- 1Department of Pediatrics, Hospital Universitario Severo Ochoa, Madrid, Spain
- 2Instituto de Investigación Sanitaria Puerta de Hierro—Segovia de Arana, Hospital Universitario Puerta de Hierro Majadahonda, Majadahonda, Spain
- 3Networked Biomedical Research Center for Infectious Diseases (CIBERINFEC), Madrid, Spain
- 4Traslational Research Network in Pediatric Infectious Diseases (RITIP), Madrid, Spain
Introduction: Moderate-to-late preterm infants constitute the majority within the preterm infant population. Most research on preterm infants has focused on very preterm children, often treating moderate-to-late preterm infants as similar to full-term infants. Our objective was to compare clinical, respiratory, cardio-metabolic and neurodevelopmental outcomes in adolescents aged 12–15 years born moderate and late preterm with a control group of the same age born full-term.
Methods: Observational cross-sectional study, comparing moderate-to-late preterm (32–36+6 weeks’ gestational age) with full-term adolescents (37–41+6 weeks’ gestational age; 75 each group). Perinatal and neonatal history were collected as well as data on respiratory evolution (ISAAC questionnaire for asthma symptoms for adolescents 13–14 years), anthropometric values, learning difficulties, behavioral test (screening questionnaire for high-performance autism spectrum disorder and evaluation test for attention deficit hyperactivity disorder), skin prick test, pulmonary function test, echocardiogram and blood pressure. A blood test with metabolic profile was conducted.
Results: Moderate-to-late preterm adolescents had more current asthma [p = 0.008, OR3 (95% CI 1.26–7.14)] and longer duration of combined treatments to control asthma (inhaled corticosteroids and anti-leukotrienes; p = 0.048). Forced vital capacity <80% was detected more often in moderate-to-late preterm patients (p = 0.013). When assessing right ventricle, moderate-to-late preterm adolescents showed better tricuspid annular plane systolic excursion z-score (p = 0.003), shortening fraction (p < 0.001) and E/A ratio z-score (p = 0.002). Regarding left ventricular assessment, moderate-to-late preterm group had smaller ventricle diastolic diameter (p = 0.04) and lower posterior wall z-score values (p = 0.037). They also showed a better S’wave z-score (p = 0.027), E wave (p = 0.005), E/A ratio (p = 0.003) and a higher septal myocardial performance index z-score (p = 0.025). Moderate-to-late preterm adolescents presented lower weight z-score (p = 0.039), body mass index z-score (p = 0.013), Waterlow weight index (p = 0.006) and higher undernutrition index [p = 0.04; OR 1.4 (95% CI 1–1.9)]. Although there were no differences in neurodevelopmental survey or behavioral tests.
Conclusion: Our findings underscore the importance of extended follow-up for this predominant group of premature infants to identify potential respiratory, cardiac and anthropometric issues that may emerge in the future.
Introduction
Preterm infants account for 10.6% of livebirths (1). Preterm birth, defined as birth before 37 weeks, is a very heterogeneous group. Moderate-to-late preterm (MLP) infants, which are defined as birth between 32 and 36 weeks’ gestation and represents 85% of all preterm births (1, 2).
Previously, MLP infants have been considered “near” to term. However, recent publications (3, 4) report that MLP births have higher rates of morbidity and mortality compared to full-term children, especially in the first year of life. In addition, most recent studies (5, 6) suggest cardiovascular, neurodevelopmental and respiratory adversity in their evolution (Figure 1). These studies have revealed that MLP birth can be associated with poor growth (7, 8), increased blood pressure (4, 9), dyslipidemia or insulin resistance (10, 11), but outcomes in the adolescence have been inconsistent.
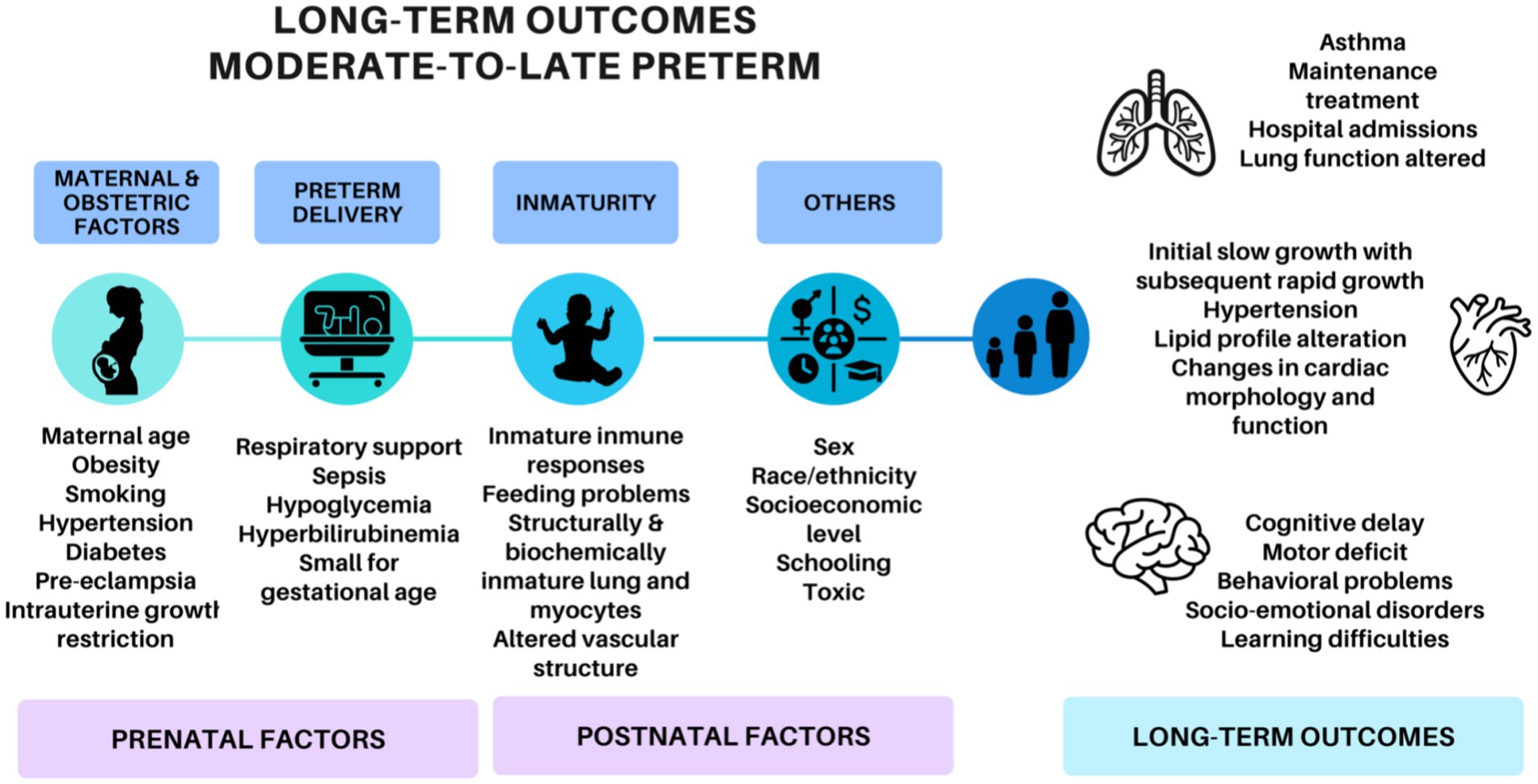
Figure 1. Factors involved in the development of the long-term outcomes of moderate-to-late preterm adolescents.
Lung development is also affected in MLP births. Compared with term, MLP children suffer more bronchiolitis, requiring hospitalization, and asthma especially in childhood (12–14). As the age of infants born MLP increases, a lower prevalence of asthma has been reported (15). It has been described in MLP infants a similar forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1), but lower mean forced expiratory flow between 25% and 75% of FVC (FEF25–75) than in term infants (15, 16). However, the impact of MLP birth on asthma prevalence and lung function during adolescence remains unclear.
The premature developing brain is exposed to an extrauterine environment during their development. Prematurity is associated to higher risk of cognitive, motor, behavioral and neurosensory deficit. In particular greater prevalence of behavioral and psychiatric disorders have been described in MLP patients (17, 18).
In all these studies, it remains unclear whether the outcomes are associated with gestational age and whether they persist into adulthood. The long-term evolution of MLP adolescents needs to be well characterized to implement specific guidelines, targeted screening, and early treatment to improve their prognosis.
