
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 24 September 2024
Sec. Pulmonary Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1390083
This article is part of the Research Topic Reviews in: Pulmonary Medicine 2023 View all 11 articles
Background: Drug-induced lung disease (DILD) is a considerable and potentially fatal adverse event with poorly understood risk factors. Large-scale, data-driven analyses investigating regional discrepancies in DILD incidence are lacking. The aim of this study was to investigate the potential association among DILD prevalence, regional differences and other factors based on large-scale data base.
Methods: This retrospective observational study analyzed spontaneous adverse event reports from the FDA Adverse Event Reporting System (FAERS) database between January 2010 and December 2020. Regional disparities in DILD incidence were assessed among reports from the United States of America (USA), the European Union (EU), and Japan (JP). Using multivariate logistic regression accounting for age, sex, and reporting years, we calculated the reporting odds ratios (RORs) with 95% confidence intervals. Subgroup analyses were performed for different types of anticancer agents, including tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs), antibody–drug conjugates (ADCs), and cytotoxic agents.
Results: Regional differences in RORs were observed for anticancer drugs in reports from JP and the EU compared with those from the USA (JP, ROR 4.432; EU, ROR 1.291) and for non-anticancer drugs (JP, ROR 3.481; EU, ROR 1.086). Significantly higher RORs were observed for all anticancer drug regimens reported in JP than in the USA (TKIs, ROR 3.274; ICIs, ROR 2.170; ADCs, ROR 2.335; cytotoxic agents, ROR 3.989). The EU reports exhibited higher RORs for TKIs and cytotoxic agents than the USA reports, with no significant differences in ICIs or ADCs (TKIs, ROR 1.679; ICIs, ROR 1.041; ADCs, ROR 1.046; cytotoxic agents, ROR 1.418).
Conclusion: The prevalence of DILD in JP, the EU, and the USA differed. These findings have important implications in evaluating the safety profiles of drugs and patient safety in drug development and clinical practice. This study is the first to identify regional differences in DILDs using a large global database.
Drug-induced lung disease (DILD) is an adverse event (AE) with a fatality rate of up to 40% (1–3). Anticancer drugs are the most common cause of DILDs, and their prevalence has increased with the use of recently developed anticancer drugs (3). Specifically, the occurrence rate of DILDs in patients administered with tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) is 5–20%, which is higher than the approximately 2% rate in patients administered with classical cytotoxic agents (2, 4). The mortality rate of DILDs associated with anticancer drugs, particularly TKIs and ICIs, is extremely high, with mortality rates of 35–40% and 15–20%, respectively (2, 5). Although DILDs have a considerable clinical impact, their associated predictive factors have not been elucidated.
Regional differences in DILD incidence have been noted in several clinical trials, with relatively high incidences reported in Asia (4). The incidence of DILDs after treatment with molecularly targeted drugs [e.g., epidermal growth factor receptor (EGFR) TKIs] in Asia is 10 times higher than that in non-Asian regions. The mortality rate of DILDs associated with molecularly targeted drugs is higher than that of DILDs associated with drugs with other mechanisms of action (1). For antibody–drug conjugates (ADCs) and ICIs, the incidence of DILDs is relatively high in Asian (Japanese) patients. In clinical studies of ICIs in Asia (Japan), regional differences in DILD prevalence have been observed, with a reported rate of 15–20% (4, 6–8).
Large-scale database analyses investigating the regional differences in DILDs have not yet been reported. The Food and Drug Administration Adverse Event Reporting System (FAERS) is the largest database of spontaneous reports on drug-related AEs worldwide. However, no previous reports have employed these types of large-scale databases to examine ethnic differences in DILD development. This study aimed to investigate the potential associations among DILD prevalence, regional differences, and other factors by using FAERS data.
This retrospective observational study was conducted using data from the FAERS database. FAERS data were downloaded through the USA Food and Drug Administration (FDA) website,1 and DILD reports issued between January 2010 and December 2020 were identified. As of January 2023, more than 14 million reports have been collected and analyzed by downloading raw data. AEs suspected to be associated with DILD in the FAERS were reviewed separately. The following information was collected: patient characteristics (date of AE, date of report to the FDA, age at AE, sex, weight, reporter, reporting country, and country of occurrence), drug information (suspected drug identification, drug name, route of administration, dose, administration date, treatment start date, and treatment end date), patient outcomes (death and hospitalization), and source of information (foreign, clinical trial, and consumer). The FAERS is a publicly accessible, anonymous database. The requirement for informed consent and approval from the institutional review board was waived.
