
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 28 June 2024
Sec. Ophthalmology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1389186
Purpose: To evaluate the visual and refractive outcomes of astigmatic cataract patients following opposite clear corneal incision (OCCI) combined with rotationally asymmetric multifocal intraocular lens (IOL) implantation.
Setting: Department of Ophthalmology, Zhongshan Hospital (Xiamen), Fudan University, People’s Republic of China.
Design: Retrospective cohort study.
Methods: This study comprised 58 cataract eyes of 54 patients with corneal astigmatism who underwent phacoemulsification and rotationally asymmetric multifocal IOL implantation which received either OCCI (OCCI group) or a single clear corneal incision (SCCI group). The follow-up period was 3 months after surgery. Distance, intermediate and near visual acuity, refractive outcomes, and corneal anterior keratometry were compared between the two groups. Vector analysis was used to evaluate astigmatism correction.
Results: Three months after surgery, the distance, intermediate and near visual acuity, and sphere remained comparable between the two groups, but a significant difference was detected in residual astigmatism and anterior corneal keratometric astigmatism. In the OCCI group, the residual astigmatism and keratometric astigmatism were −0.60 ± 0.29 D and 0.59 ± 0.28 D, respectively, which were lower than those in SCCI groups (−1.18 ± 0.47 D and 1.15 ± 0.45 D, both p < 0.05). In vector analysis, the difference vector (DV), angle of error (AoE), absolute AoE, index of success (IoS) and correction index (CI) were statistically significantly different between the two groups (p < 0.05).
Conclusion: OCCI combined with rotationally asymmetric multifocal intraocular lens implantation showed predictable and desirable efficacy in treating cataract patients with astigmatism.
Cataract is a leading cause of visual disability in the elderly. With the advances in the development of multifocal intraocular lenses (IOLs), the goal of contemporary cataract surgery is shifting toward achieving desirable refractive outcomes and visual quality, rather than simply restoring vision (1, 2). By generating several foci at different distances, multifocal IOLs are designed to reduce the dependence on spectacle after surgery by restoring distance, intermediate, and near vision. The traditional concentric refractive, diffractive, or a combination of refraction and diffraction multifocal IOLs, however, trade off the image quality for spectacle independence. Because the incoming light rays are split to produce 2 or more focal points, patients implanted with those IOLs can experience decreased contrast sensitivity, glare disability, halos, and other optical phenomena (3). Refractive rotationally asymmetrical multifocal IOLs have been introduced to reduce such side effects (4, 5). The design has been shown to improve visual outcomes and provide good patient satisfaction (6–8).
Corneal astigmatism is another refractive error that should be managed to achieve postoperative emmetropia and spectacle-free. We have demonstrated that nearly half of the cataract surgery candidates have corneal astigmatism ≥ 1.00 D, and the magnitude of astigmatism increases with age (9). Meanwhile, 78% of cataract patients over the age of 65 had astigmatism greater than 0.5D (10). According to the EUREQUO database, even for those whose postoperative absolute mean biometry prediction error (spherical equivalent refraction, SEQ) was no more than 0.5 D, 21.2% of them have postoperative cylinder more than 1.0 D (11). Studies of Steven and Shen provided that even low levels of residual astigmatism can degrade visual acuity (12, 13). Therefore, reliable corneal astigmatism control or correction in cataract surgery is critically important for better visual outcomes and patient satisfaction. There are various options for the reduction of corneal astigmatism during cataract surgery, including the position selection of cataract incision, peripheral corneal relaxing incisions, Toric intraocular lens implantation, intrastromal ring implantation and surface ablation (14–16).
Opposite clear corneal incision (OCCI) involves a pair of incisions in the clear cornea that flattens the corneal curvature in that meridian (17). Studies have shown, in terms of reducing postoperative astigmatism, that the procedure is effective and safe in combination with spherical or aspherical monofocal IOL implantation (18–20). However, it was not clear whether OCCI with rotationally asymmetrical multifocal IOL implantation can improve the optical and visual outcomes for cataract patients with astigmatism, thus far achieving the goal of spectacle-free.
In this study, we aim to investigate the efficacy and safety of the technique by comparing the visual and refractive outcomes of a group of cataract patients with or without OCCI during phacoemulsification and rotationally asymmetric multifocal IOL implantation.