The aim of this study was to characterize the clinical, respiratory (asthma evolution, skin prick test and lung function), cardio-metabolic (hypertension, morphological or functional cardiac changes, growth disturbance and metabolic disorders) and neurodevelopmental outcomes (behavioral, social and learning diseases) among adolescents born with moderate and late prematurity, in comparison to their full-term counterparts.
Methods
An observational analytic cross-sectional study was performed. All adolescents aged 12 to 15 years, with a history of MLP birth (32–366 weeks of gestational age), born from 1 January 2006 to 31 December 2007, in the Severo Ochoa University Hospital, were invited to participate in the study. A control group of adolescents aged 12 to 15 years born at term (≥37 weeks) was also included. Furthermore, moderate preterm adolescents (MP) (32–336 weeks of gestational age) were compared with late preterm and full-term adolescents (LPFT) (34–416 weeks of gestational age).
The study sample was obtained from the birth registry of the Severo Ochoa University Hospital. A list was generated with all the MLP and full-term infants, arranged in chronological order according to date of birth. All the parents of the MLP patient were contacted by telephone to inform them of the study and, if interested in participate, to arrange an appointment. Each MLP patient was matched with a full-term control, the one immediately after the MLP patient who accepted to be included in the study.
The study was approved by the Ethics Committee of the Severo Ochoa Hospital. Written informed consent was obtained from all the patients and their parents after full explanation of the study protocol. All methods were carried out in the accordance with relevance guidelines and regulations.
Perinatal and neonatal medical history were collected from medical history (newborn measurements were presented with Fenton z-score) (19). The same respiratory, cardiologic, metabolic and neurological evaluation was conducted in both, cases and controls, as described below.
The frequency of asthma, allergy, abnormal lung function, hypertension, functional cardiac changes, growth disturbance, metabolic disorders and behavioral, social and learning diseases was compared between MLP and full-term adolescents and between MP and LPFT adolescents.
Respiratory evaluation
A specific questionnaire was used to obtain information on wheezing episodes, hospital admissions, maintenance medication, and family history of respiratory disease. The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire for asthma symptoms for adolescents 13–14 years (20), previously validated and translated to Spanish, was answered by adolescents. Current asthma prevalence was estimated by the percentage of children with an affirmative answer to question number 2 (wheezing or whistling in the chest in the past 12 months), which has demonstrated the higher correlation with current asthma prevalence in validation studies (20, 21).
Skin prick test was performed to evaluate allergic sensitization for common inhaled allergens. Standardized Allergens (ALK-Abelló) were used with a positive control (10 mg/mL histamine) and negative control (glycerol-saline carrier solution). Positive test was considered when the papule diameter was greater than the positive control (22).
Lung function was evaluated by spirometry, according with the established guidelines (23) using Easy on-PC spirometer (NDD, New diagnostic design medical technologies). At least three reproducible maneuvers were performed, selecting the one with best FEV-1 and FVC values. The percentages were given with Zapletal (24) and z-score of predicted values with reference values of Global Lung Function Initiative (25). The variables collected were: FVC, FEV1, FEV1/FVC and FEF25–75. The results were normal when FEV1 and FVC were ≥80%, ratio FEV1/FVC > 80% and FEF25–75 ≥ 65% (26). Bronchodilator test was positive when FEV1 increased >12% compared to the baseline after administration of 400 mcg of salbutamol (27).
Cardiologic evaluation
Blood pressure (BP) was measured oscillometrically with EarlyVue VS30 (Philips Healthcare, EEUU). Results were presented with z-scores of National High Blood Pressure Education Working Group on High Blood Pressure in Children and Adolescents 2004 (28), being considered pathological if >95th percentile (29). Echocardiogram to evaluate the cardiac function was performed using Vivid Pro7 and 9 (General Electric Healthcare, United States). Measurements were obtained using M-mode, power-doppler, continuous-doppler and myocardial performance index (MPI) and the results were presented with z-scores (30–32).
Metabolic assessment
The follow-up included current anthropometric measurements [weight, height, body mass index presented with Carrascosa 2010 z-score (33)], Waterlow index (34) and abdominal perimeter [presented with Moreno z-score (35)]. Body mass index and abdominal perimeter were considered pathological > 2standard deviations-SD (36, 37). Laboratory tests with metabolic profile (LDL-low-density lipoprotein, HDL-high-density lipoprotein, cholesterol, triglycerides, glycated hemoglobin-HbA1c) were performed. Cholesterol values ≥ 200 mg/dL, HDL < 40 mg/dL, LDL ≥ 130 mg/dL, triglycerides ≥ 150 mg/dL and HbA1c ≥ 6.5% were considered as pathologic (37, 38).
Neurologic evaluation
Learning disabilities and social development were evaluated. Behavioral tests [Asperger Syndrome Screening Questionnaire–ASSQ (39) and attention deficit hyperactivity disorder-ADHD assessment scale (40)] were carried out. The ASSQ was considered abnormal when the score was greater than 19 points. The test ADHD was pathological if the patient obtained >30 points, >10 points in attention deficit/hyperactivity subscale or 11 points in the behavioral disorder subscale (41, 42).
Statistical analysis
To calculate the sample size, the expected prevalence of asthma in preterm adolescents was expected to be about 25–30% (43) vs. 10% (44) in the control group. The minimal sample size required, with an alpha error of 5% and a power of 80%, was 90 patients in each group. All the analysis was performed using the Statistical Package for the Social Sciences (SPSS) Version 23.0.
Absolute and relative frequencies were used to describe qualitative variables. Continuous variables were described using median and interquartile range-IQR (non-normal distribution). Comparisons were performed with Student’s test, Chi2 and Mann–Whitney test. p-value < 0.05 was regarded as statistically significant. To control for potentially confounding variables and to examine the independent contribution of the explicative variables on the likelihood of developing asthma, a backward stepwise binomial logistic regression model was built. All the variables with p-value < 0.1 were introduced in the multi-variate analysis. Adjusted odds ratios (OR) with 95% confidence intervals (CI) were calculated.
Results
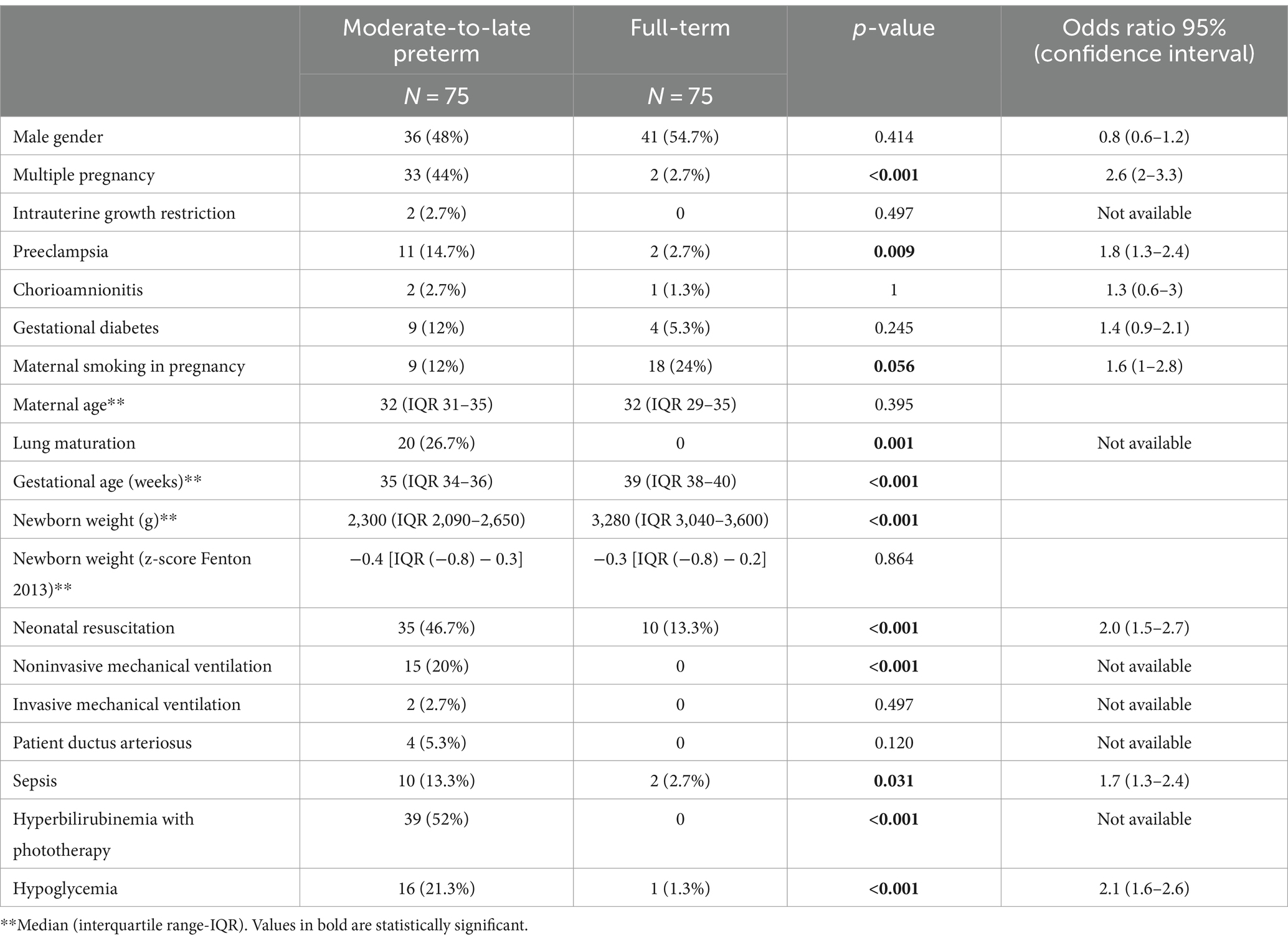
A total of 150 children (75 preterm and 75 full-term) were included, with mean age 13 years (IQR 13–14). Preterm adolescents were younger (13 years, IQR 12–13) than the full-term ones (14 years, IQR 13–14) (p < 0.001). Perinatal characteristics are presented in Table 1.
