- 1Division of Cardiology, Department of Internal Medicine, Hanyang University College of Medicine, Hanyang University Guri Hospital, Guri-si, Republic of Korea
- 2Department of Internal Medicine, Gyeongsang National University School of Medicine and Cardiovascular Center, Gyeongsang National University Changwon Hospital, Changwon-si, Republic of Korea
- 3Department of Internal Medicine, Gyeongsang National University School of Medicine and Division of Cardiology, Gyeongsang National University Hospital, Jinju-si, Republic of Korea
- 4Sinai Center for Thrombosis Research and Drug Development, Sinai Hospital of Baltimore, Baltimore, MD, United States
- 5CAU Thrombosis and Biomarker Center, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong-si, Republic of Korea
- 6Division of Cardiology, Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Republic of Korea
Objective: The long-term clinical effect of arterial stiffness in high-risk disease entities remains unclear. The prognostic implications of brachial-ankle pulse wave velocity (baPWV) were assessed using a real-world registry that included patients who underwent percutaneous coronary intervention (PCI).
Methods: Arterial stiffness was measured using baPWV before discharge. The primary outcome was net adverse clinical events (NACE), defined as a composite of all-cause death, non-fatal myocardial infarction, non-fatal stroke, or major bleeding. Secondary outcomes included major adverse cardiac and cerebrovascular events (MACCE: a composite of all-cause death, non-fatal myocardial infarction, or non-fatal stroke), and major bleeding. The outcomes were assessed over a 4-year period.
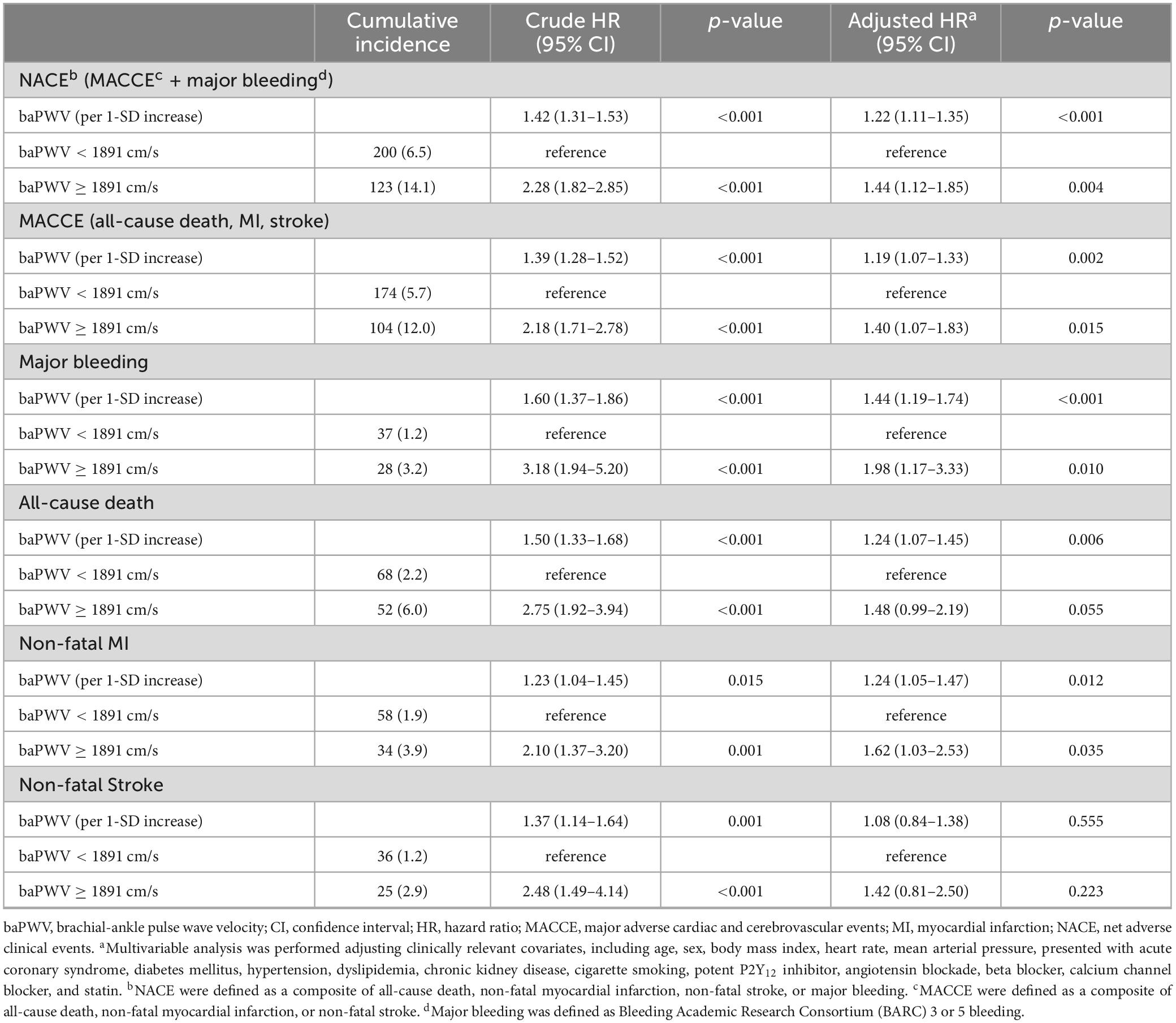
Results: Patients (n = 3,930) were stratified into high- and low-baPWV groups based on a baPWV cut-off of 1891 cm/s determined through time-dependent receiver operating characteristic curve analysis. baPWV was linearly correlated with 4-year post-PCI clinical events. The high baPWV group had a greater cumulative incidence of NACE, MACCE, and major bleeding. According to multivariable analysis, the high baPWV groups had a significantly greater risk of 4-year NACE (adjusted hazard ratio [HRadj]: 1.44; 95% confidence interval [CI]: 1.12–1.85; p = 0.004), MACCE (HRadj: 1.40; 95% CI: 1.07–1.83; p = 0.015), and major bleeding (HRadj: 1.94; 95% CI: 1.15–3.25; p = 0.012).
Conclusion: In PCI-treated patients, baPWV was significantly associated with long-term clinical outcomes, including ischemic and bleeding events, indicating its value for identifying high-risk phenotypes.
1 Introduction
Coronary artery disease (CAD) is a major global health issue, with an age-standardized prevalence rate of 3,610.2 per 100,000 people. Furthermore, it remains the leading cause of global cardiovascular (CV) disease mortality, with an age-standardized rate per 100,000 of 108.8 deaths (1). Percutaneous coronary intervention (PCI) is a widely recognized and established treatment for patients with acute coronary syndrome (ACS) and is performed selectively in patients with chronic coronary syndromes (2). Despite significant advances in interventional procedures and medical therapies, addressing the ongoing occurrence of adverse outcomes after PCI remains an important issue (3). Although traditional CV risk factors and various risk scores are currently used to target biological pathways and predict clinical outcomes, this approach does not comprehensively encompass the occurrence of future CV events (4). Cardiac biomarkers and non-invasive cardiac imaging tools play valuable roles in risk stratification and the prediction of future CV events. However, there are several concerns associated with these methods, including their cost, time requirements, potential side effects, and limited predictive power.
