
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med., 11 April 2024
Sec. Family Medicine and Primary Care
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1387935
This article is part of the Research TopicGlobal Advances in the Diagnosis, Management, and Treatment of Low Back PainView all 19 articles
Background: Spinal anesthesia (SA) is a good alternative to general anesthesia (GA) for spine surgery. Despite that, a few case series concern the use of thoracic spinal anesthesia for short-duration surgical interventions. In search of an alternative approach to GA and a better opioid-free modality, we aimed to investigate the safety, feasibility, and patient satisfaction of thoracic SA for spine surgery.
Materials and methods: We analyzed retrospectively a cohort of 24 patients operated on for a degenerative and osteoporotic pathology of the lower thoracic and lumbar spine. Data was collected from medical records, including clinical notes, operative and anesthesia records, and patient questionnaires.
Results: Twenty-one surgeries for herniated discs, two for degenerative spinal stenosis, and one for multi-level osteoporotic vertebral body fractures were performed under spinal anesthesia with intrathecal sedation. In all cases, we applied 0.5% isobaric bupivacaine and the following adjuvants: midazolam, clonidine or dexmedetomidine, and dexamethasone. We boosted the anesthesia with local ropivacaine due to inefficient sensory block in two patients. Nobody in the cohort received intravenous opioids, non-steroidal anti-inflammatory drugs, or additional sedation intraoperatively. Postoperative painkillers were upon the patient’s request. No significant complications were detected.
Conclusion: Thoracic spinal anesthesia incorporating adjuvants such as midazolam, clonidine or dexmedetomidine, and dexamethasone demonstrates not only efficient conditions for spine surgery, a favorable safety profile, high patient satisfaction, and intrathecal sedation but also effective opioid-free pain management.
Numerous studies have confirmed that spinal anesthesia (SA) is a good alternative to general (GA) for lower spine surgeries. It demonstrates a low level of intra- and postoperative complications, including cognitive impact in at-risk patients, and better postoperative pain management with reduced anti-inflammatory drugs and opioid utilization. Additionally, the SA is associated with decreased operative duration, time to ambulation, length of hospitalization, and costs compared to GA (1–5).
Anesthetic procedures at the thoracic and upper lumbar segment are far less common but are expected to offer similar advantages. The literature concerning the use of thoracic spinal anesthesia with intrathecal sedation for lumbar spine surgery is scarce. Only a few case reports and series with limited subjects have recently been published, and a widely accepted protocol is missing (6–8). Some clinicians have voiced concern about an increased risk of neurological deficits from injuring the spinal cord and difficulty in getting intrathecal access to perform spinal anesthesia in patients with degenerative vertebral pathology, especially with segmental vertebral deformities. However, some authors present results without an increased rate of complications (8, 9).
The aim of this study is to evaluate the feasibility, safety, patient satisfaction, and opioid-sparing potential of thoracic spinal anesthesia with intrathecal sedation for spine surgery.
All procedures discussed in this retrospective cohort study were conducted between March 2022 and December 2023 in the Clinic of Neurosurgery at St. Ivan Rilski University Hospital, Sofia, Bulgaria, a tertiary care facility for spinal and neurosurgical intervention. This work fulfills the STROBE checklist for reporting cohort observational studies. We analyzed a cohort of 24 patients operated on for a degenerative and osteoporotic pathology of the lower thoracic and lumbar spine.
Briefly, all patients received spinal anesthesia through a routine single-shot technique with a 22G Quincke needle in a sitting position. After identifying the intervertebral space by anatomical landmarks, 2 cm of the spinal needle was inserted by a paramedian approach. Any further insertion was performed with caution until bony contact with vertebral lamina. The spinal needle was then redirected and further advanced by 2–3 mm increments. After each advancement a check for cerebral spinal fluid backflow was performed. Once the needle was in the intrathecal space, 0.5% isobaric bupivacaine solution was applied in the range of 10–15 mg. Adjuvants, including an α-2 agonist (clonidine 10–20 mcg or dexmedetomidine 10–15 mcg), midazolam (2–3 mg), and dexamethasone (4 mg), were administered. Patients were then placed supine till the sensory block fixation and then in lateral decubitus or prone position for surgery. The level of puncture was verified by C-arm. No urethral catheters were inserted.