Respiratory health
The responses to the ISAAC questionnaire for asthma symptoms are displayed in Table 2. Current asthma (Question 2) was more frequent in the MLP group compared to the full-term one [p = 0.008, OR 3 (95% CI 1.26–7.14)] as well as in the MP group in comparison with the LPFT group [p = 0.003; OR 3.22 (95% CI 1.56–6.62)]. The frequency of asthma ever (Question 6) showed a tendency to be more common in the MLP group, although it did not reach statistical significance (p = 0.08).
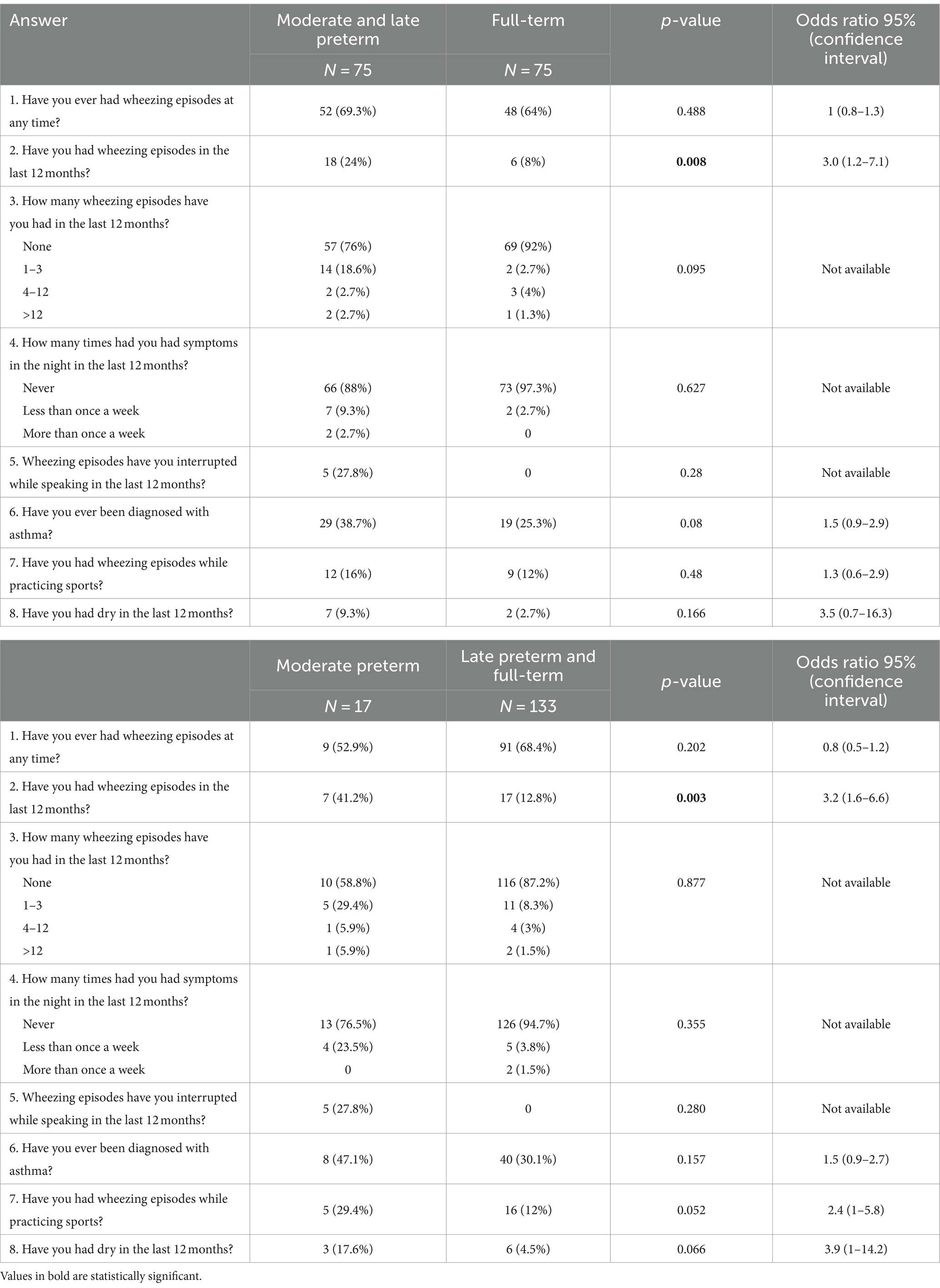
Table 2. Affirmative answers to the ISAAC Questionnaire for asthma symptoms in moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
After logistic regression, current asthma and asthma diagnosis ever in adolescents were independently associated to neonatal respiratory support and allergic sensitization (Table 3).

Table 3. Multivariate analysis of risk factors independently associated with current asthma and asthma diagnosis ever in the whole cohort of 12–15-year adolescents moderate-late preterm and full-term children.
Respiratory evolution and family history of asthma/allergic sensitization of preterm and full-term children are shown in Table 4. No differences were observed in asthma chronic treatment prescription or duration of inhaled corticosteroids or anti-leukotrienes treatment. However, MLP patients were more likely to receive longer combined treatment with both drugs simultaneously (p = 0.048). The risk of hospital admission due to respiratory causes were similar in both groups.