In recent years, interest in vascular function measurement in the CV field has increased. Arterial stiffness is a progressive vascular aging process that can be worsened by prolonged exposure to various noxious stimuli such as high blood pressure, hyperglycemia, inflammation, and oxidative stress (5). The measured arterial stiffness index has been significantly associated with the occurrence of future CV events not only in the general population but also in patients with various diseases, even after adjusting for traditional CV risk factors (6–12). Pulse wave velocity (PWV) is a reliable indicator of arterial stiffness and can be obtained by dividing the distance between two different arterial points by the difference in the arrival time of the waveform (13). Numerous studies have identified its incremental value in CV risk assessment. However, the current robust and expanding body of evidence primarily stems from studies conducted in the general population with limited information available on whether these individuals have established CV disease (9, 14, 15). In patients with significant CAD, the prognostic implication of PWV have recently been reported in several studies (7, 16–18), but most of them have limitations regarding sample size, duration of follow-up, and reliability of their results (7, 19). This real-world registry study aimed to investigate the potential association between elevated arterial stiffness measured using brachial-ankle PWV (baPWV) and long-term clinical outcomes in patients who underwent PCI.
2 Patients and methods
2.1 Study population
The study population was selected from the multicenter prospective Gyeongsang National University Hospital (G-NUH) registry (NCT04650529) (20). Consecutive patients with significant CAD who underwent PCI (Jinju and Changwon) between January 2010 and November 2018 were enrolled in this registry, which evaluated multiple vascular, hemostatic, and physiological parameters, if available. In the current analysis, we included patients who underwent baPWV measurement during index hospitalization. Of the 4,676 eligible patients, 746 with an ankle-brachial index ≤ 0.9 or >1.4 or uncontrolled arrhythmia because of insufficient accuracy. Finally, 3,930 participants were analyzed (Figure 1).
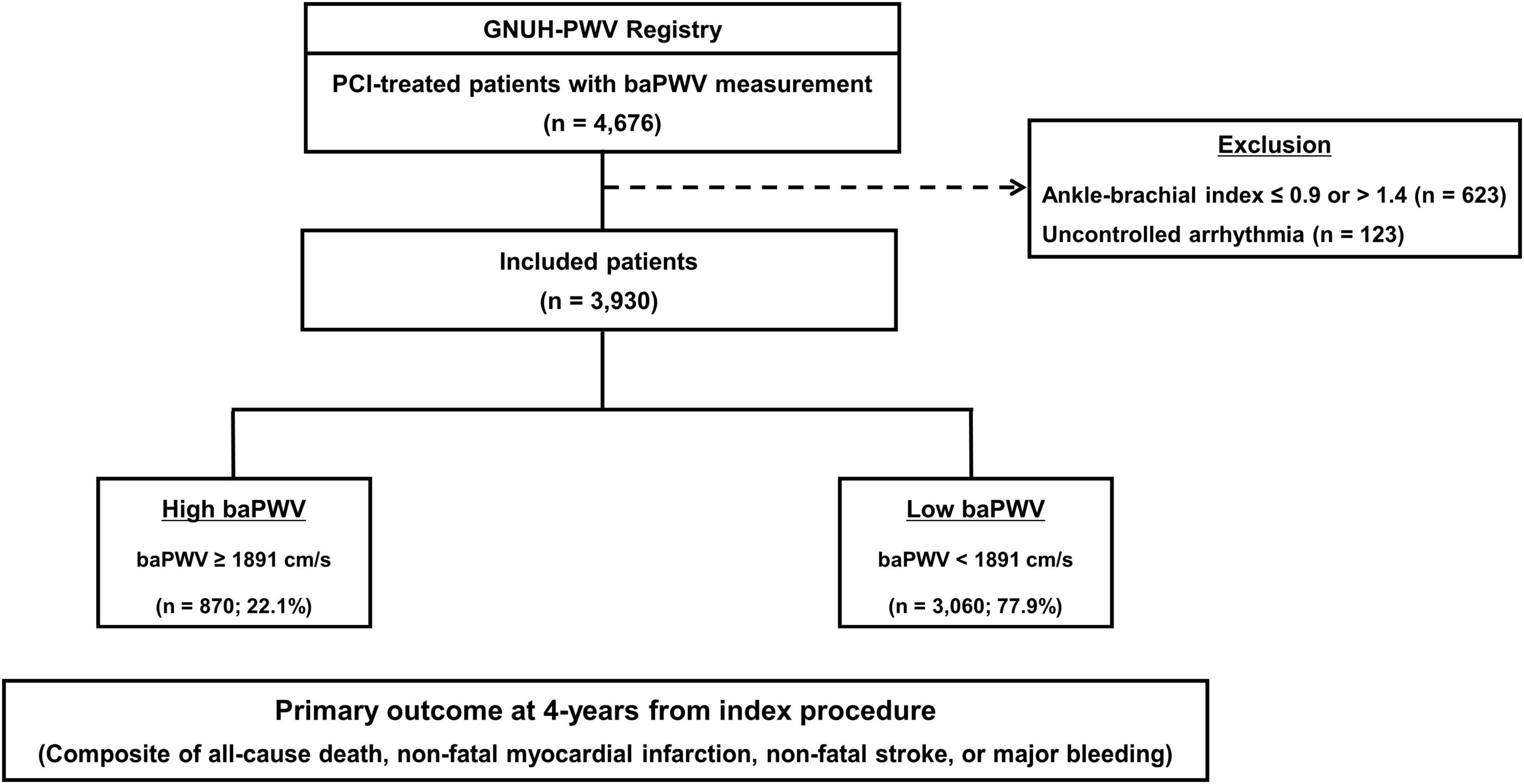
Figure 1. Flow diagram of the study. baPWV, brachial-ankle pulse wave velocity; PCI, percutaneous coronary intervention.
2.2 Patient management and procedures
The patients were treated according to standard practices at the respective hospitals. The choice of treatment strategy (stent implantation and medication) was left to the discretion of the operator based on guidelines (21, 22). Baseline demographic characteristics, CV risk factors, angiographic and procedural parameters, discharge medications, and clinical outcome data were collected through patient interviews or a review of medical records under the supervision of the principal investigator. Further information was collected via telephone, if necessary.
2.3 Brachial–ankle pulse wave velocity measurement
The baPWV measurement method has been described previously (6, 23). Briefly, the patient rested in a quiet, temperature-controlled room for at least 5 min before the examination. baPWV was measured using a commercially available volume plethysmographic device (VP-1000; Colin Co., Ltd., Komake, Japan). Pneumatic cuffs were applied to the brachial arteries and ankles bilaterally, electrocardiographic electrodes were placed on both wrists, and phonocardiographic electrodes were placed on the edge of the sternum. The baPWV was calculated by dividing the brachial-ankle distance by the transit time. Well-trained technicians blinded to the protocol evaluated the baPWV based on the approved protocol. In this study, we used the average baPWV values from the left and right measurements.
2.4 Study endpoint and definitions
The primary outcome was the occurrence of net adverse clinical events (NACE) within a 4-year period, characterized as a composite of all-cause death, non-fatal myocardial infarction (MI), non-fatal stroke, or major bleeding. Secondary outcomes included major adverse cardiac and cerebrovascular events (MACCE) and major bleeding, both of which were evaluated for up to 4 years. MACCE were defined as a composite of all-cause death, non-fatal myocardial infarction, or non-fatal stroke. Furthermore, major bleeding was specifically defined as Bleeding Academic Research Consortium (BARC) grade 3 or 5 bleeding (24). The other secondary outcomes included each MACCE component.
