- 1Shanghai Eye Diseases Prevention & Treatment Center/Shanghai Eye Hospital, School of Medicine, Tongji University, Shanghai, China
- 2National Clinical Research Center for Eye Diseases, Shanghai Engineering Research Center of Precise Diagnosis and Treatment of Eye Diseases, Shanghai, China
- 3Eye Institute and Department of Ophthalmology, Eye & ENT Hospital, Fudan University, Shanghai, China
- 4NHC Key Laboratory of Myopia and Related Eye Diseases, Chinese Academy of Medical Science, Shanghai, China
- 5Shanghai Key Laboratory of Visual Impairment and Restoration, Shanghai, China
Purpose: To compare corneal biomechanical properties and intraocular pressure (IOP) measurements in patients who underwent Descemet’s stripping with endothelial keratoplasty (DSEK) with those of the follow healthy eyes.
Methods: In this retrospective comparative study, a total of 35 eyes of 35 patients who underwent DSEK by a single surgeon from 2015.02 to 2019.12 were enrolled along with their fellow healthy eyes. Corneal biomechanical parameters were assessed at least 3 months post-DSEK using Corneal Visualization Scheimpflug Technology (CST). IOP was measured by CST, Goldmann applanation tonometry (GAT), and MacKay-Marg tonometer.
Results: Central corneal thickness (CCT) and stiffness parameter at first applanation (SP-A1) were significantly increased after DSEK when compared to the fellow eyes. In DSEK eyes, biomechanically-corrected intraocular pressure (bIOP) and MacKay-Marg IOP correlated significantly with GAT IOP measurements, with bIOP showed the lowest IOP values. All the IOP values did not correlate with CCT. However, GAT-IOP and MacKay-Marg IOP showed a positive correlation with SP-A1.
Conclusion: The corneal stiffness increased after DSEK. Central corneal thickness may have less influence than corneal biomechanics on IOP measurements in eyes after DSEK. Biomechanically-corrected IOP obtained by CST seemed to be lower than other tonometry techniques in DSEK eyes, perhaps because of correction for corneal stiffness, CCT and age.
1 Introduction
Over the last decade, Descemet’s stripping with endothelial keratoplasty (DSEK), a selective replacement of the diseased corneal endothelium, has become the most commonly performed procedure for treating corneal endothelial dysfunction (1). This technique surpass penetrating keratoplasty in terms of rapid visual recovery, preserved corneal sensation, tectonic stability, and absence of suture-related complications (1, 2). Post-DSEK intraocular pressure (IOP) elevation, one of the most common complications, accelerates primary graft failure (3). The reported incidence ranges from 16.7 to 54%, mostly due to cumulative use of corticosteroids (4–10). Therefore, as with all types of keratoplasty, IOP monitoring after DSEK remains essential.
However, an accurate measurement of IOP after DSEK remains challenging. For example, Goldmann applanation tonometer (GAT) is considered as the gold standard for IOP measurement, but a growing body of research suggests that it is inevitably affected by corneal biomechanics and central corneal thickness (CCT) (11, 12), which is affected by the additional donor graft in DSEK (13, 14). Tonopen is a hand-held electronic tonometer based on the MacKay-Marg principle, but flattens the cornea in a smaller area compared to GAT, thus reducing the difference between the flattening pressure and the real IOP (15). It has been reported that Tono-Pen XL was less affected by CCT in eyes that underwent penetrating keratoplasty (16).
Corneal biomechanics is the study of corneal deformation in response to external forces. It can be analyzed in vivo with both the Ocular Response Analyzer (ORA) and the Corvis ST Tonometer (CST). In particular, CST can provide assessments of specific changes in the corneal elastic properties based on its ultra-high-speed Scheimpflug technology (17). It has been reported that CST is more sensitive to corneal biomechanical changes after cataract surgery than the ORA (18, 19). Recently, biomechanically-corrected intraocular pressure (bIOP), a newly released CST parameter, was corrected for corneal stiffness, CCT and age (20).
The purpose of our study was to investigate the effects of DSEK on corneal biomechanics via CST and to explore the relationship between corneal biomechanics and IOP obtained by CST, GAT, and MacKay-Marg tonometer.