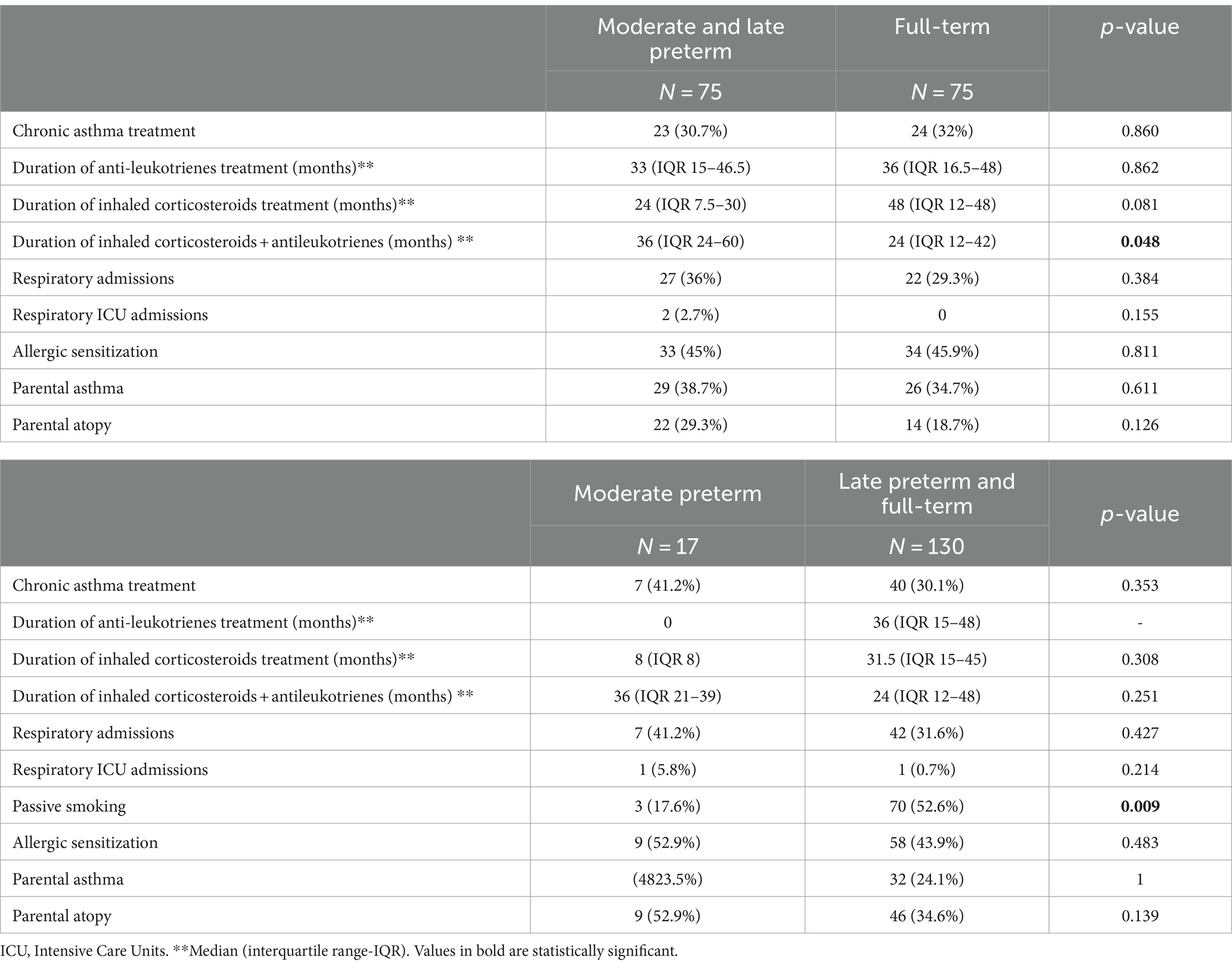
Table 4. Comparison of respiratory evolution during the follow up of moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at 12–15 years of age.
Overall, spirometry measurements were within normal limits in both groups. A higher proportion of children with FVC < 80% was observed in the MLP group (p = 0.013). Additionally, when comparing MP with LPFT adolescents, FVC < 80% and FEF25–75 < 65% were more often found in the MP group (p = 0.021 and p = 0.046, respectively). Data are represented in Table 5.

Table 5. Lung function comparisons between moderate-to-late preterm and full-term counterparts and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
After bivariate analysis, the variables associated with lower lung function (FEV1 z-score, FEV1 < 80%, FVC z-score and FVC < 80%) were: MLP, male gender, current asthma, asthma diagnosis ever, wheezing with exercises and chronic asthma treatment (Table 6).
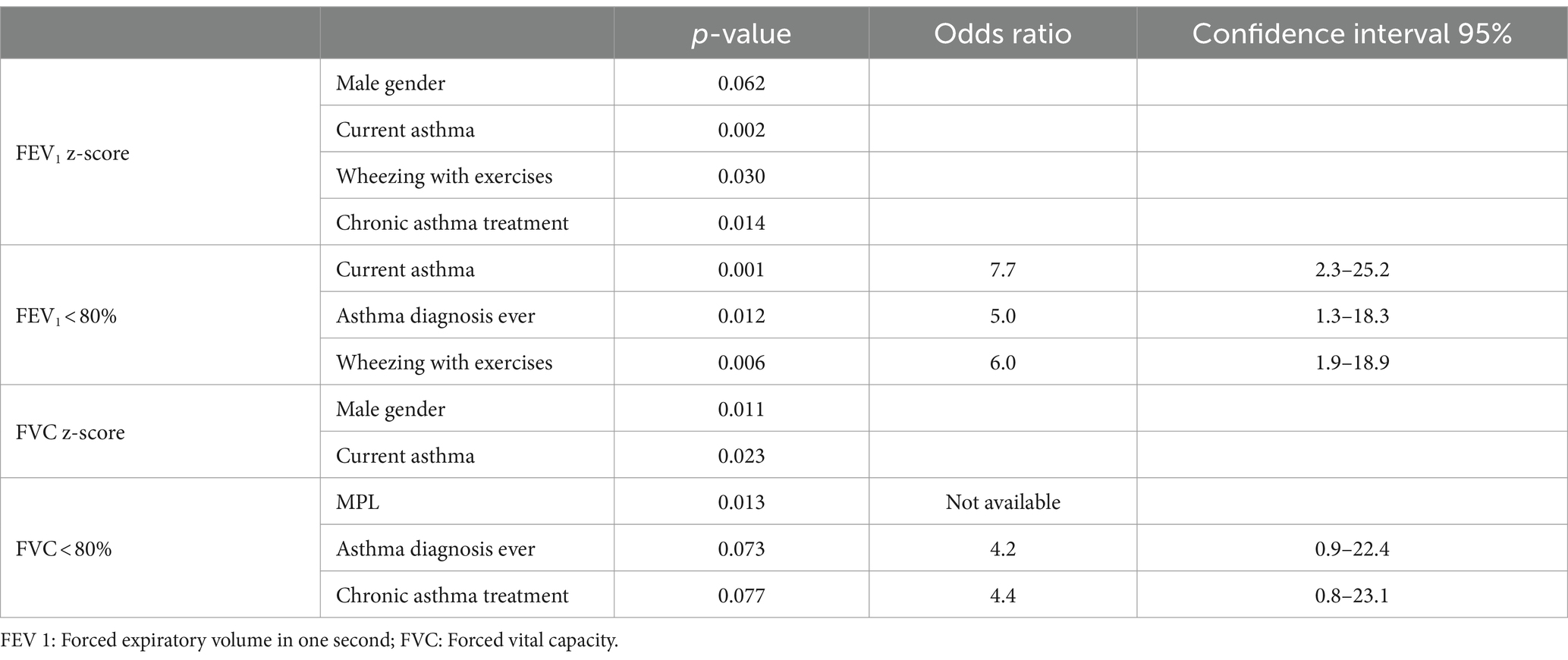
Table 6. Variables associated to lower lung function values in moderate-to-late preterm and full-term adolescents (12–15 years of age).
Cardiometabolic results
BP values were similar in MLP and full-term adolescents, with no differences in the prevalence of hypertension. In relation to metabolic disease as dyslipidemia or diabetes, lower HDL values were found only in one MLP and in 4 full-term adolescents. Hypertriglyceridemia was detected in one full-term patient and hypercholesterolemia and high LDL values in only one MLP adolescent. No child presented pathological HbA1c hemoglobin values or metabolic disease (Table 7).