Postoperative pain management consisted of non-steroidal anti-inflammatory drugs (NSAID) on demand, including 1 g of paracetamol or 50 mg of dexketoprofen. Opioids were given if sufficient analgesia wasn’t achieved with the previous.
We used the Ramsay Sedation Scale (RSS) as a tool to evaluate the intraoperative level of consciousness, Table 1 (10). Pain intensity was assessed by the Visual Analogue Scale (VAS) presented by a straight line with points ranging from 0 (“no pain at all”) to 10 (“the worst possible pain”). It was measured at the 6th and 24th hour after the puncture for SA. Information about the level of patient satisfaction was retrieved from specific questionnaires designed by our group and given to all patients who underwent surgery under loco- regional anesthesia on the day of hospital discharge. The questionnaires included 3 questions, each with three possible answers, Table 2. Every patient with a sum of fewer than 7 points was considered satisfied, whereas we accepted a result of 7 as borderline.
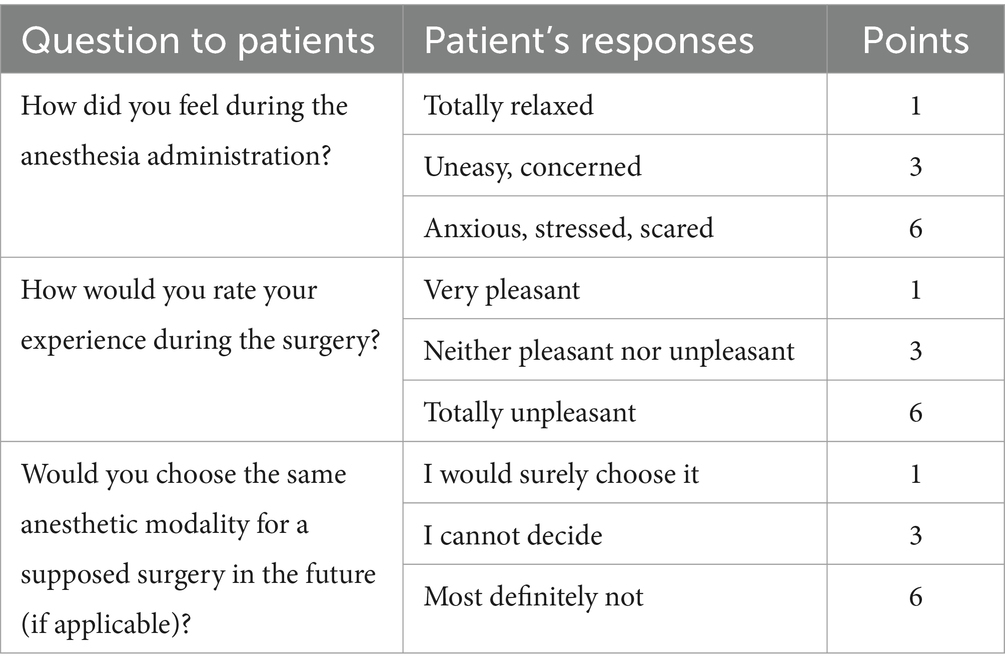
Table 2. Patient satisfaction questionnaire, designed by our group, consists of 3 questions with three answers each.
Procedural time, puncture level, drug amounts, sensory blockade and sedation levels, patient and surgeon satisfaction, and postoperative usage of painkillers were analyzed for each case. Data was collected from medical records, including clinical notes, operative and anesthesia records and questionnaires.
