- 1Department of Medical Oncology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, China
- 2Department of Medical Oncology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 3Department of Medical Oncology, Qingdao Shibei Changqing Hospital, Qingdao, Shandong Province, China
- 4Department of Chemotherapy, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province, China
- 5Department of Breast and Thyroid Surgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 6Department of General Surgery, People’s Hospital of Rizhao, Rizhao, Shandong Province, China
- 7Department of Breast and Thyroid Surgery, Rizhao Traditional Chinese Medical Hospital, Rizhao, Shandong Province, China
- 8Department of Breast and Thyroid Surgery, People’s Hospital of Juxian, Rizhao, Shandong Province, China
- 9Department of Pharmacy, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, China
Background: Trastuzumab emtansine (T-DM1) has been approved worldwide for treating metastatic breast cancer (mBC) in patients who have received first-line therapy, shown disease progression, and are human epidermal growth factor receptor 2 (HER2)-positive. T-DM1 received approval in China to treat early-stage breast cancer (BC) in 2020 and for mBC in 2021. In March 2023, T-DM1 was included in medical insurance coverage, significantly expanding the eligible population.
Materials and methods: This post-marketing observational study aimed to assess the safety and effectiveness of T-DM1 in real-world clinical practice in China. This study enrolled 31 individuals with HER2-positive early-stage BC and 70 individuals with HER2-positive advanced BC from 8 study centers in Shandong Province, China. The T-DM1 dosage was 3.6 mg/kg injected intravenously every 3 weeks until the disease advanced or the drug toxicity became uncontrollable, whichever occurred earlier. Additionally, efficacy and safety information on T-DM1 were collected.
Results: During the 7-month follow-up period, no recurrence or metastases were observed in patients who had early-stage BC. The disease control rate was 31.43% (22/70) in patients with advanced BC. The most common adverse effect of T-DM1 was thrombocytopenia, with an incidence of 69.31% (70/101), and the probability of Grade ≥ 3 thrombocytopenia was 11.88% (12/101).
Conclusion: This real-world study demonstrated that T-DM1 had good efficacy and was well tolerated by both HER2-positive early-stage BC and mBC patients.
1 Introduction
Breast cancer (BC) is a common malignancy among women worldwide, and 70–80% of patients with early-stage non-metastatic disease can potentially be cured. According to current treatments, advanced BC with distant metastases is generally considered to have a poor prognosis (1). BC is highly heterogeneous, and treatment strategies vary according to the molecular characteristics, including human epidermal growth factor receptor 2 (HER2) activation, expression of hormone receptors, gene mutations, and immune microenvironment markers (2).
Approximately 20% of BC cases over-expressing HER2 (3) have a poorer prognosis and short overall survival (OS) (4). In the last two decades, various HER2-targeted therapies have been developed, including humanized monoclonal antibodies such as trastuzumab (5) or pertuzumab (6), as well as tyrosine kinase inhibitors (TKIs) such as lapatinib (7) or neratinib (8). Currently, the most authoritative treatment for patients with metastatic disease includes the “dual HER-2 blockade” therapy using trastuzumab and pertuzumab plus paclitaxel (9). This treatment regimen improves progression-free survival (PFS) and OS. Nevertheless, despite these achievements, HER2-positive metastatic BC (mBC) remains incurable.
The introduction of trastuzumab has significantly affected the prognosis of individuals with HER2-positive BC and influenced its diagnosis and treatment approaches. It represents a significant breakthrough in the drug treatment of BC (10, 11). The combination of trastuzumab with other chemotherapeutic agents improves the prognosis of patients with metastatic diseases and decreases cancer recurrence (12–14). Despite the superior efficacy of trastuzumab, most patients with advanced disease develop resistance to this drug; therefore, there remains an ongoing requirement to discover new drugs that specifically target progressive HER2-amplified diseases (15). HER2-directed therapy works best when combined with cytotoxic chemotherapy; hence, a novel antibody–drug conjugate (ADC) has been developed (15).
Trastuzumab emtansine (T-DM1) comprises trastuzumab and the tubulin inhibitor, DM1, which are bound via a stable, non-lysable, non-reducing thioether linker. DM1 can prevent the assembly of mitotic functional spindles by effectively binding to tubulin, thus depolymerizing it, and can induce cell cycle arrest and apoptosis. T-DM1 possesses the same mechanism of action as trastuzumab, which effectively blocks the HER2 signaling pathway (16). The 2012 EMILIA study provided evidence for the effectiveness of T-DM1 as a second-line treatment for HER2-positive mBC (17). T-DM1 was approved by the Food and Drug Administration in the following year as a standalone therapy for treating mBC (18). In 2019, the KATHERINE study laid the groundwork for using T-DM1 to manage early-stage BC (19). This study investigated the effectiveness and safety of T-DM1 in real-world clinical practice, specifically for treating early-stage or advanced HER2-positive BC in China.
2 Materials and methods
2.1 Study population
This Phase 4, multicentric, observational, clinical study was conducted in eight sites in the Shandong Province of China from 1 March 2023 to 28 June 2023. Our research was approved by the Medical Ethical Committee of Qilu Hospital of Shandong University. All participants signed a comprehensive informed consent document, which included the purpose and procedures of the study. The study was conducted according to the principles of Good Clinical Practice.
This study had specific inclusion criteria. It included individuals who demonstrated HER2 overexpression with an immunohistochemistry (IHC) score of 3+. Alternatively, those with a 2+ IHC score and positive results in fluorescence in situ hybridization testing were also considered eligible for participation. Patients with pathologically confirmed unilateral, measurable invasive mBC who received chemotherapy, specifically trastuzumab and a taxane drug were included. Patients also comprised those with non-invasive primary BC who had completed at least six cycles of paclitaxel adjuvant chemotherapy, as well as those with residual invasive diseases detected in the surgical specimens of breast or axillary lymph nodes. Other eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, successful recuperation from any treatment-related toxicities, left ventricular ejection fraction (LVEF) ≥50%, absolute neutrophil count ≥1,500 cells/mm3, hemoglobin ≥90 g/L, platelet count ≥100,000 cells/mm3, aspartate aminotransferase and alanine aminotransferase ≤2.5 × upper limits of normal (ULN), and total bilirubin ≤1.5 × ULN.
The exclusion criteria included anti-HER2 ADC therapy, chemotherapy, hormone therapy, radiotherapy, or BC surgery within 3 weeks before the screening process; a history of symptomatic chronic heart failure or treatment for severe arrhythmia; severe systemic disease; HIV or hepatitis B infection; pregnancy or breastfeeding; allergy to T-DM1; and any other medical or psychiatric condition deemed unsuitable for the study by the investigator.
2.2 Treatment methods
T-DM1 administration involved an intravenous infusion of 3.6 mg/kg over 90 min during the initial cycle. Subsequently, a dose of 3.6 mg/kg was administered over 30 min every 3 weeks until the investigator detected uncontrolled toxicity or disease progression, whichever occurred earlier. Uncontrolled toxicity refers to a serious treatment-related adverse event (AE) that prevents the continued use of a therapeutic drug. Before each infusion, premedication was administered, which included analgesics/antipyretics and antihistamines. The study allowed the use of other supportive medications, such as ondansetron or palonosetron, as well as palliative care during the study.
2.3 Efficacy and toxicity
According to the RECIST 1.1 (20) guidelines, tumor response was evaluated after 6 weeks of treatment or earlier if clear signs of disease progression appeared rapidly. The primary outcomes were disease control rate (DCR) and disease-free survival (DFS). DCR generally refers to the percentage of cases with remission and stable lesions in the number of evaluable cases after treatment. The DCR was calculated by summing the rates of complete response (CR), partial response (PR), and stable disease (SD). CR means that the patient has been treated for the tumor in multiple ways, that the majority of the lesions have disappeared, that no new lesions have formed, and that the tumor marker examination has continued normally for more than 4 weeks. PR means that the maximum size reduction of the target lesion is ≥30% and maintained for at least 4 weeks. SD means that the sum of the large diameter of the patient’s target lesions does not shrink by more than 30%, or the increase does not exceed 20%. DFS refers to the time from a clinically proven CR to local recurrence or distant metastasis.
Toxicities were evaluated and categorized as per the National Cancer Institute Common Toxicity Criteria, version 5.0. The deadline for data collection for the study was 5 June 2023.
