- 1Lübeck Institute of Experimental Dermatology, University of Lübeck, Lübeck, Germany
- 2Department of Dermatology, University Hospital Schleswig-Holstein Lübeck, Lübeck, Germany
- 3Independent Researcher, Groß Grönau, Germany
- 4Institute and Comprehensive Centre for Inflammation Medicine, University-Hospital Schleswig-Holstein, Lübeck, Germany
- 5Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel
- 6Unit of Dermatology and Skin Research Laboratory, Barch Padeh Medical Center, Poriya, Israel
Objectives: This study investigated psoriatic arthritis (PsA) risk across varied psoriasis manifestations, considering sex and ethnicity.
Methods: Using TriNetX, a federated database encompassing over 120 million electronic health records (EHRs), we performed global retrospective cohort studies. Psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), and generalized pustular psoriasis (GPP) cohorts were retrieved using ICD-10 codes. Propensity score matching, incorporating age, sex, and ethnicity, was employed. An alternative propensity matching model additionally included established PsA risk factors.
Results: We retrieved data from 486 (Black or African American-stratified, GPP) to 35,281 (Pso) EHRs from the US Collaborative Network. Significant PsA risk variations emerged: Pso carried the highest risk [hazard ratio (HR) 87.7, confidence interval (CI) 63.4–121.1, p < 0.001], followed by GPP (HR 26.8, CI 6.5–110.1, p < 0.0001), and PPP (HR 15.3, CI 7.9–29.5, p < 0.0001). Moreover, we identified significant sex- and ethnicity-specific disparities in PsA development. For instance, compared to male Pso patients, female Pso patients had an elevated PsA risk (HR 1.1, CI 1.1–1.2, p = 0.002). Furthermore, White Pso patients had a higher likelihood of developing PsA compared to their Black or African American counterparts (HR 1.3, CI 1.04–1.7, p = 0.0244). We validated key findings using alternative propensity matching strategies and independent databases.
Conclusion: This study delineates nuanced PsA risk profiles across psoriasis forms, highlighting the pivotal roles of sex and ethnicity. Integrating these factors into PsA risk assessments enables tailored monitoring and interventions, potentially impacting psoriasis patient care quality.
1 Introduction
Psoriasis is a common chronic inflammatory disease, predominately affecting the skin. Psoriatic disease most commonly manifests as psoriasis vulgaris (Pso), which affects 1–3% of the worldwide population (1). Less common psoriasis types are pustulosis palmoplantaris (PPP) and generalized pustular psoriasis (GPP). Compared to Pso, PPP has a lower prevalence of 0.6 to 8.9 cases per 10,000 people (2), while GPP is considered an orphan disease with a reported prevalence ranging from 0.02 to 1.4 cases per 10,000 people (3). Despite significant advancements in the treatment of psoriasis, this disease still imposes a major medical burden (4).
One major contributor to the disease burden of Pso is psoriatic arthritis (PsA). PsA manifests in 8.3 to 30% of Pso patients (5–7), but is most likely underdiagnosed (8, 9). In a prospective study, the risk of PsA amounted to 10.1% in 464 Pso patients during an 8-year follow-up (10). Hence, the risk of PsA in Pso is well-established. However, the reported associations between the two diseases vary greatly, there is only scant data from case–control studies, and with few exceptions (11), the impact of sex and ethnicity remains largely understudied (11, 12). Similarly to Pso, PPP is associated with a significant comorbidity, which predominantly includes PsA. The reported risk of comorbid PsA in PPP is lower when compared to Pso, with 6.31 cases per 1,000 patients as opposed to 11.49 cases per 1,000 patients (13). In a small-scale observational study, 14% of PPP patients were also diagnosed with PsA (14). Collectively, PsA coincides with PPP in 8.6 to 26% of cases (15). Thus, there is a substantial body of evidence supporting an association of PPP with PsA. However, no cohort studies have been performed to determine the risk of PsA in PPP, and data regarding sex- and ethnic-specific risks or controlling for the bias potentially imposed by PsA risk factors, is missing. In GPP, a recent study from Japan highlighted a highly significant association with PsA in GPP patients (16). In this study, however, all GGP patients had an additional diagnosis of prior Pso. In a multicenter retrospective investigation, 24/102 (23.5%) GPP patients were additionally diagnosed with PsA (17). In a single-center retrospective observational study from Italy enrolling 140 GPP patients, 8% had additional PsA (18). A large multicenter retrospective cohort study originating from China included 744 GPP patients, of which 3.1% were diagnosed with PsA (19). Hence, previous studies point towards a possible association of GPP with PsA. However, the reported prevalence showed a high variability, ranging from 3.1 to 23.5%. Furthermore, the temporal relationship between this presumed association remains unknown.
Overall, managing PsA poses significant challenges, primarily focusing on disease progression control without joint restoration (20). Therefore, early detection and timely intervention hold considerable potential to preserve patient well-being. Moreover, for Pso patient populations at an elevated risk of developing PsA, treatments with the capacity to prevent its onset may be especially advantageous (2, 21). Thus, we aimed here to determine PsA risk in patients diagnosed with different forms of psoriasis. For this, a retrospective cohort study using a large scale federated database of electronic health records (EHRs) was used.
2 Materials and methods
2.1 Study design and database
A global population-based retrospective cohort study with propensity-score matching was performed following previously published protocols (22–26). We retrieved electronic health records (EHRs) from individuals who had been diagnosed with psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), or generalized pustular psoriasis (GPP), as well as control subjects who did not have any of these conditions. The grouping criteria were established using ICD-10 codes. Subsequently, we assessed the risk of developing psoriatic arthritis (PsA, ICD-10:L40.5) in the US Collaborative Network, comparing the risk for PsA in individuals with Pso, PPP, or GPP against non-psoriasis controls. To validate our findings, we conducted similar analyses within the EMEA and LATAM Collaborative Networks of TriNetX. Next, we compared the risk for PsA development among individuals with Pso, PPP, and GPP within the US Collaborative Network. Lastly, within the same network, we explored sex- and ethnicity-specific PsA development risks (Figure 1). The data used in this investigation was gathered between February and May 2023 from the US (91 million patients from 57 healthcare organizations), EMEA (15 million patients from 24 healthcare organizations located in the United Kingdom, Sweden, Finland, Spain, Belgium, Germany, Italy, Poland, Hungary, Bulgaria and Israel), and LATAM (26 million patients from 24 healthcare organizations located in Brazil) Collaborative Networks on the TriNetX platform. If indicated, analysis was performed in May 2024. As part of a collaboration between the University Clinic of Schleswig-Holstein (UKSH) and TriNetX, UKSH researchers have access to the TriNetX network.