The study cohort included 24 patients (11 females and 13 males) with a mean age of 49.6 years (range 21–88 years). All patients were grade I or II according to the physical status classification system of the American Society of Anesthesiologists (ASA). They suffered from disc herniations at the lumbar level, except two with degenerative spinal stenosis and one with multi-level osteoporotic compression vertebral fractures at the thoracolumbar junction. Patients’ data and details regarding the surgical intervention, spinal anesthesia, and early clinical outcome are extensively presented in Table 3.
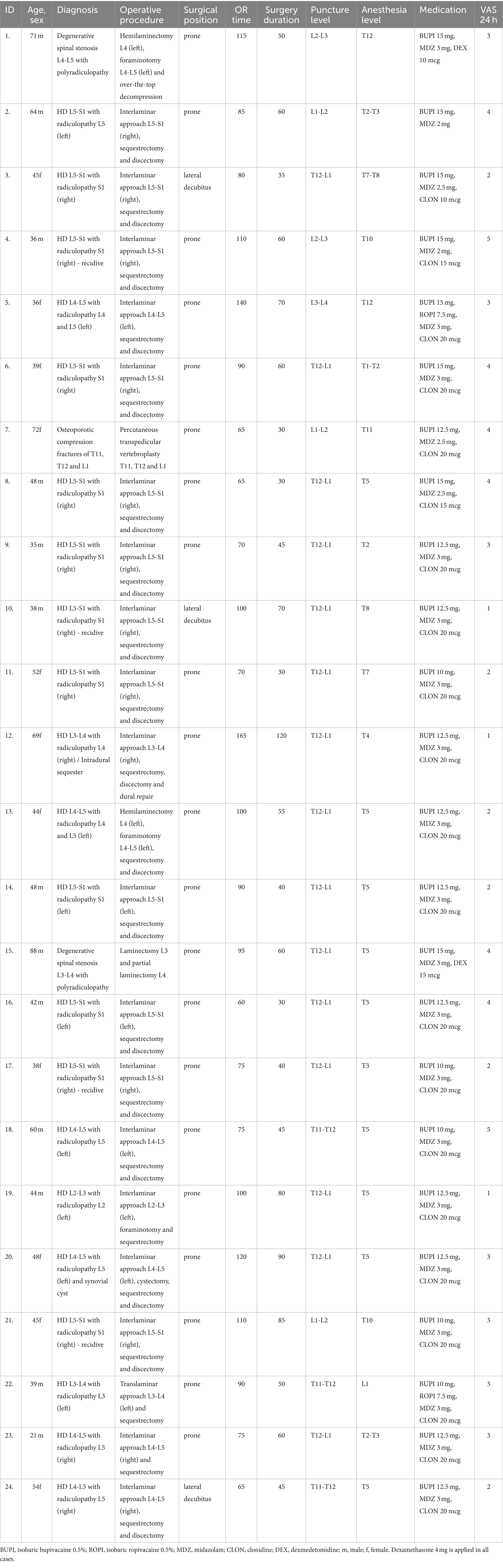
Table 3. Patients’ data and details regarding the surgical intervention, spinal anesthesia, and early clinical outcome are.
The mean time spent by the patient in the operating room was 92 min (range 60–165 min, SD ± 26 min), and the surgical duration was 56 min (range 30–120 min, SD ± 22 min). In six cases, the point of access was at or below the L1-L2 level, whereas all the remaining dural punctures were in the T11-L1 segment, with the T12-L1 level being the most common in 15 procedures. The drug amounts were adjusted individually based on the puncture level, patient demographics, and comorbidities. We applied up to 12.5 mg of bupivacaine above L1-L2 with a single-shot technique and 15 mg at lower access points. Two patients (ID No 5 and 22) required an additional local application of 7.5 mg of ropivacaine due to an inefficient sensory block. In all cases, the spinal anesthesia was successful. Sedation, lasting approximately 45 min, was achieved at levels between 2 and 3 according to the Ramsay Sedation Scale in all cases. Nobody in the cohort received opioids, NSAIDs, or additional intravenous sedation intraoperatively.