2 Materials and methods
2.1 Patients
This retrospective comparative study included 35 patients aged 18–85 years old, who underwent DSEK for the treatment of corneal endothelial decompensation and had healthy contralateral eyes by one surgeon at the Eye & ENT Hospital of Fudan University (Shanghai, China). Exclusion criteria for the post-DSEK eyes were as follows: (1) operative eyes had corneal stromal layer surgery history or trauma, (2) graft detachment with air injection, (3) persistent epithelial defect, (4) graft failure or endothelial immunologic rejection within 3 months postoperative, (5) operative eyes undergone any other ocular surgery during follow-up period. Exclusion criteria for the healthy fellow eyes were corneal abnormalities such as guttae, edema, scars; glaucoma; history of ocular surgery or other ocular abnormalities. This study was approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University (Approval No. 2015020) and adhered to the tenets of the Declaration of Helsinki.
2.2 Assessments
All the participants underwent a routine examination including slit lamp biomicroscopy and best corrected visual acuity to check the exclusion criteria. Corneal bio-mechanical properties were assessed by a single investigator using the Corvis ST (CST; Oculus, Wetzlar, Germany). As previously described, this instrument releases a rapid air puff of air onto the cornea and captures the entire corneal deformation process using an ultra-high-speed Scheimpflug camera (17). The corneal response is divided into an inward applanation (flattening), deformation to maximum concavity, and the second outward applanation as the cornea returns to its original shape. The recorded images were analyzed via the built-in CST software (ver. 1.3r1538).
In addition to the CST test, IOP was also measured by GAT and MacKay-Marg tonometer (TonoPen AVIA; Reichert Inc., Buffalo, New York) according to manufacturers’ instructions. For MacKay-Marg tonometer measurements, only values with a coefficient of variation of 5% or less were accepted. A total of three consecutive measurements were obtained and averaged for each patient.
2.3 Statistical analyses
Statistical analyses were performed using SPSS 22.0 software. All statistics were presented as mean ± standard deviation. The Kolmogorov–Smirnov test was used to assess the normality of the data. If the data were normally distributed, a two-tailed paired Student’s t-test with Bonferroni correction was used for multiple comparisons, otherwise a Wilcoxon signed rank test was used for the statistical analysis with the contralateral eye as the control. One way ANOVA with Bonferroni correction was used to compare the differences between three IOP measurements. Bland–Altman analysis was performed to demonstrate the agreement between the three IOP measurements. The correlation between IOP and corneal biomechanical parameters was analyzed by Pearson’s correlation coefficient. A p < 0.05 was considered statistically significant.
3 Results
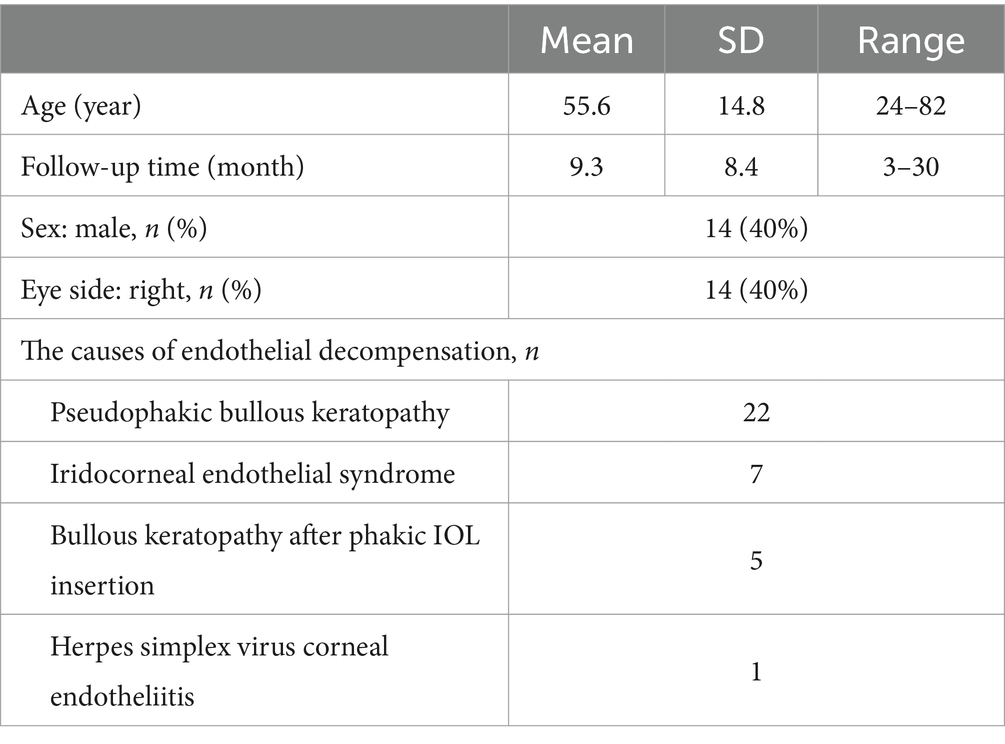