Clinical outcomes were defined according to the recommendations of the Academic Research Consortium (25). Non-fatal MI was defined as the recurrence of symptoms with the presence of electrocardiographic changes or imaging evidence of new loss of viable myocardium or new regional wall motion abnormalities in associated with an increase in cardiac biomarker levels above the upper limit of normal. Peri-procedural MI was not included as a clinical outcome. Stroke was defined as a neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause requiring hospitalization and was confirmed by a neurologist based on imaging results. All clinical events were evaluated by an independent event adjudication committee. After discharge from the index hospitalization, patients were routinely followed up at 1, 6, and 12 months after the index procedure and annually thereafter.
2.5 Statistical analysis
All categorical variables are presented as numbers and percentages, while continuous variables are presented as the means with standard deviations or medians with interquartile ranges (IQRs) depending on their distribution, which was assessed using the Kolmogorov–Smirnov test. Categorical variables were compared using the chi-squared test or Fisher’s exact test, whereas continuous variables were compared using Student’s t test or Mann–Whitney test.
To determine the optimal cut-off value of baPWV for predicting the primary outcome, we used a time-dependent receiver operating characteristic (ROC) curve analysis. Time-dependent ROC curve analysis was performed using sensitivity and 1 - specificity, which were obtained from various cut-off values of baPWV at 4 years. Youden’s index was used to calculate the optimal cut-off value. Additionally, we classified baPWV into three categories based on a previous study for sensitivity analysis: <1400, ≥1400 & <1800, and ≥1800 cm/s (26).
The associations between baPWV as a continuous variable and the risk of clinical outcomes were fitted using a restricted cubic spline curve with five knots. Cumulative event rates were estimated with the Kaplan–Meier curves and compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for primary and secondary outcomes based on the baPWV group were calculated using a Cox proportional hazard regression model. Multivariable Cox proportional hazard models were constructed considering clinically relevant variables such as age, sex, body mass index (BMI), heart rate, mean arterial pressure (systolic blood pressure × 1/3 + diastolic blood pressure × 2/3), presentation with ACS, cigarette smoking, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, and the use of potent P2Y12 inhibitors, angiotensin blockades, beta-blockers, calcium channel blockers, and statins. The reduced models were constructed through a backward elimination procedure using Akaike’s information criterion. Furthermore, subgroup analysis was performed using multivariable Cox proportional hazard models with specifications including age (≤65, >65 years), sex, ACS status, medical history of hypertension and diabetes mellitus, initial estimated glomerular filtration rate (eGFR, <60 or ≥60 mL/min/1.73 m2), and left ventricular ejection fraction (LVEF, <50 or ≥50%). The proportional hazards assumption for the variables in the model was assessed using scaled Schoenfeld residuals. Variable inflation factors (VIFs) were calculated for the variables in the multivariable models, and all the VIFs were below 2, indicating that multicollinearity was not a concern.
All the statistical analyses were conducted using the open-source statistical software R (version 4.2.3)1 and R-studio (version 2023.03.1)2 and the statistical packages, rms, descr, survival, tableone, survminer, ggplot2, timeROC, and plotRCS. All tests were two-tailed, and a p value of <0.05 was considered significant.
3 Results
3.1 baPWV and baseline characteristics
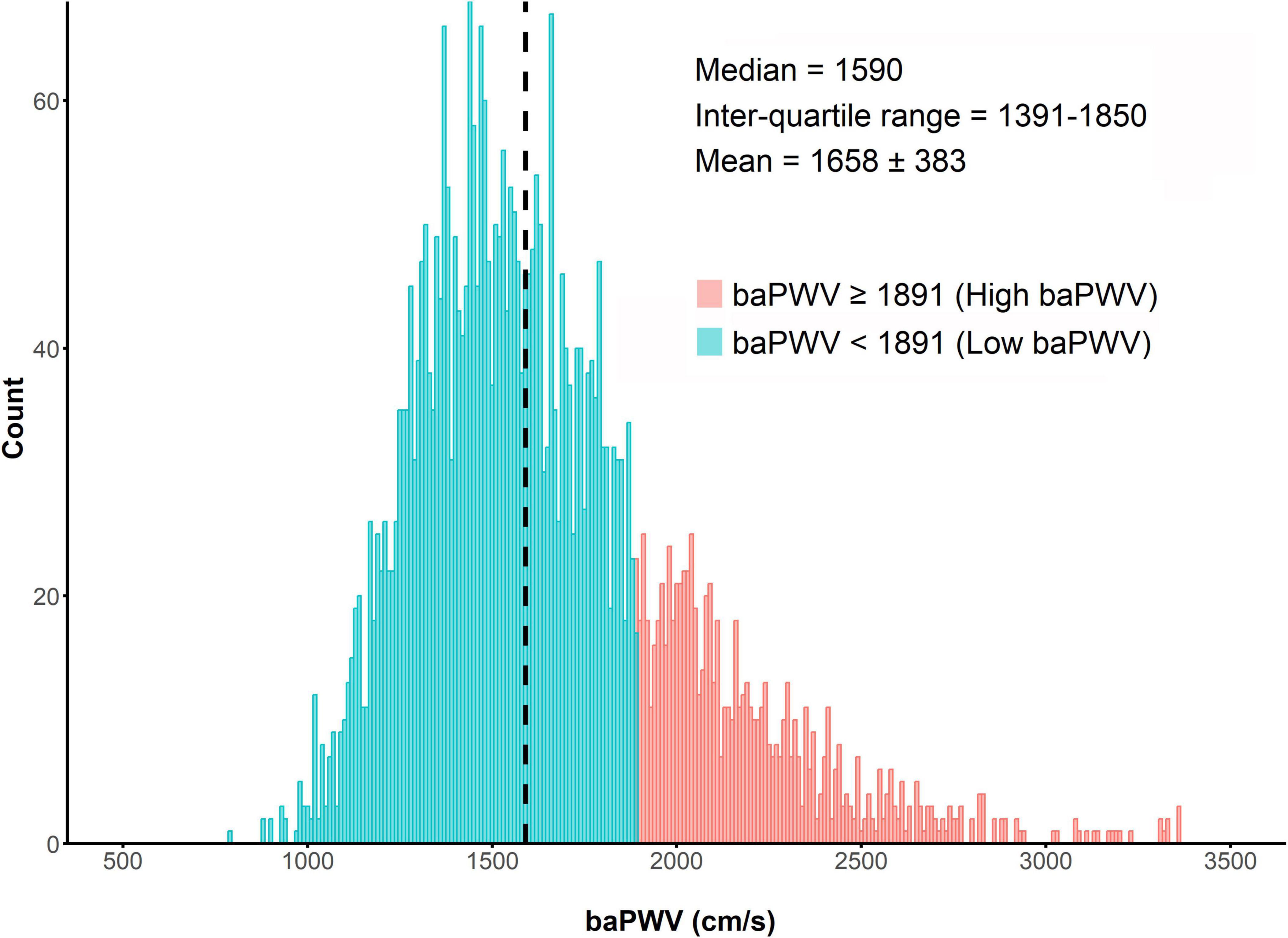
The distribution of baPWV values among the enrolled patients (n = 3,930) is shown in Figure 2. The median baPWV was 1590 cm/s (intertertile range: 1391, 1850). Supplementary Figure 1 shows the optimal cut-off for baPWV in predicting the primary outcome, which was identified as baPWV = 1891 cm/s. Patients were categorized into two groups according to the baPWV phenotype: 1) the high baPWV group (baPWV ≥ 1891 cm/s; n = 870); and 2) the low baPWV group (baPWV < 1891 cm/s; n = 3,060).