Hemodynamic stability was maintained throughout the whole period of anesthesia, with a mean drop of the systolic blood pressure of 28 mmHg (range 10–50 mmHg, SD ± 19 mmHg). The mean drop of mean arterial pressure (MAP) was 15 mmHg (range 0–43 mmHg, SD ± 14 mmHg), corresponding to 18.1% (range 0–38.2%, SD ± 18.3%). One patient (ID No: 15, 88 year-old, degenerative spinal stenosis with laminectomy) developed a drop of MAP of 38.2% which required the use of a vasopressor (10 mg ephedrine intravenously).
Four patients had 6, fifteen had 5, five had 3, and two had 7 points on patient satisfaction scores assessed by our proprietary questionnaire. Thus, the satisfaction rate was 91.7%. The rest were borderline. Twenty patients reported that they would choose the same anesthetic modality in the future, whereas four could not decide. All patients reported an overall positive experience in the operating room.
The median reported VAS score both at 6th post-puncture hour was 2 (range 1–3) and 24th hour was 3 (range 1–5). Twenty-one out of 24 patients reported the need for postoperative analgesia with an NSAID. In all of them it occurred in the morning of surgery and during movement. In none of the cases opioids were required. All patients were ambulated on the same day and were discharged on postoperative days between 1 and 3.
The surgical conditions evaluated by the operator were optimal in all performed interventions, further supporting the feasibility of this technique. No intraoperative liquorrhea related to the spinal anesthesia was evident. No transient or permanent neurologic deficit was registered after dissipation of the sensory blockade. One patient developed transient urinary retention and a globus vesicalis, which was resolved after the insertion of a urinary catheter. No major complications related to the anesthesia or surgery were observed.
It is believed that spinal anesthesia is unsuitable and even contraindicated for patients with pathology of the spine mainly because of the normal anatomy compromise and the unpredictability of the local anesthetic spread. In this article, we present a cohort of 24 patients who underwent spine surgery for degenerative disorders and osteoporotic fractures under SA. The anesthesia was successful in all cases without major surgical or procedural complications.
Nevertheless, spinal anesthesia has been used for vertebral surgery, and large numbers of patients were treated, but dural punctures were typically performed at the lumbar spine (4, 5, 11–13). On the one hand, as Saifuddin et al. noted, the location of conus medullaris in a large adult population was shown to range from the middle third of T12 to the upper third of L3, mean at the lower third of L1 (14), which is a zone of risk for any interventions. On the other hand, Duniec et al. reported that the concordance rate between clinical examination and using assessment of level identification for the lumbar puncture is 64% among patients undergoing spinal anesthesia for lower limb surgery (15). Because of the uncertain and insufficient coverage of the sensory blockade in the cranial direction for interventions at the lumbar spine, we adopted the lower thoracic dural puncture technique. Our data shows that the difference in only one level of puncture (L1-L2 compared to T12-L1) provides a significant increase (5 dermatome levels) of local anesthetic spread without increasing the risk of conus medullaris injury. Using our protocol as described, the somatosensory block consistently reached a level between T2 and T7 (mean at T5 dermatome) for access points at T12-L1 and above. The lower puncture sites achieved a level up to T10, which was insufficient for completely anesthetizing the skin in the upper border of the surgical incision. Thus, it mandates the need for supplemental local anesthetic skin infiltration by the surgeon. The observed sensory block patterns suggest that the spread of the anesthesia correlates with the level of puncture rather than the concentration and volume of the local anesthetic used.
The use of intrathecal sedation with midazolam and an α-2 agonist (either clonidine or dexmedetomidine) not only mitigated their hemodynamic and respiratory drive suppression effects, compared to when applied intravenously but also provided patient comfort during the procedure (7). This approach offers better hemodynamic stability than traditional SA without adjuvants with a lesser mean drop of MAP. The last provides an opportunity for its use in the elderly or comorbid patients. Vital signs are more stable than when emerging from GA and during the immediate postoperative period, which may be beneficial for patients with severe cardiac illness (16, 17). We confirm these findings with only one case at the age of 88 with a temporary and not clinically significant drop of MAP.