As shown in Table 1, a total of 35 patients, 14 males and 21 females, who underwent DSEK in one eye were enrolled in this study, with a mean age of 55.7 ± 14.8 years (range, 24–82 years). The mean followed-up was 9.3 ± 8.4 months (3–30 months). The initial causes of corneal endothelial decompensation were peudophakic bullous keratopathy (n = 22), iridocorneal endothelial syndrome (n = 7), bullous keratopathy after phakic IOL insertion (n = 5), and herpes simplex virus corneal endotheliitis (n = 1). All enrolled subjects maintained clear corneas, insignificant edema, and no graft failure during follow-up.
Corneal biomechanical results are summarized in Table 2. Overall, statistical differences were observed between the two groups in applanation velocity (A1V; p = 0.004) and stiffness parameter at first applanation (SP-A1) (p < 0.0001). The SP-A1 is a novel indicator for corneal stiffness (21). Significantly higher SP-A1 values were found in operated eyes compared to the contra-lateral eyes (149.84 ± 29.22 mmHg/mm vs. 125.4 ± 20.18 mmHg/mm). CCT was significantly increased in operated eyes compared to normal eyes (660.5 ± 99.81 μm vs. 565.56 ± 57.78 μm, p < 0.0001). A slight but not significant decrease in peak distance (PD) and an increase in integrated radius (IR) were observed. These results indicate an increased corneal stiffness after DSEK.

Table 2. Corneal biomechanical parameters and different intraocular pressure measurements in post-DSEK eyes and contralateral healthy eyes.
The results of the IOP measurements are summarized in Table 2 with no statistical differences between the operated eyes and the contralateral eyes. All IOP values in the operated eyes measured by Corvis ST, GAT, and Tonopen AVIA were positively correlated with each other (Figures 1A–C). In post-DSEK eyes, bIOP showed the lowest IOP, while GAT IOP and MacKay-Marg IOP were similar as shown in Table 3. Bland–Altman analysis showed the 95% limits of agreement between these three tonometers in the post-DSEK eye group (Figures 1D–F). The range of MacKay-Marg—Corvis ST difference (95% LoA, −10.2 to +6.3 mmHg) was the highest, followed by GAT-Corvis ST difference (95% LoA, −4.8 to +9.4 mmHg).

Figure 1. Correlation and agreement between the different tonometry methods in post-DSEK eyes. (A–C) Scatter graphs show the correlations of intraocular pressure measurements obtained by Corvis ST, Goldmann applanation, and MacKay-Marg tonometer (Tonopen AVIA), respectively. (D–F) Bland–Altman plots show the agreement of intraocular pressure measurements obtained by Corvis ST, Goldmann applanation, and MacKay-Marg tonometer, respectively.
As shown in Table 4, correlation analyses were performed between the corneal bio-mechanical parameters and the IOP values of the post-DSEK eyes measured by Corvis ST, GAT, and MacKay-Marg tonometer. Most of the biomechanical parameters were strongly correlated with IOPs obtained from the three tonometry techniques. SP-A1 was positively correlated to GAT-IOP and MacKay-Marg IOP, whereas bIOP was not affected by SP-A1, suggesting that corneal stiffness had no impact on bIOP measurement. Of note, no significant correlation was found between CCT and any of these tonometers.

Table 4. Association between Corneal Biomechanical Parameters and different Intraocular Pressure measurements in post-DSEK eyes.