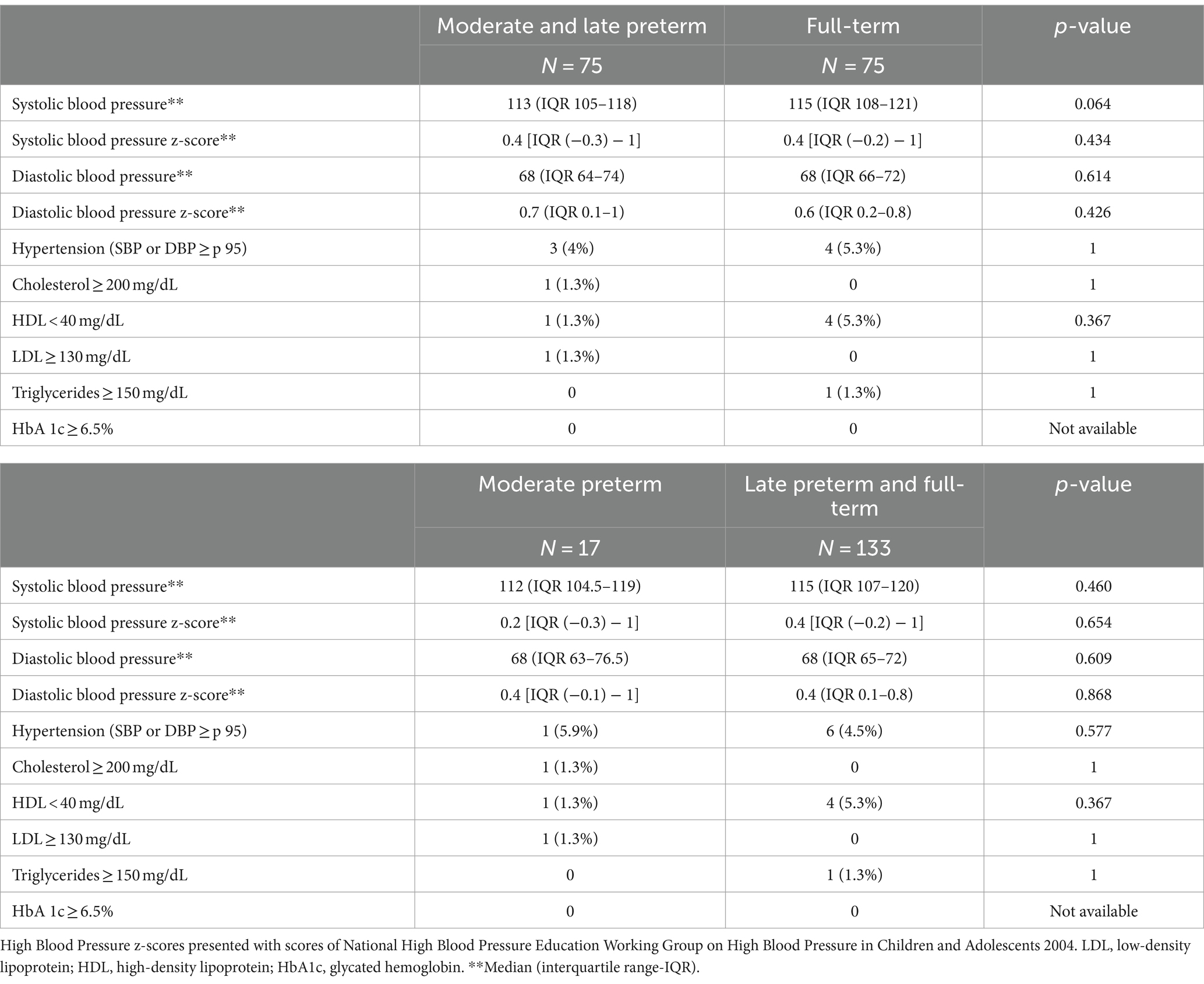
Table 7. Blood pressure and metabolic disease in moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
Right ventricular function and morphology data are showed in Table 8. MLP adolescents had better TAPSE (p = 0.025), TAPSE z-score (p = 0.003), shortening fraction (p < 0.001) and E/A z-score (p = 0.002) than full-term ones. When MP adolescents were compared with LPFT, a smaller right ventricular diastolic diameter and their z-score were observed (p = 0.006 and p = 0.046, respectively). However, no differences regarding right ventricular function were observed.
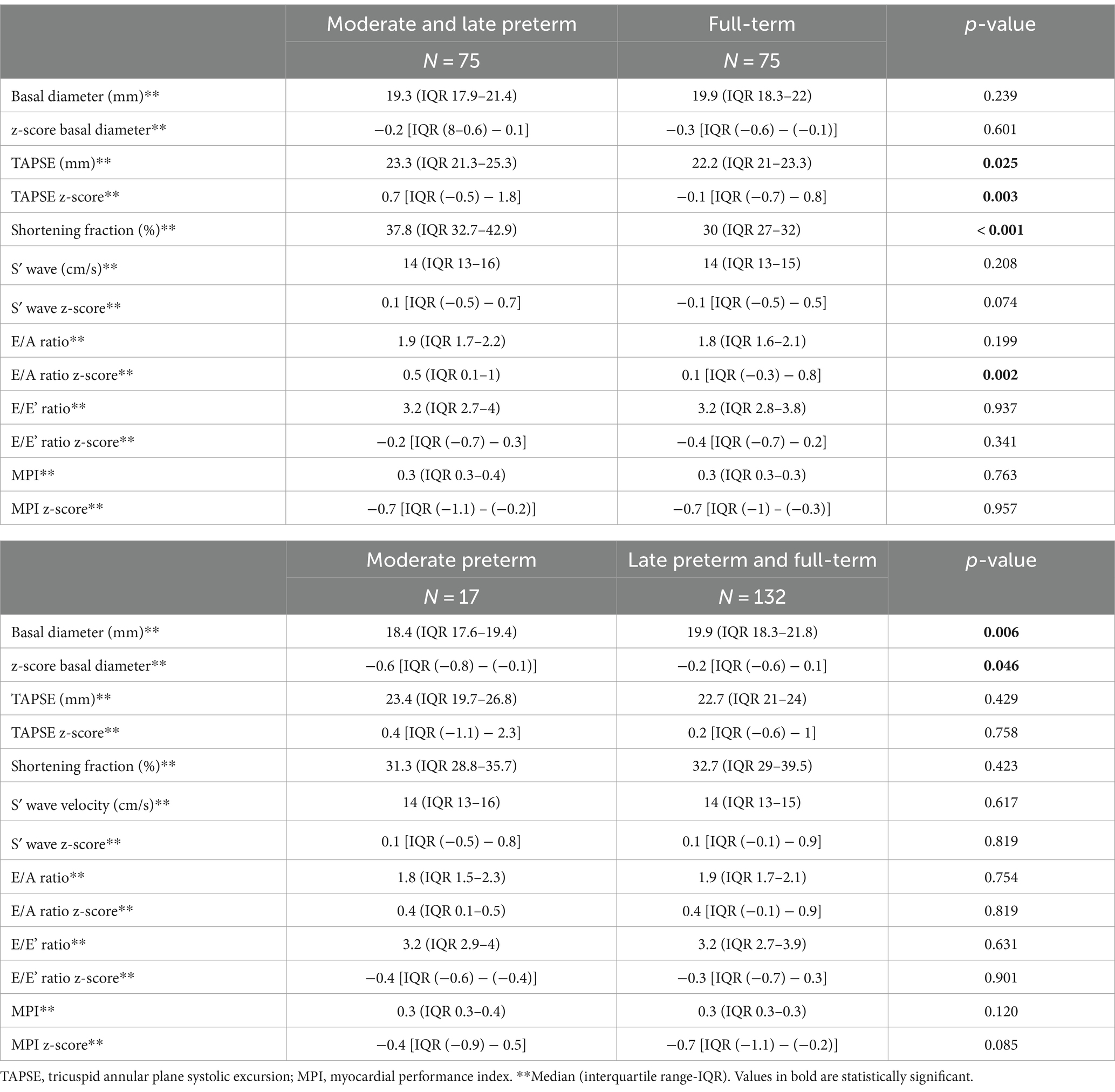
Table 8. Right ventricular function and morphology in moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
Regarding the left ventricular assessment, MLP adolescents had smaller ventricular diastolic diameter compared to full-term children (p = 0.04) and lower posterior wall z-score values (p = 0.037). They also had better S′ wave z-score (p = 0.027), E’ wave z-score (p = 0.005), E/A ratio (p = 0.003) and higher septal MPI z-score (p = 0.025). When comparing MP vs. LPFT, no differences in left ventricular morphology and function were found. Data obtained in the assessment of left ventricle are shown in Table 9.