Importantly, none of the patients in our cohort required any additional sedation different from the described. Furthermore, no intra-procedural opioids were administered for pain management, indicating adequate analgesia without the need for traditional opioid-based approaches and even the use of NSAIDs. In our study, a good level of sedation lasted approximately 45 min. All patients reported an overall positive experience during surgery and an excellent satisfaction rate. This observation is supported by other authors using both benzodiazepines and dexmedetomidine (17).
Few articles present patient and surgeon satisfaction when comparing SA to GA (18, 19). We carefully prepared our patient satisfaction questionnaire to provide insight into the overall patient experience with the modality and compare pre- and postoperative patient comfort. The procedures were explained in great detail, and directions were given to all the patients. They were instructed to signal the anesthetist or the surgeon if any discomfort occurred because of stress, fear, pain, body position, etc. None of the patients had any of the mentioned complaints. To note, despite being lightly sedated, they responded well to commands and were cooperative overall. No involuntary movements were observed, which can create difficulties for the surgeon working under magnification.
In our study, all surgeries were performed by the same team. The operators were asked to evaluate the surgical conditions in terms of ease of obtaining the surgical field, patient positioning, operative room stay, and the feasibility of the intervention. In contrast with Sadrolsadat et al. (20) study, which showed SA had no advantages over GA, our surgical team evaluated the conditions as optimal. This confers with the findings of McLain et al. (21) with a focus on easier patient positioning, shorter operative room stay, and better facility management than with GA to further support the spinal anesthesia feasibility.
As many authors advocate, we also support the opioid-free options for anesthesia in spine surgery (22). No intrathecal or intravenous opioids were used in our cohort, and the postoperative painkillers were on demand. Patients were instructed to demand medications if pain level rises above VAS score 4 or discomfort is high. The staff was instructed to be vigilant about subjects requiring additional analgesia and/or complaining of insufficiency of analgesia by NSAIDs and the need for opioids. Out of the protocol, the patients were also asked at discharge to describe when and how the highest level of pain occurred, with the majority reporting pain at the surgical skin incision, only when moving, and in the following morning after the procedure, not exceeding VAS score 5. Adequate analgesia was achieved in all cases only with NSAIDs, while four patients did not need any painkillers.
Early ambulation was achieved in all 24 patients without any complications or neurologic deficits, which again highlights the safety and efficacy of thoracic spinal anesthesia with intrathecal sedation. We could not find any other study investigating these circumstances. In none of the patients, a urinary catheter was inserted before surgery, and fluid administration was cautious. Nevertheless, one patient (female, 34 years) developed a globus vesicalis, which was treated successfully, and no micturition disturbances were reported.
While our study provides valuable insights, certain limitations should be acknowledged. The relatively small sample size and the absence of a control group warrant caution in generalizing the results to broader patient populations. All patients being ASA I-II limits the findings to patients without severe comorbidities. However, it would be specifically appropriate for the high-risk groups. Therefore, further research is needed to explore the applicability and safety of thoracic spinal anesthesia in patients with more significant health challenges. Building on the positive outcomes observed in this study, future research should consider prospective trials with larger sample sizes to validate further the safety, efficacy, and cost-effectiveness of thoracic spinal anesthesia. Exploring the long-term effects, particularly concerning postoperative recovery and complications, would contribute to a more comprehensive understanding of its applicability in diverse clinical scenarios.