4 Discussion
Adequate management of IOP elevation after DSEK requires accurate measurement of intraocular pressure. Understanding the changes in corneal biomechanics after DSEK will help to better understand IOP measurement. In this study, we investigated the effects of DSEK surgery on corneal biomechanics using Corvis ST. As expected, CCT was significantly increased after DSEK compared to the fellow eyes. Significant enhancement of other CST parameters including A1T, A1V, A2T, and SP-A1 were also observed. In addition, we compared the newly released CST parameter, bIOP, with IOP values measured by two traditional and widely used tonometers, GAT and MacKay-Marg tonometer (Tonopen AVIA). The IOP measured by CST showed the lowest value compared to GAT and MacKay-Marg both in post-DSEK eyes and in fellow healthy eyes. In the light of some previous works that have investigated post-DSEK IOP measurement using techniques such as GAT and Tono-Pen (22), this study, to our knowledge, is the first approach to measure post-DSEK IOP using CST and to analyze the association of corneal biomechanical properties with IOP measurement after DSEK.
The new parameter SP-A1 was introduced to represent corneal stiffness, which was believed to have no correlation with corneal volume and age, and may reflect the changes of corneal stiffness closer to the real value (21). Corneas with higher ocular stiffness will have a higher value of SP-A1 (21). In this study, SP-A1 was significantly increased after DSEK, indicating the increased corneal stiffness. One potential explanation is that the transplanted cornea with additional donor grafts was significantly thicker than that of normal eyes, which required greater flattening pressure to induce corneal flattening reaction corresponding to prolonged first applanation time (A1T), reduced first applanation velocity (A1V), and shortened second applanation time (A2T) during corneal rebound. Although a corneal endodermis contributes little to corneal biomechanical properties, it is critical to keep the stroma from edema. As a hydrated tissue, the water content of the cornea has a critical effect on its biomechanical properties including tissue stiffness. Previous in vitro studies using unconfined compression tests and uniaxial tensile experiments have found corneal hydration level to be negative contributor to corneal tensile and compressive stiffness (23, 24). Meanwhile, SP-A1 was believed negatively correlated with corneal curvature, which was often decreased after DSEK due to grafts (25, 26). Some other variables may also be associated with SP-A1. For instance, an increase in IR and a decrease in PD may be responsible for the increase in corneal stiffness. In this study, post-DSEK PD and IR were found minimally decreased and increased without significant difference, respectively, suggesting that these variables may be less sensitive to corneal biomechanical measurements after DSEK compared to SP-A1. In the older version of CST, the highest concavity deformation amplitude (HCDA) and highest concavity radius (HCR) were obtained to analysis the ocular rigidity. High HCDA value and low HCR were associated with low ocular rigidity, corresponding to prolonged A1T, decreased A1V, and shortened A2T (27). However, no statistical difference in HCDA and HCR was found between post-DSEK eyes and the contralateral eyes in this study, which was in consistent with the previous results from Maeda et al. (28), indicating that SP-A1 may be a more sensitive parameter to reflect corneal stiffness than HCDA in DSEK eyes.
Previous studies found that eyes after DSEK had lower stiffness than normal eyes, including corneal hysteresis and corneal resistance factor assessed by ORA (13). However, Faramarzi et al. (14) showed the same conclusion as we did, that corneal biomechanics after DSEK were significantly increased and were comparable to the fellow healthy eyes using ORA. These conflicting results may be explained by differences in patient characteristics, graft thickness, and corneal edema status. It would also be necessary to further investigate the relationship between ORA and CST measurements applied to eyes after keratoplasty.
To determine the validity and reliability of the newly released parameter, bIOP obtained by CST in routine clinical practice, IOP measurements provided by this and two other widely used tonometry devices, GAT and MacKay-Marg tonometer (TonoPen AVIA), were recorded and compared in this study. Although the three devices showed high agreement, bIOP-CST showed an underestimated tendency in post-DSEK eyes compared to GAT (−2.3 mmHg, p = 0.001) and MacKay-Marg tonometer (−1.9 mmHg, p = 0.015). Further agreement analysis using Bland–Altman plots also revealed negative agreement between CST and the other two tonometers, suggesting that 70% of post DSEK eyes and 30% of healthy fellow eyes showed underestimated IOP difference between tonometers of more than ±3 mmHg. Thus, bIOP measurement by CST may not be interchangeable with GAT or MacKay-Marg tonometer. In agreement with our results, Karmiris et al. (29) reported smaller bIOP-CST values than GAT-IOP values in 113 adults. Similarly, Matsuura et al. (17), Hong et al. (30), and Vinciguerra et al. (31) reported underestimated bIOP values compared with GAT-IOP in patients with glaucoma. Considering that GAT-IOP is usually overestimated due to corneal edema and stiffness change after DSEK, we speculate that CST-IOP may be closer to the actual IOP value than GAT. On the other hand, several studies demonstrated conflicting evidence that CST tended to overestimate IOP values compared to those obtained by GAT (32–36).