We investigated AEs reported between January 2011 and December 2020 in patients aged 20–100 years at the time of AE occurrence. In the FAERS, the Medical Dictionary of Regulatory Activities organ system classification and preferred term level were used to describe suspected AEs.
The investigators (respiratory physicians) reviewed all reported AEs and defined DILD items that could be clinically determined as DILDs. A comprehensive inventory of DILD items was compiled (Supplementary Table S1).
All reported drugs were reviewed by the investigators and classified as either anticancer or non-anticancer. Anticancer drugs were further classified into TKIs, ICIs, ADCs, antibodies, cytotoxins, hormones, and others according to their mechanisms of action (Supplementary Table S2). The causal relationship between a combined regimen (drugs with different modes of action) remains unclear, and only monotherapeutic anticancer drugs or drugs with the same known mechanisms of action were included in the analyses.
Regional differences in DILD incidence were examined using data from the USA, the European Union (EU), and Japan (JP) reports. The names of the reporting countries were collected from the FAERS data, and the EU included member countries as of August 2022.2 Members of the EU were identified and listed by country codes, and the program automatically identified matching records.
Patient characteristics in each region were collected as follows. The median and the interquartile range were calculated for continuous patient characteristics. For categorical patient characteristics, frequencies were tabulated.
The risk of DILDs in the EU and JP compared with that in the USA was assessed using the reporting odds ratio (ROR), defined as the ratio of the odds in reports from the JP or the EU to that in the USA, and the odds were defined as the ratio of the presence of DILD to the absence of DILDs in each region. An ROR close to one indicated no difference in the frequency of DILDs between the EU (or JP) and the USA reports. When the ROR was >1, the risk of DILDs in the EU (or JP) reports was higher than that in the USA reports.