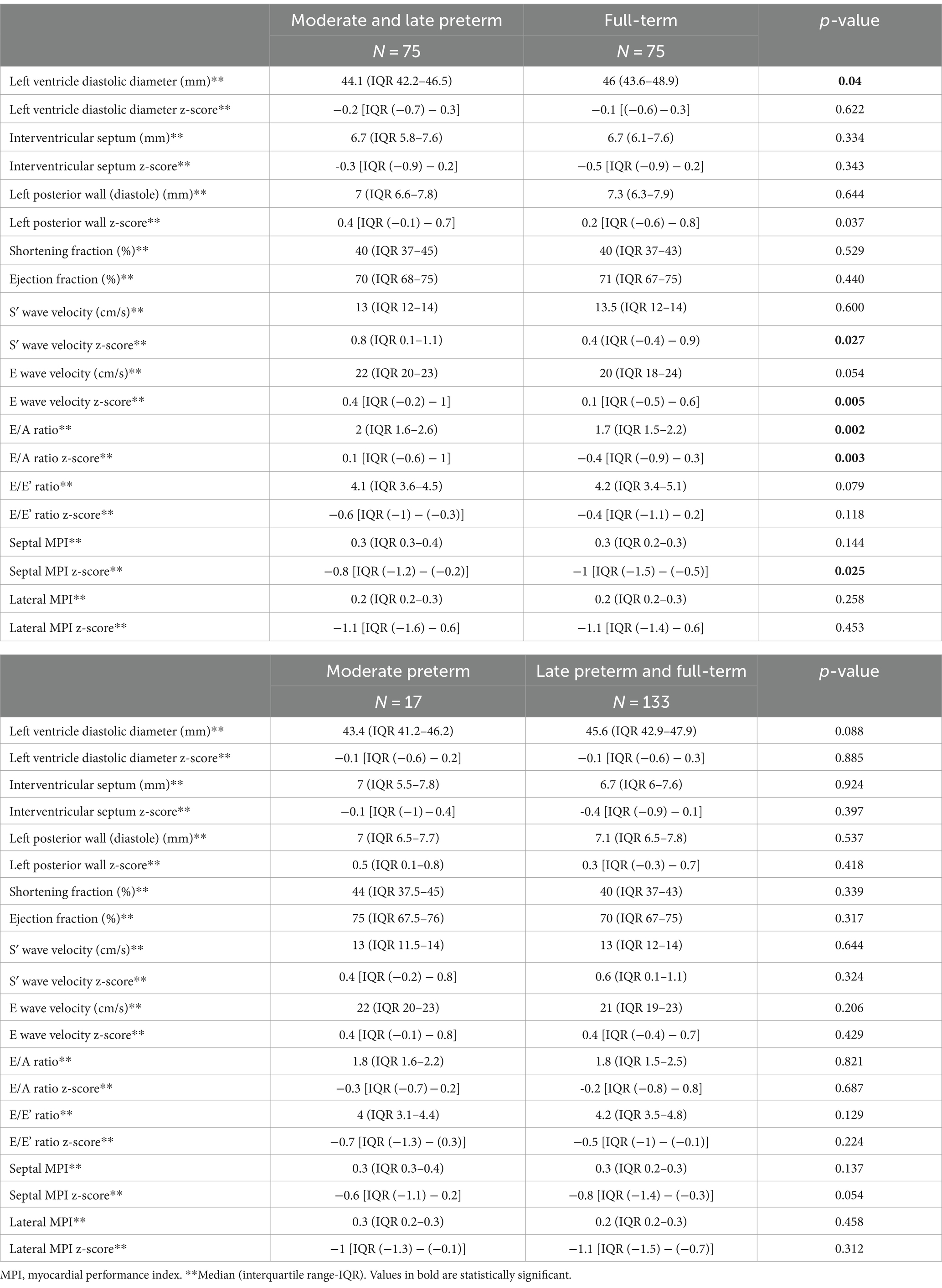
Table 9. Left ventricular morphology and function in moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
Anthropometric data
No significant differences could be detected between both groups regarding the anthropometric measurements at birth, according to their gestational age (presented with Fenton 2013 z-score). In the follow-up, MLP adolescents had lower weight (p < 0.001) and lower weight z-score (p = 0.039) than the full-term ones. They also showed lower body mass index (p = 0.002) and lower body mass index z-score (p = 0.013) than the control group. No differences were found in height or abdominal circumference between the two groups. The Waterlow weight index was lower in the MLP group (p = 0.006) and there was a higher percentage of MLP patients with undernutrition index [p = 0.04; OR 1.4 (95% CI 1–1.9)]. These differences were also observed when comparing the MP patients with the group composed of LPFT. MP adolescents also had lower weight (p = 0.022), weight z-score (p = 0.017), body mass index (p = 0.011), body mass index z-score (p = 0.011) and lower Waterlow weight index (p = 0.039). Anthropometrics data of both group of adolescents are shown in Table 10.
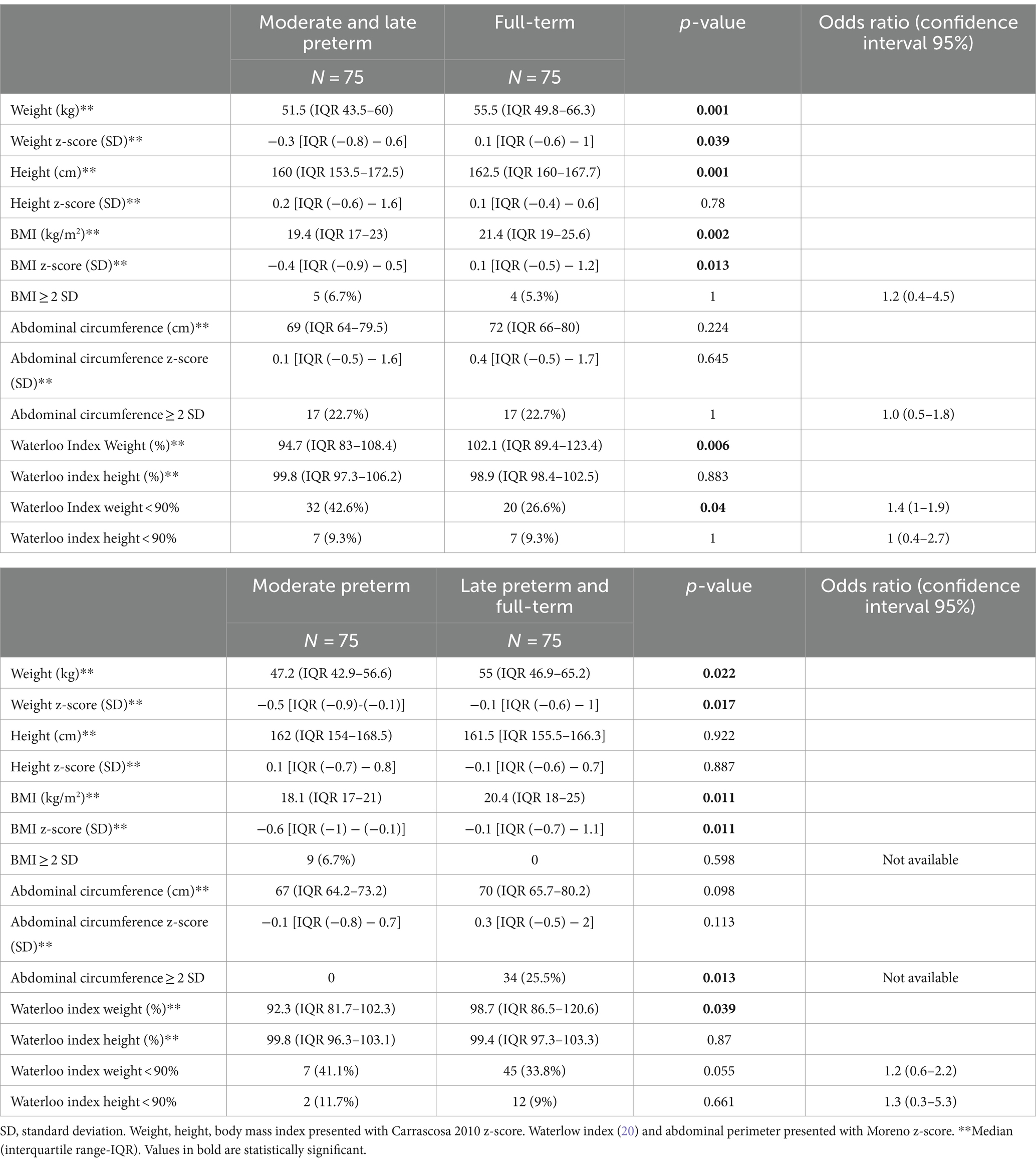
Table 10. Comparison of anthropometrics characteristics of moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
After logistic regression, the variables independently associated with undernutrition at 12–15 years of age (Waterloo index weight < 90%) were: male gender [p < 0.001; OR 4.65 (95% CI 2.17–9.98)] and MLP birth [p = 0.016; OR 2.5 (95% CI 1.19–5.25)].
Neurological
Neurodevelopmental outcomes are shown in Table 11. No differences were found in the neurodevelopmental and behavioral tests, although MP infants, in comparison with LPFT ones, reported more social problems (p < 0.001).

Table 11. Neurodevelopmental outcome of moderate-to-late preterm vs. full-term adolescents and in moderate preterm vs. late preterm and full-term at the follow up (12–15 years of age).
Discussion
To our knowledge, this is the first study to evaluate the global development of MLP patients in adolescence and to compare it with children of the same age born at term. Our results show that MLP adolescents are not the same as full-term, with some differences, but also with some similarities.