Tonopen is a versatile tonometer with potential advantages in assessing IOP in the presence of corneal scarring or edema. Chang et al. (37) and Ohana et al. (38) reported that Tonopen XL is a reliable tool for measuring IOP after DSEK. Tonopen AVIA is a new hand-held flattening tonometer with higher sensitivity than the Tonopen XL. To our knowledge, the present study is the first report to measure post-DSEK IOP using Tonopen AVIA. Bland–Altman plots showed high agreement between Tonopen AVIA and GAT with a mean difference of 0.4 mmHg in post-DSEK eyes. The flattening range of the Tonopen AVIA flattening probe is only 1 mm in diameter, which is considered to be less affected by CCT and is particularly suitable for the limited measurement of GAT (15). In this study, the high correlation and consistency of GAT and Tonopen AVIA measurements in both post-DSEK eyes and normal eyes might be due to the similar applanation technique. Of course, these results do not necessarily mean that the IOP obtained by GAT and Tonpen AVIA were absolutely accurate, because GAT is no longer a gold standard for measuring post-keratoplasty IOP.
In this study, although the GAT-IOP was the highest among all, it had no correlation with CCT, which could not indicate that the thickened cornea after DSEK made the GAT-IOP higher. Consistent with our findings, Clemmenssen and Hjortdal (13) analyzed the relationship between GAT-IOP and CCT in both FECD and DSEK eyes, and found that corneal thickening had no effect on GAT-IOP. At the same time, MacKay-Marg tonometer and Corvis ST IOP measurements were also not correlated with CCT. It is widely accepted that the biomechanical properties of the cornea have great influence on IOP measurements. As mentioned above, corneal endothelial transplantation changed host corneal structure, directly affecting corneal stiffness shown by corneal biomechanical parameters including AT1, AT2, AV1 and SP-A1. However, corneal endothelial grafts have little morphological effect on the structures that produce or outflow aqueous humor, and are therefore much less likely to in turn affect the real IOP directly. Thus, we speculate that the IOP change after DSEK may have a very small effect on corneal stiffness which affects IOP measurement. Correlation analyses showed that GAT-IOP and MacKay-Marg IOP were correlated with these biomechanical parameters, while Corvis ST IOP was not correlated with SP-A1. As for the bIOP calculated by Corvis ST which is corrected for corneal biomechanical changes, it may lead to lower IOP values in Corvis ST than in the other two tonometry systems.
This study also has some limitations. To enhance the strength of the paired comparison, we included patients with four types of typical monocular endothelial decompensation and healthy fellow eyes. Thus, this study design resulted in a rather small sample size, and was difficult to exclude confounding factors for the preoperative biomechanical properties of the different pathological corneas. In addition, this study did not evaluate the preoperative biomechanical properties of the operated eyes. As a result, changes in biomechanics and IOP both pre- and postoperatively could not be analyzed. With the innovation of Corvis ST, we are also planning to purchase the newest version and include newly released parameters for corneal stiffness such as CBI, SSI and TBI in our future studies using both Corvis ST and Pentacam.