A logistic regression model was used to calculate the DILDs and 95% confidence intervals (CIs) for the EU and JP reports relative to those for the USA reports. Multivariate analysis was performed using a logistic regression model to adjust for confounding variables, including sex (male/female), age (10-year range), and year of occurrence (2010–2014 or 2015–2020). To assess the risk of DILDs by drug type, we performed the same analysis for drug types (i.e., non-anticancer and anticancer drugs) with a reasonable prevalence in single agents or agents with the same mechanism of action (TKIs, ICIs, ADCs, and cytotoxic agents) in subgroup analyses. All analyses were performed using SAS version 9.4.
We identified patients with AEs and DILDs in reports from the USA [3,057,102 and 73,137 (2.39%), respectively], EU [934,576 and 29,533 (3.16%), respectively], and JP [228,064 and 22,463 (9.84%), respectively]. Sex-reporting bias was also observed. The reports indicated relatively older age in JP, fewer fatalities and more consumer reports in the USA, and more patients with onset within 90 days in JP. No other significant differences in characteristics were found among the reports from the three regions (Table 1). No significant difference was noted in the reporting proportion (DILD events per total AEs) based on the reported years (Figure 1).
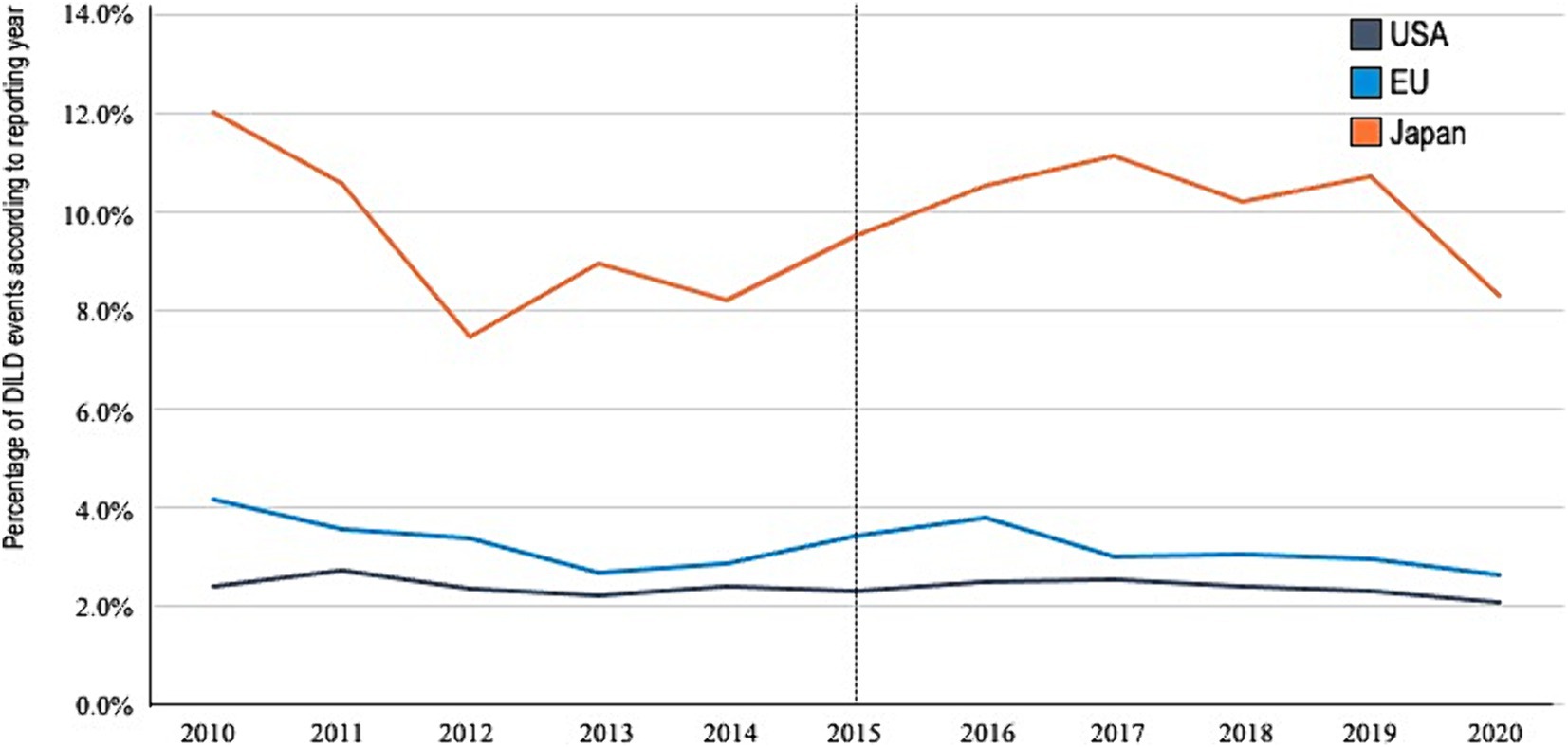
Figure 1. Reporting percentage by year. DILDs, drug-induced lung disease; US, United States; EU, European Union; JP, Japan.
Univariate analysis results (Table 2) indicated that the incidence of DILDs was relatively low in females and increased with age. As shown in Figure 2 and Table 3, multivariate analysis revealed a higher reporting proportion and RORs of non-anticancer drugs in JP than in the USA. By contrast, no difference in DILD incidence rates associated with non-anticancer drugs was found between reports from the EU and USA. Significant differences in RORs were also observed across anticancer drugs in reports from JP and the EU compared with those from the USA (JP, ROR 4.432 [95% CI, 4.366–4.499]; EU, ROR 1.291 [95% CI, 1.274–1.308]). The fatality rates associated with DILDs in the USA, EU, and JP reports were 6.1, 12.4, and 15.5%, respectively, with a significantly higher proportion of DILDs in the EU and JP reports than in the USA reports.