In this study, patients received phacoemulsification and rotationally asymmetric multifocal intraocular lens implantation (Lentis Comfort LS-313 MF15, TELEON GmbH, Berlin, Germany) from 1 December 2022 to 30 April 2023 at Zhongshan Hospital (Xiamen), Fudan University were reviewed. The inclusion criteria were as follows: (1) Age 50 years or older; (2) Preoperative magnitude of manifest astigmatism ≥ 0.5D; (3) Preoperative magnitude of corneal astigmatism between 0.50 and 3.00 D; (4) Preoperative corrected distance visual acuity (CDVA) was 1.0 LogMAR or better.
The exclusion criteria were as follows: (1) patients with concomitant ophthalmic or systemic diseases affecting visual acuity, such as macular degeneration, retinal vascular diseases, glaucoma, or neurological disease; (2) missing key data on the pre- or post-operative measurement.
In total, 58 eyes of 54 patients were enrolled in this study. These patients were separated into two groups based on whether they underwent the OCCI or single clear corneal incision (SCCI) surgical procedure. The study was conducted in agreement with the tenets of the Declaration of Helsinki and was approved by the Review Board of Zhongshan Hospital (Xiamen), Fudan University (B2023-106R).
All patients underwent bio-measurement (IOL-Master 500, Carl Zeiss Meditec.AG) and had their IOL target refraction calculated by Barrett Universal II Formula preoperatively.1 Levofloxacin eye drops (Ofloxacin, Santen) were used 4 days prior to surgery. Following surgery, tobramycin dexamethasone eye drops and cream (Tobradex, Alcon) were used to provide anti-microbial and anti-inflammatory action for two weeks.
All surgeries were performed by an expert surgeon (Dr. Yao P). Proparacaine hydrochloride eye drops (Alcaine, Alcon) were administered to induce surface anesthesia. The surgical procedures differed between the two groups when performing corneal incisions, as described below.
In the OCCI group, limbal marks were made before surgery at 3 and 9 o’clock positions on corneal while patients sitting in front of the slit lamp and looking straightly ahead to avoid cyclotorsion. Patients underwent topography examination (OCULUS 77000) before surgery, and the axis position of maximum keratometry from axial map was selected as main incision position.
The main incision was performed at the crosspoint of the steep K axis and corneal limbal near the right hand (2.4 mm in width). An assisted incision was performed beside the main incision near the left hand (1 mm in width). Viscoelastic material was injected from the assisted incision to maintain the anterior-chamber depth. Continuous central circular capsulorhexis (approximately 5.5 mm in diameter) was performed. Hydrodissection and phacoemulsification were performed to remove the lens nucleus (Centurion, Alcon), followed by aspiration of the cortex. The IOL was implanted in the capsular bag through main incision using a Viscoject Bio 2.2 injector (Medicel AG, Altenrhein, Switzerland). The position of the intraocular lens (IOL) was adjusted by using a hook to ensure that the distance vision function area of the IOL was aligned with the optical axis, while the near vision function area was positioned at the sub-nasal region (Figure 1). The OCCI was made on the opposite position of the main incision at steep meridian using the same knife (2.4 mm in width). The irrigation-aspiration system was used to remove residual cortex. Finally, only the phacoemulsification incision and the assisted incision were hydrated at the end of the surgery.