In conclusion, the corneal biomechanical stiffness was increased after DSEK compared to normal eyes. Central corneal thickness may be less important than corneal biomechanics in measuring IOP in eyes after DSEK. The biomechanically-corrected IOP obtained by CST seems lower than other tonometry techniques in DSEK eyes, suggesting that IOP measurement with more than one tonometry may be necessary for confirming the IOP elevation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Eye & ENT Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. SW: Funding acquisition, Investigation, Supervision, Validation, Writing – original draft. LT: Investigation, Writing – review & editing. YL: Investigation, Writing – review & editing. JH: Supervision, Writing – review & editing. YW: Supervision, Writing – review & editing. JX: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82301178 and 82201175), the Clinical Research Program of Shanghai Municipal Commission of Health and Family Planning (20234Y0107), and the Shanghai Sailing Program (20YF1405100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Price, MO, Gupta, P, Lass, J, and Price, FW Jr. EK (DLEK, DSEK, DMEK): new frontier in cornea surgery. Annu Rev Vis Sci. (2017) 3:69–90. doi: 10.1146/annurev-vision-102016-061400
2. Price, MO, Mehta, JS, Jurkunas, UV, Price, FW, and Price, FW Jr. Corneal endothelial dysfunction: evolving understanding and treatment options. Prog Retin Eye Res. (2021) 82:100904. doi: 10.1016/j.preteyeres.2020.100904
3. Suh, LH, Yoo, SH, Deobhakta, A, Donaldson, KE, Alfonso, EC, Culbertson, WW, et al. Complications of Descemet’s stripping with automated endothelial keratoplasty: survey of 118 eyes at one institute. Ophthalmology. (2008) 115:1517–24. doi: 10.1016/j.ophtha.2008.01.024
4. Allen, MB, Lieu, P, Mootha, VV, Bowman, RW, Petroll, WM, Tong, L, et al. Risk factors for intraocular pressure elevation after Descemet stripping automated endothelial keratoplasty. Eye Contact Lens. (2010) 36:223–7. doi: 10.1097/ICL.0b013e3181e6ae30
5. Chan, EW, Wong, TT, Htoon, HM, HO, CL, Tan, DT, and Mehta, JS. De novo ocular hypertension after Descemet stripping endothelial keratoplasty: comparative 3-year incidence, risk factors, and outcomes. Clin Ophthalmol. (2013) 7:1829–41. doi: 10.2147/OPTH.S50584
6. Elalfy, M, Maqsood, S, Soliman, S, Hegazy, SM, Hannon, AA, Gatzioufas, Z, et al. Incidence and risk factors of ocular hypertension/glaucoma after Descemet stripping automated endothelial keratoplasty. Clin Ophthalmol. (2021) 15:2179–88. doi: 10.2147/OPTH.S299098
7. Kaleem, M, Ridha, F, Shwani, Z, Swenor, B, Goshe, J, and Singh, A. Rates of intraocular pressure elevation and use of topical antihypertensive medication after Descemet stripping automated endothelial keratoplasty. Cornea. (2017) 36:669–74. doi: 10.1097/ICO.0000000000001205
8. Maier, AK, Klamann, MK, Torun, N, Gonnermann, J, Schroeter, J, Joussen, AM, et al. Intraocular pressure elevation and post-DSEK glaucoma after Descemet’s stripping endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. (2013) 251:1191–8. doi: 10.1007/s00417-012-2203-5
9. Ozeki, N, Yuki, K, Shiba, D, Shimmura, S, Murat, D, and Tsubota, K. Intraocular pressure elevation after Descemet’s stripping endothelial keratoplasty. Jpn J Ophthalmol. (2012) 56:307–11. doi: 10.1007/s10384-012-0149-0
10. Sandhu, S, Petsoglou, C, Grigg, J, and Veillard, AS. Elevated intraocular pressure in patients undergoing penetrating keratoplasty and Descemet stripping endothelial Keratoplasty. J Glaucoma. (2016) 25:390–6. doi: 10.1097/IJG.0000000000000251
11. Kwon, TH, Ghaboussi, J, Pecknold, DA, and Hashash, Y. Role of corneal biomechanical properties in applanation tonometry measurements. J Refract Surg. (2010) 26:512–9. doi: 10.3928/1081597X-20090814-02
12. Martinez-De-La-Casa, JM, Garcia-Feijoo, J, Vico, E, Fernandez-Vidal, A, Benitez DEL Castillo, JM, Wasfi, M, et al. Effect of corneal thickness on dynamic contour, rebound, and goldmann tonometry. Ophthalmology. (2006) 113:2156–62. doi: 10.1016/j.ophtha.2006.06.016
13. Clemmensen, K, and Hjortdal, J. Intraocular pressure and corneal biomechanics in Fuchs' endothelial dystrophy and after posterior lamellar keratoplasty. Acta Ophthalmol. (2014) 92:350–4. doi: 10.1111/aos.12137
14. Faramarzi, A, Feizi, S, Najdi, D, Ghiasian, L, and Karimian, F. Changes in corneal biomechanical properties after Descemet stripping automated endothelial keratoplasty for pseudophakic bullous keratopathy. Cornea. (2016) 35:20–4. doi: 10.1097/ICO.0000000000000684
15. Bhan, A, Browning, AC, Shah, S, Hamilton, R, Dave, D, and Dua, HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. (2002) 43:1389–92.