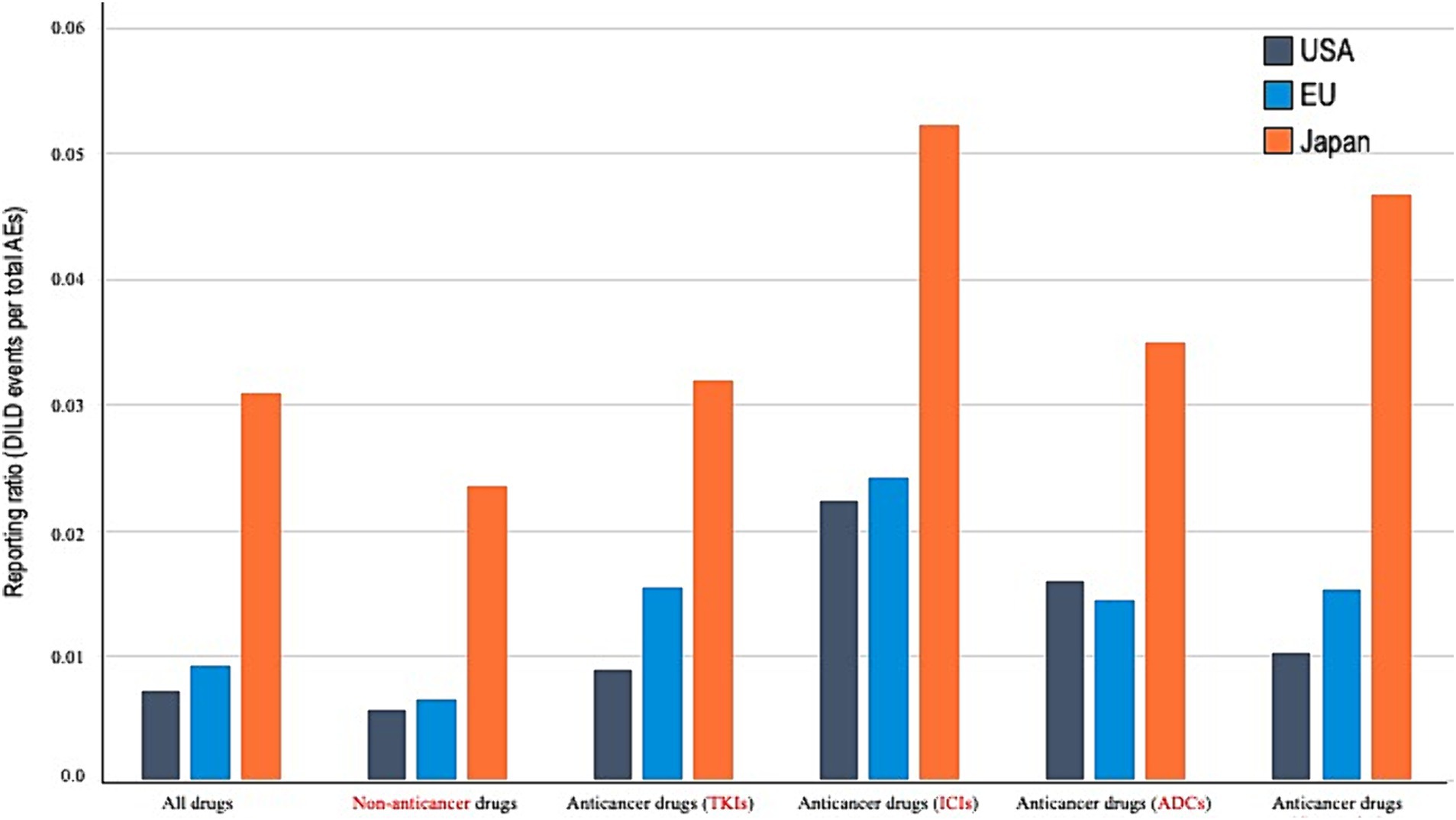
Figure 2. Reporting proportion according to the drug types. AE, adverse event; DILDs, drug-induced lung disease; US, United States; EU, European Union; JP, Japan; TKIs, tyrosine kinase inhibitors; ICIs, immune checkpoint inhibitors; ADCs, antibody–drug conjugates; cytotoxics, cytotoxic agents.
The results of multivariate analysis for each single-agent regimen of anticancer drugs showed that the RORs in reports from JP were significantly higher than those in reports from the USA (TKIs, ROR 3.274 [95% CI, 3.105–3.451]; ICIs, ROR 2.170 [95% CI, 2.025–2.325]; ADCs, ROR 2.335 [95% CI, 1.666–3.272]; and cytotoxic agents, ROR 3.989 [95% CI, 3.776–4.215]). Reports from the EU had higher RORs than those from the USA for TKIs and cytotoxic agents; no statistically significant differences were noted between the RORs of ICIs and ADCs (TKIs, ROR 1.679 [95% CI, 1.594–1.769]; ICIs, ROR 1.041 [95% CI, 0.965–1.123]; ADCs, ROR 1.046 [95% CI, 0.763–1.434]; and cytotoxic agents, ROR 1.418 [95% CI, 1.354–1.485]).
This study is the first to identify regional differences in DILDs using a large, global database. This analysis revealed that the prevalence of DILDs was significantly higher in JP than in the USA for all anticancer and non-anticancer drugs. This study confirmed the previously reported higher prevalence of DILDs in clinical trials due to TKIs and ADCs as well as the previously unreported higher prevalence of DILDs due to ICIs in JP than in Western countries (4, 6–8). The EU had higher RORs than the US for TKIs and cytotoxic agents, and no significant differences in RORs were observed between ICIs and ADCs.