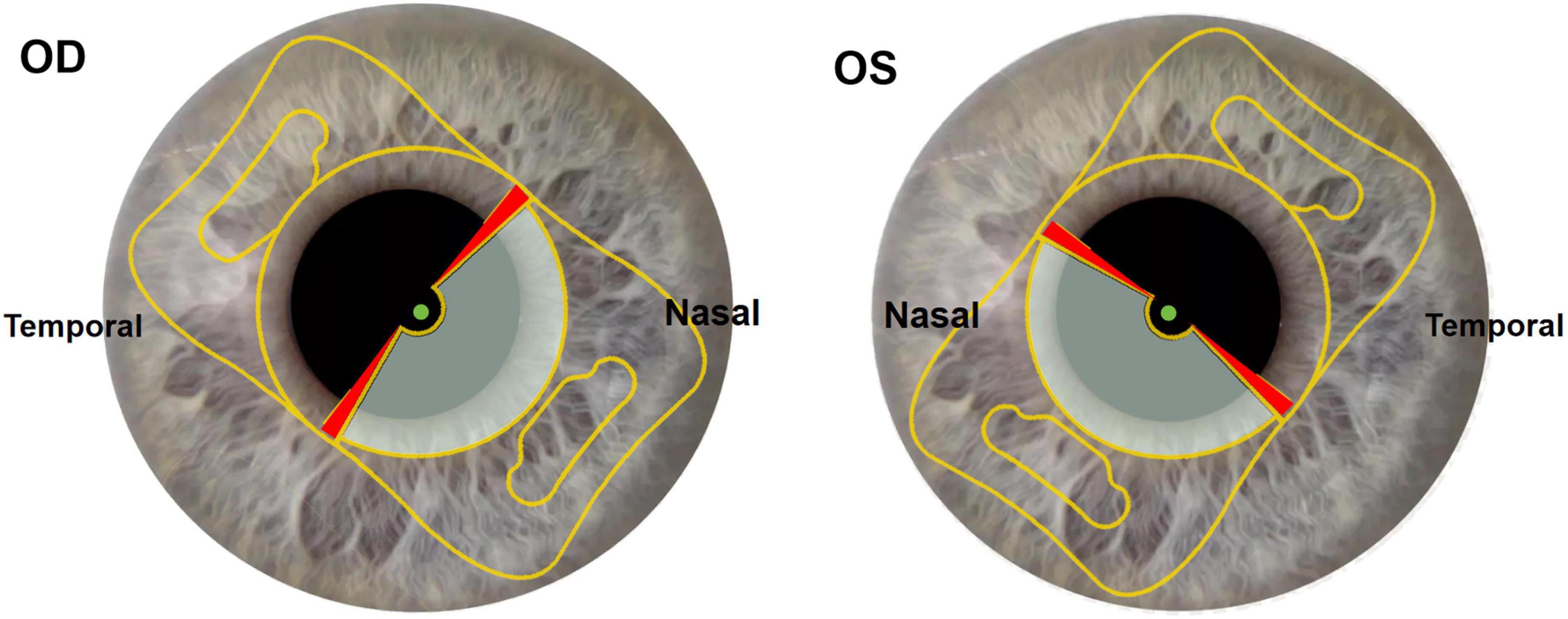
Figure 1. Slit lamp sample images of multifocal IOL implantation position. Gray part of optical zone: near vision function area. Transparent part of optical zone: distance vision function area. Red part of optical zone: transition region. Green point: intersection of visual axis and corneal anterior surface located in distance vision function area. OD: right eye. OS: left eye.
In the SCCI group, limbal marks were not required. The main and assisted incisions were made at the sites of 11 and 1 o’clock (2.4 and 1 mm in width, respectively). The remaining surgical procedures were identical to those of the OCCI group, excluding the OCCI.
Before surgery, all patients underwent measurements of corrected distance visual acuity (CDVA), manifest refraction, and keratometric astigmatism (IOL-master 500, Carl Zeiss Meditec AG). Three months post-surgery, in addition to reassessing CDVA, manifest refraction and keratometric astigmatism, we further evaluated uncorrected distance visual acuity (UDVA) and uncorrected near visual acuity (UNVA). All visual acuity outcomes were analyzed and reported in LogMAR units. The distance for near visual acuity was set at 30 cm and 60 cm. The changes of manifest refraction astigmatism and keratometric astigmatism were analyzed by using vector analysis method to assess the efficacy of astigmatism correction (21). Astigmatism was divided into three types according to cylinder axis: with the rule (WTR), against the rule (ATR) and obilque. WTR was defined as a negative cylinder from 0° to 29° or 151° to 180°, ATR between 90° ± 29° (61° and 119°), oblique between 120° and 150° and 30° and 60°. Astigmatism data was converted from the spectacle plane to the corneal plane.
Vector analysis was performed by ASSORT calculator,2 which was provided by Dr. Alpins in the official website of International Society Refractive Surgery (ISRS). The parameters of vector analysis were calculated as follows:
Target induced astigmatism (TIA): the astigmatism change that was supposed to be induced by surgery.
Surgically induced astigmatism (SIA): the astigmatism change that was induced actually by surgery.
Difference vector (DV): the vector difference of TIA and SIA.
Magnitude of error (ME): the arithmetic difference of SIA and TIA.
Angle of error (AoE): the angle difference of axis between TIA and SIA.
Correction index (CI): the ratio of SIA to TIA, this parameter could notice an astigmatic undercorrection or overcorrection when CI < 1 or > 1.
Index of success (IoS): the ratio of DV and TIA. A result of astigmatism correction was more desirable when IoS was closer to 0.
All data analysis were performed by using SPSS Statistic (version 27, IBM Corp). The normality of all data was first checked by Kolmogorov-Smirnov test. Normally distributed data was compared using the independent Student’s t-test, and non-normally distributed data was analyzed by Mann-Whitney U-test. A p-value less than 0.05 was considered statistically significant.