2.4 Statistical analysis
We used chi-square tests or Fisher’s exact test to assess the difference in the efficacy of T-DM1 between early-stage and advanced BC patients. Statistical analyses were performed using Statistical Product and Service Solutions 24.0. GraphPad Prism was used to plot the Kaplan–Meier survival curves. The log-rank test was used to analyze the PFS. Results with a p-value of ≤0.05 were considered statistically significant.
3 Results
3.1 Patient information
This study included 101 women with HER2-positive B, and T-DM1 treatment was administered. The median age was 51 years (range, 21–74). Hormone receptor positivity was observed in 44 cases (43.56%). Distant metastasis was reported in 70 cases (69.31%), including 38 (54.29%) with visceral metastasis and 10 (26.32%) with brain metastasis. The baseline characteristics are shown in Table 1. All patients were previously treated with paclitaxel and trastuzumab.
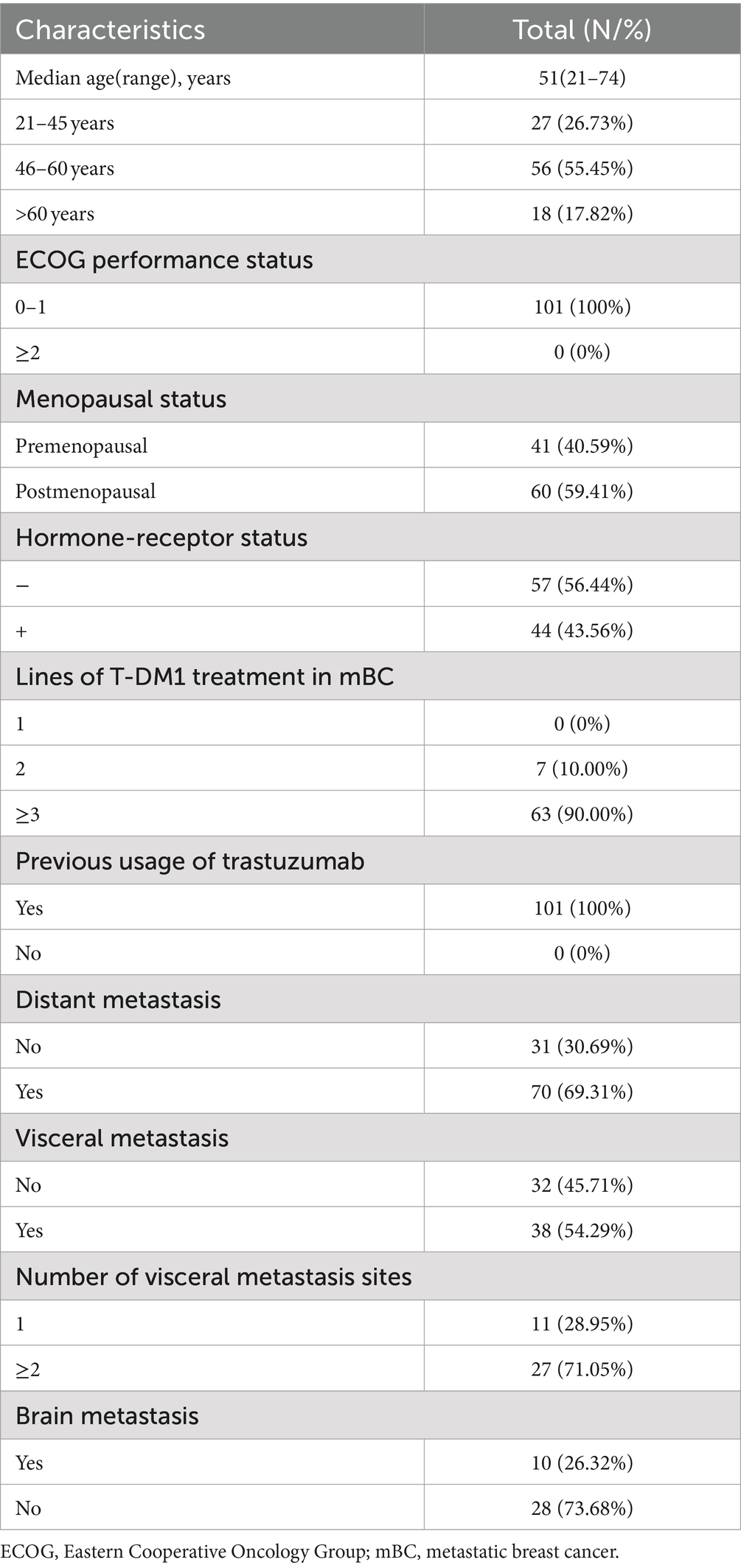
Table 1. Baseline clinicopathological and disease characteristics of 101 human epidermal growth factor receptor 2-positive breast cancer patients.
3.2 Treatment administration
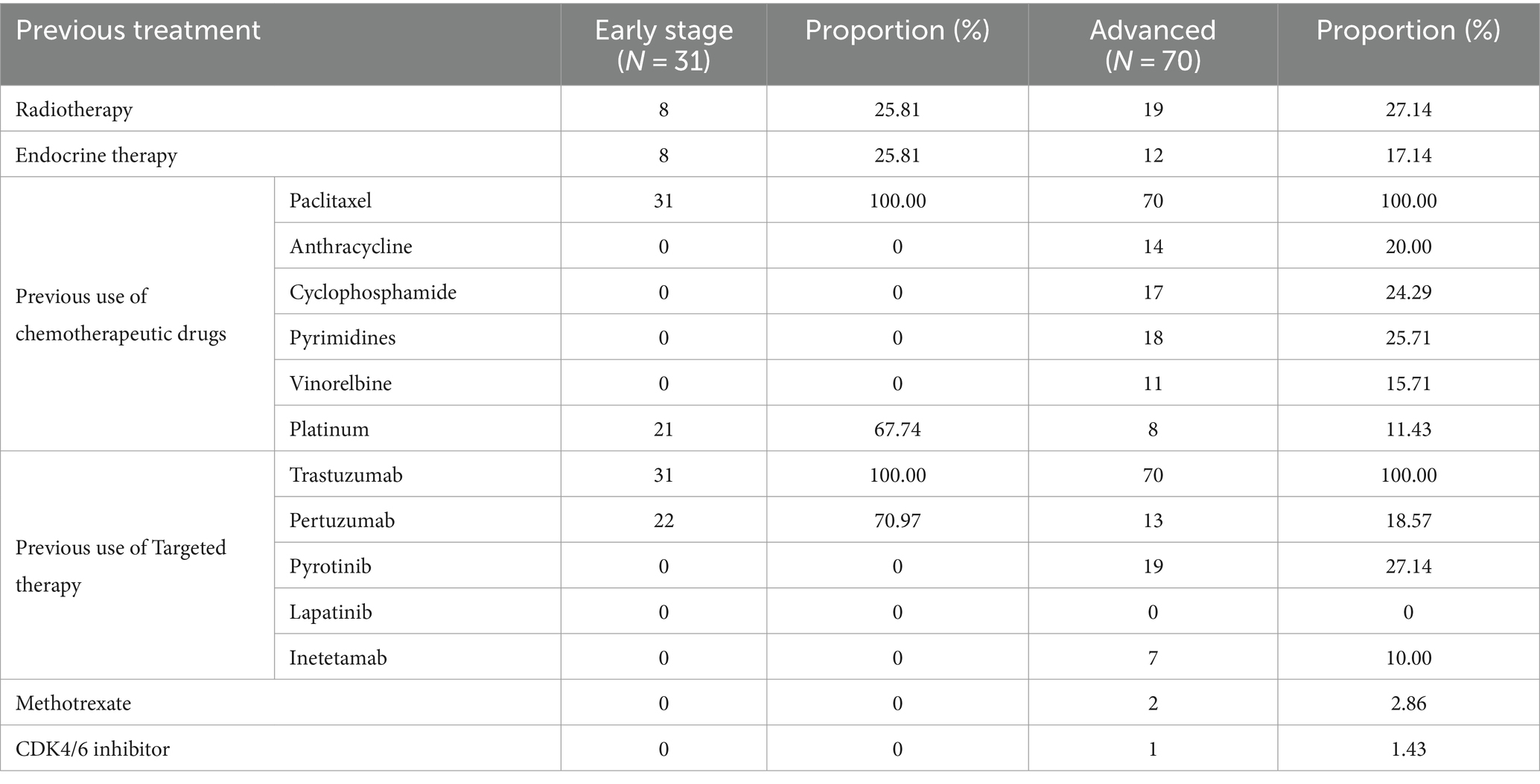
Table 2 summarizes the previous treatment methods.
