and definitive histological examination among breast cancer patients: a retrospective survival study. Front Oncol. (2022) 12:847858. doi: 10.3389/fonc.2022.847858
13. Tamaki Y. One-step nucleic acid amplification (OSNA): where do we go with it? Int J Clin Oncol. (2017) 22:3–10. doi: 10.1007/s10147-016-1030-9
14. Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. (2007) 13:4807–16. doi: 10.1158/1078-0432.CCR-06-2512
15. Li D, Xu X, Chen J, Chen J, Yang B, Yang W, et al. Utility of one-step nucleic acid amplification (OSNA) assay in detecting breast cancer metastases of sentinel lymph nodes in a Chinese population. Breast Cancer. (2013) 22:135–40. doi: 10.1007/s12282-013-0461-7
16. Raia-Barjat T, Trombert B, Khaddage A, Douchet C, Seffert P, Peoc'h M, et al. OSNA (one-step nucleic acid amplification) sentinel lymph node intraoperative molecular analysis in breast cancer: a cost-benefit analysis. Med Oncol. (2014) 31:322. doi: 10.1007/s12032-014-0322-z
17. Shi F, Liang Z, Zhang Q, Wang C, Liu X. The performance of one-step nucleic acid amplification assay for intraoperative detection of sentinel lymph node macrometastasis in breast cancer: an updated meta-analysis. Breast. (2018) 39:39–45. doi: 10.1016/j.breast.2018.03.005
18. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. (2006) 6:31. doi: 10.1186/1471-2288-6-31
20. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
21. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
22. Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. (2002) 31:88–95. doi: 10.1093/ije/31.1.88
23. Visser M, Jiwa M, Horstman A, Brink AATP, Pol RP, van Diest P, et al. Intra-operative rapid diagnostic method based on CK19 mRNA expression for the detection of lymph node metastases in breast cancer. Int J Cancer. (2008) 122:2562–7. doi: 10.1002/ijc.23451
24. Schem C, Maass N, Bauerschlag DO, Carstensen MH, Löning T, Roder C, et al. One-step nucleic acid amplification-a molecular method for the detection of lymph node metastases in breast cancer patients; results of the German study group. Virchows Archiv. (2009) 454:203–10. doi: 10.1007/s00428-008-0703-9
25. Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K, et al. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. (2009) 15:2879–84. doi: 10.1158/1078-0432.CCR-08-1881
26. Bernet L, Cano R, Martinez M, Dueñas B, Matias-Guiu X, Morell L, et al. Diagnosis of the sentinel lymph node in breast cancer: a reproducible molecular method: a multicentric Spanish study. Histopathology. (2011) 58:863–9. doi: 10.1111/j.1365-2559.2011.03836.x
27. Khaddage A, Berremila SA, Forest F, Clemenson A, Bouteille C, Seffert P, et al. Implementation of molecular intra-operative assessment of sentinel lymph node in breast cancer. Anticancer Res. (2011) 31:585–90.