Fifty-four patients (58 eyes) were enrolled in this study, including 26 females (48.1%). The patients’ age was 69.8 ± 8.8 (range 51–86) years old. The SCCI group consisted of 28 patients (30 eyes), with 15 females (53.6%), and the age was 70.2 ± 7.4 (range 54–86) years old. The age of the 26 patients (28 eyes) in the OCCI group was 69.3 ± 10.1 (range 51–83) years old, with 11 females (42.3%). The age (t = −0.38, p = 0.71) and gender (X2 = 1.04, p = 0.71) were balanced across the two groups. Differences in axial length, CDVA, manifest refraction outcomes, keratometric astigmatism and scotopic pupil diameter between the two groups were comparable (Table 1). All surgeries were performed uneventfully, and no complications were observed during or after the procedure.
Three months after surgery, no complications were detected in either group. The UDVA, CDVA, 60 cm-UNVA, and 30 cm-UNVA were 0.22 ± 0.18, 0.11 ± 0.11, 0.27 ± 0.16, 0.48 ± 0.17 separately in the OCCI group, and 0.20 ± 0.10, 0.12 ± 0.10, 0.26 ± 0.68 and 0.43 ± 0.78 in the SCCI group, respectively. There was no statistically significant difference in these measures between the two groups (Table 2).
As for manifest refractive outcomes and anterior corneal curvature, the OCCI group had significantly lower postoperative manifest astigmatism (−0.60 ± 0.29 D vs. −1.18 ± 0.47 D, P < 0.01) and anterior corneal keratometric astigmatism (0.59 ± 0.28 D vs. 1.15 ± 0.45 D, P < 0.01). However, there was virtually no difference in the residual spherical refractive error between the 2 groups (Table 2).
Manifest astigmatism was analyzed by vector analysis (Table 3). The DV in the OCCI group was less than that in the SCCI group (0.59 ± 0.29 vs. 1.15 ± 0.46, p < 0.001). The AoE and absolute AoE in the OCCI group (3.17 ± 17.25° and 8.82 ± 11.33°) were also less than those in the SCCI group (−12.17 ± 34.10° and 20.52 ± 22.86°). The differences between the two groups were statistically significant (p = 0.04 and p = 0.004, respectively). The IoS in the OCCI group was less than that in the SCCI group (0.60 ± 0.22 vs. 1.10 ± 0.37, P < 0.001). However, the differences in the SIA, ME and CI between the two groups were not considered statistically significant (p = 0.17, 0.16, and 0.08, respectively).
We also analyzed the distribution of residual astigmatism of the two groups. When compared with the SCCI group, residual astigmatisms of the OCCI group were more centralized in the double angle plot (Figures 2B, D). In the OCCI group, the 95% CI of astigmatism distribution reduced noticeably after surgery, and the astigmatism axial distribution was similar to the preoperative state (Figures 2A, B). In the SCCI group, however, the 95% CI of distribution was similar to the preoperative state, and the axial distribution changed evidently after surgery (Figures 2C, D).

Figure 2. Astigmatism double angle plot, prior to and following surgery. The 95% confidence ellipse area of astigmatism distribution was similar in the OCCI group (A) and the SCCI group (C) preoperatively. Three months after surgery, the 95% confidence ellipse area of astigmatism distribution in the OCCI group (B) decreased significantly, while the SCCI group (D) remained similar to its preoperative distribution. OCCI, opposite clear corneal incision; SCCI, single clear corneal incision.
Astigmatic measures of vector analyze were shown in Figures 3A–D for OCCI group and a, b, c, d for SCCI group. In addition, we found that the slope was smaller in the OCCI group than in the SCCI group in the scatter diagram of TIA & SIA (Figures 3E, e). In the OCCI group, the angle of error was within ± 5° in 25% of eyes, and within ± 15° in 75% of eyes, compared to 18 and 39% in the SCCI group, respectively (Figures 3F, f).