3.3 Clinical efficacy
The efficacy in patients with diverse clinicopathologic and disease features until the cutoff date is shown in Table 3. Among the patients with advanced BC disease, the DCR did not differ significantly between those with different clinicopathologic and disease characteristics treated with T-DM1 (Figure 1A). The DCR in patients with advanced disease was 31.43% (22/70). However, the median PFS (mPFS) was longer in patients who had used pertuzumab (188 days; 95% CI, 178.40–197.60) than in those who had not (182 days; 95% CI, 123.35–240.66), without a statistically significant difference (p = 0.92; Figure 1B). Similarly, the mPFS was longer in patients who had used pyrotinib (188 days; 95% CI, 177.77–198.23) than in those who had not (129 days; 95% CI, 58.44–199.56), without a statistically significant difference (p = 0.49; Figure 1C). Furthermore, the mPFS was longer in patients with visceral metastases (183 days; 95% CI, 177.62–188.38) than in those without visceral metastases (129 days; 95% CI, 37.53–220.47), without a statistically significant difference (p = 0.38; Figure 1D). The mPFS of patients without brain metastases (183 days; 95% CI, 176.74–189.26) was longer than that of patients with brain metastases (120 days), without a statistically significant difference (p = 0.84; Figure 1E). Until the cutoff date, no patients with early-stage BC who were followed up for 7 months showed disease progression after T-DM1 treatment.
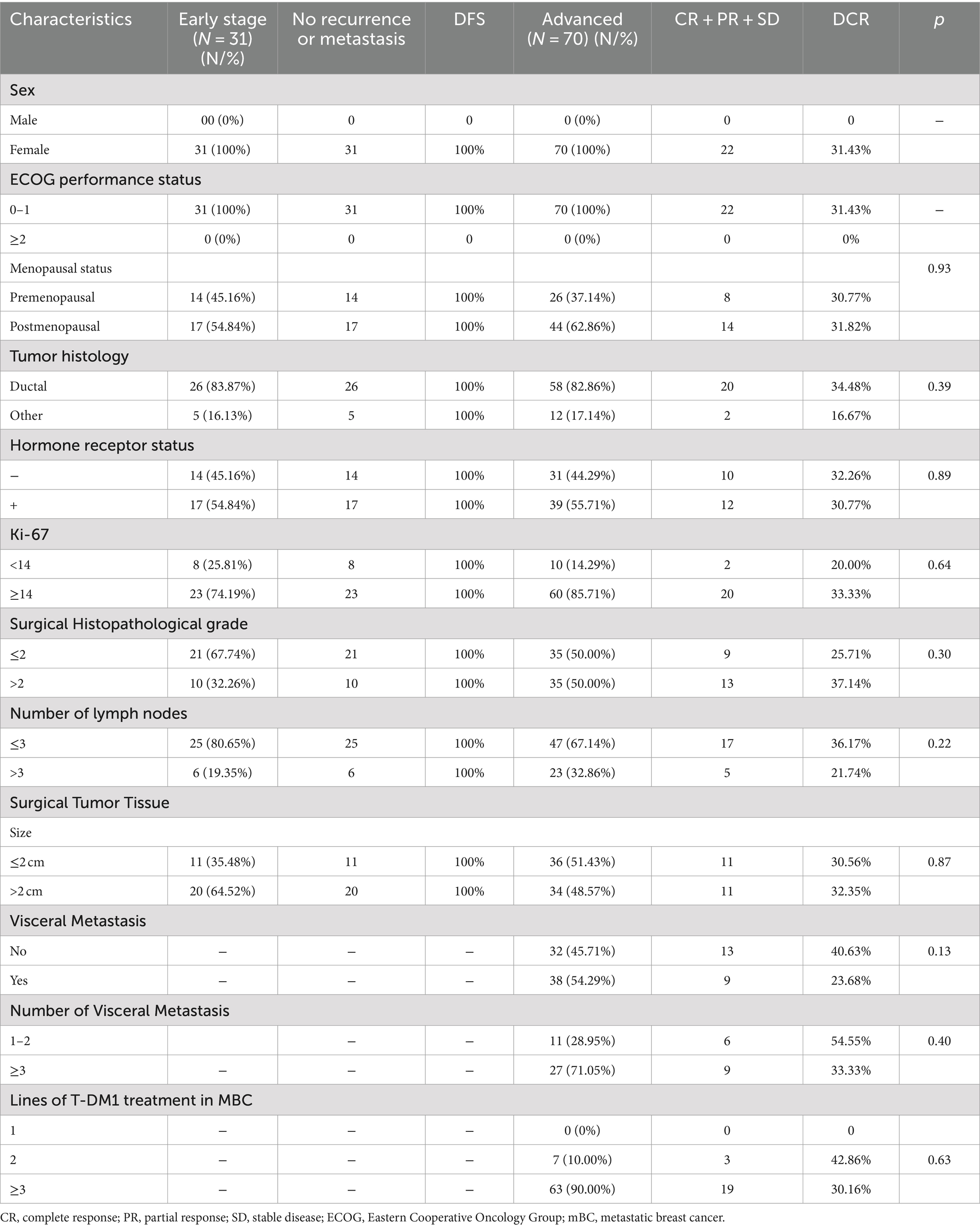
Table 3. Evaluation of efficacy in patients with different clinicopathologic and disease characteristics.
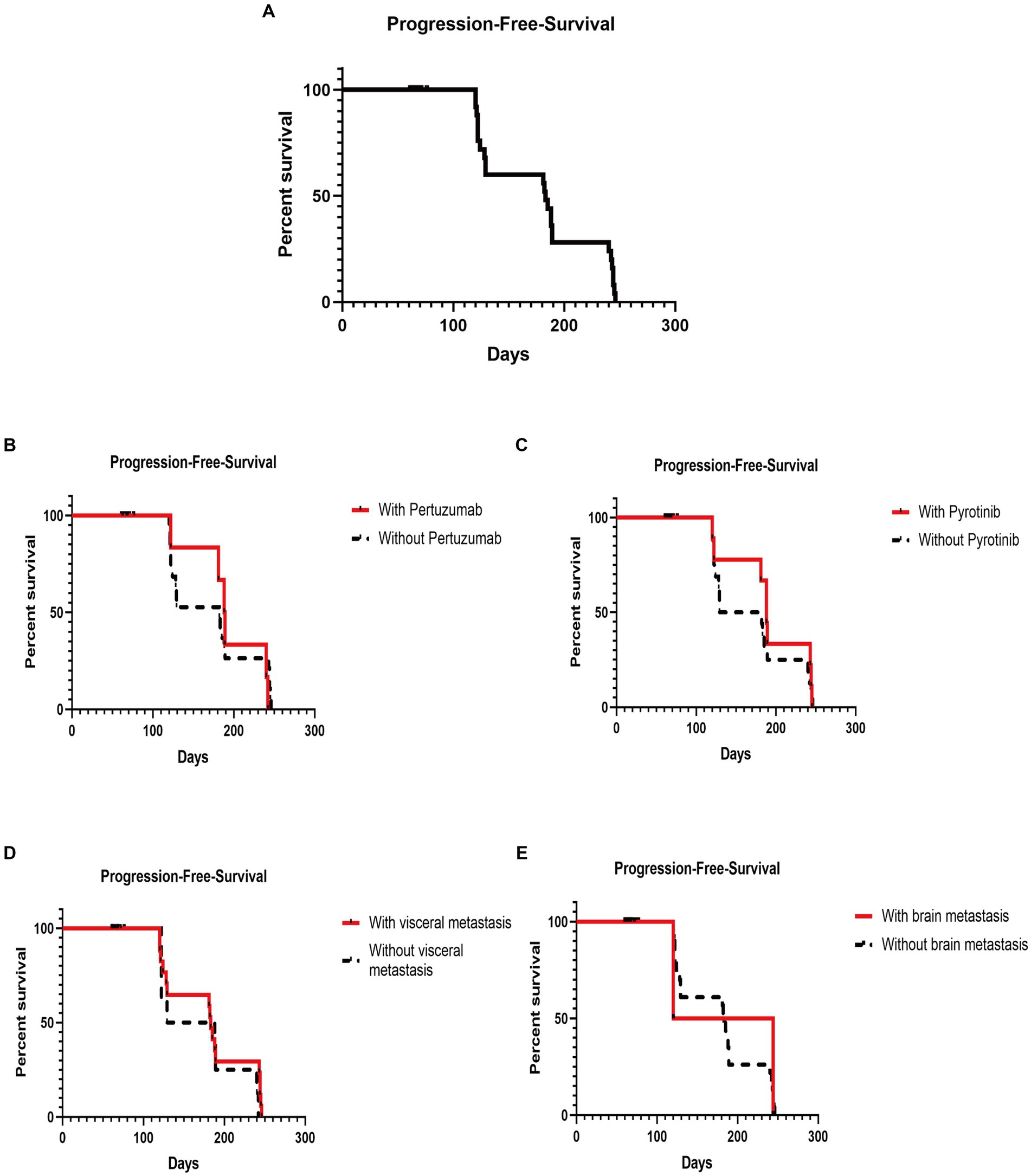
Figure 1. Kaplan–Meier plot of progression-free survival and log-rank analysis of the predictors of trastuzumab emtansine treatment. (A) Kaplan–Meier plot of the PFS of all patients subjected to T-DM1 treatment; (B) Kaplan–Meier plot of the PFS of patients with and without previous pertuzumab treatment; (C) Kaplan–Meier plot of the PFS of patients with and without exposure to pyrotinib; (D) Kaplan–Meier plot of the PFS of patients with and without visceral metastasis; (E) Kaplan–Meier plot of the PFS of patients with and without brain metastasis (PFS, progression-free survival; T-DM1, trastuzumab emtansine).