28. Feldman S, Krishnamurthy S, Gillanders W, Gittleman M, Beitsch PD, Young PR, et al. A novel automated assay for the rapid identification of metastatic breast carcinoma in sentinel lymph nodes. Cancer. (2011) 117:2599–607. doi: 10.1002/cncr.25822
29. Snook KL, Layer GT, Jackson PA, de Vries CS, Shousha S, Sinnett HD, et al. Multicentre evaluation of intraoperative molecular analysis of sentinel lymph nodes in breast carcinoma. Br J Surg. (2011) 98:527–35. doi: 10.1002/bjs.7347
30. Sun X, Fu X, Han W, Zhao Y, Liu H, Sheng Z. Dedifferentiation of human terminally differentiating keratinocytes into their precursor cells induced by basic fibroblast growth factor. Biol Pharmaceut Bullet. (2011) 34:1037–45. doi: 10.1248/bpb.34.1037
31. Le Frère-Belda MA, Bats AS, Gillaizeau F, Poulet B, Clough KB, Nos C, et al. Diagnostic performance of one-step nucleic acid amplification for intraoperative sentinel node metastasis detection in breast cancer patients. Int J Cancer. (2011) 130:2377–86. doi: 10.1002/ijc.26291
32. Wang Y, Ou-yang T, Wu J, Liu Y, Cao X, Sun X, et al. Comparative study of one-step nucleic acid amplification assay, frozen section, and touch imprint cytology for intraoperative assessment of breast sentinel lymph node in Chinese patients. Cancer Sci. (2012) 103:1989–93. doi: 10.1111/cas.12001
33. Buglioni S, Di Filippo F, Terrenato I, Casini B, Gallo E, Marandino F, et al. Quantitative molecular analysis of sentinel lymph node may be predictive of axillary node status in breast cancer classified by molecular subtypes. PLoS ONE. (2013) 8:e58823. doi: 10.1371/journal.pone.0058823
34. Osako T, Iwase T, Kimura K, Horii R, Akiyama F. Sentinel node tumour burden quantified based on cytokeratin 19 mRNA copy number predicts non-sentinel node metastases in breast cancer: molecular whole-node analysis of all removed nodes. Eur J Cancer. (2013) 49:1187–95. doi: 10.1016/j.ejca.2012.11.022
35. Sagara Y, Ohi Y, Matsukata A, Yotsumoto D, Baba S, Tamada S, et al. Clinical application of the one-step nucleic acid amplification method to detect sentinel lymph node metastasis in breast cancer. Breast Cancer. (2013) 20:181–6. doi: 10.1007/s12282-011-0324-z
36. Banerjee SM, Michalopoulos NV, Williams NR, Davidson T, El Sheikh S, McDermott N, et al. Detailed evaluation of one step nucleic acid (OSNA) molecular assay for intra-operative diagnosis of sentinel lymph node metastasis and prediction of non-sentinel nodal involvement: experience from a London Teaching Hospital. Breast. (2014) 23:378–84. doi: 10.1016/j.breast.2014.02.001
37. Bettington M, Lakhani SR, Ung OA. Is the one-step nucleic acid amplification assay better for intra-operative assessment of breast sentinel nodes? ANZ J Surg. (2014) 84:725–9. doi: 10.1111/ans.12497
38. Chaudhry A, Williams S, Cook J, Jenkins M, Sohail M, Calder C, et al. The real-time intra-operative evaluation of sentinel lymph nodes in breast cancer patients using One Step Nucleic Acid Amplification (OSNA) and implications for clinical decision-making. Eur J Surg Oncol. (2014) 40:150–7. doi: 10.1016/j.ejso.2013.12.007
39. Jara-Lazaro AR, Hussain IHM, Thike AA, Wong CY, Ho GH, Yong WS, et al. Assessment of suitability of the one step nucleic acid amplification (OSNA) assay as an intraoperative procedure for detection of metastasis in sentinel lymph nodes of breast cancer. J Clin Pathol. (2014) 67:1032–7. doi: 10.1136/jclinpath-2014-202361
40. Pathmanathan N, Renthawa J, French JR, Edstrom-Elder E, Hall G, Mahajan H, et al. Intraoperative sentinel lymph node assessment in breast cancer: a comparison of rapid diagnostic method based on CK19 mRNA expression and imprint cytology. ANZ J Surg. (2014) 84:730–4. doi: 10.1111/ans.12668
41. Terada M, Niikura N, Tsuda B, Masuda S, Kumaki N, Tang X, et al. Comparative study of the one-step nucleic acid amplification assay and conventional histological examination for the detection of breast cancer sentinel lymph node metastases. Tokai J Exp Clin Med. (2014) 39:122–7.