Thoracic spinal anesthesia incorporating adjuvants such as midazolam, clonidine or dexmedetomidine, and dexamethasone demonstrates not only efficient conditions for spine surgery, a favorable safety profile, high patient satisfaction, and intrathecal sedation but also effective opioid-free pain management. Thus, our findings imply that this is an appropriate alternative to the general anesthesia for spine surgery. Future research should further investigate and validate the potential of the technique, including its cost-effectiveness, and explore the optimal surgical and pain management strategies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
NB: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. DF: Formal analysis, Project administration, Resources, Supervision, Visualization, Writing – review & editing. PV: Investigation, Project administration, Resources, Writing – original draft. DY: Formal analysis, Resources, Supervision, Writing – review & editing. SB: Investigation, Resources, Validation, Writing – original draft. RT: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sykes, DAW, Tabarestani, TQ, Chaudhry, NS, Salven, DS, Shaffrey, CI, Michael Bullock, W, et al. Awake spinal fusion is associated with reduced length of stay, opioid use, and time to ambulation compared to general anesthesia: a matched cohort study. World Neurosurg. (2023) 176:e91–e100. doi: 10.1016/j.wneu.2023.05.001
2. Finsterwald, M, Muster, M, Farshad, M, Saporito, A, Brada, M, and Aguirre, JA. Spinal versus general anesthesia for lumbar spine surgery in high risk patients: perioperative hemodynamic stability, complications and costs. J Clin Anesth. (2018) 46:3–7. doi: 10.1016/j.jclinane.2018.01.004
3. Khattab, MFM, Sykes, DAW, Abd-El-Barr, MM, Waguia, R, Montaser, A, Ghamry, SE, et al. Spine surgery under awake spinal anesthesia: an Egyptian experience during the COVID-19 pandemic. Neurosurg Focus. (2021) 51:E6. doi: 10.3171/2021.9.FOCUS21456
4. Lessing, NL, Edwards, CC, Dean, CL, Waxter, OH, Lin, C, Curto, RA, et al. Spinal anesthesia for geriatric lumbar spine surgery: a comparative case series. Int J Spine Surg. (2020) 14:713–21. doi: 10.14444/7103
5. Sekerak, R, Mostafa, E, Morris, MT, Nessim, A, Vira, A, and Sharan, A. Comparative outcome analysis of spinal anesthesia versus general anesthesia in lumbar fusion surgery. J Clin Orthop Trauma. (2021) 13:122–6. doi: 10.1016/j.jcot.2020.11.017
6. Jang, I, Shin, IW, Ok, SH, Park, KE, Sohn, JT, Lee, HK, et al. Spinal anesthesia and intrathecal clonidine decrease the hypnotic requirement of Propofol. Reg Anesth Pain Med. (2010) 35:145–7. doi: 10.1097/AAP.0b013e3181c75c05
7. Vincenzi, P, Stronati, M, Isidori, P, Iuorio, S, Gaudenzi, D, Boccoli, G, et al. Opioid-free segmental thoracic spinal anesthesia with intrathecal sedation for breast and axillary surgery: report of four cases. Local Reg Anesth. (2022) 15:23–9. doi: 10.2147/LRA.S358157
8. Ozyurt, G, Basagan-Mogol, E, Bilgin, H, and Tokat, O. Spinal anesthesia in a patient with severe thoracolumbar kyphoscoliosis. Tohoku J Exp Med. (2005) 207:239–42. doi: 10.1620/tjem.207.239
9. Aissaoui, Y, Bahi, M, El Khader, A, El Barni, R, and Belhadj, A. Thoracic spinal anaesthesia for abdominal surgery in a humanitarian military field hospital: a prospective observational study. BMJ Mil Health. (2024) 170:26–30. doi: 10.1136/bmjmilitary-2022-002075
10. Ramsay, MA, Savege, TM, Simpson, BR, and Goodwin, R. Controlled sedation with alphaxalone-alphadolone. Br Med J. (1974) 2:656–9.