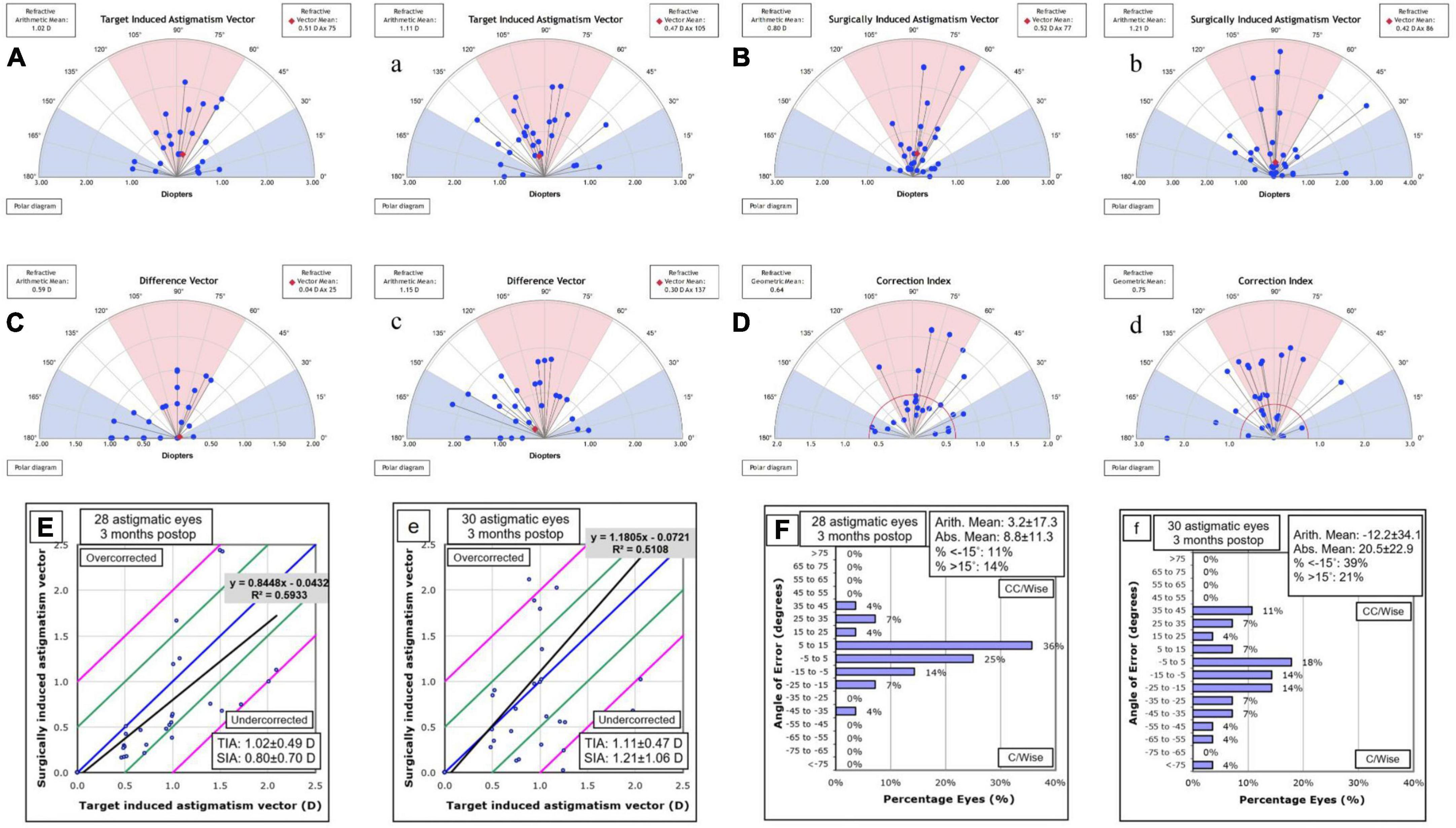
Figure 3. Vector analysis of corneal astigmatism in the OCCI group (A,B,C,D,E,F) and the SCCI group (a,b,c,d,e,f). The astigmatism axis distribution of surgically induced astigmatism (SIA) was more similar to target induced astigmatism (TIA) in the OCCI group than in the SCCI group (A,a). Additionally, the distributions of difference vector (DV) and correction index (CI) were closer to the preferred values (i.e., 0 and 1, respectively) in the OCCI group compared to the SCCI group (C,c). The scatterplots of TIA against SIA indicate that the OCCI group were more predictable than the SCCI group (E,e). As for the angle of error, it was within the range of ± 15° for 75% of eyes in the OCCI group, while it was only 39% in the SCCI group (F,f). OCCI, opposite clear corneal incision; SCCI, single clear corneal incision.
In the current study, we found that the opposite clear corneal incision (OCCI) effectively reduces residual astigmatism following multifocal IOL implantation. Patients receiving OCCI exhibited desirable visual acuity outcomes and superior refractive profiles compared to those with conventional incisions.