3.4 Safety
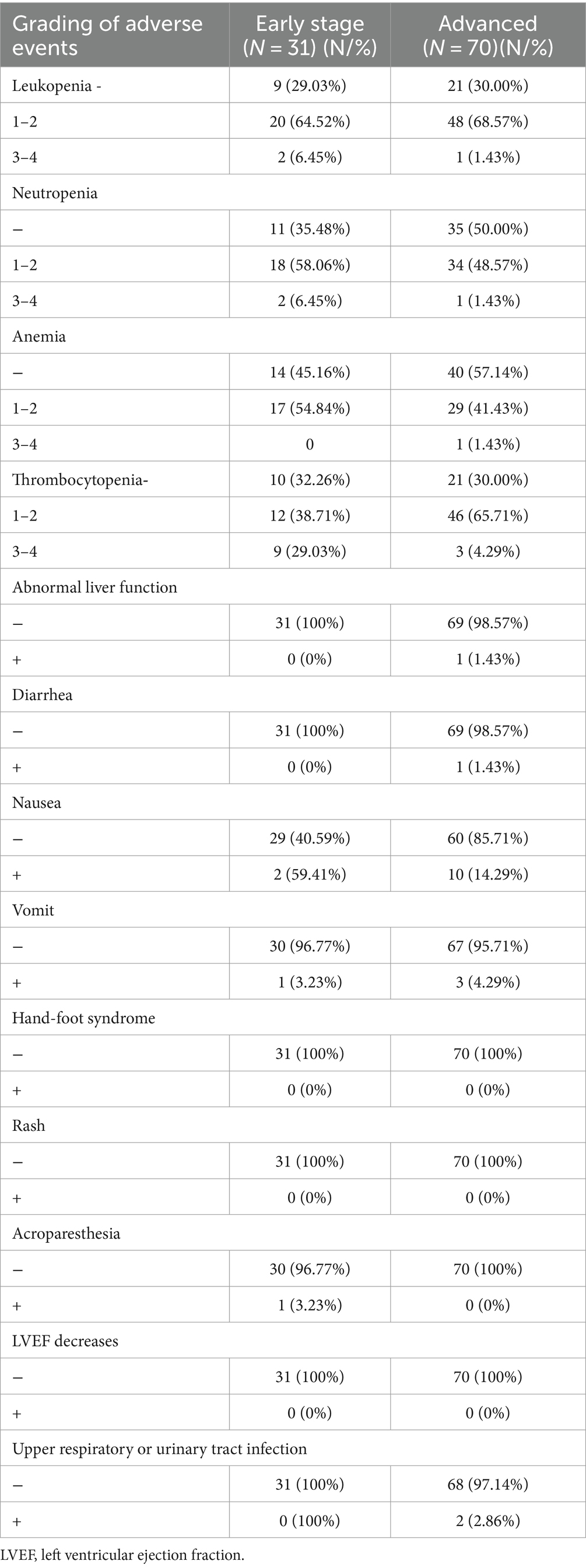
We evaluated 101 patients for toxicity. The overall toxicity rate was 70% (49/70) in the advanced BC group and 71% (22/31) in the early-stage BC group.
Platelet counts decreased in 70% (49/70) of patients in the advanced BC group and 67.74% (21/31) of patients in the early-stage BC group. Patients with platelets <50*10^9/L accounted for 4.29% (3/70) and 29.03% (9/31) of the advanced BC and early-stage BC groups, respectively. The following AEs were commonly reported in association with treatment: diarrhea (1/101, 0.99%), neutropenia (55/101, 54.46%), and nausea and vomiting (16/101, 15.84%). Hematological toxicity and gastrointestinal toxicity were the most common. Details of AE occurrences are shown in Table 4. These AEs are generally tolerated and manageable. AEs can be improved to Grade 1 or 2 after therapy with recombinant human thrombopoietin injection and/or aprepitant, ondansetron, and others, without the interruption of the T-DM1 therapy. Two patients in the early-stage BC group experienced a reduction in the platelet count, leading to a dose reduction of T-DM1. Additionally, one patient in the advanced BC group had their T-DM1 dose reduced due to nausea and diarrhea.
4 Discussion
The major outcomes of this study demonstrate that T-DM1 exhibits favorable efficacy in patients with early-stage HER2-positive BC, which corroborates the findings of previous clinical trials (19, 21). The DCR is not ideal in advanced BC, which could be due to the administration of T-DM1 as the second-line therapy. The decrease in the platelet count was most significant in terms of the safety of T-DM1 administration (22). The DCR was higher for hormone receptor-negative HER2-positive mBC than for hormone receptor-positive HER2-positive mBC (33.33% versus 28.21%) without a statistically significant difference between these two subgroups.
T-DM1 treatment showed a greater survival benefit in patients who had previously used pertuzumab or pyrotinib compared to those who had not. A trend toward better outcomes with T-DM1 was observed in patients with visceral metastases but no brain metastases as compared to those without metastases. However, no statistical difference was observed in these results, which could be associated with the fact that the average number of treatment lines for advanced BC treated with T-DM1 was more than five. Key evidence supporting continuous HER2 inhibition after trastuzumab treatment has been reported in BC patients. BC patients showing disease progression after treatment with trastuzumab and anthracyclines or taxanes were included in the EGF100151 study (23). In a randomized study, patients were assigned to receive lapatinib plus capecitabine or capecitabine monotherapy. The PFS of the group receiving combination treatment was 8.4 months. In the GBG26 study, the addition of trastuzumab to capecitabine extended the mPFS to 8.2 months (24). Other trials have also demonstrated the importance of continued anti-HER2 therapy (25–27). The T⁃DM1 drug comprises a combination of the targeted therapeutic drug trastuzumab and the cytotoxic drug DM1. The targeting effect of trastuzumab and the anti-tumor effect of DM1 are exerted simultaneously, and the anti-tumor drugs are delivered to the target cells for a better therapeutic effect (28–32). The Emilia trial showed that the PFS of the T-DM1 group could be nearly 9.6 months following the failure of trastuzumab (22). In our study, the mPFS of patients with disease progression after trastuzumab and/or TKIs has not been determined.