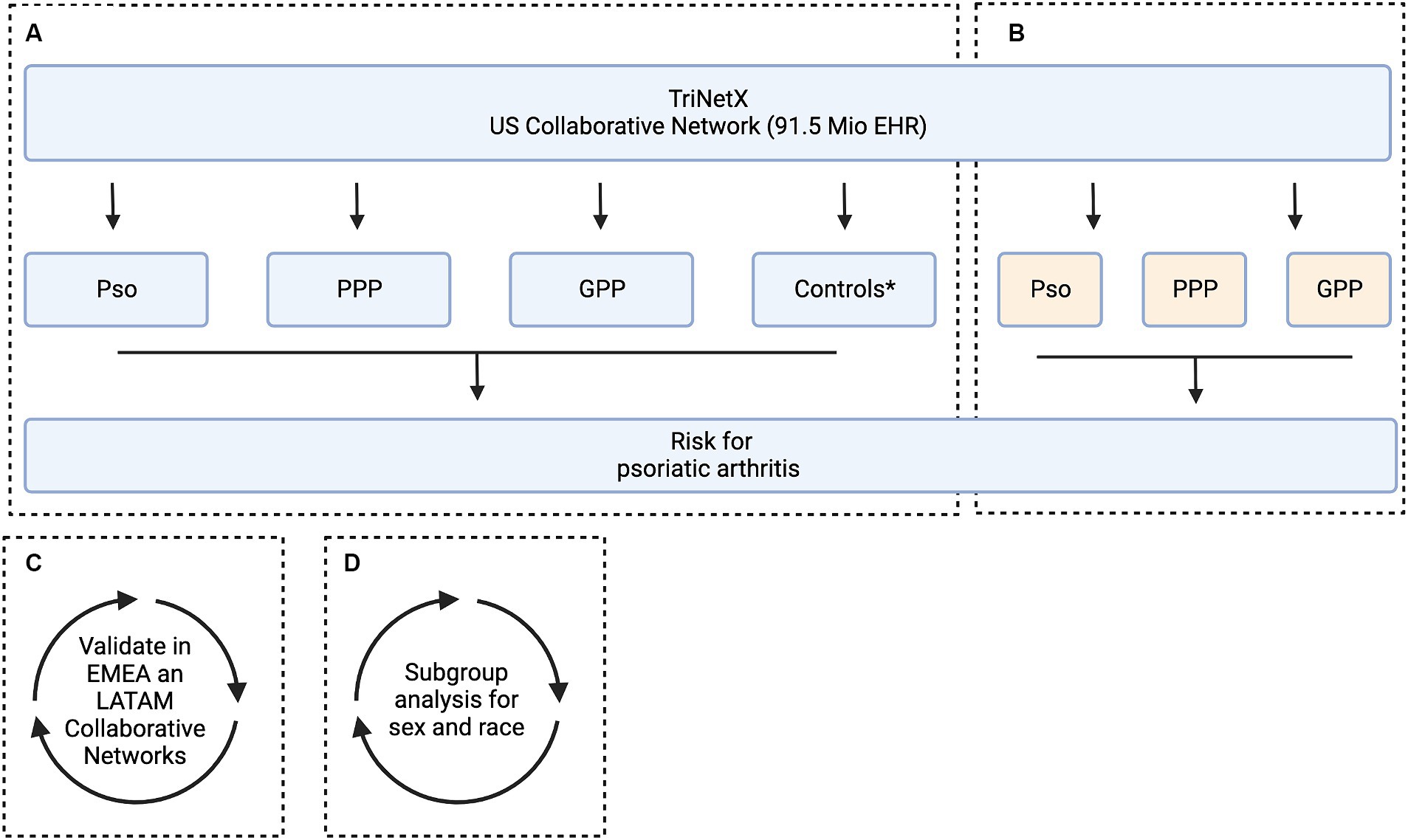
Figure 1. Study flow chart. The aim of this study was to assess the risk of developing psoriatic arthritis among patients diagnosed with psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), and generalized pustular psoriasis (GPP). This analysis includes subgroup stratification based on sex and ethnicity. For this, electronic health records (EHRs) indicative of Pso, PPP, and GPP were retrieved from the US Collaborative Network of TriNetX. For each patient cohort, an equally sized, propensity matched control cohort was built. We used two alternative strategies for propensity matching. In Model 1, matching was based on age, sex, and ethnicity, while in Model 2, risk factors for psoriatic arthritis were additionally considered. (A) In the initial analysis, the risk to develop psoriatic arthritis was contrasted between each matching case and control group. Subsequently two analyses were performed in parallel. (B) To investigate potential variations in the risk of developing psoriatic arthritis across different clinical presentations of psoriasis, we conducted a comparative analysis between Pso, PPP, and GPP. (C) To validate our findings, the analyses assessing the risk of developing psoriatic arthritis among patients diagnosed with Pso, PPP, and GPP was repeated in two independent Collaborative Networks within TriNetX, namely EMEA and LATAM.
2.2 Ethics statement
This retrospective study is exempt from informed consent. The data reviewed is a secondary analysis of existing data, does not involve intervention or interaction with human subjects, and is de-identified per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. This formal determination by a qualified expert refreshed on December 2020.
2.3 Study population and definition of eligible patients
EHRs of cases and controls were retrieved from the database based on ICD-10 codes. The study included the following groups: individuals with Pso (defined by ICD-10:L40.0, excluding ICD-10:L40.1 and L40.3), PPP (defined by ICD-10:L40.3, excluding ICD-10:L40.0 and L40.1), and GPP (defined by ICD-10:L40.1, excluding ICD10:L40.0 and L40.3), as well as controls who did not exhibit any manifestation of psoriasis in any form (Supplementary Table S1).
2.4 Covariates
To allow for better comparability when contrasting the risk for subsequent diagnosis of PsA, propensity-score matching (PSM) was performed by establishing covariate matrixes. We used two different models for propensity matching. In the first model (Model 1), age at index, female sex, and ethnicity were used as variables for propensity matching. In the second model, (Model 2), known risk factors of PsA were additionally used for propensity matching (27, 28). These included: Hyperlipidemia (ICD-10:E78.5), overweight and obesity (ICD-10:E66), disorders of the thyroid gland (ICD-10:E00-07), past (ICD-10:Z87.891) or present (ICD-10:F17) nicotine dependence, depression (ICD-10:F32.A), hyperuricemia (ICD-10:E79.0), and panuveitis (ICD-10:H44.11). Each matrix row order was randomized after data retrieval. A propensity score for each patient was generated by logistic regression using the Python package “scikit-learn.” Matching was performed 1:1 using the greedy nearest neighbor approach with a cut-off distance of 0.1 pooled standard deviations of the logit of the propensity-score. Baseline characteristics were re-evaluated and reported after matching, while differences were compared by t-test for continuous variables and z-test for binary or categorical variables.
2.5 Statistical analysis
For statistical analysis, the index event was set as the diagnosis of any of the indicated psoriatic diseases, or the reported healthcare encounter in the control group. Outcomes any time after the index event were considered in the analysis. Outcomes prior to the diagnosis of each index event were excluded. Relative risks and risk differences were calculated. Survival analyses were performed using the Kaplan–Meier method (KM). KM-curves were compared using the Log-rank test; p-values of less than 0.05 were considered significant. Nelson–Aalen plots were utilized to test the proportionality assumption. A univariate Cox proportional hazards regression was used to express hazard ratios (HRs).
3 Results
3.1 Cohorts
After propensity matching for age at index, gender, and ethnicity (Model 1), we retrieved 35,281, 9,639, and 2,281 EHRs from the US Collaborative Network for Pso, PPP, and GPP, respectively. For each patent cohort, an equally sized, propensity-matched control group was compiled. No significant differences among the matched cases and control groups were noted (Table 1). In the EMEA Collaborative Network, data from 9,834 Pso, 611 PPP, and 250 GPP EHRs were retrieved, with an equal number of propensity-matched controls for each of the patient groups. Again, no differences regarding age, sex, and ethnicity (Model 1) were noted (Supplementary Table S2). In the LATAM Collaborative Network, less EHRs for each patient cohort were present. We retrieved 674, 23, and 16 EHRs for Pso, PPP, and GPP, respectively. Comparison to the equally-sized control groups showed no differences in the parameters used for propensity matching (Supplementary Table S3). To account for potential bias from risk factors of psoriatic arthritis (27, 28), these were included in the propensity matching for Model 2. Because of the relatively low number of retrieved EHRs in the EMEA and LATAM Collaborative Networks of TriNetX, this analysis was solely performed with data retrieved from the US Collaborative Network. After implementing propensity matching in Model 2, we obtained 35,812, 7,788, and 2,408 EHRs from the US Collaborative Network for Pso, PPP, and GPP, respectively. For each patient cohort, an equally-sized, propensity-matched control group was constructed. No discrepancies were observed among cases and control groups for any of the factors used for propensity matching (Table 2).
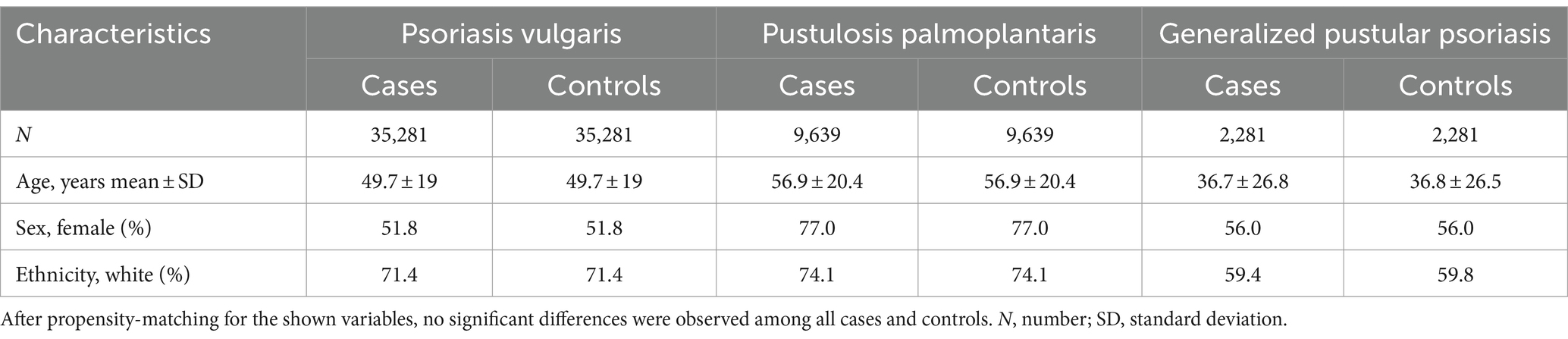
Table 1. Demographics and comorbidities of the study population in the US Collaborative Network (propensity matching Model 1).