Modern cataract surgery strives to achieve spectacle-free vision, liberating patients from the constraints of cumbersome eyewear such as thick glasses and bifocals. This ultimately translates into enhanced comfort, convenience, and visual clarity. Correcting presbyopia and astigmatism is crucial for this objective.
The IOLs (Lentis Mplus LS-313 MF15) used in the study feature a rotational asymmetry design with a near add of +1.50 D. The design’s advantage lies in its ability to provide optimal visual outcomes at near to intermediate distances without compromising distance visual acuity, additionally exhibiting a low incidence of photic phenomena (4–8). We found that the patients in both groups had relatively good uncorrected distant, intermediate visual acuity and acceptable near vision (Table 2). This significantly reduces their reliance on spectacles. Throughout the follow-up period, no patients reported glare, halos, dysphotopsia or other postoperative optical symptoms.
In the OCCI group, placing incisions at the steep keratometry meridian significantly reduced the residual astigmatism compared to the SCCI group. Two-thirds of the eyes in the SCCI group had postoperative astigmatism exceeding 0.5 D, with one-third exceeding 1.0 D. Conversely, three-fourths of OCCI eyes had postoperative astigmatism below 0.5 D, with none exceeding 1.0 D (Figure 4). Vector analysis further support OCCI’s efficacy. Both difference vector (DV) and index of success (IoS) were statistically significantly smaller in the OCCI group, and the polar plots showed a more centralized distributions of astigmatic indices. These findings align with studies using similar incision techniques for monofocal IOLs (22–24), confirming the efficacy of corneal curvature optimized incision planning with multifocal IOLs. Combined with the astigmatism analysis, OCCI emerges as a readily available and cost-effective alternative to expensive toric multifocal IOLs for mild to moderate astigmatism correction.

Figure 4. Refractive and keratometric outcomes of the OCCI group (A,B,C) and SCCI group (a,b,c). The percentage of eyes achieving the ± 0.50 D target spherical equivalent refraction (SEQ) was 70%, and the ± 1.00 D target was 83% in the OCCI group (A), indicating greater satisfaction compared to the SCCI group (a). In the OCCI group, astigmatism decreased significantly following surgery in both manifest refractive (B) and keratometric outcomes (C), compared to the SCCI group, which showed no changes (b,c). OCCI, opposite clear corneal incision; SCCI, single clear corneal incision.
Intriguingly, the improved astigmatism correction observed with the OCCI did not translate directly into a difference in uncorrected visual acuity (UCVA) across distance, intermediate and near distances between the two groups (Table 2). This discrepancy between astigmatic and visual outcomes can be attributed to several factors.
First, while the difference in residual astigmatism between the two groups was statistically significant, it was relatively small (only around 0.5 D). Moreover, approximately 40% of the eyes in both groups exhibited with-the-rule (WTR) astigmatism postoperatively. It has been reported that cylindrical correction offers limited visual acuity improvement for WTR astigmatism when the magnitude of astigmatism is lower than −0.75 D (25). The combination of the relatively small astigmatic reduction and the mixed effects of astigmatic orientation may partly explain the comparable visual outcomes.
Second, patients with multifocal IOLs demonstrate some tolerance for residual astigmatism (12). And it is particularly true for low levels of astigmatism. Hayashi observed that when astigmatism was less than 1.0 D, eyes implanted with a multifocal IOLs performed better in terms of the visual acuity at both distance and near (26). Additionally, studies have suggested that a lower IOL addition shows better astigmatic defocus tolerability (27, 28). The optical design of IOLs may also influence astigmatic tolerance. McNeely reported high tolerance for residual astigmatism with the Mplus LS-312 MF30, which shares the rotationally asymmetric multifocal design as the MF15 IOL used in the current study, albeit with a higher IOL addition (29).
Third, the current study only measured visual acuity as visual outcomes, which may not fully capture the impact of astigmatism on other aspects of visual performance, such as contrast sensitivity, functional vision, and patient satisfaction (12, 26, 29). Other factors can also affect visual acuity after multifocal IOL implantation, including high order aberrations (30). This may further explain why a significant difference was observed in astigmatism correction but not in visual acuity. Further studies examining these parameters are warranted to fully evaluate the relationship between residual astigmatism and overall visual wellbeing in patients with multifocal IOLs.