16. Fabian, ID, Barequet, IS, Skaat, A, Rechtman, E, Goldenfeld, M, Roberts, CJ, et al. Intraocular pressure measurements and biomechanical properties of the cornea in eyes after penetrating keratoplasty. Am J Ophthalmol. (2011) 151:774–81. doi: 10.1016/j.ajo.2010.11.007
17. Matsuura, M, Murata, H, Fujino, Y, Yanagisawa, M, Nakao, Y, Tokumo, K, et al. Relationship between novel intraocular pressure measurement from Corvis ST and central corneal thickness and corneal hysteresis. Br J Ophthalmol. (2020) 104:563–8. doi: 10.1136/bjophthalmol-2019-314370
18. Hirasawa, K, Nakakura, S, Nakao, Y, Fujino, Y, Matsuura, M, Murata, H, et al. Changes in corneal biomechanics and intraocular pressure following cataract surgery. Am J Ophthalmol. (2018) 195:26–35. doi: 10.1016/j.ajo.2018.07.025
19. Wallace, HB, Misra, SL, Li, SS, and Mckelvie, J. Biomechanical changes in the cornea following cataract surgery: a prospective assessment with the corneal visualisation Scheimpflug technology. Clin Experiment Ophthalmol. (2019) 47:461–8. doi: 10.1111/ceo.13451
20. Joda, AA, Shervin, MM, Kook, D, and Elsheikh, A. Development and validation of a correction equation for Corvis tonometry. Comput Methods Biomech Biomed Engin. (2016) 19:943–53. doi: 10.1080/10255842.2015.1077515
21. Fernandez, J, Rodriguez-Vallejo, M, Martinez, J, Tauste, A, Salvestrini, P, and Pinero, DP. New parameters for evaluating corneal biomechanics and intraocular pressure after small-incision lenticule extraction by Scheimpflug-based dynamic tonometry. J Cataract Refract Surg. (2017) 43:803–11. doi: 10.1016/j.jcrs.2017.03.035
22. De Padua Soares Bezerra, B, Chan, E, Chakrabarti, R, and Vajpayee, RB. Intraocular pressure measurement after corneal transplantation. Surv Ophthalmol. (2019) 64:639–46. doi: 10.1016/j.survophthal.2019.02.011
23. Hatami-Marbini, H, and Etebu, E. Hydration dependent biomechanical properties of the corneal stroma. Exp Eye Res. (2013) 116:47–54. doi: 10.1016/j.exer.2013.07.016
24. Hatami-Marbini, H, and Rahimi, A. Evaluation of hydration effects on tensile properties of bovine corneas. J Cataract Refract Surg. (2015) 41:644–51. doi: 10.1016/j.jcrs.2014.07.029
25. Jayaswal, R, Alexander, P, and Maharajan, VS. Analysis of post-DSEK corneal profile and relationship to hyperopic shift. J Cataract Refract Surg. (2009) 35:2036. doi: 10.1016/j.jcrs.2008.12.051
26. Zhang, Y, Wang, Y, Li, L, Dou, R, Wu, W, Wu, D, et al. Corneal stiffness and its relationship with other corneal biomechanical and nonbiomechanical parameters in myopic eyes of Chinese patients. Cornea. (2018) 37:881–5. doi: 10.1097/ICO.0000000000001605
27. Perez-Rico, C, Gutierrez-Ortiz, C, Gonzalez-Mesa, A, Zandueta, AM, Moreno-Salgueiro, A, and Germain, F. Effect of diabetes mellitus on Corvis ST measurement process. Acta Ophthalmol. (2015) 93:e193–8. doi: 10.1111/aos.12530
28. Maeda, N, Ueki, R, Fuchihata, M, Fujimoto, H, Koh, S, and Nishida, K. Corneal biomechanical properties in 3 corneal transplantation techniques with a dynamic Scheimpflug analyzer. Jpn J Ophthalmol. (2014) 58:483–9. doi: 10.1007/s10384-014-0344-2