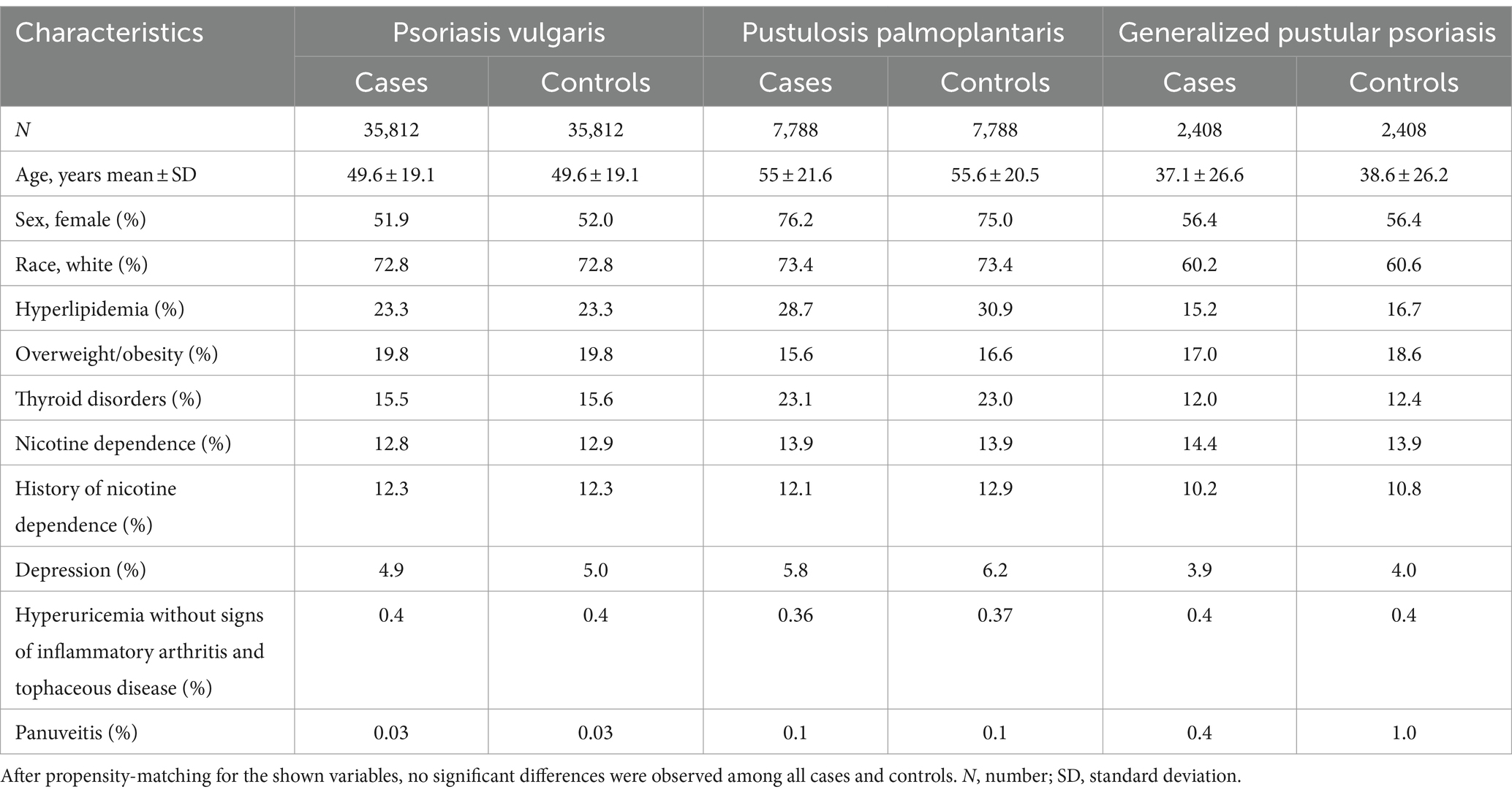
Table 2. Demographics and comorbidities of the study population in the US Collaborative Network (propensity matching Model 2).
3.2 Overall risk for psoriatic arthritis in psoriasis vulgaris, generalized pustular psoriasis and pustulosis palmoplantaris
3.2.1 Contrasting PsA risk between Pso, GPP, and PPP to that of non-psoriasis controls
We further contrasted the risk to develop psoriatic arthritis in each of the patient cohorts (Pso, PPP, and GPP) to that of the corresponding control groups not affected by any form of psoriasis. For this analysis, we selected the US Collaborative Network of TriNetX because it included considerably more EHRs fulfilling our predefined in- and exclusion criteria. In the initial analysis, propensity matching was limited to age, sex, and ethnicity to maximize the inclusion of a large number of cases. As expected, our analysis revealed an elevated risk of PsA development subsequent to the diagnosis of Pso, PPP, or GPP (Figures 2A–D and Table 3). Notably, significant disparities emerged among the various presentations of psoriasis. The highest risk of PsA was observed in patients with Pso (HR 87.7, 95% CI 63.4–121.1, p < 0.0001). While both PPP (HR 15.3, 95% CI 7.9–29.5, p < 0.0001) and GPP (HR 26.8, 95% CI 6.5–110.1, p < 0.0001) were associated with substantially heightened probabilities of developing PsA compared to their respective control groups, these risks were notably lower than those observed in individuals with Pso.
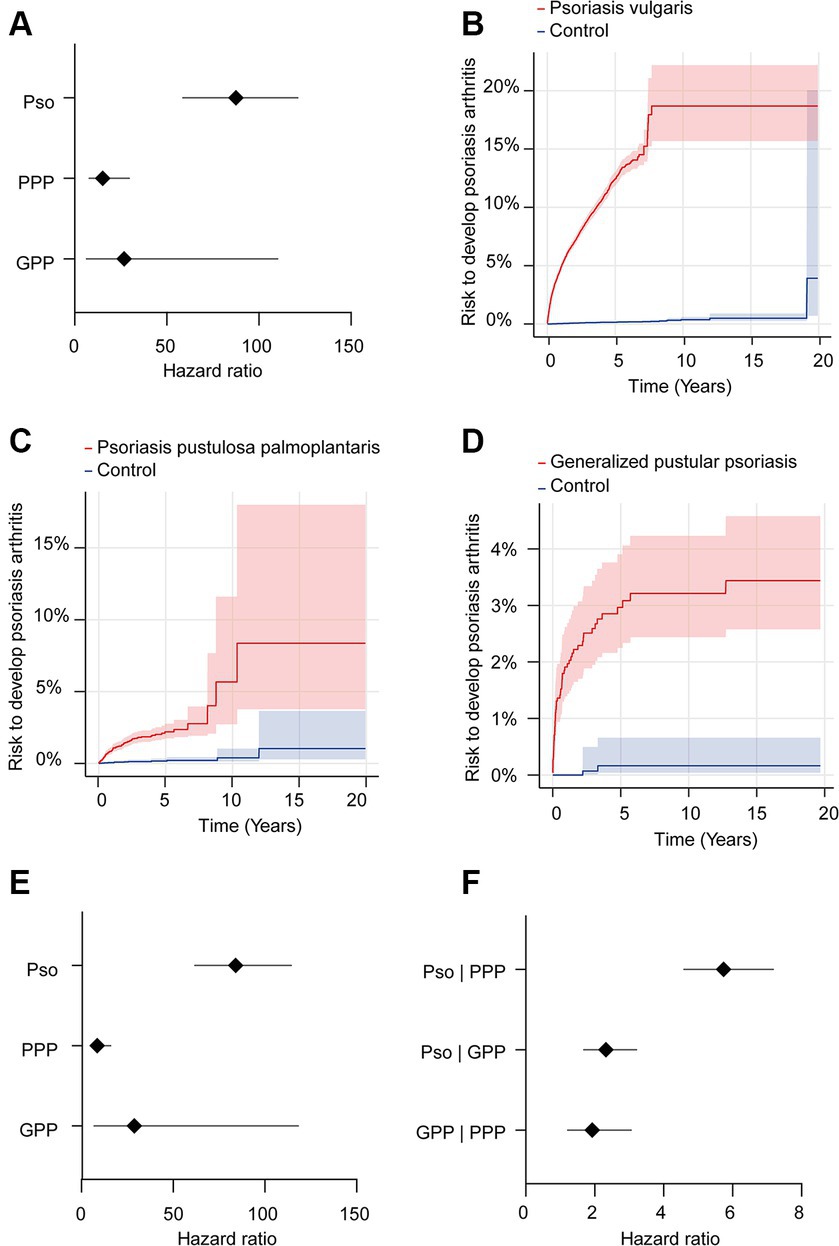
Figure 2. Risk of developing psoriatic arthritis in psoriasis vulgaris, pustulosis palmoplantaris or generalized pustular psoriasis. We assessed the risk of developing psoriatic arthritis within patient cohorts diagnosed with psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), or generalized pustular psoriasis (GPP), comparing them to corresponding control groups without any form of psoriasis using electronic health records from the US Collaborative Network of TriNetX. Propensity matching in the initial analysis was confined to age, sex, and ethnicity to allow for better comparability. (A) We document an elevated risk of psoriatic arthritis following Pso, PPP, or GPP diagnosis. Diamonds represent hazard ratio (HR) and error bars correspond to the 95% confidence interval (CI). Only significant results are displayed. (B–D) Nelson–Aalen plots contrast the risk of psoriatic arthritis of (B). Pso (HR 87.7, CI 63.4–121.1, p < 0.0001), (C) PPP (HR 15.3, CI 7.9–29.5, p < 0.0001), and (D) GPP (HR 26.8, CI 6.5–110.1, p < 0.0001) to that of the appropriate controls. Solid lines represent the HR and lighter shaded areas represent the CI. (E) To address the potential bias introduced by known risk factors for psoriatic arthritis, they were considered for propensity matching. Again, all psoriasis manifestations demonstrated consistent associations with increased psoriatic arthritis risk. Pso maintained the highest risk (HR 84.1, CI 61.8–114.3, p < 0.0001), while PPP and GPP displayed substantial risk elevations, with HRs of 8.7 (CI 4.8–15.8, p < 0.0001) and 258.8 (CI 7.0–1,118, p < 0.0001), respectively. (F) To directly compare PsA risks among different psoriasis manifestations, we contrasted this risk between patients with Pso-PPP, Pso-GPP and GPP-PPP. Here, Pso demonstrated a significantly greater risk of developing psoriatic arthritis compared to those with PPP (HR 5.7, CI 4.5–7.1, p < 0.0001) and GPP (HR 2.3, CI 1.7–3.2, p < 0.0001). In comparison to PPP, we documented a greater risk of psoriatic arthritis development in individuals with GPP (HR 1.9, CI 1.2–3.1, p = 0,0042).