The limitations of this study should also be mentioned here. Firstly, due to the retrospective non-randomized design, although the baseline characteristics were balanced between the two groups, potential confounders cannot be fully controlled for. Secondly, only anterior corneal curvature was assessed in the study. The posterior curvature is also an important component of corneal refraction. However, the keratometric astigmatism was consistent with the manifest refractive measurements, both prior to and following surgery. Thirdly, as mentioned earlier, only the visual acuity and refractive outcomes were measured and analyzed in the present study, in addition to the relatively shorter follow-up, further studies should be conducted to include other visual-related measurements, such as contrast sensitivity, wavefront parameters, and patient satisfaction or scales of vision-related quality of life.
In conclusion, the current study confirms that incisions placing at steep K meridian can safely reduce residual astigmatism after cataract surgery. Corneal curvature optimized incision planning, specifically opposite clear corneal incision, combined with rotationally asymmetric multifocal intraocular lens implantation provides optimal visual and refractive outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Review Board of Zhongshan Hospital (Xiamen), Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XQ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. XW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. YW: Formal analysis, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing. YH: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. ZC: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. YY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Xiamen Municipal Natural Science Foundation (No.3502Z20227277). It was also supported by the Science and Technology Project of Xiamen (No.3502Z20224ZD1076).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rosen E, Ali JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J Cataract Refract Surg. (2016) 42:310–28. doi: 10.1016/j.jcrs.2016.01.014
2. Mencucci R, Favuzza E, Ribeiro F. Editorial: Addressing the unmet needs of cataract patients: When quality of vision can make the difference in quality of life. Front Med. (2023) 10:1232243. doi: 10.3389/fmed.2023.1232243
3. Alio JL, Plaza-Puche AB, Javaloy J, Ayala M, Moreno LJ, Piñero DP. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens. Ophthalmology. (2012) 119:555–63. doi: 10.1016/j.ophtha.2011.08.036
4. Ali JL, Plaza-Puche AB, Piñero DP, Javaloy J, Ayala MJ. Comparative analysis of the clinical outcomes with 2 multifocal intraocular lens models with rotational asymmetry. J Cataract Refract Surg. (2011) 37:1605–14. doi: 10.1016/j.jcrs.2011.03.054
5. Ali JL, Piñero DP, Plaza-Puche AB, Chan MJR. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. (2011) 37:241–50. doi: 10.1016/j.jcrs.2010.08.043
6. Berrow EJ, Wolffsohn JS, Bilkhu PS, Dhallu S. Visual performance of a new bi-aspheric, segmented, asymmetric multifocal IOL. J Refract Surg. (2014) 30:584–8. doi: 10.3928/1081597X-20140814-01
7. Vargas V, Ferreira R, Barrio JLAD, Ali JL. Visual outcomes, patient satisfaction, and light distortion analysis after blended implantation of rotationally asymmetric multifocal intraocular lenses. J Refract Surg. (2020) 36:796–803. doi: 10.3928/1081597X-20200902-01
8. Oshika T, Arai H, Inoue Y, Fujita Y. Five-year clinical outcomes of low-add-power segmented rotationally asymmetrical intraocular lens. Ophthalmol Ther. (2023) 12:1649–56. doi: 10.1007/s40123-023-00703-2
9. Guan Z, Yuan F, Yuan YZ, Niu WR. Analysis of corneal astigmatism in cataract surgery candidates at a teaching hospital in Shanghai, China. J Cataract Refract Surg. (2012) 38:1970–7. doi: 10.1016/j.jcrs.2012.07.025
10. Day AC, Dhariwal M, Keith MS, Ender F, Vives CP, Miglio C, et al. Distribution of preoperative and postoperative astigmatism in a large population of patients undergoing cataract surgery in the UK. Br J Ophthalmol. (2019) 103:993–1000. doi: 10.1136/bjophthalmol-2018-312025
11. Lundström M, Dickman M, Henry Y, Manning S, Tassignon MJ, Young D, et al. Risk factors for refractive error after cataract surgery: Analysis of 282 811 cataract extractions reported to the European registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. (2018) 44:447–52. doi: 10.1016/j.jcrs.2018.01.031
12. Schallhorn SC, Hettinger KA, Pelouskova M, Teenan D, Venter JA, Hannan SJ, et al. Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J Cataract Refract Surg. (2021) 47:991–8.
13. Shen W, Zhuo B, Zhang L, Shen J, Ma D, Yang J. Effect of astigmatism on visual outcomes after multifocal intraocular lens implantation: A systematic review and meta-analysis. Front Med. (2023) 10:1214714. doi: 10.3389/fmed.2023.1214714
14. Rubenstein JB, Raciti M. Approaches to corneal astigmatism in cataract surgery. Curr Opin Ophthalmol. (2013) 24:30–4.