The EMILIA study further demonstrated that T-DM1 not only exhibited improved efficacy but also a favorable safety profile. Specifically, the TDM-1 group showed a decreased incidence of Grade ≥ 3 severe AEs, with thrombocytopenia being the most commonly reported (22). Furthermore, the TH3RESA clinical trial was conducted to compare the efficacy of T-DM1 therapy with physician choice therapy (TPC). The study aimed to evaluate the efficacy of T-DM1 in patients who had undergone prior treatment with a minimum of two HER2-targeted therapies, such as lapatinib and trastuzumab (33). Most patients had metastatic disease and had previously undergone at least four prior treatments, and most patients in the TPC group had been treated with a regimen containing trastuzumab. A significant improvement in PFS was observed with T-DM1 compared to TPC (mPFS, 6.2 months versus 3.3 months). In the last analysis of OS, patients treated with T-DM1 showed a significantly longer median OS than that of the control group (median OS, 22.7 months versus 15.8 months). Consistent with the findings of the EMILIA study, thrombocytopenia was identified as a more common AE associated with T-DM1 treatment (34). In the TDM4450g study, patients with HER2-positive mBC or recurrent locally advanced BC were assigned to receive either T-DM1 or trastuzumab plus docetaxel as the first-line treatment. T-DM1 has a better safety profile and fewer serious AEs compared to other treatment regimens (35).
Additionally, the KATHERINE trial study validated T-DM1 for treating residual aggressive HER2-positive BC. In the study, 5.7% of the patients developed thrombocytopenia (19, 36). The efficacy of T-DM1 has also been demonstrated in other clinical trials (37–51). For example, T-DM1 was active and well tolerated in a population with brain metastases from BC, and metronomic temozolomide in combination with standard dose T-DM1 has shown low-grade toxicity and potential activity in the secondary prevention of HER2+ brain metastases (41, 52), Moreover, thrombocytopenia has been a dose-limiting side effect that can lead to treatment discontinuation (53, 54).
Regarding the treatment safety in this study, the most common Grade ≥ 3 AE among the people analyzed in Shandong Province, China, was peripheral thrombocytopenia. Satisfactory relief could be achieved after administering thrombopoietin to stimulate platelet production, and most patients could achieve a good platelet count tolerance in subsequent courses. Other common AEs related to T-DM1 are nausea, elevated transaminase, diarrhea, and vomiting, most of which were of Grade 1–2 severity. These findings align with those of previous studies (30, 36). Therefore, the AEs of T-DM1 in the real world are tolerable and easily controlled (55). Notably, thrombocytopenia can be caused by impaired platelet production as well as reduced platelet survival in circulation (56). T-DM1 can inhibit the production of megakaryocyte platelets (57–59). T-DM1 is absorbed by megakaryocytes, which inhibits megakaryocyte differentiation, disrupts platelet formation by inducing abnormal tubulin organization, and inhibits microtubule dynamic instability. However, clinical studies have also shown that the platelet survival rate of patients treated with T-DM1 has a statistically significant and gradual downward trend, and T-DM1 directly decreases the patients’ platelet circulation time and function (60).
Our data also included patients with Grade 4 thrombocytopenia. We examined the genes of two patients with very low platelet counts, and both revealed mutation sites, namely MDR121 exon G2677 homozygous mutation, CYP3A4 heterozygous mutation, and CYP3A5 heterozygous mutation. Several studies have found that there are two molecular “efflux pumps” in tumor cell membranes, namely P-glycoprotein (P-gp) and multi-drug resistance-associated protein. These pumps are responsible for expelling therapeutic drugs from the cancer cells, leading to the phenomenon called multi-drug resistance (61, 62). Among them, P-gp is the transporter of the adenosine triphosphate-binding cassette, encoded by the ABCB1/MDR1 gene (63, 64). Multi-mutation analysis revealed that MDR1 had a large genetic variation, indicating that single-nucleotide polymorphisms (SNPs) may significantly affect the expression and function of P-gp transporters (65–67). Of the SNPs reported in the MDR1 gene, G2677T/A in exon 21 has been extensively studied and determined to be of functional significance. There are racial differences in the localization of this gene region (68–70).The G2677T/A SNP of exon 21 leads to 893Ser (G2677T) and the much rarer 893Thr (G2677A), which can alter transporter function or expression (71, 72). Furthermore, G2677 has prognostic significance in disease progression (73). A particular study revealed a strong association between the G2677T/A SNP and the efficacy of paclitaxel treatment in patients with ovarian cancer. Patients with pure mutations had a high probability of responding to paclitaxel therapy. The frequency of the T or A allele was also higher in the group of patients with a better prognosis than in those with a poor prognosis (74). Moreover, several studies have shown that the MDR1 genotype is strongly associated with the efficacy and toxicity of chemotherapy in BC (75, 76). For example, adriamycin is affected by the ABCB1 transporter (72, 77). CYP3A4 and CYP3A5 are members of the human cytochrome P450 gene family, located on chromosome 7, and primarily involved in the metabolism of drugs. They break down drugs into molecules that can be absorbed by major tissues. Genetic abnormalities affect the activity of the gene-encoded protein, which in turn affects the metabolism of the drug in the human body, leading to too high or too low drug concentration in the blood, which results in a too high or too low therapeutic effect and even drug side effects. For example, cyclophosphamide is an active drug activated by a variety of cytochrome P450 enzymes, including CYP3A5 (78). These enzyme and transporter genes all have genetic variations to some degree, characterized by SNPs. In summary, we hypothesize that T-DM1 and its active metabolites are substrates for MDR1-encoded glycoprotein (P-gp). Furthermore, the liver enzymes CYP3A4 and CYP3A5 are also trapped in the drug metabolism of T-DM1, and patients with mutations in these two genes are susceptible to AEs associated with T-DM1, especially thrombocytopenia.
The findings of this real-world study corroborated the data from the EMILIA study and the KATHERINE study. The mechanism of thrombocytopenia revealed the mutation site of the metabolic enzyme.
This retrospective study has some limitations. First, the sample size was relatively small, which may have introduced a bias in the results. Second, the questionnaire survey and telephonic follow-up for AE analysis could introduce subjectivity. Third, the follow-up period was relatively short. Therefore, further validation of these findings is warranted through large prospective cohort studies.
5 Conclusion
The main AE of T-DM1 as the second-line therapy for HER2-positive mBC was thrombocytopenia in the Chinese population. It was overall well tolerated and exhibited promising anti-tumor activity, even in patients in whom previous trastuzumab and pertuzumab therapy had failed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethical Committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The treatments used are guideline-based.
Author contributions
MH: Formal analysis, Writing – original draft. WZ: Writing – review & editing. PW: Data curation, Writing – review & editing. WL: Data curation, Writing – review & editing. HC: Data curation, Writing – review & editing. ZY: Data curation, Writing – review & editing. GP: Data curation, Writing – review & editing. HG: Data curation, Writing – review & editing. LS: Data curation, Writing – review & editing. JC: Data curation, Writing – review & editing. LL: Visualization, Writing – review & editing. YH: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (grant number: 81600092), the Natural Science Foundation of Shandong Province (no. ZR2023QH292), and the horizontal project Molecular Characterization of Breast Cancer with ESR1 Gene Mutation and Relevance to Treatment (260101120023BL).