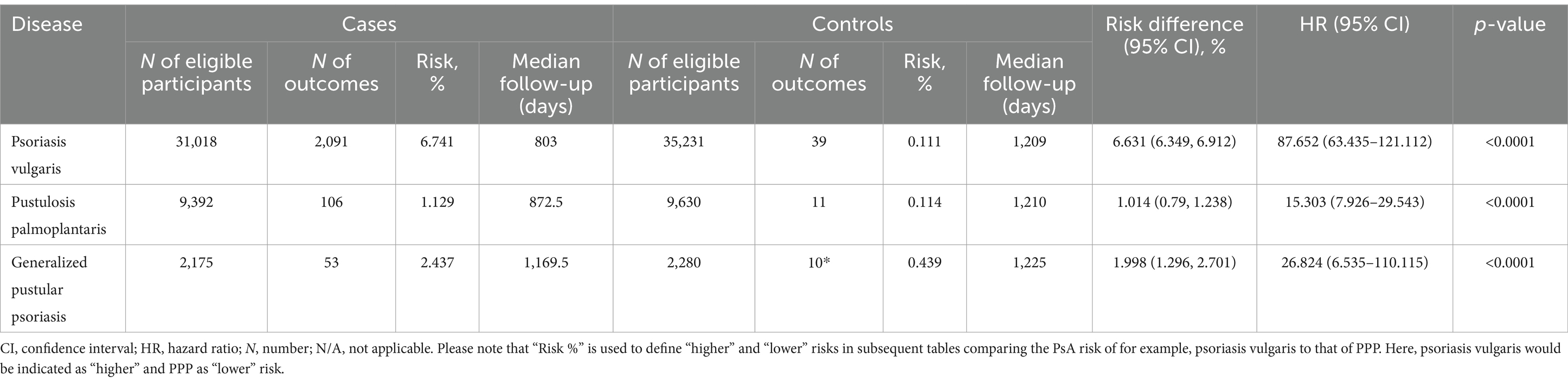
Table 3. Risk of psoriatic arthritis development in varied psoriasis manifestations with propensity matching for age, sex and ethnicity.
Subsequently, to mitigate potential bias introduced by established risk factors for PsA, we conducted a secondary analysis that incorporated these factors into the propensity matching. In this subsequent analysis, we corroborated the findings from the preceding investigation. Specifically, within the model additionally considering known risk factors of PsA, we observed a consistent association between all psoriasis manifestations and an elevated risk of PsA (Figure 2E and Table 4). Notably, Pso exhibited the highest risk (HR 84.1, CI 61.8–114.3, p < 0.0001), while PPP and GPP also demonstrated substantially increased risks, with HRs of 8.7 (CI 4.8–15.8, p < 0.0001) and 258.8 (CI 7.0–1,118, p < 0.0001), respectively.
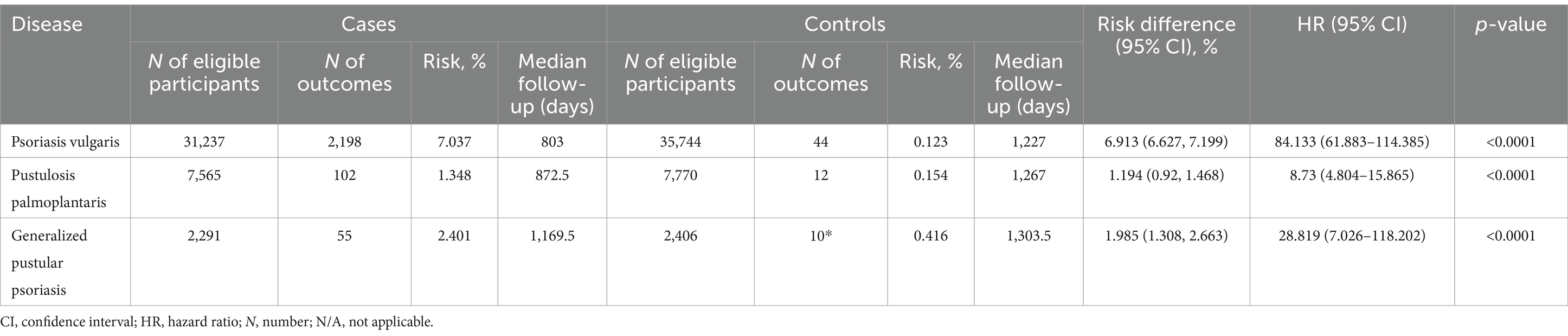
Table 4. Risk of psoriatic arthritis development in varied psoriasis manifestations with propensity matching for age, sex, ethnicity, and known risk factors of psoriatic arthritis.
To independently validate our findings, we conducted an investigation into the risk of PsA in cohorts of Pso, PPP, and GPP obtained from the EMEA and LATAM Collaborative Networks. Due to the limited number of EHRs available in these Networks, our analysis was confined to Model 1. Within the EMEA Collaborative Network, Pso, consistent with previous findings, was associated with an increase in the risk of subsequent PsA (HR 181.8, CI 58.4–565.3, p < 0.0001). For PPP, HR and CI could not be calculated due to the absence of outcomes in the control group, nevertheless we observed a statistically significant increased risk for PsA (p < 0.0001). Conversely, GPP did not demonstrate a statistically significant risk of PsA (Supplementary Table S4). In the LATAM Collaborative Network, Pso also displayed an increased risk of PsA, with an HR of 20.8 (CI 2.8–154.5, p < 0.0001). However, data regarding PPP and GPP cannot be considered due to the insufficient number of EHRs in both cases and controls, as well as the limited number of outcomes (Supplementary Table S5).
3.2.2 Contrasting PsA risk between Pso, PPP and GPP
Our earlier findings highlighted a notable disparity in the risk of subsequent PsA development, with Pso exhibiting the highest risk while PPP displayed the lowest risk. To perform a direct comparison of PsA risk among different psoriasis manifestations, we conducted pairwise comparisons between Pso and PPP, Pso and GPP, and GPP and PPP. These comparisons were performed after propensity matching the respective cohorts for age, sex, and ethnicity (Table 5). In accordance with our descriptive observations, individuals diagnosed with Pso demonstrated a significantly greater risk of developing PsA compared to those with PPP (HR 5.7, CI 4.5–7.1, p < 0.0001) and GPP (HR 2.3, CI 1.7–3.2, p < 0.0001, Figure 2F and Table 6). The direct comparison of psoriatic arthritis risk between GPP and PPP also mirrored our descriptive findings. In comparison to PPP, we documented a greater risk of PsA development in individuals with GPP (HR 1.9, CI 1.2–3.1, p = 0,0042, Figure 2F and Table 6).