In relation to the respiratory evolution, MLP adolescents, exhibited, in our study, higher prevalence of current asthma, with a threefold increased risk compared to full-term children, particularly at lower gestational ages. Furthermore, the MLP group was more likely to undergo longer combined chronic asthma treatment. In summary, these data might indicate that moderate and late birth should be acknowledged as a risk factor for asthma development, not only in the early years of life, but also in adolescence. Several studies have described higher prevalence of asthma in MLP births especially during childhood (4, 43, 45–47). However, the duration of this increased risk is a matter of controversy. Kotecha et al. (48) reported similar respiratory morbidity, including asthma, in 37 born MLP and 34 born full-term at 13–14 years of age. In contrast, Thunqvist et al. (49) found that female subjects, born moderate to late preterm, reported significantly more respiratory symptoms at both, 8 and 16 years of age, than females born term. Additionally, according to the meta-analysis by Been et al. (50), the strength of the association between preterm birth and wheezing disorders is similar between children aged younger and older 5 years. This heterogeneity in the results may be related to various factors, especially the different gestational age of the included patients and their varied age at the time of follow-up, as well as the diversity in the definition of asthma used by different authors. We must also consider other factors that may affect the development of asthma such as a history of asthma, respiratory syncytial virus infection, genetics or the role of inflammasome as an immunomodulator (51). As previously described, we did not find any differences in allergic sensitization rates based on gestational age (14, 16).
Although the rate of asthma admissions did not differ between the preterm and full-term groups, MLP patients did experience higher rate of intensive care unit admissions than full-term children. Our data, according to other reports (12, 16, 45, 52) suggest that, compared to full-term, MLP infants may experience more severe asthma exacerbations (52, 53).
Regarding asthma treatment, no differences were found when the proportion of patients under chronic treatment was compared (30.7 and 32%). However, the duration of combined inhaled corticosteroid/anti-leukotriene treatment was significantly longer in MLP patients, suggesting, a more severe asthma course among MLP adolescents. Other studies have also described similar percentages of chronic asthma treatment in MLP children, such as Perez-Tarazona et al. (16), (28.4%) or Yaacoby-Bianu et al. (54). In contrast, only 8% of moderate preterm children and 5.7% of late preterm in the cohort of Haataja et al. (45) was prescribed asthma treatment.
In relation to lung function, airway obstruction has been described in MLP births during childhood (14, 55). However, the lung function data during school age are controversial (48, 49, 54, 56) and in adolescence most studies (5, 15, 16, 57) indicate that FVC and FEV1 values are comparable to those observed in full-term, with only FEF25–75 values slightly lower or at the lower limit of normality. Overall, the results of pulmonary function tests in our study showed no significant differences between MLP and full-term children. However, the MP group showed lower FEV1 values when compared with LPFT. Furthermore, the probability of FVC values less than 80% and FEF25–75 less than 65% was seven and three times higher, respectively, in the MP group. Similar results were observed in previously reports (15, 16, 57). The presence of normal lung function in late adolescents preterm might be related to the pulmonary plasticity described in this group as age progresses (56). On the other hand, the mild lung function impairment is maintained in the most premature (57, 58) with the MP adolescents having, in our cohort, lower FEV1 and FEF25–75 z-score with a higher percentage of FVC less than 80% and FEF25–75 less than 65%. These data suggest that, albeit mild, late preterm infants maintain a mild obstructive and restrictive pulmonary pattern in adolescence, that deserves ongoing monitoring over time to confirm its progression and to implement preventive pulmonary rehabilitation measures aimed at improving thoracic mobility (59, 60).
In our series, no patient had a diagnosis of bronchopulmonary dysplasia. However, Manti el al (61) did not find significant differences in lung function values between very preterm patients (<32 weeks of gestational age) with or without bronchopulmonary dysplasia, at preschool age.
Prematurity has extensively been described as a cardiovascular risk factor (9, 62–64). In our study, no differences in BP values could be demonstrated between MLP and full-term children, even when analyzing separately MP children as a group of greater vulnerability. Several prior publications (4, 9, 64, 65) have noted minimal differences in BP, particularly among infants under 32 weeks, with even smaller variances observed in late preterm infants, primarily identified through continuous blood pressure monitoring. In addition to gestational age, there may be other factors such as a history of small for gestational age (63), female sex (9, 64) as well as other cardiovascular risk factors (obesity, sedentary lifestyle, metabolic disorders, etc.) and prenatal factors (pre-eclampsia, use of corticosteroids or fetal growth, among others) that may influence the increase in BP levels (63–65).
A reduction in cardiac cavity size, coupled with an increase in mass, diminished stroke volume, and decreased end-diastolic volume, along with observed alterations in myocardial deformation and reduced relaxation, have been noted in the hearts of very premature infants (11, 66, 67). These factors collectively contribute to hypertrophy rather than the hyperplasia seen in the third trimester of gestation. Consequently, these alterations lead to shifts in cardiac morphology and affect both systolic and diastolic functions, particularly in the left ventricle. It is important to note that not all studies have reported the same extent of cardiac remodeling, and variations may depend on factors such as perinatal and postnatal care practices, a history of pulmonary hypertension or obesity (64, 66, 68). Regarding cardiac morphology and function in MLP group, our observations revealed a diminished size of the right ventricle with maintained function, consistent with the findings reported by Lewandoski et al. (66). They similarly observed a reduced right ventricle size paired with a larger ventricular mass, though their results exhibited a more pronounced effect. This variation might be partially attributed to the heightened sensitivity of magnetic resonance in detecting changes in ventricular morphology compared to the ultrasound scan utilized in our study. Additionally, the individuals included by Lewandoski et al. (66) were young adults, potentially exposed to various cardiovascular risk factors not yet present in our adolescent patients. Lastly, it is noteworthy that Lewandoski et al. (66) incorporated, not only preterm patients, but also those classified as small for gestational age, a condition with well-documented negative cardiovascular effects. On the other hand, our results are consistent with those published by Arroyas et al. (57), who detected no differences in the morphological assessment of the right ventricle in MLP group. They included patients of the same age than ours and used the same imaging technique (ultrasound) as in our study.
With respect to right ventricular function, Arroyas et al. (57) found a trend toward lower systolic function and worse diastolic function in very premature infants and in those with bronchopulmonary dysplasia. However, these trends were not evident in our study population.
In the evaluation of left ventricle morphology, we observed slightly smaller size z-score values in MLP adolescents and larger septal and posterior wall z-score values. This aligns with studies on preterm infants, which have described a correlation between ventricular size and gestational age, indicating smaller sizes in the more premature infants (66, 69). Additionally, an increase in septal and posterior wall z-score values was noted, a feature independent of blood pressure (66).
In the assessment of the left ventricle function, we observed better diastolic function data in MLP children compared with term children (E-wave velocity, E/A ratio and their respective z-score values). However, no differences were detected in systolic function values, except for the S-wave ́z-score. Notably, these results were not observed in the MP group. Similarly, global left ventricle function (septal MPI index) exhibited higher z-score values in both MLP and MP adolescents. It is crucial to underscore that despite the statistical significance of these differences, they probably lack clinical relevance, as all patients exhibited figures considered normal for their respective age. Our findings on left ventricle function contrast with previously available publications (11, 57, 66, 69) where poorer systolic and diastolic function data were reported in very preterm children. However, it is essential to consider several factors that distinguish these studies from ours. Firstly, the study population in these previous studies consisted of very preterm infants, with an age range between 18 and 40 years, which is significantly older than our cohort. Also, these patients belonged to an era characterized by neonatal care practices markedly different from those of today (such as the use of mechanical ventilation, surfactation or corticosteroids, prenatal factors that influence cardiac remodeling), as well as their patients had a higher cardiovascular risk, since they exhibited hypercholesterolemia, hypertriglyceridemia and insulin resistance, factors not present in our adolescent patients. Additionally, the cardiological assessments were conducted using magnetic resonance imaging, offering a different and potentially more sensitive approach compared to our study.
From an anthropometric point of view, MLP adolescents had less weight and body mass index, without differences in their height when compared to full-term children. The growth of MLP children has been studied in the different stages of development, showing a slower growth in the neonatal and school period (4, 7), followed by a progressive catch-up. When this catch-up occurs is still uncertain, although our results are similar to the findings of Bergmann et al. (8), who observed a slower growth in their premature infants. As far as we know, there are no available data about the nutritional status of MLP children in adolescence. According to our results MLP adolescents have 1.5 times more undernutrition than their full-term pairs. These alterations in anthropometric data had been previously described in selected MP births, but as mentioned above, they are described here for the first time in MLP group.