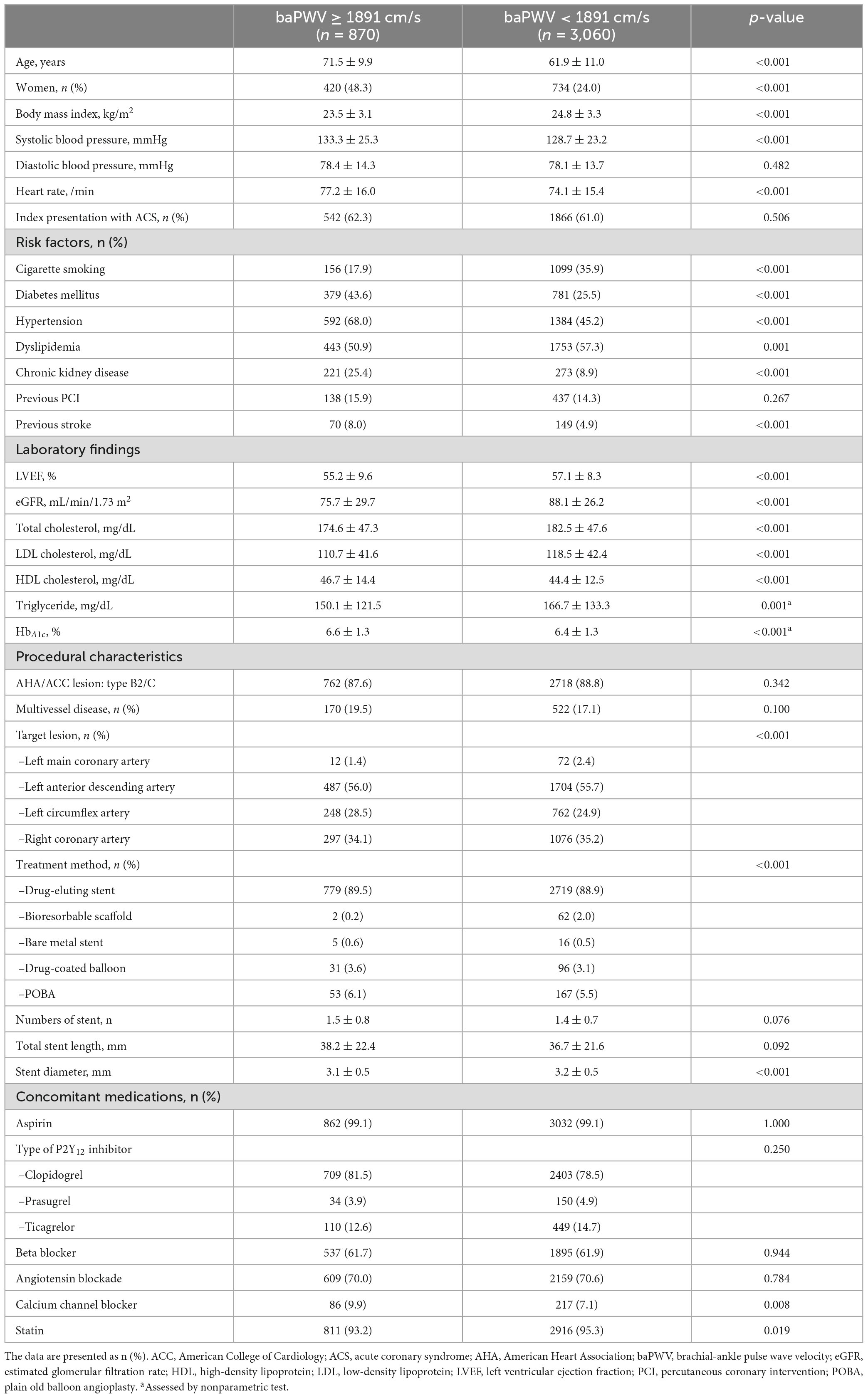
The baseline characteristics of the enrolled patients according to baPWV are presented in Table 1. The overall age was 64.0 ± 11.5 years, and approximately 70% were men. Approximately 60% of the cohort initially presented with ACS, and most of the patients were treated with drug-eluting stents. The high baPWV group was more likely to be older and have a greater prevalence of female sex and CV comorbidities (e.g., diabetes mellitus, hypertension, chronic kidney disease, and previous stroke) than the low baPWV group, whereas the low baPWV group was more likely to have a greater incidence of cigarette smoking and dyslipidemia than the high baPWV group. Prescriptions for concomitant medications were mostly similar between the groups.
3.2 baPWV and clinical outcomes
The median follow-up duration was 3.72 years (IQR: 1.56–6.47 years). During the follow-up period of up to 4 years, 323 (8.2%) NACE occurred in all patients. baPWV was linearly correlated with the risk of clinical events after PCI. baPWV was significantly associated with the risk of NACE (HR per 100 cm/s increase, 1.095; 95% CI, 1.074–1.118; p < 0.001), MACCE (HR per 100 cm/s increase, 1.091; 95% CI, 1.067–1.115; p < 0.001), and major bleeding (HR per 100 cm/s increase, 1.130; 95% CI, 1.085–1.176; p < 0.001) (Figure 3). When comparing clinical outcomes according to the baPWV cut-off value, patients with high baPWV had greater rates of NACE (unadjusted HR: 2.28; 95% CI: 1.82–2.85; p < 0.001), MACCE (unadjusted HR: 2.18; 95% CI: 1.71–2.78; p < 0.001), and major bleeding (unadjusted HR: 3.18; 95% CI: 1.94–5.20; p < 0.001) at the 4-year follow-up than did those with low baPWV (Figure 4).