Previous clinical trials have shown that Asians, specifically Japanese, are susceptible to DILDs after treatment with certain drugs, and the incidence of DILDs caused by gefitinib, an EGFR-TKI, is relatively high in the Asian population (9). Similar trends have been reported for ADCs and TKIs, with Asians having a relatively high risk of developing DILDs (3). In the present study, a discernible discrepancy in the incidence rates of DILDs between reports from JP and the USA was observed. This disparity has been observed in the contexts of TKIs and ADCs. In terms of ICIs, a few previous studies have reported ethnic discrepancies in DILD prevalence that, as delineated in previous reports, ranges between 14.5 and 19.0% (7, 10, 11). This study elucidated a notable distinction in the incidence rates of DILDs across various drug types, including ICIs, when comparing reports from JP and the USA, using a large-scale database. A similar trend surfaced when comparing reports from JP, China, South Korea, and ASEAN countries with those from the EU and the USA (Supplementary Table S3). These findings implicate inherent factors, such as genomic variances and comorbid conditions, as potential drivers of discrepancies in DILD prevalence after treatment with anticancer drugs, including ICIs, between Asian and non-Asian populations.
This study investigated the differences in reporting propensity from JP, the EU, and the USA by comparing the reports from the three regions to ensure data robustness. The data among the three regions were possibly influenced by biases in reporting, healthcare access, language, and DILD severity. These biases were considered when interpreting the results of this study. We investigated reporter bias by examining the differences in reporting between the EU and the USA, considering reporting bias from regions other than the USA. The RORs of ICIs and ADCs showed only minor differences between the EU and USA, whereas clear differences were observed between the USA and JP. The high proportion of healthcare professionals as reporters in both the EU and Japan contribute to the higher number of severe cases being reported from these regions (Table 1). Regional differences in mortality from DILDs were notably higher in JP and the EU than in the USA. This result indicates that reporting relatively mild cases of DILDs does not result in a reporting bias that would increase the prevalence in JP and the EU. Moreover, significant DILDs that needed to be reported were recorded regardless of the region and country, including outside the USA. These findings eliminated reporting bias and strongly suggested ethnic differences in DILD incidence resulting from several agents.
The mechanisms by which anticancer drugs induce DILDs must be elucidated for the prevention and treatment of this AE. The results of the present study showed that the prevalence of DILD differed with each drug, indicating that the mechanism underlying DILD development differs for each drug. Two main mechanisms of DILD development have been proposed: direct drug-induced damage and immunological mechanisms. Several hypotheses on the mechanism of direct injury state that drugs induce the release of cytotoxic reactive oxygen species in the lungs, leading to the degeneration of alveolar cells and pulmonary macrophages owing to increased phospholipid accumulation in alveolar cells (1, 2, 12–15). In terms of immunological mechanisms, direct haptenic modification of tissue-resident proteins by immune cells and deposition of antigen–antibody complexes have been proposed (8, 12, 16). Few studies reported on the mechanism of DILD development using tissue samples from affected patients. Further research on each mode of action should be conducted using blood and tissue samples and various omics analyses to identify predictive biomarkers for DILD development, especially in Asians, where DILD is more common. Our team has initiated a study analyzing the immunological profile and genetic background of DILD tissue samples.
In conclusion, the prevalence of DILD was higher in JP than in the USA and EU across all anticancer drug regimens. These findings underscore the importance of considering differences in the prevalence of DILD in JP, the EU, and the USA when evaluating the safety profiles of drug development and patient safety in clinical practice.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or the patients'/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RS: Data curation, Formal analysis, Methodology, Writing – review & editing. TK: Writing – review & editing. YK: Writing – review & editing. MO: Writing – review & editing. NY: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Kan Yonemori for his contribution to the team building.