Acknowledgments
The authors would like to thank all the researchers who participated in the data collection and the entities providing financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harbeck, N, Penault-Llorca, F, Cortes, J, Gnant, M, Houssami, N, Poortmans, P, et al. Breast Cancer. Nat Rev Dis Prim. (2019) 5:66. doi: 10.1038/s41572-019-0111-2
2. Hong, R, and Xu, B. Breast Cancer: An up-to-date review and future perspectives. Cancer communications (London, England). (2022) 42:913–36. doi: 10.1002/cac2.12358
3. Slamon, DJ, Clark, GM, Wong, SG, Levin, WJ, Ullrich, A, and McGuire, WL. Human breast Cancer: correlation of relapse and survival with amplification of the her-2/Neu oncogene. Science (New York, NY). (1987) 235:177–82. doi: 10.1126/science.3798106
4. Rubin, I, and Yarden, Y. The basic biology of Her2. Annals of Oncol: Official J European Society for Medical Oncol. (2001) 12:S3–8. doi: 10.1093/annonc/12.suppl_1.s3
5. Carter, P, Presta, L, Gorman, CM, Ridgway, JB, Henner, D, Wong, WL, et al. Humanization of an anti-P185her2 antibody for human Cancer therapy. Proc Natl Acad Sci USA. (1992) 89:4285–9. doi: 10.1073/pnas.89.10.4285
6. Franklin, MC, Carey, KD, Vajdos, FF, Leahy, DJ, de Vos, AM, and Sliwkowski, MX. Insights into Erbb signaling from the structure of the Erbb2-Pertuzumab complex. Cancer Cell. (2004) 5:317–28. doi: 10.1016/s1535-6108(04)00083-2
7. Xia, W, Mullin, RJ, Keith, BR, Liu, LH, Ma, H, Rusnak, DW, et al. Anti-tumor activity of Gw572016: a dual tyrosine kinase inhibitor blocks Egf activation of Egfr/Erbb2 and downstream Erk1/2 and Akt pathways. Oncogene. (2002) 21:6255–63. doi: 10.1038/sj.onc.1205794
8. Chan, A, Delaloge, S, Holmes, FA, Moy, B, Iwata, H, Harvey, VJ, et al. Neratinib after Trastuzumab-based adjuvant therapy in patients with Her2-positive breast Cancer (Extenet): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2016) 17:367–77. doi: 10.1016/s1470-2045(15)00551-3
9. Swain, SM, Baselga, J, Kim, SB, Ro, J, Semiglazov, V, Campone, M, et al. Pertuzumab, Trastuzumab, and docetaxel in Her2-positive metastatic breast Cancer. N Engl J Med. (2015) 372:724–34. doi: 10.1056/NEJMoa1413513
10. Rossi, M, Carioli, G, Bonifazi, M, Zambelli, A, Franchi, M, Moja, L, et al. Trastuzumab for Her2+ metastatic breast Cancer in clinical practice: cardiotoxicity and overall survival. European J Cancer (Oxford, England: 1990). (2016) 52:41–9. doi: 10.1016/j.ejca.2015.09.012
11. Maximiano, S, Magalhães, P, Guerreiro, MP, and Morgado, M. Trastuzumab in the treatment of breast Cancer. BioDrugs: Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy. (2016) 30:75–86. doi: 10.1007/s40259-016-0162-9
12. Moasser, MM . The oncogene Her2: its signaling and transforming functions and its role in human Cancer pathogenesis. Oncogene. (2007) 26:6469–87. doi: 10.1038/sj.onc.1210477
13. Choong, GM, Cullen, GD, and O'Sullivan, CC. Evolving standards of care and new challenges in the management of Her2-positive breast Cancer. CA Cancer J Clin. (2020) 70:355–74. doi: 10.3322/caac.21634
14. Pernas, S, and Tolaney, SM. Clinical trial data and emerging strategies: Her2-positive breast Cancer. Breast Cancer Res Treat. (2022) 193:281–91. doi: 10.1007/s10549-022-06575-7
15. Swain, SM, Shastry, M, and Hamilton, E. Targeting Her2-positive breast Cancer: advances and future directions. Nat Rev Drug Discov. (2023) 22:101–26. doi: 10.1038/s41573-022-00579-0
16. Lewis Phillips, GD, Li, G, Dugger, DL, Crocker, LM, Parsons, KL, Mai, E, et al. Targeting Her2-positive breast Cancer with Trastuzumab-Dm1, an antibody-cytotoxic drug conjugate. Cancer Res. (2008) 68:9280–90. doi: 10.1158/0008-5472.Can-08-1776
17. Verma, S, Miles, D, Gianni, L, Krop, IE, Welslau, M, Baselga, J, et al. Trastuzumab Emtansine for Her2-positive advanced breast Cancer. N Engl J Med. (2012) 367:1783–91. doi: 10.1056/NEJMoa1209124
18. Amiri-Kordestani, L, Blumenthal, GM, Xu, QC, Zhang, L, Tang, SW, Ha, L, et al. Fda approval: ado-Trastuzumab Emtansine for the treatment of patients with Her2-positive metastatic breast Cancer. Clinical Cancer Res: Official J American Association for Cancer Res. (2014) 20:4436–41. doi: 10.1158/1078-0432.Ccr-14-0012
19. von Minckwitz, G, Huang, CS, Mano, MS, Loibl, S, Mamounas, EP, Untch, M, et al. Trastuzumab Emtansine for residual invasive Her2-positive breast Cancer. N Engl J Med. (2019) 380:617–28. doi: 10.1056/NEJMoa1814017
20. Eisenhauer, EA, Therasse, P, Bogaerts, J, Schwartz, LH, Sargent, D, Ford, R, et al. New response evaluation criteria in solid Tumours: revised Recist guideline (version 1.1). European J Cancer (Oxford, England: 1990). (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
21. Filho, OM, Viale, G, Stein, S, Trippa, L, Yardley, DA, Mayer, IA, et al. Impact of Her2 heterogeneity on treatment response of early-stage Her2-positive breast Cancer: phase ii neoadjuvant clinical trial of T-Dm1 combined with Pertuzumab. Cancer Discov. (2021) 11:2474–87. doi: 10.1158/2159-8290.Cd-20-1557
22. Diéras, V, Miles, D, Verma, S, Pegram, M, Welslau, M, Baselga, J, et al. Trastuzumab Emtansine versus Capecitabine plus Lapatinib in patients with previously treated Her2-positive advanced breast Cancer (Emilia): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:732–42. doi: 10.1016/s1470-2045(17)30312-1
23. Geyer, CE, Forster, J, Lindquist, D, Chan, S, Romieu, CG, Pienkowski, T, et al. Lapatinib plus Capecitabine for Her2-positive advanced breast Cancer. N Engl J Med. (2006) 355:2733–43. doi: 10.1056/NEJMoa064320
24. von Minckwitz, G, Schwedler, K, Schmidt, M, Barinoff, J, Mundhenke, C, Cufer, T, et al. Trastuzumab beyond progression: overall survival analysis of the Gbg 26/big 3-05 phase iii study in Her2-positive breast Cancer. European J Cancer (Oxford, England: 1990). (2011) 47:2273–81. doi: 10.1016/j.ejca.2011.06.021
25. Martin, M, Holmes, FA, Ejlertsen, B, Delaloge, S, Moy, B, and Iwata, H. Neratinib after Trastuzumab-based adjuvant therapy in Her2-positive breast Cancer (Extenet): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2017) 18:1688–700. doi: 10.1016/s1470-2045(17)30717-9
26. Spector, N . Treatment of metastatic Erbb2-positive breast Cancer: options after progression on Trastuzumab. Clin Breast Cancer. (2008) 8:S94–9. doi: 10.3816/cbc.2008.s.005
27. Ihnenfeld Arciénega, I, Imesch, P, Fink, D, and Dedes, KJ. Prolonged complete remission of metastatic Her2-positive breast Cancer after continuous Trastuzumab treatment: a case report and review of the literature. Target Oncol. (2015) 10:297–301. doi: 10.1007/s11523-014-0350-9
28. Ferraro, E, Drago, JZ, and Modi, S. Implementing antibody-drug conjugates (Adcs) in Her2-positive breast Cancer: state of the art and future directions. Breast Cancer Res: BCR. (2021) 23:84. doi: 10.1186/s13058-021-01459-y
29. Rinnerthaler, G, Gampenrieder, SP, and Greil, R. Her2 directed antibody-drug-conjugates beyond T-Dm1 in breast Cancer. Int J Mol Sci. (2019) 20:1115. doi: 10.3390/ijms20051115
30. Najjar, MK, Manore, SG, Regua, AT, and Lo, HW. Antibody-drug conjugates for the treatment of Her2-positive breast Cancer. Gen Dent. (2022) 13:2065. doi: 10.3390/genes13112065