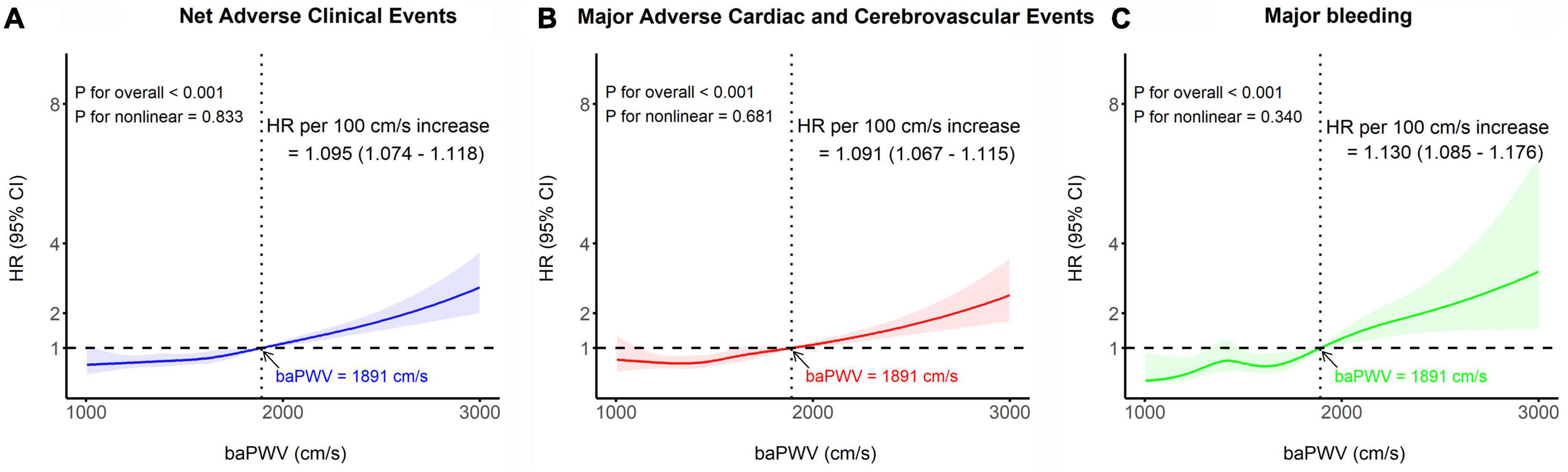
Figure 3. Restricted cubic spline analyses depicting the continuous association between baPWV and the risk of panel (A) NACE, (B) MACCE, and (C) major bleeding. The solid line represents the relative hazard in relation to the cut-off value of baPWV (1891 cm/s), with the shaded area indicating the 95% confidence intervals. baPWV, brachial-ankle pulse wave velocity; CI, confidence interval; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular event; NACE, net adverse clinical event.
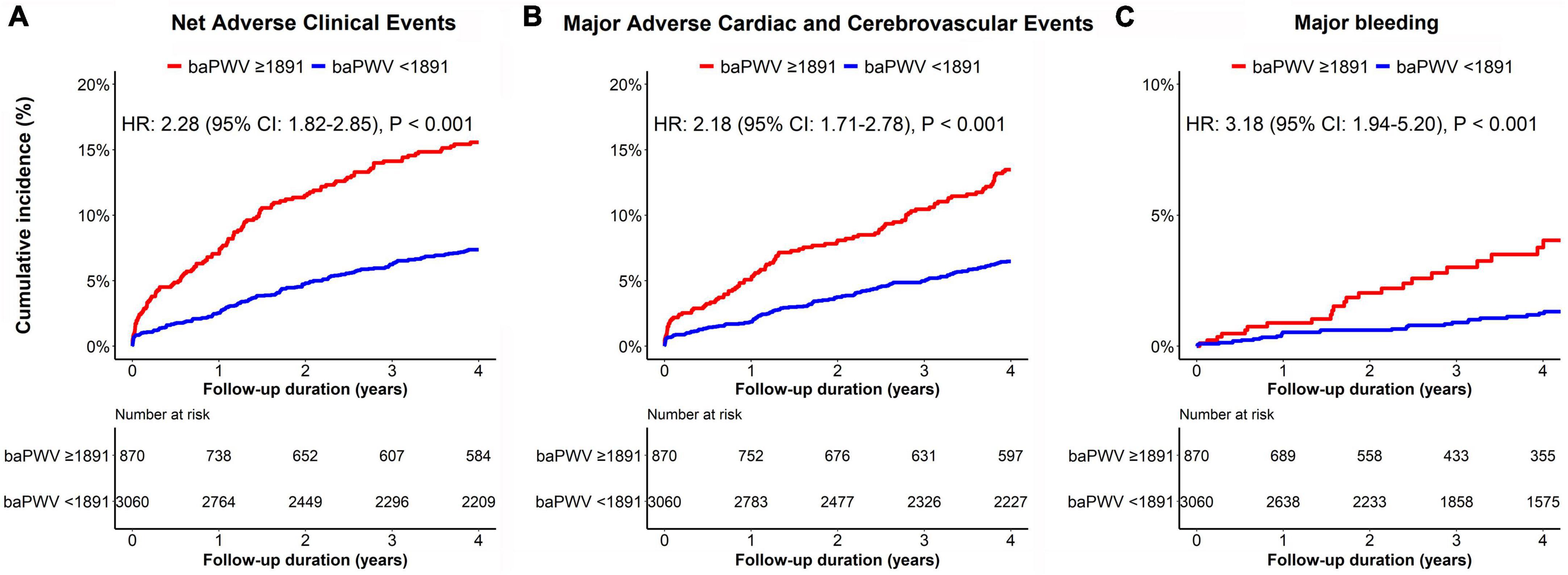
Figure 4. Kaplan–Meier curves for the cumulative incidence of panel (A) NACE, (B) MACCE, and (C) major bleeding according to the baPWV groups. The red line represents clinical events from patients with high baPWV (≥1891 cm/s), while the blue line represents clinical events from subjects with low baPWV (<1891 cm/s). baPWV, brachial-ankle pulse wave velocity; MACCE, major adverse cardiac and cerebrovascular event; NACE, net adverse clinical event.
After adjusting for clinically relevant variables, patients with high baPWV consistently had a greater risk of clinical events including NACE (adjusted HR 1.44; 95% CI,1.12–1.85; p = 0.004), MACCE (adjusted HR 1.40; 95% CI, 1.07–83; p = 0.015) and major bleeding (adjusted HR 1.94; 95% CI, 1.15–3.25; p = 0.012) than did those with low baPWV. The baPWV was also independently associated with the risk of clinical event occurrence as a continuous variable (per 1-SD increase) (Table 2). In the sensitivity analysis with baPWV classified into three categories, we observed a trend of higher risk of NACE with increasing baPWV categories; however, this did not reach statistical significance (Supplementary Table 1).
We evaluated the clinical impact of high baPWV on the occurrence of clinical events across subgroups (Figure 5). Compared to patients with baPWV values lower than the cutoff, patients with baPWV values higher than the cutoff showed a consistently worse prognosis in terms of NACE, MACCE, and major bleeding across all subgroups, with no significant interactions. In particular, clinical impact of high vs. low baPWV phenotype on NACE occurrence was consistently significant, regardless of age (>65 vs. ≤65 years old) (Supplementary Figure 2).