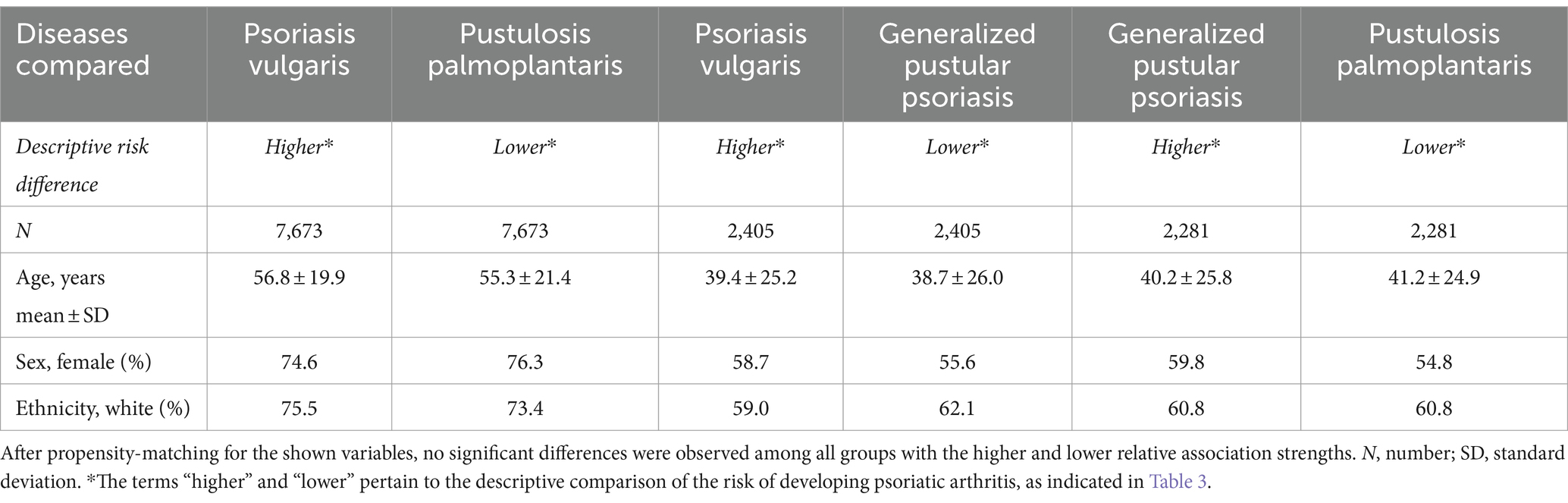
Table 5. Demographics of the study population in relative association analysis in the US Collaborative Network.
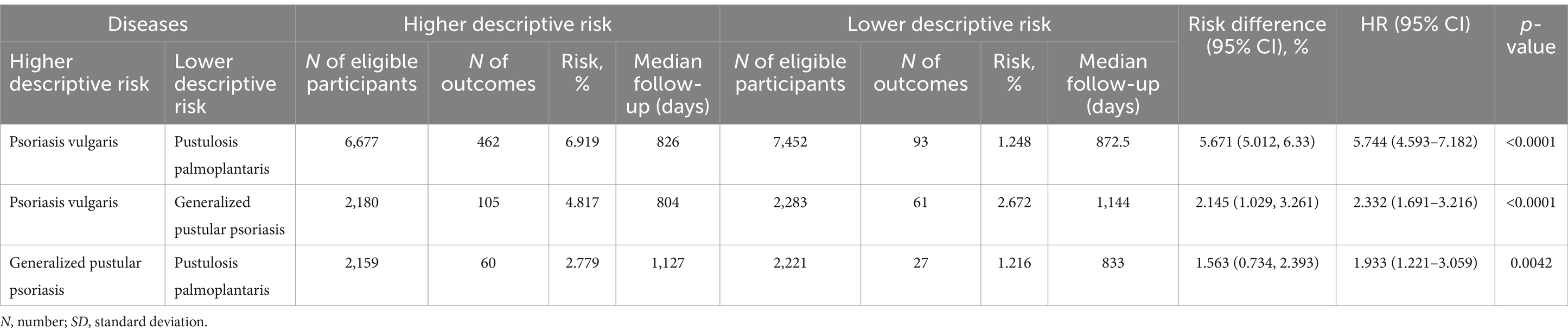
Table 6. Comparative risk of psoriatic arthritis development amongst varied psoriasis manifestations.
3.3 Sex-specific risks for psoriatic arthritis in psoriasis vulgaris, generalized pustular psoriasis and pustulosis palmoplantaris
3.3.1 Contrasting PsA risk between Pso, PPP, and GPP to that of non-psoriasis controls
To explore the possibility of sex-specific differences for PsA risk subsequent to diagnosis of Pso, PPP, or GPP, we conducted a comparative analysis of this risk within subgroups defined by female or male sex among cases and controls. Due to the limited availability of EHRs in the EMEA and LATAM Collaborative Networks, these analyses were conducted using the US Collaborative Network. As our previous analyses yielded consistent results between both models for propensity matching, we adopted Model 1 (excluding sex) for propensity matching.
Depending on psoriasis manifestation and sex, we retrieved 1,038 to 16,787 cases and controls per group. Following propensity matching, cases and controls for all investigations were similar in regard to age and White ethnic proportion (Supplementary Table S6). The analysis stratified to EHRs indicating female sex demonstrated an increased risk of PsA after the diagnosis of Pso, PPP, or GPP (Figure 3A and Supplementary Table S7). As observed in the non-stratified analysis, striking inequalities were present among the various presentations of psoriasis. More specifically, the highest risk of PsA was observed in female patients with Pso (HR 78.4, CI 51.8–118.6, p < 0.0001). In female PPP (HR 9.3, CI 4.8–17.8, p < 0.0001) and GPP (HR 21.0, CI 5.1–87.1, p < 0.0001) patients the risk of PsA was also increased, but to a lesser extent compared to Pso (Figure 3A and Supplementary Table S7). Risk for PsA was also increased in male individuals diagnosed with Pso (HR 76.7, CI 47.8–123.1, p < 0.0001), PPP (HR 14.8, CI 3.5–62.5, p < 0.0001), and GPP (HR 4.4, CI 1.3–15.3, p < 0.0001, Figure 3A and Supplementary Table S8). Considering the discernible variations in psoriatic arthritis risk among female and male patients diagnosed with Pso, PPP, or GPP, we proceeded to contrast the relative risk of PsA between female and male Pso patients. The demographics after propensity matching for age and ethnicity for this comparison are depicted in Supplementary Table S9. The cumulative risk of developing PsA was 7.8% among female patients diagnosed with Pso, in contrast to 6.6% among their male counterparts. Hence, female Pso patients exhibit a marginally higher, yet statistically significant increased risk to develop psoriatic arthritis (HR 1.1, CI 1.1–1.2, p = 0.002, Figure 3B and Supplementary Table S10). Because of the limited number of cases and outcomes, we refrained from conducting a similar analysis for PPP and GPP.
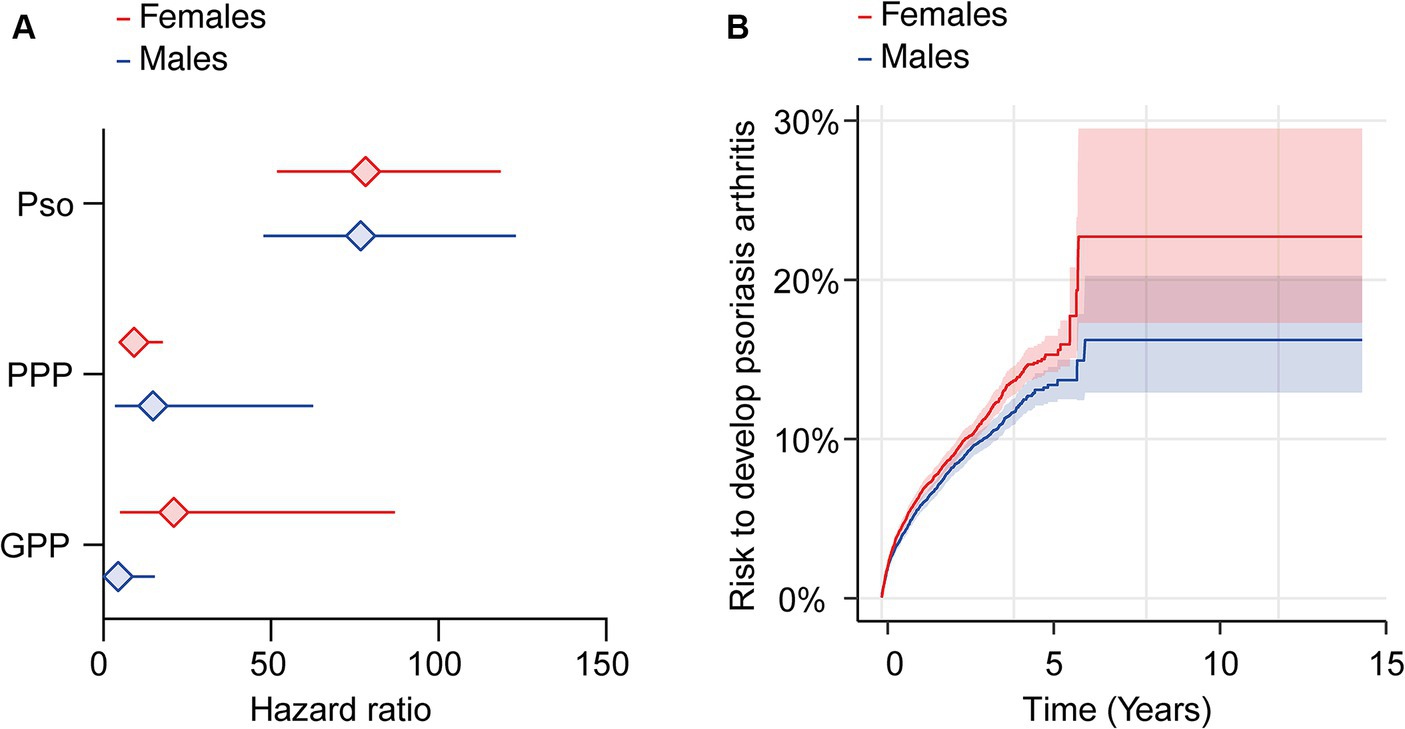
Figure 3. Sex-specific risks for the development of psoriatic arthritis following a diagnosis of psoriasis vulgaris, pustulosis palmoplantaris or generalized pustular psoriasis. We assessed the risk of developing psoriatic arthritis within sex-stratified (female or male) patient cohorts diagnosed with psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), or generalized pustular psoriasis (GPP), comparing them to corresponding control groups without any form of psoriasis using electronic health records from the US Collaborative Network of TriNetX. Propensity matching in the initial analysis was confined to age and ethnicity to allow for better comparability. (A) We document an elevated risk of psoriatic arthritis following Pso, PPP, or GPP diagnosis in both female (red) and male (blue) patients. Diamonds represent hazard ratio (HR) and error bars correspond to the 95% confidence interval (CI). Only significant results are displayed. (B) The Nelson–Aalen plot contrasts the risk of psoriatic arthritis in female (red) and male (blue) Pso patients. Compared to male Pso patients, the risk of psoriatic arthritis in their female counterparts is significantly greater (HR 1.1, CI 1.1–1.2, p = 0.002). Solid lines represent the HR and lighter shaded areas represent the CI.