31. Hunter, FW, Barker, HR, Lipert, B, Rothé, F, Gebhart, G, Piccart-Gebhart, MJ, et al. Mechanisms of resistance to Trastuzumab Emtansine (T-Dm1) in Her2-positive breast Cancer. Br J Cancer. (2020) 122:603–12. doi: 10.1038/s41416-019-0635-y
32. Yamazaki, CM, Yamaguchi, A, Anami, Y, Xiong, W, Otani, Y, Lee, J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. (2021) 12:3528. doi: 10.1038/s41467-021-23793-7
33. Krop, IE, Kim, SB, González-Martín, A, LoRusso, PM, Ferrero, JM, Smitt, M, et al. Trastuzumab Emtansine versus treatment of Physician's choice for pretreated Her2-positive advanced breast Cancer (Th3resa): a randomised, open-label, phase 3 trial. Lancet Oncol. (2014) 15:689–99. doi: 10.1016/s1470-2045(14)70178-0
34. Krop, IE, Kim, SB, Martin, AG, LoRusso, PM, Ferrero, JM, Badovinac-Crnjevic, T, et al. Trastuzumab Emtansine versus treatment of Physician's choice in patients with previously treated Her2-positive metastatic breast Cancer (Th3resa): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. (2017) 18:743–54. doi: 10.1016/s1470-2045(17)30313-3
35. Hurvitz, SA, Dirix, L, Kocsis, J, Bianchi, GV, Lu, J, Vinholes, J, et al. Phase ii randomized study of Trastuzumab Emtansine versus Trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast Cancer. J Clin Oncol. (2013) 31:1157–63. doi: 10.1200/jco.2012.44.9694
36. Mamounas, EP, Untch, M, Mano, MS, Huang, CS, Geyer, CE Jr, von Minckwitz, G, et al. Adjuvant T-Dm1 versus Trastuzumab in patients with residual invasive disease after neoadjuvant therapy for Her2-positive breast Cancer: subgroup analyses from Katherine. Annals of Oncol: official J European Society for Medical Oncol. (2021) 32:1005–14. doi: 10.1016/j.annonc.2021.04.011
37. de Haas, SL, Slamon, DJ, Martin, M, Press, MF, Lewis, GD, Lambertini, C, et al. Tumor biomarkers and efficacy in patients treated with Trastuzumab Emtansine + Pertuzumab versus standard of care in Her2-positive early breast Cancer: An open-label, phase iii study (Kristine). Breast Cancer Res: BCR. (2023) 25:2. doi: 10.1186/s13058-022-01587-z
38. Hurvitz, SA, Martin, M, Jung, KH, Huang, CS, Harbeck, N, Valero, V, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in human epidermal growth factor receptor 2-positive breast Cancer: three-year outcomes from the phase iii Kristine study. J Clin Oncol. (2019) 37:2206–16. doi: 10.1200/jco.19.00882
39. Harbeck, N, Nitz, UA, Christgen, M, Kümmel, S, Braun, M, Schumacher, C, et al. De-escalated neoadjuvant Trastuzumab-Emtansine with or without endocrine therapy versus Trastuzumab with endocrine therapy in Hr+/Her2+ early breast Cancer: 5-year survival in the Wsg-Adapt-Tp trial. J Clin Oncol. (2023) 41:3796–804. doi: 10.1200/jco.22.01816
40. Perez, EA, Barrios, C, Eiermann, W, Toi, M, Im, YH, Conte, P, et al. Trastuzumab Emtansine with or without Pertuzumab versus Trastuzumab plus Taxane for human epidermal growth factor receptor 2-positive, advanced breast Cancer: primary results from the phase iii Marianne study. J Clin Oncol. (2017) 35:141–8. doi: 10.1200/jco.2016.67.4887
41. Jenkins, S, Zhang, W, Steinberg, SM, Nousome, D, Houston, N, Wu, X, et al. Phase I study and cell-free DNA analysis of T-Dm1 and metronomic Temozolomide for secondary prevention of Her2-positive breast Cancer Brain metastases. Clinical Cancer Res: Official JAmerican Association for Cancer Res. (2023) 29:1450–9. doi: 10.1158/1078-0432.Ccr-22-0855
42. Hatschek, T, Foukakis, T, Bjöhle, J, Lekberg, T, Fredholm, H, Elinder, E, et al. Neoadjuvant Trastuzumab, Pertuzumab, and docetaxel vs Trastuzumab Emtansine in patients with Erbb2-positive breast Cancer: a phase 2 randomized clinical trial. JAMA Oncol. (2021) 7:1360–7. doi: 10.1001/jamaoncol.2021.1932
43. Wuerstlein, R, Ellis, P, Montemurro, F, Antón Torres, A, Delaloge, S, Zhang, Q, et al. Final results of the global and Asia cohorts of Kamilla, a phase Iiib safety trial of Trastuzumab Emtansine in patients with Her2-positive advanced breast Cancer. ESMO open. (2022) 7:100561. doi: 10.1016/j.esmoop.2022.100561
44. Waks, AG, Keenan, TE, Li, T, Tayob, N, Wulf, GM, Richardson, ET 3rd, et al. Phase Ib study of Pembrolizumab in combination with Trastuzumab Emtansine for metastatic Her2-positive breast Cancer. J Immunother Cancer. (2022) 10:e005119. doi: 10.1136/jitc-2022-005119
45. Bellon, JR, Tayob, N, Yang, DD, Tralins, J, Dang, CT, Isakoff, SJ, et al. Local therapy outcomes and toxicity from the Atempt trial (Tbcrc 033): a phase ii randomized trial of adjuvant Trastuzumab Emtansine versus paclitaxel in combination with Trastuzumab in women with stage I Her2-positive breast Cancer. Int J Radiat Oncol Biol Phys. (2022) 113:117–24. doi: 10.1016/j.ijrobp.2021.12.173
46. Cortés, J, Diéras, V, Lorenzen, S, Montemurro, F, Riera-Knorrenschild, J, Thuss-Patience, P, et al. Efficacy and safety of Trastuzumab Emtansine plus Capecitabine vs Trastuzumab Emtansine alone in patients with previously treated Erbb2 (Her2)-positive metastatic breast Cancer: a phase 1 and randomized phase 2 trial. JAMA Oncol. (2020) 6:1203–9. doi: 10.1001/jamaoncol.2020.1796
47. Krop, IE, Im, SA, Barrios, C, Bonnefoi, H, Gralow, J, Toi, M, et al. Trastuzumab Emtansine plus Pertuzumab versus Taxane plus Trastuzumab plus Pertuzumab after anthracycline for high-risk human epidermal growth factor receptor 2-positive early breast Cancer: the phase iii Kaitlin study. J Clin Oncol. (2022) 40:438–48. doi: 10.1200/jco.21.00896
48. Krop, IE, LoRusso, P, Miller, KD, Modi, S, Yardley, D, Rodriguez, G, et al. A phase ii study of Trastuzumab Emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast Cancer who were previously treated with Trastuzumab, Lapatinib, an anthracycline, a Taxane, and Capecitabine. J Clin Oncol. (2012) 30:3234–41. doi: 10.1200/jco.2011.40.5902
49. Martin, M, Fumoleau, P, Dewar, JA, Albanell, J, Limentani, SA, Campone, M, et al. Trastuzumab Emtansine (T-Dm1) plus docetaxel with or without Pertuzumab in patients with Her2-positive locally advanced or metastatic breast Cancer: results from a phase Ib/Iia study. Annals of Oncol: Official J European Society for Medical Oncol. (2016) 27:1249–56. doi: 10.1093/annonc/mdw157
50. Miller, KD, Diéras, V, Harbeck, N, Andre, F, Mahtani, RL, Gianni, L, et al. Phase Iia trial of Trastuzumab Emtansine with Pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast Cancer. J Clin Oncol. (2014) 32:1437–44. doi: 10.1200/jco.2013.52.6590
51. Burris, HA 3rd, Rugo, HS, Vukelja, SJ, Vogel, CL, Borson, RA, Limentani, S, et al. Phase ii study of the antibody drug conjugate Trastuzumab-Dm1 for the treatment of human epidermal growth factor receptor 2 (Her2)-positive breast Cancer after prior Her2-directed therapy. J Clin Oncol. (2011) 29:398–405. doi: 10.1200/jco.2010.29.5865
52. Montemurro, F, Delaloge, S, Barrios, CH, Wuerstlein, R, Anton, A, Brain, E, et al. Trastuzumab Emtansine (T-Dm1) in patients with Her2-positive metastatic breast Cancer and Brain metastases: exploratory final analysis of cohort 1 from Kamilla, a single-arm phase Iiib clinical trial(☆). Annals of Oncol: Official J European Society for Medical Oncol. (2020) 31:1350–8. doi: 10.1016/j.annonc.2020.06.020