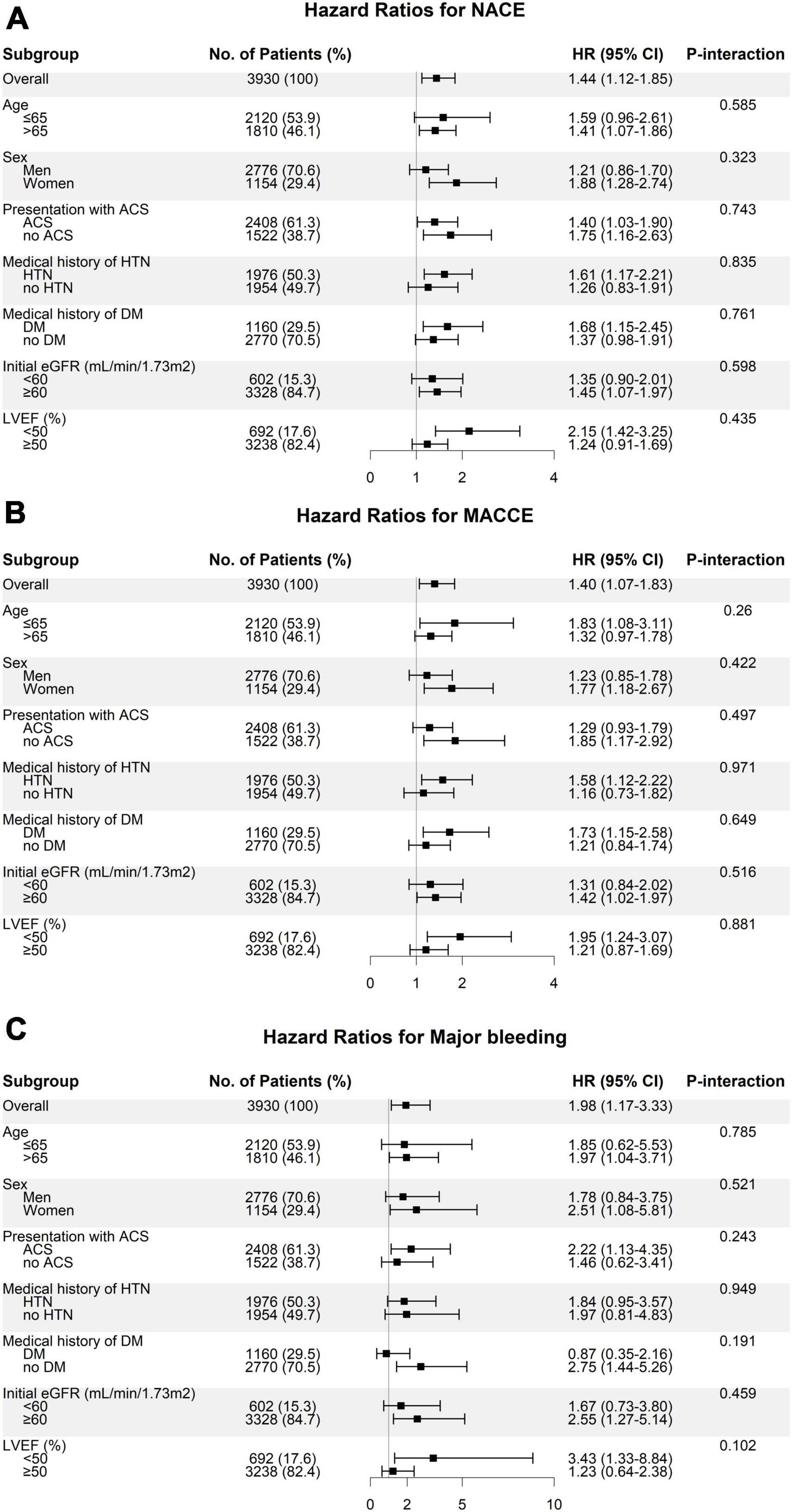
Figure 5. Subgroup analysis of the risk of panel (A) NACE, (B) MACCE, and (C) major bleeding according to the baPWV groups. Hazard ratios represent the risk of clinical outcomes in patients with high baPWV (≥1891 cm/s) compared to subjects with low baPWV (<1891 cm/s). ACS, acute coronary syndrome; baPWV, brachial-ankle pulse wave velocity; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular event; NACE, net adverse clinical event.
4 Discussion
To the best of our knowledge, the present study is the largest to evaluate the long-term association between the arterial stiffness index and clinical outcomes in high-risk patients with CAD. This is the first demonstration of a close link between baPWV and ischemic and bleeding events in patients who underwent PCI. The key findings were as follows: (1) the 4-year risks of ischemic and bleeding events increased with increasing baPWV values; (2) the optimal cutoff value of baPWV for predicting future clinical events was 1891 cm/s; and (3) patients with baPWV values higher than the cutoffs consistently exhibited a greater risk for clinical outcomes including NACE, MACCE, and major bleeding, even after adjusting for clinically relevant covariates.
Arterial stiffness serves as an early marker of functional and structural changes in the arterial walls. Extensive research has established that elevated arterial stiffness is an independent risk factor for atherosclerotic CV disease and has prognostic value beyond conventional risk factors. This association is observed not only in the general population but also in patients with various CV conditions (6–9).
Recent studies have highlighted an association between arterial stiffness and coronary atherosclerosis. However, these studies primarily demonstrated the correlation between the presence/severity of CAD and baPWV (7, 16, 27, 28). There is limited evidence regarding the long-term prognostic implications of baPWV in these populations (29–33). The current study provides the largest-sized clinical evidence to date demonstrating a link between baseline baPWV and long-term clinical outcomes in high-risk patients with CAD, specifically those who underwent PCI. Notably, this analysis also revealed a novel finding by establishing a significant relationship between baseline baPWV and the occurrence of major bleeding, in addition to ischemic events.
Several hypotheses have been proposed to explain the mechanisms underlying these findings. First, arterial stiffness may reflect the cumulative deleterious effects of CV risk factors on the arterial wall over a long period of time. Previous studies have consistently reported that PWV is closely influenced by major risk factors for CV events, including abnormal lipid phenotype, insulin resistance, and blood pressure. Our study also showed that patients with high PWV had more traditional CV risk factors and comorbidities than those with low PWV (26, 34, 35). Second, arterial stiffness is associated with endothelial dysfunction, platelet activation, and plaque vulnerability; the latter are significantly associated with the development and prognosis of CAD (36, 37). Third, arterial stiffness may influence the hemodynamic indices of coronary-vascular system that are associated with poor prognosis in patients with CAD. For example, arterial stiffness is positively associated with myocardial wall stress and negatively associated with coronary perfusion and coronary flow reserve (38–40). Finally, arterial stiffness may reflect the degree of vascular fragility (41, 42). Endothelial cells play a role in all major hemostatic pathways following vascular injury, limiting clot formation to areas where hemostasis is needed to restore vascular integrity. Although dual antiplatelet therapy is essential for reducing the risk of ischemic events after PCI, its potency can also increase the risk of bleeding. Therefore, an increase in arterial stiffness may be considered an indicator of vascular vulnerability related to risk of bleeding during antithrombotic therapy.
This study has several important clinical implications. First, PWV has been widely recognized in arterial hypertension guidelines as an indicator of target organ damage and a predictor of CV events (14, 43). Our findings provide valuable evidence for extending the clinical application of PWV to other disease groups, particularly those with high-risk profile such as CAD. Second, despite a significant reduction in the incidence of recurrent events after PCI compared to that in the past, it is still notably high (44). As identifying these high-risk groups remains essential, baPWV may be a promising marker for identifying patients at an elevated risk of recurrent events following successful PCI. The improved identification of high-risk patients will enable better risk stratification and more effective preventive therapies. In high-risk patients with elevated PWV, PWV can serve as a target or monitoring tool for interventions aimed at lowering CV risk. Pharmacological approaches such as antihypertensive drugs, statins, and nitrates, as well as non-pharmacological strategies (e.g., weight reduction, exercise, reduced salt intake, and moderate alcohol consumption), may offer potential benefits in managing this high-risk phenotype (13). However, further studies are needed to confirm whether reducing the PWV using these approaches directly improves the clinical prognosis of patients with CV disease. Further research is required to explore the prognostic utility of a PWV-guided approach for secondary prevention in patients with CAD who have undergone PCI.