3.3.2 Contrasting PsA risk between psoriasis vulgaris, GPP and PPP
Likewise, in respect to the comparisons made for the different clinical manifestations of psoriasis, we also compared the PsA risk between Pso, GPP and PPP here. Our sex-stratified analyses corroborated the previous non-sex-stratified results. Specifically, in EHRs stratified for female sex, the PsA risk was highest in Pso, followed by GPP, and lowest in PPP. These differences were statistically significant when directly comparing the PsA risk between any two clinical manifestations of psoriasis. Similar findings were observed in EHRs stratified by male sex. Detailed demographics and results can be found in Supplementary Tables S11–S14.
3.4 Ethnicity-specific risk for psoriatic arthritis in psoriasis vulgaris, generalized pustular psoriasis and pustulosis palmoplantaris
3.4.1 Contrasting PsA risk between Pso, GPP, and PPP to that of non-psoriasis controls
Next, to investigate potential ethnic disparities in the likelihood of developing PsA following diagnoses of Pso, PPP, or GPP, we carried out a comparative risk analysis within subgroups categorized by either White or Black or African American ethnicity among both cases and controls. Due to the restricted number of EHRs fulfilling our in- and exclusion criteria in the EMEA and LATAM Collaborative Networks, along with limited ethnicity coding in these networks, we limited these analyses to the US Collaborative network. Given the consistent outcomes observed in our earlier analyses using both models for propensity matching, we used Model 1 (excluding ethnicity) for propensity matching.
Depending on psoriasis manifestation and ethnicity, we retrieved between 486 and 23,043 cases and controls per group. Following propensity matching, cases and controls for all investigations were similar regarding age and sex (Supplementary Table S15). When we stratified the analysis based on EHRs indicating Black or African American ethnicity, we observed an elevated risk of PsA following the diagnosis of Pso, PPP, or GPP (Figure 4A and Supplementary Table S16). Similar to our findings in the non-stratified analysis, disparities were evident among the different psoriasis presentations. Specifically, the highest risk of PsA was noted in Black or African American patients with Pso (HR 167.8, CI 23.2–1,214.2, p < 0.0001). Black or African American PPP patients also exhibited an increased risk, albeit to a lesser extent compared to Pso (HR 16.1, CI 2.0–128.8, p = 0.0005). For GPP, HR and CI could not be calculated due to the absence of outcomes in the control group; nevertheless, a statistically significant increased risk for PsA was observed (p < 0.0085, Figure 4A and Supplementary Table S16). Similarly, the risk for PsA was elevated among White individuals diagnosed with Pso (HR 59.4, CI 43.1–81.7, p < 0.0001), PPP (HR 8.0, CI 4.3–14.8, p < 0.0001), and GPP (HR 20.7, CI 5.0–85.6, p < 0.0001, Figure 4A and Supplementary Table S17). Given the noticeable differences in PsA risk among Black or African American and White patients diagnosed with Pso, PPP, or GPP, we proceeded to compare its relative risk among these two ethnic groups. The demographic characteristics after propensity matching for age and sex for this comparison are provided in Supplementary Table S18. The cumulative risk of developing PsA was 5.5% among Black or African American patients diagnosed with Pso, in contrast to 7.2% among their White propensity matched controls. Thus, White Pso patients exhibited a slightly higher, yet statistically significant, increased risk of developing psoriatic arthritis (HR 1.3, CI 1.04–1.7, p = 0.024, Figure 4B and Supplementary Table S19). Due to the limited number of cases and outcomes, we refrained from conducting a similar analysis for PPP and GPP.
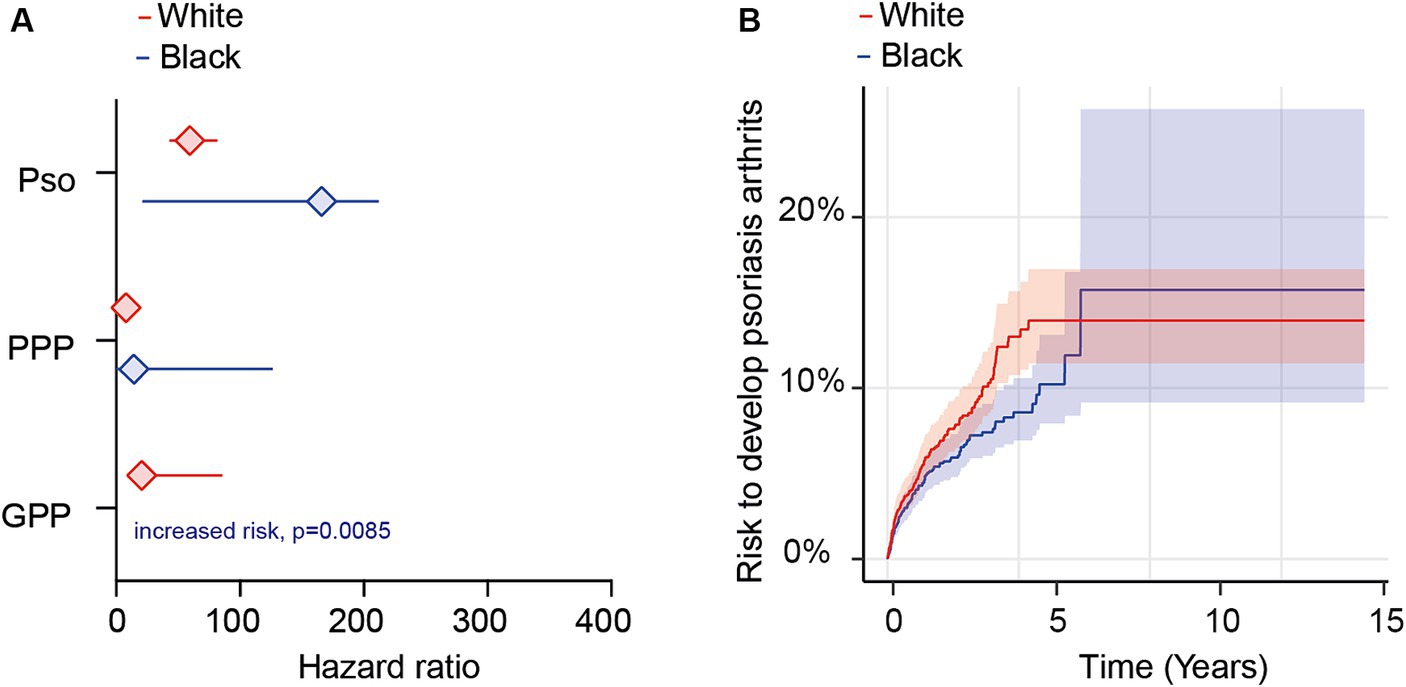
Figure 4. Ethnicity-specific risks for the development of psoriatic arthritis following a diagnosis of psoriasis vulgaris, pustulosis palmoplantaris or generalized pustular psoriasis. We assessed the risk of developing psoriatic arthritis within ethnicity-stratified (Black or African American/White) patient cohorts diagnosed with psoriasis vulgaris (Pso), pustulosis palmoplantaris (PPP), or generalized pustular psoriasis (GPP), comparing them to corresponding control groups without any form of psoriasis using electronic health records from the US Collaborative Network of TriNetX. Propensity matching in the initial analysis was confined to age and sex to allow for better comparability. (A) We document an elevated risk of psoriatic arthritis following Pso, PPP, or GPP diagnosis in both Black or African American (blue) and White (red) patients. Diamonds represent hazard ratio (HR) and error bars correspond to the 95% confidence interval (CI). Only significant results are displayed. (B) The Nelson–Aalen plot contrasts the risk of psoriatic arthritis in Black or African American (red) and White (blue) Pso patients. Compared to Black or African American Pso patients, the risk of psoriatic arthritis in their White counterparts is significantly greater (HR 1.3, CI 1.1–3.2, p = 0.024). Solid lines represent the HR and lighter shaded areas represent the CI.