42. Wang Y-S, Liu Y-H, Ou-Yang T, Yang X-H, Wu J, Su F-X, et al. GeneSearch™ BLN assay could replace frozen section and touch imprint cytology for intra-operative assessment of breast sentinel lymph nodes. Breast Cancer. (2014) 21:583–9. doi: 10.1007/s12282-012-0437-z
43. Hao X, Liu Y, Li X, Kang H, Qu X, He J, et al. An intra-operative RT-LAMP method allows rapid and reliable detection of sentinel lymph node metastasis in breast cancer patients. Virchows Archiv. (2014) 466:169–76. doi: 10.1007/s00428-014-1693-4
44. Takamoto K, Shimazu K, Naoi Y, Shimomura A, Shimoda M, Kagara N, et al. One-step nucleic acid amplification assay for detection of axillary lymph node metastases in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. (2016) 23:78–86. doi: 10.1245/s10434-015-4693-y
45. Shigematsu H, Ozaki S, Yasui D, Zaitsu J, Taniyama D, Saitou A, et al. Comparison of CK-IHC assay on serial frozen sections, the OSNA assay, and in combination for intraoperative evaluation of SLN metastases in breast cancer. Breast Cancer. (2017) 25:191–7. doi: 10.1007/s12282-017-0811-y
46. Shimazu K, Tanei T, Tamaki Y, Saeki T, Osaki A, Hasebe T, et al. Performance of a new system using a one-step nucleic acid amplification assay for detecting lymph node metastases in breast cancer. Med Oncol. (2019) 36:54. doi: 10.1007/s12032-019-1277-x
47. Pina H, Salleron J, Gilson P, Husson M, Rouyer M, Leroux A, et al. Intraoperative prediction of non-sentinel lymph node metastases in breast cancer using cytokeratin 19 mRNA copy number: a retrospective analysis. Mol Clin Oncol. (2022) 16:58. doi: 10.3892/mco.2022.2491
48. Goda H, Nakashiro KI, Oka R, Tanaka H, Wakisaka H, Hato N, et al. One-step nucleic acid amplification for detecting lymph node metastasis of head and neck squamous cell carcinoma. Oral Oncol. (2012) 48:958–63. doi: 10.1016/j.oraloncology.2012.03.026
49. Urrutia G, Laurito S, Marzese DM, Gago F, Orozco J, Tello O, et al. Epigenetic variations in breast cancer progression to lymph node metastasis. Clin Exp Metastasis. (2015) 32:99–110. doi: 10.1007/s10585-015-9695-4
50. Leidenius MHK, Krogerus LA, Toivonen TS, von Smitten KJA. The feasibility of intraoperative diagnosis of sentinel lymph node metastases in breast cancer. J Surg Oncol. (2003) 84:68–73. doi: 10.1002/jso.10296
51. Bharath S, Sharma D, Yadav SK, Shekhar S, Jha CK. A systematic review and meta-analysis of touch imprint cytology and frozen section biopsy and their comparison for evaluation of sentinel lymph node in breast cancer. World J Surg. (2022) 47:478–88. doi: 10.1007/s00268-022-06800-w
Keywords: OSNA, CK19, breast cancer, sentinel lymph nodes, metastasis, meta-analysis
Citation: Liu M, Wang W and Wang Y (2024) The diagnostic performance of the one-step nucleic acid amplification assay for the detection of sentinel lymph node metastases in cytokeratin 19-positive breast cancer: a PRISMA-compliant meta-analysis. Front. Med. 11:1391621. doi: 10.3389/fmed.2024.1391621
Received: 26 February 2024; Accepted: 20 August 2024;
Published: 09 September 2024.
Edited by:
Daniel Matias Regiart, University of São Paulo, BrazilReviewed by:
Zheng Wang, Shanghai Jiao Tong University, ChinaFenglin Dong, The First Affiliated Hospital of Soochow University, China
Gang Sun, Xinjiang Cancer Center/Key Laboratory of Oncology of Xinjiang Uyghur Autonomous Region, China
Copyright © 2024 Liu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufang Wang, d3lmMjAxMjEyMzFAMTYzLmNvbQ==
 Meirong Liu1
Meirong Liu1 Yufang Wang
Yufang Wang