53. Krop, IE, Beeram, M, Modi, S, Jones, SF, Holden, SN, Yu, W, et al. Phase I study of Trastuzumab-Dm1, an Her2 antibody-drug conjugate, given every 3 weeks to patients with Her2-positive metastatic breast Cancer. J Clin Oncol. (2010) 28:2698–704. doi: 10.1200/jco.2009.26.2071
54. Yardley, DA, Krop, IE, LoRusso, PM, Mayer, M, Barnett, B, Yoo, B, et al. Trastuzumab Emtansine (T-Dm1) in patients with Her2-positive metastatic breast Cancer previously treated with chemotherapy and 2 or more Her2-targeted agents: results from the T-pas expanded access study. Cancer J (Sudbury, Mass). (2015) 21:357–64. doi: 10.1097/ppo.0000000000000144
55. Liu, F, Ke, J, and Song, Y. T-Dm1-induced thrombocytopenia in breast Cancer patients: new perspectives. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. (2020) 129:110407. doi: 10.1016/j.biopha.2020.110407
56. Swain, F, and Bird, R. How I approach new onset thrombocytopenia. Platelets. (2020) 31:285–90. doi: 10.1080/09537104.2019.1637835
57. Thon, JN, Devine, MT, Jurak Begonja, A, Tibbitts, J, and Italiano, JE Jr. High-content live-cell imaging assay used to establish mechanism of Trastuzumab Emtansine (T-Dm1)--mediated inhibition of platelet production. Blood. (2012) 120:1975–84. doi: 10.1182/blood-2012-04-420968
58. Uppal, H, Doudement, E, Mahapatra, K, Darbonne, WC, Bumbaca, D, Shen, BQ, et al. Potential mechanisms for thrombocytopenia development with Trastuzumab Emtansine (T-Dm1). Clinical Cancer Res: Official J American Association for Cancer Res. (2015) 21:123–33. doi: 10.1158/1078-0432.Ccr-14-2093
59. Zhao, H, Gulesserian, S, Ganesan, SK, Ou, J, Morrison, K, Zeng, Z, et al. Inhibition of megakaryocyte differentiation by antibody-drug conjugates (Adcs) is mediated by macropinocytosis: implications for Adc-induced thrombocytopenia. Mol Cancer Ther. (2017) 16:1877–86. doi: 10.1158/1535-7163.Mct-16-0710
60. Ansary, AM, Stolla, M, Corson, J, Cundy, A, Bailey, SL, Pellham, E, et al. Effect of ado-Trastuzumab Emtansine on autologous platelet kinetics and function. JCO Precis Oncol. (2022) 6:e2200237. doi: 10.1200/po.22.00237
61. Kartal-Yandim, M, Adan-Gokbulut, A, and Baran, Y. Molecular mechanisms of drug resistance and its reversal in Cancer. Crit Rev Biotechnol. (2016) 36:716–26. doi: 10.3109/07388551.2015.1015957
62. Ren, F, Shen, J, Shi, H, Hornicek, FJ, Kan, Q, and Duan, Z. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian Cancer. Biochim Biophys Acta. (2016) 1866:266–75. doi: 10.1016/j.bbcan.2016.10.001
63. Gottesman, MM . Mechanisms of Cancer drug resistance. Annu Rev Med. (2002) 53:615–27. doi: 10.1146/annurev.med.53.082901.103929
64. Żesławska, E, Kincses, A, Spengler, G, Nitek, W, Wyrzuc, K, Kieć-Kononowicz, K, et al. The 5-aromatic Hydantoin-3-acetate derivatives as inhibitors of the tumour multidrug resistance efflux pump P-glycoprotein (Abcb1): synthesis, crystallographic and biological studies. Bioorg Med Chem. (2016) 24:2815–22. doi: 10.1016/j.bmc.2016.04.055
65. Potocnik, U, Glavac, MR, Golouh, R, and Glavac, D. The role of P-glycoprotein (Mdr1) polymorphisms and mutations in colorectal Cancer. Pflugers Arch - Eur J Physiol. (2001) 442:R182–3. doi: 10.1007/s004240100017
66. Li, YH, Wang, YH, Li, Y, and Yang, L. Mdr1 gene polymorphisms and clinical relevance. Yi chuan xue bao = Acta genetica Sinica. (2006) 33:93–104. doi: 10.1016/s0379-4172(06)60027-9
67. Potocnik, U, Glavac, D, and Dean, M. Common germline Mdr1/Abcb1 functional polymorphisms and haplotypes modify susceptibility to colorectal cancers with high microsatellite instability. Cancer Genet Cytogenet. (2008) 183:28–34. doi: 10.1016/j.cancergencyto.2008.01.023
68. Kelland, L . The resurgence of platinum-based Cancer chemotherapy. Nat Rev Cancer. (2007) 7:573–84. doi: 10.1038/nrc2167
69. Sharom, FJ . Abc multidrug transporters: structure, function and role in Chemoresistance. Pharmacogenomics. (2008) 9:105–27. doi: 10.2217/14622416.9.1.105
70. Miyata, K, Nakagawa, Y, Kimura, Y, Ueda, K, and Akamatsu, M. In vitro and in vivo evaluations of the P-glycoprotein-mediated efflux of Dibenzoylhydrazines. Toxicol Appl Pharmacol. (2016) 298:40–7. doi: 10.1016/j.taap.2016.03.008
71. Tanabe, M, Ieiri, I, Nagata, N, Inoue, K, Ito, S, Kanamori, Y, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (Mdr)-1 gene. J Pharmacol Exp Ther. (2001) 297:1137–43.
72. Leith, CP, Kopecky, KJ, Chen, IM, Eijdems, L, Slovak, ML, McConnell, TS, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins Mdr1/P-glycoprotein, Mrp1, and Lrp in acute myeloid leukemia: a southwest oncology group study. Blood. (1999) 94:1086–99.
73. Karageorgopoulou, S, Kostakis, ID, Gazouli, M, Markaki, S, Papadimitriou, M, Bournakis, E, et al. Prognostic and predictive factors in patients with metastatic or recurrent cervical Cancer treated with platinum-based chemotherapy. BMC Cancer. (2017) 17:451. doi: 10.1186/s12885-017-3435-x
74. Gréen, H, Söderkvist, P, Rosenberg, P, Horvath, G, and Peterson, C. Mdr-1 single nucleotide polymorphisms in ovarian Cancer tissue: G2677t/a correlates with response to paclitaxel chemotherapy. Clinical Cancer Res: Official J American Association for Cancer Res. (2006) 12:854–9. doi: 10.1158/1078-0432.Ccr-05-0950
75. Bray, J, Sludden, J, Griffin, MJ, Cole, M, Verrill, M, Jamieson, D, et al. Influence of pharmacogenetics on response and toxicity in breast Cancer patients treated with doxorubicin and cyclophosphamide. Br J Cancer. (2010) 102:1003–9. doi: 10.1038/sj.bjc.6605587
76. Zhou, Z, Chen, Q, Zuo, D, Wang, H, Hua, Y, and Cai, Z. Abcb1 (Rs1128503) polymorphism and response to chemotherapy in patients with malignant tumors-evidences from a Meta-analysis. Int J Clin Exp Med. (2015) 8:265–72.
77. Fairchild, CR, Ivy, SP, Kao-Shan, CS, Whang-Peng, J, Rosen, N, Israel, MA, et al. Isolation of amplified and overexpressed DNA sequences from Adriamycin-resistant human breast Cancer cells. Cancer Res. (1987) 47:5141–8.
78. Roy, P, Yu, LJ, Crespi, CL, and Waxman, DJ. Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and Ifosfamide activation based on Cdna-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos. (1999) 27:655–66.
Keywords: trastuzumab emtansine, antibody–drug conjugate, T-DM1, DCR, platelet
Citation: He M, Zhao W, Wang P, Li W, Chen H, Yuan Z, Pan G, Gao H, Sun L, Chu J, Li L and Hu Y (2024) Efficacy and safety of Trastuzumab Emtansine in treating human epidermal growth factor receptor 2-positive metastatic breast cancer in Chinese population: a real-world multicenter study. Front. Med. 11:1383279. doi: 10.3389/fmed.2024.1383279
Edited by:
Udhaya Kumar, Baylor College of Medicine, United StatesReviewed by:
Wajeeha Razaq, University of Oklahoma Health Sciences Center, United StatesChristopher Hillyar, University of Oxford, United Kingdom
Copyright © 2024 He, Zhao, Wang, Li, Chen, Yuan, Pan, Gao, Sun, Chu, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Hu, aHV5dXNkdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Miao He
Miao He Wen Zhao
Wen Zhao Peng Wang3
Peng Wang3 Hanhan Chen
Hanhan Chen Li Li
Li Li Yu Hu
Yu Hu