3.4.2 Contrasting PsA risk between psoriasis vulgaris, GPP and PPP
The ethnicity-stratified analyses regarding PsA risk in EHRs indicating Black or African American, or alternatively White ethnicity, corroborated the previous non-ethnicity-stratified results. Specifically, in EHRs stratified for Black or African American ethnicity, the PsA risk was highest in Pso, followed by GPP, and lowest in PPP. These differences were statistically significant when directly comparing the PsA risk between any two clinical manifestations of psoriasis. Similar findings were observed in EHRs stratified by White ethnicity. However, due to the low sample size (N = 428) and low number of outcomes (n = 10), no significant difference was found between Black and White EHRs when contrasting GPP and PPP. Detailed demographics and results can be found in Supplementary Tables S20–S23.
4 Discussion
We demonstrate here that psoriasis patients’ risk to develop PsA varies greatly depending on their disease variant, sex, and ethnicity. Specifically, the likelihood of PsA development ranged from 1.1 to 7.4%. The lowest risk for PsA was observed in Black or African American PPP patients, while the highest risk was seen in female Pso patients. Similarly, striking differences were noted when focusing on sex- and ethnicity-stratified cohorts within Pso, PPP, and GPP patients. For example, the likelihood of PsA development ranged from 5.5% (Black or African American) to 7.4% (female) in Pso patients. PsA prevalence following PPP diagnosis ranged from 1.1% (Black or African American) to 1.5% (male). In GPP, the prevalence of subsequent psoriatic arthritis ranged from 1.5% (male) to 3.2% (female). These results underscore the importance of stringently implementing sex- and ethnic-specific analyses in epidemiology research. In the past, ethnicity and sex have largely been understudied epidemiologically, especially for the topic addressed herein (11, 12, 29).
4.1 Impact of sex on PsA risk
Overall, the previously reported PsA risk in individual studies shows high variability in the male-to-female ratio, spanning from 0.42 to 2.75 (12). In a prospective, population-based annual incidence study of early arthritis, including PsA, from Sweden, 8 out of 100,000 women and 3 out of 100,000 men were diagnosed with PsA. This translates into the highest reported risk for female psoriasis patients to develop PsA. The relatively low number of PsA patients, however, may have introduced a relevant bias (30). In addition to this study, two additional investigations supported the notion of a higher PsA risk in females compared to males (12). However, these results were contradicted by an Argentinian study which evaluated the risk of PsA development in around 140,000 people presenting to a university hospital-based health management organization in Buenos Aires over a 6-year period. Here, a total of 35 patients developed PsA, including 12 female and 23 male patients (31). The greater male PsA risk observed here has also been reported in several other studies. Here again, small sample size in all those studies, ranging from 147 to 329 PsA patients, should be considered as a potential source of bias (32–35). Lastly, a study from the US showed no sex differences in PsA prevalence in a cohort of 65 patients diagnosed with this disease (36). Our study retrieved 31,018 EHRs with a diagnosis of Pso, of which 2,091 were subsequently diagnosed with PsA in the cohort not stratified for sex or ethnicity (Table 3). In our sex-stratified analysis, 1,082 of the 14,453 female Pso patients were diagnosed with PsA, while only 894 of the 13,552 male Pso patients received a PsA diagnosis (Supplementary Tables S9, S10). Consequently, any potential bias resulting from the limited number of outcomes in our study would be relatively low. Additionally, upon direct comparison of PsA risk between male and female Pso patients, a higher risk was evident in the latter group (Supplementary Table S12). Thus, overall, females Pso patients exhibit a heightened risk to develop PsA. This trend remains consistent for GPP, with 3.2% of female GPP patients subsequently developing PsA. However, this pattern is not seen in PPP, where the risk of PsA remained relatively similar between female and male PPP patients. Nevertheless, it is imperative to exercise caution in interpreting these findings due to the relatively low number of PsA cases within the PPP and GPP groups (Supplementary Tables S9, S10).
In regard to clinical translation, our study data enables the stratification of psoriasis patients into distinct PsA risk groups. Within high-risk categories, leveraging established PsA risk factors alongside the potential integration of novel biomarkers holds promise for enhancing PsA risk prediction. The findings support the implementation of a more nuanced approach involving frequent PsA evaluations and earlier initiation of targeted biological treatments, which may potentially reduce the likelihood of PsA development (2, 21).
4.2 Limitations
This study has several limitations to be appreciated. Primarily, cohorts were identified using ICD-10 codes, which to a certain degree include coding inaccuracies. This issue likely applies to our study because the observed prevalence of PsA was lower than has been reported elsewhere. Thus, PsA is most likely under-diagnosed within the HCOs contributing data to TriNetX (9). As this is a systemic issue, the relative differences reported here remain valid. In defining psoriasis cohorts by including one variant of the disease while simultaneously excluding any other variants, we partially addressed this bias. We were also unable to consider the severity of psoriasis, since ICD-10:L40.70, the code for “Moderate to severe psoriasis,” was not accessible. Furthermore, this study did not include medications because we aimed to include as many EHRs as possible. Thus, the potential impact of medications has not been addressed and will be the focus of subsequent endeavors. When comparing PsA risk of psoriasis patients versus non-psoriasis controls, an assessment bias (i.e., patients with psoriasis are more often screened for the presence of PsA) could potentially confound the results. Lastly, due to the study’s retrospective design, differences in PsA risks found here cannot be causally attributed to underlying psoriasis.
5 Conclusion
In conclusion, our data provides comprehensive insights into the sex- and ethnicity-specific risks associated with development of PsA after a diagnosis of Pso, PPP, or GPP. Our findings underscore the opportunity for more precise PsA monitoring tailored to individual patient characteristics and specific psoriasis variants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
BG: Investigation, Writing – original draft, Writing – review & editing. KB: Visualization, Writing – review & editing. AV: Visualization, Writing – review & editing. ML: Investigation, Writing – review & editing. HZ: Investigation, Writing – review & editing. DD: Investigation, Writing – review & editing. DT: Investigation, Supervision, Writing – review & editing. KK: Conceptualization, Investigation, Writing – review & editing. RL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Cluster of Excellence “Precision Medicine in Chronic Inflammation” (EXC 2167), the Research Training Group “Autoimmune Pre-Disease” (GRK 2633), the Individual Research Grant LU 877/25-1, all from the Deutsche Forschungsgemeinschaft and the Schleswig-Holstein Excellence-Chair Program from the State of Schleswig Holstein.