NY received institutional research funds from Astellas, Chugai, Eisai, Taiho, BMS, Pfizer, Novartis, Eli Lilly, AbbVie, Daiichi-Sankyo, Bayer, Boehringer Ingelheim, Kyowa Kirin, Takeda, ONO, Janssen Pharma, MSD, MERCK, GSK, Sumitomo Pharma, Chiome Bioscience, Otsuka, Carna Biosciences, Genmab, Shionogi, TORAY, KAKEN, AstraZeneca, Cmic, InventisBio, and Rakuten Medical; personal fee from Eisai, Takeda, Boehringer Ingelheim, Cmic, Chugai, MERCK, and Healios as advisory role; and personal honoraria from ONO, Chugai, Daiichi-Sankyo, and Eisai. TK declares grants from PACT Pharma, Chugai, Daiichi-Sankyo, AstraZeneca, Novartis, Eli Lilly, Takeda, Janssen, and Zymeworks and honoraria from Sysmex and Chugai.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2024.1390083/abstract#supplementary-material
1. Rosenow, EC, Myers, JL, Swensen, SJ, and Pisani, RJ. Drug-induced pulmonary disease. Chest. (1992) 102:239–50. doi: 10.1378/chest.102.1.239
2. Saito, Y, and Gemma, A. Current status of DILD in molecular targeted therapies. Int J Clin Oncol. (2012) 17:534–41. doi: 10.1007/s10147-012-0494-5
3. Conte, P, Ascierto, PA, Patelli, G, Danesi, R, Vanzulli, A, Sandomenico, F, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. (2022) 7:100404. doi: 10.1016/j.esmoop.2022.100404
4. Skeoch, S, Weatherley, N, Swift, AJ, Oldroyd, A, Johns, C, Hayton, C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. (2018) 7:356. doi: 10.3390/jcm7100356
5. Wang, DY, Salem, JE, Cohen, JV, Chandra, S, Menzer, C, Ye, F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
6. Nishino, M, Giobbie-Hurder, A, Hatabu, H, Ramaiya, NH, and Hodi, FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
7. Suzuki, Y, Karayama, M, Uto, T, Fujii, M, Matsui, T, Asada, K, et al. Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: a multicenter prospective study. J Thorac Oncol. (2020) 15:1317–27. doi: 10.1016/j.jtho.2020.04.002
8. Tarantino, P, Modi, S, Tolaney, SM, Cortés, J, Hamilton, EP, Kim, SB, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. (2021) 7:1873–81. doi: 10.1001/jamaoncol.2021.3595
9. Kudoh, S, Kato, H, Nishiwaki, Y, Fukuoka, M, Nakata, K, Ichinose, Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. (2008) 177:1348–57. doi: 10.1164/rccm.200710-1501OC
10. Fukihara, J, Sakamoto, K, Koyama, J, Ito, T, Iwano, S, Morise, M, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death-1 inhibitors. Clin Lung Cancer. (2019) 20:442–450.e4. doi: 10.1016/j.cllc.2019.07.006
11. Suresh, K, Voong, KR, Shankar, B, Forde, PM, Ettinger, DS, Marrone, KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. (2018) 13:1930–9. doi: 10.1016/j.jtho.2018.08.2035
12. Matsuno, O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. (2012) 13:39. doi: 10.1186/1465-9921-13-39
13. de Goeij, BE, and Lambert, JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol. (2016) 40:14–23. doi: 10.1016/j.coi.2016.02.008
14. Kumagai, K, Aida, T, Tsuchiya, Y, Kishino, Y, Kai, K, and Mori, K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting ab-drug conjugate, in monkeys. Cancer Sci. (2020) 111:4636–45. doi: 10.1111/cas.14686
15. Polakis, P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. (2016) 68:3–19. doi: 10.1124/pr.114.009373
16. Rapoport, BL, Shannon, VR, Cooksley, T, Johnson, DB, Anderson, L, Blidner, AG, et al. Pulmonary toxicities associated with the use of immune checkpoint inhibitors: an update from the immuno-oncology subgroup of the neutropenia, infection and myelosuppression study group of the multinational association for supportive care in cancer. Front Pharmacol. (2021) 12:743582. doi: 10.3389/fphar.2021.743582
Keywords: drug-induced lung disease, FDA adverse event reporting system, ethnic diversity, interstitial lung disease, real-world data
Citation: Sato J, Sadachi R, Koyama T, Katsuya Y, Okada M and Yamamoto N (2024) Regional diversity in drug-induced lung diseases among the USA, European Union, and Japan. Front. Med. 11:1390083. doi: 10.3389/fmed.2024.1390083
Received: 22 February 2024; Accepted: 13 September 2024;
Published: 24 September 2024.
Edited by:
Koji Sakamoto, Nagoya University, JapanReviewed by:
Rudolf Maria Huber, Ludwig Maximilian University of Munich, GermanyCopyright © 2024 Sato, Sadachi, Koyama, Katsuya, Okada and Yamamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Sato, anVuc2F0bzJAbmNjLmdvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.