11. Tetzlaff, JE, Dilger, JA, Kodsy, M, al-Bataineh, J, Yoon, HJ, and Bell, GR. Spinal anesthesia for elective lumbar spine surgery. J Clin Anesth. (1998) 10:666–9. doi: 10.1016/S0952-8180(98)00112-3
12. Pierce, JT, Kositratna, G, Attiah, MA, Kallan, MJ, Koenigsberg, R, Syre, P, et al. Efficiency of spinal anesthesia versus general anesthesia for lumbar spinal surgery: a retrospective analysis of 544 patients. Local Reg Anesth. (2017) 10:91–8. doi: 10.2147/LRA.S141233
13. Sarkar, S, Banerji, A, Chattopadhyaya, A, and Banerjee, S. Lumbar spine instrumented fusion surgery under spinal anaesthesia versus general anaesthesia-a retrospective study of 239 cases. J Clin Orthop Trauma. (2021) 18:205–8. doi: 10.1016/j.jcot.2021.04.026
14. Saifuddin, A, Burnett, SJ, and White, J. The variation of position of the conus medullaris in an adult population. A magnetic resonance imaging study. Spine. (1998) 23:1452–6. doi: 10.1097/00007632-199807010-00005
15. Duniec, L, Nowakowski, P, Kosson, D, and Łazowski, T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. (2013) 45:1–6. doi: 10.5603/AIT.2013.0001
16. Kahveci, K, Doger, C, Ornek, D, Gokcinar, D, Aydemir, S, and Ozay, R. Perioperative outcome and cost-effectiveness of spinal versus general anesthesia for lumbar spine surgery. Neurol Neurochir Pol. (2014) 48:167–73. doi: 10.1016/j.pjnns.2014.05.005
17. Kim, H, Kim, Y, Bae, J, Yoo, S, Lim, YJ, and Kim, JT. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg Anesth Pain Med. (2024) 49:110–6. doi: 10.1136/rapm-2023-104415
18. Zorrilla-Vaca, A, Healy, RJ, and Mirski, MA. Patient, surgeon, and anesthesiologist satisfaction: who has the priority? J Neurosurg Anesthesiol. (2018) 30:191–3. doi: 10.1097/ANA.0000000000000488
19. Capdevila, X, Aveline, C, Delaunay, L, Bouaziz, H, Zetlaoui, P, Choquet, O, et al. Factors determining the choice of spinal versus general anesthesia in patients undergoing ambulatory surgery: results of a multicenter observational study. Adv Ther. (2020) 37:527–40. doi: 10.1007/s12325-019-01171-6
20. Sadrolsadat, SH, Mahdavi, AR, Moharari, RS, Khajavi, MR, Khashayar, P, Najafi, A, et al. A prospective randomized trial comparing the technique of spinal and general anesthesia for lumbar disk surgery: a study of 100 cases. Surg Neurol. (2009) 71:60–5. doi: 10.1016/j.surneu.2008.08.003
21. McLain, RF, Kalfas, I, Bell, GR, Tetzlaff, JE, Yoon, HJ, and Rana, M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: a case-controlled analysis of 400 patients. J Neurosurg Spine. (2005) 2:17–22. doi: 10.3171/spi.2005.2.1.0017
Keywords: thoracic spinal anesthesia, intrathecal midazolam, intrathecal clonidine, intrathecal dexmedetomidine, intrathecal sedation, spine surgery
Citation: Boykov N, Ferdinandov D, Vasileva P, Yankov D, Burev S and Tanova R (2024) Thoracic spinal anesthesia with intrathecal sedation for lower back surgery: a retrospective cohort study. Front. Med. 11:1387935. doi: 10.3389/fmed.2024.1387935
Received: 18 February 2024; Accepted: 29 March 2024;
Published: 11 April 2024.
Edited by:
Plamen Todorov Todorov, Plovdiv Medical University, BulgariaReviewed by:
Yavor Enchev, Medical University of Varna, BulgariaCopyright © 2024 Boykov, Ferdinandov, Vasileva, Yankov, Burev and Tanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilyan Ferdinandov, ZmVyZGluYW5kb3ZAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.