Conflict of interest
DT has received honoraria or fees for serving on advisory boards, as a speaker, as a consultant from AbbVie, Amgen, Almirall, Beiersdorf, Bristol-Meiers-Squibb, Boehringer Ingelheim, Galapagos, Leo Pharma, Merck Sharp & Dohme, Morphosys, Lilly, Novartis, Janssen-Cilag, Pfizer, Regeneron, Sanofi, Hexal, Sun Pharmaceuticals, and UCB and grants from Leo Pharma and Novartis. RL has received honoraria for speaking or consulting or has obtained research grants from Monasterium Laboratories, Novartis, Lilly, Bayer, Dompe, Synthon, Argen-X, and Incyte during the last 3 years.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1385491/full#supplementary-material
References
1. Griffiths, CEM, Armstrong, AW, Gudjonsson, JE, and Barker, JNWN. Psoriasis. Lancet. (2021) 397:1301–15. doi: 10.1016/S0140-6736(20)32549-6
2. Ramcharran, D, Strober, B, Gordon, K, DeKlotz, C, Fakharzadeh, S, Yang, YW, et al. The epidemiology of palmoplantar pustulosis: an analysis of multiple health insurance claims and electronic health records databases. Adv Ther. (2023) 40:5090–101. doi: 10.1007/s12325-023-02669-w
3. Puig, L, Choon, SE, Gottlieb, AB, Marrakchi, S, Prinz, JC, Romiti, R, et al. Generalized pustular psoriasis: a global Delphi consensus on clinical course, diagnosis, treatment goals and disease management. J Eur Acad Dermatol Venereol. (2023) 37:737–52. doi: 10.1111/jdv.18851
4. Ujiie, H, Rosmarin, D, Schön, MP, Ständer, S, Boch, K, Metz, M, et al. Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front Med. (2022) 9:875492. doi: 10.3389/fmed.2022.875492
5. Rech, J, Sticherling, M, Stoessel, D, Biermann, MHC, Häberle, BM, and Reinhardt, M. Psoriatic arthritis epidemiology, comorbid disease profiles and risk factors: results from a claims database analysis. Rheumatol Adv Pract. (2020) 4:rkaa033. doi: 10.1093/rap/rkaa033
6. Reich, K, Krüger, K, Mössner, R, and Augustin, M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. (2009) 160:1040–7. doi: 10.1111/j.1365-2133.2008.09023.x
7. Ritchlin, CT, Colbert, RA, and Gladman, DD. Psoriatic arthritis. N Engl J Med. (2017) 376:957–70. doi: 10.1056/NEJMra1505557
8. Villani, AP, Rouzaud, M, Sevrain, M, Barnetche, T, Paul, C, Richard, MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. (2015) 73:242–8. doi: 10.1016/j.jaad.2015.05.001
9. Mease, PJ, Gladman, DD, Papp, KA, Khraishi, MM, Thaçi, D, Behrens, F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/north American dermatology clinics. J Am Acad Dermatol. (2013) 69:729–35. doi: 10.1016/j.jaad.2013.07.023
10. Eder, L, Haddad, A, Rosen, CF, Lee, KA, Chandran, V, Cook, R, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. (2016) 68:915–23. doi: 10.1002/art.39494
11. Shwe, S, Nguyen, C, and Bhutani, T. Racial disparities in clinical trials of biologic treatments for psoriatic arthritis. J Am Acad Dermatol. (2022) 87:910–2. doi: 10.1016/j.jaad.2021.08.038
12. Bragazzi, NL, Bridgewood, C, Watad, A, Damiani, G, and McGonagle, D. Sex-based medicine meets psoriatic arthritis: lessons learned and to learn. Front Immunol. (2022) 13:849560. doi: 10.3389/fimmu.2022.849560
13. Kim, DH, Lee, JY, Cho, SI, and Jo, SJ. Risks of comorbidities in patients with palmoplantar pustulosis vs. patients with psoriasis vulgaris or pompholyx in Korea. JAMA Dermatol. (2022) 158:650–60. doi: 10.1001/jamadermatol.2022.1081
14. Oktem, A, Uysal, PI, Akdoğan, N, Tokmak, A, and Yalcin, B. Clinical characteristics and associations of palmoplantar pustulosis: an observational study. An Bras Dermatol. (2020) 95:15–9. doi: 10.1016/j.abd.2019.04.008
15. Brunasso, AMG, and Massone, C. Recent advances in palmoplantar pustulosis. Fac Rev. (2021) 10:62. doi: 10.12703/r/10-62
16. Hayama, K, Iwasaki, R, Tian, Y, and Fujita, H. Factors associated with generalized pustular psoriasis progression among patients with psoriasis vulgaris in Japan: results from a claims database study. J Dermatol. (2023) 50:1531–8. doi: 10.1111/1346-8138.16949
17. Ohata, C, Tsuruta, N, Yonekura, K, Higashi, Y, Saito, K, Katayama, E, et al. Clinical characteristics of Japanese pustular psoriasis: a multicenter observational study. J Dermatol. (2022) 49:142–50. doi: 10.1111/1346-8138.16217
18. Gisondi, P, Bellinato, F, and Girolomoni, G. Clinical characteristics of patients with pustular psoriasis: a single-center retrospective observational study. Vaccines. (2022) 10:1171. doi: 10.3390/vaccines10081171
19. Wang, HM, Xu, JM, and Jin, HZ. Characteristics and burdens of disease in patients from Beijing with generalized pustular psoriasis and palmoplantar pustulosis: multicenter retrospective cohort study using a regional database. Am J Clin Dermatol. (2023) 17:425–43. doi: 10.1007/s40257-023-00807-2
20. Ogdie, A, Coates, LC, and Gladman, DD. Treatment guidelines in psoriatic arthritis. Rheumatology. (2020) 59:i37–46. doi: 10.1093/rheumatology/kez383
21. Acosta Felquer, ML, LoGiudice, L, Galimberti, ML, Rosa, J, Mazzuoccolo, L, and Soriano, ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis. (2022) 81:74–9. doi: 10.1136/annrheumdis-2021-220865
22. Kridin, K, Mruwat, N, Amber, KT, and Ludwig, RJ. Risk of infections in patients with pemphigus treated with rituximab versus azathioprine or mycophenolate mofetil: a large-scale global cohort study. Br J Dermatol. (2023) 188:499–505. doi: 10.1093/bjd/ljac118
23. Kasperkiewicz, M, Vorobyev, A, Bieber, K, Kridin, K, and Ludwig, RJ. Risk of comorbid autoimmune diseases in patients with immunobullous disorders: a global large-scale cohort study. J Am Acad Dermatol. (2023) 89:1269–71. doi: 10.1016/j.jaad.2023.07.1030
24. Boch, K, Zirpel, H, Thaci, D, Mruwat, N, Zillikens, D, Ludwig, RJ, et al. Mortality in eight autoimmune bullous diseases: a global large-scale retrospective cohort study. J Eur Acad Dermatol Venereol. (2022) 37:e535–7. doi: 10.1111/jdv.18700
25. Kridin, K, and Ludwig, RJ. Isotretinoin and the risk of psychiatric disturbances—a global study shedding new light on a debatable story. J Am Acad Dermatol. (2022) 88:388–94. doi: 10.1016/j.jaad.2022.10.031
26. Kridin, K, and Ludwig, RJ. Isotretinoin and the risk of inflammatory bowel disease and irritable bowel syndrome: a large-scale global study. J Am Acad Dermatol. (2023) 88:824–30. doi: 10.1016/j.jaad.2022.12.015
27. Tsuruta, N, Imafuku, S, and Narisawa, Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol. (2017) 44:1349–52. doi: 10.1111/1346-8138.13968
28. Scher, JU, Ogdie, A, Merola, JF, and Ritchlin, C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. (2019) 15:153–66. doi: 10.1038/s41584-019-0175-0
29. Takeshita, J, Chau, J, Duffin, KC, and Goel, N. Promoting diversity, equity, and inclusion for psoriatic diseases. J Rheumatol. (2022) 49:48–51. doi: 10.3899/jrheum.211330
30. Söderlin, MK, Börjesson, O, Kautiainen, H, Skogh, T, and Leirisalo-Repo, M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis. (2002) 61:911–5. doi: 10.1136/ard.61.10.911
31. Soriano, ER, Rosa, J, Velozo, E, Schpilberg, M, Imamura, PM, Diaz, J, et al. Incidence and prevalence of psoriatic arthritis in Buenos Aires, Argentina: a 6-year health management organization-based study. Rheumatology. (2011) 50:729–34. doi: 10.1093/rheumatology/keq369
32. Wilson, FC, Icen, M, Crowson, CS, McEvoy, MT, Gabriel, SE, and Kremers, HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. (2009) 36:361–7. doi: 10.3899/jrheum.080691
33. Karmacharya, P, Crowson, CS, Bekele, D, Achenbach, SJ, Davis, JM, Ogdie, A, et al. The epidemiology of psoriatic arthritis over five decades: a population-based study. Arthritis Rheumatol. (2021) 73:1878–85. doi: 10.1002/art.41741
34. Nossent, JC, and Gran, JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol. (2009) 38:251–5. doi: 10.1080/03009740802609558
35. Savolainen, E, Kaipiainen-Seppänen, O, Kröger, L, and Luosujärvi, R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol. (2003) 30:2460–8.
Keywords: psoriasis, arthritis, risk, pustular psoriasis, pustulosis palmoplantaris, psoriatic arthritis, TriNetX, cohort study
Citation: Gershater B, Bieber K, Vorobyev A, Ludwig MA, Zirpel H, De Luca DA, Thaci D, Kridin K and Ludwig RJ (2024) Differential risks of psoriatic arthritis development in patients with varied psoriasis manifestations: a sex- and ethnicity-specific analysis. Front. Med. 11:1385491. doi: 10.3389/fmed.2024.1385491
Edited by:
Sarah Ohrndorf, Charité University Medicine Berlin, GermanyReviewed by:
Florica Sandru, Elias University Emergency Hospital, RomaniaEnrique Roberto Soriano, Italian Hospital of Buenos Aires, Argentina
Copyright © 2024 Gershater, Bieber, Vorobyev, Ludwig, Zirpel, De Luca, Thaci, Kridin and Ludwig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralf J. Ludwig, cmFsZi5sdWR3aWdAdWtzaC5kZQ==
†These authors have contributed equally to this work
 Bernard Gershater
Bernard Gershater Katja Bieber
Katja Bieber Artem Vorobyev
Artem Vorobyev Marlene A. Ludwig3
Marlene A. Ludwig3 Henner Zirpel
Henner Zirpel David A. De Luca
David A. De Luca Diamant Thaci
Diamant Thaci Khalaf Kridin
Khalaf Kridin Ralf J. Ludwig
Ralf J. Ludwig