This study has several limitations. First, the data were derived from a prospective, two-center observational registry. Despite rigorous adjustments for known risk factors, there were potentially unmeasured confounding factors. Second, although approximately 4,000 patients were consecutively included in the analysis, there was a possibility of selection bias because we excluded relatively common comorbidities such as peripheral artery disease and arrhythmia, which could affect the accuracy of baPWV. However, this study included a large sample size with a comparable representation of all-comers and prospective data collection by well-trained coordinators. Third, important variables such as antiplatelet agent compliance and post-PCI smoking cessation were not available, which could have influenced the observed outcomes. Fourth, the use of various medications, such as antiplatelet agents, statins, and antihypertensive agents, during or after PCI may have influenced the baPWV measurements. Finally, baPWV values were based on baseline measurements, and follow-up baPWV values were not considered.
5 Conclusion
The arterial stiffness index, as assessed using baPWV, was significantly associated with long-term adverse clinical outcomes, including both ischemic and bleeding events, in patients with CAD who underwent PCI. These findings suggest that baPWV measurements may serve as a valuable tool for risk stratification and prognostic assessment in high-risk patients with CAD, providing important clinical implications for the management and prevention of future CV events. Further research is warranted to explore the potential benefits of interventions that target arterial stiffness in this patient population.
Data availability statement
The datasets used in this study are not publicly available due to restrictions related to hospital-based data. Requests for access to the datasets should be directed to Y-HJ, Z29vZG9jdG9yQG5hdmVyLmNvbQ==.
Ethics statement
The studies involving humans were approved by the respective hospitals of institutional registry. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study used anonymized institutional registry data (NCT04650529).
Author contributions
BK: Writing – original draft, Data curation, Formal analysis. J-HS: Writing – original draft, Methodology, Formal analysis, Visualization. J-HA: Data curation, Writing – review and editing. MK: Data curation, Validation, Writing – review and editing. K-HK: Data curation, Writing – review and editing. JB: Methodology, Writing – review and editing. YC: Data curation, Writing – review and editing. J-SK: Data curation, Writing – review and editing. YP: Data curation, Writing – review and editing. S-JH: Data curation, Writing – review and editing. UT: Data curation, Writing – review and editing. PG: Data curation, Writing – review and editing. J-YH: Conceptualization, Writing – review and editing. Y-HJ: Conceptualization, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research fund from Hanyang University (HY- 202400000001252).
Conflict of interest
PG has received grants and personal fees from Bayer HealthCare, Otitopic, Amgen, and Janssen, US World-Meds; grants from Instrumentation Laboratory, Hikari Dx, Haemonetics, Medicure, and Idorsia Pharmaceuticals; and personal fees from UpToDate and has patents “Detection of Restenosis Risk in Patients Issued” and “Assessment of Cardiac Health and Thrombotic Risk in a Patient.” Y-HJ has received honoraria for lectures from Daiichi Sankyo, Sanofi-Aventis, Amgen, Han-mi Pharmaceuticals, and Dae-woong Pharmaceuticals; and research grants or support from Yuhan Pharmaceuticals, Han-mi Pharmaceuticals, Sam-jin Pharmaceuticals, Biotronik, and U&I Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding agencies.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1384981/full#supplementary-material
Abbreviations
ACS, acute coronary syndrome; ANOVA, analysis of variance; ARC, Academic Research Consortium; baPWV, brachial-ankle pulse wave velocity; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; CI, confidence interval; CV, cardiovascular; HBR, High Bleeding Risk; HR, hazard ratio; IQR, interquartile range; MACCE, major adverse cardiac and cerebrovascular event; MI, myocardial infarction; NACE, net adverse clinical events; PCI, percutaneous coronary intervention; PWV, pulse wave velocity; ROC, receiver operating characteristic; VIFs, variance inflation factors.
Footnotes
References
1. Mensah G, Fuster V, Murray C, Roth G, Mensah G, Abate Y, et al. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol. (2023) 82(25):2350–473. doi: 10.1016/j.jacc.2023.11.007
2. Angiolillo D, Galli M, Collet J, Kastrati A, O’Donoghue M. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. (2022) 17(17):e1371–96.
3. Alkhouli M, Alqahtani F, Kalra A, Gafoor S, Alhajji M, Alreshidan M, et al. Trends in characteristics and outcomes of patients undergoing coronary revascularization in the United States, 2003-2016. JAMA Netw Open. (2020) 3(2):e1921326. doi: 10.1001/jamanetworkopen.2019.21326
4. Simard T, Jung R, Di Santo P, Harnett D, Abdel-Razek O, Ramirez F, et al. Modifiable risk factors and residual risk following coronary revascularization: insights from a regionalized dedicated follow-up clinic. Mayo Clin Proc Innov Qual Outcomes. (2021) 5(6):1138–52. doi: 10.1016/j.mayocpiqo.2021.09.001
5. Lee G, Kim H. Incremental value of the measures of arterial stiffness in cardiovascular risk assessment. Rev Cardiovasc Med. (2022) 23(1):6.
6. Park J, Sharman J, Li Y, Munakata M, Shirai K, Chen C, et al. Expert consensus on the clinical use of pulse wave velocity in Asia. Pulse. (2022) 10(1–4):1–18. doi: 10.1159/000528208
7. Kim H, Kim S. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. (2019) 6:41. doi: 10.3389/fcvm.2019.00041
8. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55(13):1318–27.
9. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. (2010) 31(19):2338–50. doi: 10.1093/eurheartj/ehq165
10. Willum-Hansen T, Staessen J, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. (2006) 113(5):664–70.
11. Mitchell G, Hwang S, Vasan R, Larson M, Pencina M, Hamburg N, et al. Arterial stiffness and cardiovascular events: the framingham heart study. Circulation. (2010) 121(4):505–11.
12. Kim J, Kim S, Kim I, Kim J, Kim B, Kim M, et al. Arterial stiffness is an independent predictor for risk of mortality in patients with type 2 diabetes mellitus: the REBOUND study. Cardiovasc Diabetol. (2020) 19(1):143. doi: 10.1186/s12933-020-01120-6
13. Cavalcante J, Lima J, Redheuil A, Al-Mallah M. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. (2011) 57(14):1511–22.
14. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. (2018) 39(33):3021–104.
15. Boutouyrie P, Tropeano A, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. (2002) 39(1):10–5. doi: 10.1161/hy0102.099031
16. Prskalo Z, Brizić I, Markota D, Markota I, Boban M, Tomic M, et al. Arterial stiffness in patients with coronary artery disease: relation with in-stent restenosis following percutaneous coronary intervention. BMC Cardiovasc Disord. (2016) 16:128. doi: 10.1186/s12872-016-0305-4
17. Hametner B, Wassertheurer S, Mayer C, Danninger K, Binder R, Weber T. Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography. Hypertension. (2021) 77(2):571–81.
18. Fang Y, Zhong Q. Investigation of the value of carotid-femoral pulse wave velocity and coronary artery lesions in prognosis of percutaneous coronary intervention patients. Am J Transl Res. (2021) 13(6):6646–53.