15. Rocha-de-Lossada C, Rodríguez-Vallejo M, Rodríguez-Calvo-de-Mora M, Ribeiro FJ, Fernández J. Managing low corneal astigmatism in patients with presbyopia correcting intraocular lenses: A narrative review. BMC Ophthalmol. (2023) 23:254. doi: 10.1186/s12886-023-03003-2
16. D’Oria F, Alio JL, Martinez-Abad A, Luis I, Pablo L, Abdelghany AA. Astigmatic change as a predictor of intrastromal corneal ring segment late extrusion. J Cataract Refract Surg. (2022) 48:401–7. doi: 10.1097/j.jcrs.0000000000000774
18. Mendicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer-Blasco T, Montés-Mic R. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. (2009) 35:451–8.
19. Ren Y, Fang X, Fang A, Wang L, Jhanji V, Gong X. Phacoemulsification with 3.0 and 2.0 mm opposite clear corneal incisions for correction of corneal astigmatism. Cornea. (2019) 38:1105–10.
20. Tadros A, Habib M, Tejwani D, Lany H, Thomas P. Opposite clear corneal incisions on the steep meridian in phacoemulsification: Early effects on the cornea. J Cataract Refract Surg. (2004) 30:414–7. doi: 10.1016/S0886-3350(03)00649-7
22. Binayi Faal N, Ojaghi H, Sadeghieh Ahari S. Paired opposite 4 mm clear corneal incisions on steep meridian during phacoemulsification. J Curr Ophthalmol. (2021) 33:400–7. doi: 10.4103/joco.joco_205_20
23. Razmjoo H, Koosha N, Vaezi MH, Rahimi B, Peyman A. Corneal astigmatism change and wavefront aberration evaluation after cataract surgery: “Single” versus “paired opposite” clear corneal incisions. Adv Biomed Res. (2014) 3:163. doi: 10.4103/2277-9175.139126
24. Vats S, Kumari L, Goenka R, Agrawal M, Mishra S. Pattern of astigmatism using partial coherence interferometry in patients of different age groups undergoing cataract surgery. Oman J Ophthalmol. (2022) 15:295–8. doi: 10.4103/ojo.ojo_345_21
25. Tan QQ, Wen BW, Liao X, Tian J, Lin J, Lan CJ. Optical quality in low astigmatic eyes with or without cylindrical correction. Graefes Arch Clin Exp Ophthalmol. (2020) 258:451–8.
26. Hayashi K, Hayashi H, Nakao F, Hayashi F. Influence of astigmatism on multifocal and monofocal intraocular lenses. Am J Ophthalmol. (2000) 130:477–82.
27. Altinkurt E, Muftuoglu O. Comparison of three different diffractıve multifocal intraocular lenses with a +2.5, +3.0, and +3.75 diopter additıon power. Saudi J Ophthalmol. (2019) 33:353–62. doi: 10.1016/j.sjopt.2019.09.007
28. Carones F. Residual astigmatism threshold and patient satisfaction with bifocal, trifocal and extended range of vision intraocular lenses (IOLs). Open J Ophthalmol. (2017) 7:1–7.
29. McNeely RN, Pazo E, Millar Z, Richoz O, Nesbit A, Moore TCB, et al. Threshold limit of postoperative astigmatism for patient satisfaction after refractive lens exchange and multifocal intraocular lens implantation. J Cataract Refract Surg. (2016) 42:1126–34. doi: 10.1016/j.jcrs.2016.05.007
Keywords: corneal curvature, astigmatism, cataract surgery, incision position, multifocal intraocular lens
Citation: Qin X, Yao P, Wu X, Wu Y, Hong Y, Chen Z and Yuan Y (2024) Visual and refractive outcomes of opposite clear corneal incision combined with rotationally asymmetric multifocal intraocular lens implantation. Front. Med. 11:1389186. doi: 10.3389/fmed.2024.1389186
Received: 21 February 2024; Accepted: 14 June 2024;
Published: 28 June 2024.
Edited by:
Kazutaka Kamiya, Kitasato University, JapanReviewed by:
Francesco D’Oria, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, ItalyCopyright © 2024 Qin, Yao, Wu, Wu, Hong, Chen and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanzhi Yuan, eXVhbi55dWFuemhpQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.