29. Karmiris, E, Tsiripidis, K, Gartaganis, PS, Totou, S, Vasilopoulou, MG, Patelis, A, et al. Comparison of intraocular pressure obtained by Goldmann applanation tonometer, Corvis ST and an airpuff tonometer in healthy adults. Eur J Ophthalmol. (2022) 32:951–959. doi: 10.1177/11206721211069227
30. Hong, J, Xu, J, Wei, A, Deng, SX, Cui, X, Yu, X, et al. A new tonometer—the Corvis ST tonometer: clinical comparison with noncontact and Goldmann applanation tonometers. Invest Ophthalmol Vis Sci. (2013) 54:659–65. doi: 10.1167/iovs.12-10984
31. Vinciguerra, R, Rehman, S, Vallabh, NA, Batterbury, M, Czanner, G, Choudhary, A, et al. Corneal biomechanics and biomechanically corrected intraocular pressure in primary open-angle glaucoma, ocular hypertension and controls. Br J Ophthalmol. (2020) 104:121–6. doi: 10.1136/bjophthalmol-2018-313493
32. Knauf, D, Seitz, B, Schiessl, G, Zemova, E, and Flockerzi, E. Analysis of various modalities for intraocular pressure measurement in relation to keratoconus severity in 246 eyes of the Homburg Keratoconus Center. Cornea. (2023) 42:829–36. doi: 10.1097/ICO.0000000000003170
33. Lanza, M, Sbordone, S, Tortori, A, Gironi Carnevale, UA, Melillo, P, and Simonelli, F. Evaluating intraocular pressure after myopic photorefractive keratectomy: a comparison of different Tonometers. J Glaucoma. (2022) 31:406–12. doi: 10.1097/IJG.0000000000002023
34. Martinez-Sanchez, MI, Bolivar, G, Sideroudi, H, and Teus, MA. Effect of prostaglandin analogues on the biomechanical corneal properties in patients with open-angle glaucoma and ocular hypertension measured with dynamic Scheimpflug analyzer. Graefes Arch Clin Exp Ophthalmol. (2022) 260:3927–33. doi: 10.1007/s00417-022-05752-0
35. Smedowski, A, Weglarz, B, Tarnawska, D, Kaarniranta, K, and Wylegala, E. Comparison of three intraocular pressure measurement methods including biomechanical properties of the cornea. Invest Ophthalmol Vis Sci. (2014) 55:666–73. doi: 10.1167/iovs.13-13172
36. Ye, Y, Yang, Y, Fan, Y, Lan, M, Yu, K, and Yu, M. Comparison of biomechanically corrected intraocular pressure obtained by Corvis ST and Goldmann applanation tonometry in patients with open-angle glaucoma and ocular hypertension. J Glaucoma. (2019) 28:922–8. doi: 10.1097/IJG.0000000000001348
37. Chang, DT, Pantcheva, MB, and Noecker, RJ. Corneal thickness and intraocular pressure in edematous corneas before and after Descemet stripping with automated endothelial keratoplasty. Cornea. (2010) 29:1125–30. doi: 10.1097/ICO.0b013e3181d25cbd
Keywords: corneal biomechanics, DSEK, intraocular pressure, Corvis ST, tonometer
Citation: Chen H, Wu S, Tian L, Li Y, Hong J, Wang Y and Xu J (2024) Intraocular pressure measurement and association with corneal biomechanics in patients underwent Descemet’s stripping with endothelial keratoplasty: a comparative study. Front. Med. 11:1384694. doi: 10.3389/fmed.2024.1384694
Edited by:
Matthew A. Reilly, The Ohio State University, United StatesCopyright © 2024 Chen, Wu, Tian, Li, Hong, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulan Wang, dWxhbndhbmdAMTYzLmNvbQ==; Jianjiang Xu, amlhbmppYW5neHVAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huiyu Chen
Huiyu Chen Suqian Wu
Suqian Wu Lijia Tian
Lijia Tian Yue Li
Yue Li Jiaxu Hong
Jiaxu Hong Yulan Wang
Yulan Wang Jianjiang Xu
Jianjiang Xu