19. Sequí-Domínguez I, Cavero-Redondo I, Álvarez-Bueno C, Pozuelo-Carrascosa D. Nuñez de Arenas-Arroyo S, Martínez-Vizcaíno V. accuracy of pulse wave velocity predicting cardiovascular and all-cause mortality. a systematic review and meta-analysis. J Clin Med. (2020) 9(7):2080.
20. Lee S, Kim H, Ahn J, Kang M, Kim K, Bae J, et al. Prognostic impact of hypercoagulability and impaired fibrinolysis in acute myocardial infarction. Eur Heart J. (2023) 44(19):1718–28. doi: 10.1093/eurheartj/ehad088
21. Neumann F, Sousa-Uva M, Ahlsson A, Alfonso F, Banning A, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165.
22. Lawton J, Tamis-Holland J, Bangalore S, Bates E, Beckie T, Bischoff J, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(3):e4–17.
23. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. (2002) 25(3):359–64.
24. Mehran R, Rao S, Bhatt D, Gibson C, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123(23):2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
25. Garcia-Garcia H, McFadden E, Farb A, Mehran R, Stone G, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research Consortium-2 consensus document. Circulation. (2018) 137(24):2635–50.
26. Wu Z, Jiang Y, Zhu Q, Zhang H, Li Z, Wang J, et al. Combined evaluation of arterial stiffness and blood pressure promotes risk stratification of peripheral arterial disease. JACC Asia. (2023) 3(2):287–97. doi: 10.1016/j.jacasi.2023.02.001
27. Cainzos-Achirica M, Rampal S, Chang Y, Ryu S, Zhang Y, Zhao D, et al. Brachial-ankle pulse wave velocity is associated with coronary calcium in young and middle-aged asymptomatic adults: The Kangbuk Samsung health study. Atherosclerosis. (2015) 241(2):350–6. doi: 10.1016/j.atherosclerosis.2015.05.031
28. Torii S, Arima H, Ohkubo T, Fujiyoshi A, Kadota A, Takashima N, et al. Association between pulse wave velocity and coronary artery calcification in Japanese men. J Atheroscler Thromb. (2015) 22(12):1266–77.
29. Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, et al. Combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol. (2014) 64(3):179–84. doi: 10.1016/j.jjcc.2014.01.004
30. Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, et al. Brachial - ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. (2005) 69(7):815–22. doi: 10.1253/circj.69.815
31. Akkus O, Sahin D, Bozkurt A, Nas K, Ozcan K, Illyés M, et al. Evaluation of arterial stiffness for predicting future cardiovascular events in patients with ST segment elevation and non-ST segment elevation myocardial infarction. ScientificWorldJournal. (2013) 2013:792693. doi: 10.1155/2013/792693
32. Ki Y, Choi D, Lee Y, Lim L, Song H, Koh Y. Predictive value of brachial-ankle pulse wave velocity for long-term clinical outcomes after percutaneous coronary intervention in a Korean cohort. Int J Cardiol. (2014) 175(3):554–9. doi: 10.1016/j.ijcard.2014.06.032
33. Park H, Kim H, Kang M, Kim K, Koh J, Park J, et al. Predictive value of the combination of brachial-ankle pulse wave velocity and ankle-brachial index for cardiovascular outcomes in patients with acute myocardial infarction. Coron Artery Dis. (2020) 31(2):157–65. doi: 10.1097/MCA.0000000000000777
34. Wu Z, Wang J, Zhang H, Pan H, Li Z, Liu Y, et al. Longitudinal association of remnant cholesterol with joint arteriosclerosis and atherosclerosis progression beyond LDL cholesterol. BMC Med. (2023) 21(1):42. doi: 10.1186/s12916-023-02733-w
35. Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. (2021) 20(1):134. doi: 10.1186/s12933-021-01330-6
36. Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J. (2010) 74(1):24–33. doi: 10.1253/circj.cj-09-0534
37. Beaussier H, Masson I, Collin C, Bozec E, Laloux B, Calvet D, et al. Carotid plaque, arterial stiffness gradient, and remodeling in hypertension. Hypertension. (2008) 52(4):729–36.
38. Leung M, Meredith I, Cameron J. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am J Physiol Heart Circ Physiol. (2006) 290(2):H624–30. doi: 10.1152/ajpheart.00380.2005
39. Fukuda D, Yoshiyama M, Shimada K, Yamashita H, Ehara S, Nakamura Y, et al. Relation between aortic stiffness and coronary flow reserve in patients with coronary artery disease. Heart. (2006) 92(6):759–62.
40. Klug G, Feistritzer H, Reinstadler S, Krauter L, Mayr A, Mair J, et al. Association of aortic stiffness with biomarkers of myocardial wall stress after myocardial infarction. Int J Cardiol. (2014) 173(2):253–8. doi: 10.1016/j.ijcard.2014.02.038
41. Acampa M, Camarri S, Lazzerini P, Guideri F, Tassi R, Valenti R, et al. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int J Cardiol. (2017) 243:466–70. doi: 10.1016/j.ijcard.2017.03.129
42. Rosenblum H, Pinsino A, Zuver A, Javaid A, Mondellini G, Ji R, et al. Increased aortic stiffness is associated with higher rates of stroke, gastrointestinal bleeding and pump thrombosis in patients with a continuous flow left ventricular assist device. J Card Fail. (2021) 27(6):696–9. doi: 10.1016/j.cardfail.2021.02.009
43. Lee H, Shin J, Kim G, Park S, Ihm S, Kim H, et al. 2018 Korean society of hypertension guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertension. (2019) 25(1):20. doi: 10.1186/s40885-019-0124-x
Keywords: coronary artery disease, arterial stiffness, pulse wave velocity, percutaneous coronary intervention, clinical outcome
Citation: Kim BS, Ahn J-H, Shin J-H, Kang MG, Kim K-H, Bae JS, Cho YH, Koh J-S, Park Y, Hwang S-J, Tantry US, Gurbel PA, Hwang J-Y and Jeong Y-H (2024) Long-term prognostic implications of brachial-ankle pulse wave velocity in patients undergoing percutaneous coronary intervention. Front. Med. 11:1384981. doi: 10.3389/fmed.2024.1384981
Received: 11 February 2024; Accepted: 16 May 2024;
Published: 07 June 2024.
Edited by:
Udhaya Kumar, Baylor College of Medicine, United StatesReviewed by:
Zhiyuan Wu, Harvard University, United StatesMohd Mughees, The University of Texas MD Anderson Cancer Center, United States
Anton Camaj, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2024 Kim, Ahn, Shin, Kang, Kim, Bae, Cho, Koh, Park, Hwang, Tantry, Gurbel, Hwang and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeong-Hun Shin, Y2FyZGlvLmh5YXBleEBnbWFpbC5jb20=; Young-Hoon Jeong, Z29vZG9jdG9yQG5hdmVyLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Byung Sik Kim
Byung Sik Kim Jong-Hwa Ahn2†
Jong-Hwa Ahn2† Jeong-Hun Shin
Jeong-Hun Shin Min Gyu Kang
Min Gyu Kang Kye-Hwan Kim
Kye-Hwan Kim Jin-Sin Koh
Jin-Sin Koh Udaya S. Tantry
Udaya S. Tantry Paul A. Gurbel
Paul A. Gurbel Young-Hoon Jeong
Young-Hoon Jeong