Catch-up growth has been associated with higher prevalence of obesity in this group of age and possibly, higher cardiovascular risk in adulthood (9, 70). In our study, both groups, MLP and full-term, presented high percentage of pathological abdominal circumference (71), although the percentage of MLP patients with pathological body mass index (>2SD) was similar to the general population (71). However, it is important to highlight that these children have not yet made the catch-up growth and therefore, the risk of obesity might increase later in life.
In our series, no metabolic alterations were found in the MLP group. There has been much controversy in previous publications on this issue. Some studies have described some alterations in the lipid profile, in glycemic values, with increased insulin resistance, and higher frequency of metabolic syndrome (9, 10). However, these studies included young adults, adolescents or even smoking adults as well as patients with different risk factors such as very premature births. In contrast, other studies with a design similar to ours have also reported no metabolic alterations in preterm infants, aligning with our findings (62–64).
Focusing on the neurodevelopment, behavioral, socioemotional and learning difficulties have been described in preterm compared to term infants at 36 months of life (72). Although significant risk factors related to severe prematurity certainly exist, the comparison between the two groups (term and late preterm) demonstrates a similar development pathway in our study, although a higher percentage of social problems was detected in MLP patients. We did not find significant differences related to learning difficulties or need for school or out-of-school support between both groups. This result contrasts with data from other authors (17, 73, 74) who described lower school performance in MLP infants. However, it is worth it considering that these learning difficulties have been observed mainly at school age, but there have not been many follow-up studies in adolescence. According to our results, Alterman et al. (75) confirm previous studies, finding greater learning difficulties at lower gestational age but in the early stages; however, in adolescence only very preterm children had lower academic performance. On the other hand, cognitive problems detected through intelligence questionnaires or other standardized tests are more precise than those reported by the parents as in our study.
In our cohort, MLP adolescents exhibited a notably low prevalence of issues in social relationships, 4%, in contrast to findings reported in other studies (18, 76, 77), such as the study by Palumbi et al. (18), which reported a prevalence of around 30%. The elevated prevalence rates observed in the study by Palumbi et al. (18) could be attributed to the selective sampling of MLP individuals with neuropsychiatric disorders, along with the utilization of diagnostic tests specifically designed to identify these disorders in the studies conducted by Johnson et al. (76) and Polić et al. (77). However, significant differences in social development were observed in the comparison of MP group, suggesting an increase in problems in social relationship with decreasing gestational age.
According to other publications (78, 79) 8% of our MLP adolescents had scores considered diagnostic of ADHD compared to 3% of terms adolescents. Some publications (78, 80, 81) have indicated a higher frequency at lower gestational age, with a risk up to 1.5 times higher in MLP infants compared to full-term. These data were not confirmed in our sample, probably due to the use of different diagnostic scales between studies and different age of the patients.
Few studies refer to the subtypes of ADHD. Preterm birth has been mainly related to inattention disorders (72, 82, 83) with higher risk detected with more severe neonatal pathology and lower gestational age. However, in our sample of MLP adolescents, no significant increase in attention disorders was observed, nor there was a higher risk at a lower gestational age. It is important to consider that the characteristics of the disorder may vary based on gender, age, or developmental stage assessed during the evaluation, warranting further research for the complete validation of this screening scale (41, 84).
The primary limitation of our study was the sample size, which, although larger than in some other studies, proved insufficient for detecting certain differences, particularly in the domain of cardiovascular assessment and in identifying the most vulnerable subgroup of MPs. Additionally, patient recruitment had to be conducted in two stages due to a temporary interruption caused by the confinement measures implemented during the SARS-CoV-2 pandemic. Furthermore, the study faced limitations associated with the requirement for specific diagnostic tests or more sensitive imaging techniques to detect subtle differences.
In summary, despite all possible limitations, our findings provide an overview of the clinical situation of adolescent MLP and suggest that MLP patients should be regarded as a population at heightened risk of developing pathologies, mainly respiratory, minimal alterations on cardiological assessment and possibly anthropometric changes after growth, which may emerge later in life, potentially resulting in significant morbidity and mortality. Therefore, close follow-up of this majority group of preterm infants from the neonatal period to adulthood could be of interest, especially or those with additional risk factors. Prolonged monitoring of this predominant cohort of preterm infants may allow the identification of subtle alterations compared to term and very preterm infants, facilitating early implementation of interventions to mitigate the risk of future morbidity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comité de Ética de la Investigación con medicamento (CEIm), Hospital Universitario Severo Ochoa, Leganés, Madrid, Spain. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PA-L: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. SR-G: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. IO: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MG-G: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received partial support from a grant provided by the XI Convocatoria Proyectos de Investigación de la Fundación Universidad Alfonso X El Sabio (2019). There was no additional external funding received for this study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chawanpaiboon, S, Vogel, JP, Moller, A-B, Lumbiganon, P, Petzold, M, Hogan, D, et al. Global, regional and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
2. Carrapato, MRG, Pereira, T, Silva, C, Rodrigues, J, Monteiro, I, Azevedo, A, et al. Late preterms: are they all the same? J Matern Fetal Neonatal Med. (2020) 33:1780–5. doi: 10.1080/14767058.2018.1527897
3. Álvarez, P, Burón, E, Izquierdo, E, Maniega, MA, and Blanco, A. Morbilidad de los niños prematuros en edad escolar (I): alteraciones neurosensoriales, psicointelectivas y de conducta. Acta Pediatr Esp. (2011) 69:317–24.
4. Álvarez, P, Burón, E, and Blanco, A. Morbilidad de los niños prematuros en edad escolar (II): patología respiratoria, alteraciones del crecimiento y presión arterial. Acta Pediatr Esp. (2018) 20:121–31.
5. Natarajan, G, and Shankaran, S. Short-and long-term outcomes of moderate and late preterm infants. Am J Perinatol. (2016) 33:305–17. doi: 10.1055/s-0035-1571150
6. Vohr, B. Long-term outcomes of moderately preterm, late preterm and early term infants. Clin Perinatol. (2013) 40:739–51. doi: 10.1016/j.clp.2013.07.006
7. Nagasaka, M, Morioka, I, Yokota, T, Fujita, K, Kurokawa, D, Koda, T, et al. Incidence of short stature at 3 years of age in late preterm infants: a population-based study. Arch Dis Child. (2015) 100:250–4. doi: 10.1136/archdischild-2014-307045
8. Bergmann, RL, Bergmann, KE, Richter, R, Schlaud, M, Henrich, W, and Weichert, A. Growth attainment in German children born preterm and cardiovascular risk factors in adolescence. Analysis of the population representative KiGGS data. J Perinat Med. (2017) 45:619–26. doi: 10.1515/jpm-2016-0294
9. Sipola-Leppänen, M, Vääräsmäki, M, Tikanmäki, M, Hovi, P, Miettola, S, Ruokonen, A, et al. Cardiovascular risk factors in adolescents born preterm. Pediatrics. (2014) 134:e1072–81. doi: 10.1542/peds.2013-4186
10. Raju, TNK, Buist, AS, Blaisdell, CJ, Moxey-Mims, M, and Saigal, S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. (2017) 106:1409–37. doi: 10.1111/apa.13880
11. Lewandowski, AJ, Raman, B, Bertagnolli, M, Mohamed, A, Williamson, W, Pelado, JL, et al. Association of preterm birth with myocardial fibrosis and diastolic dysfunction in young adulthood. J Am Coll Cardiol. (2021) 78:683–92. doi: 10.1016/j.jacc.2021.05.053
12. Harju, M, Keski-Nisula, L, Georgiadis, L, Räisänen, S, Gissler, M, and Heinonen, S. The burden of childhood asthma and late preterm and early term births. J Pediatr. (2014) 164:295–9.e1. doi: 10.1016/j.jpeds.2013.09.057
13. Boyle, EM, Poulsen, G, Field, DJ, Kurinczuk, JJ, Wolke, D, Alfirevic, Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. (2012) 344:e896. doi: 10.1136/bmj.e896
14. Moreno-Galdó, A, Pérez-Yarza, EG, Ramilo, O, Rubí, T, Escribano, A, Torres, A, et al. Recurrent wheezing during the first 3 years of life in a birth cohort of moderate-to-late preterm infants. Pediatr Allergy Immunol. (2020) 31:124–32. doi: 10.1111/pai.13134
15. Vrijlandt, EJLE, Reijneveld, SA, Aris-Meijer, JL, and Bos, AF. Respiratory health in adolescents born moderately-late preterm in a community-based cohort. J Pediatr. (2018) 203:429–36. doi: 10.1016/j.jpeds.2018.07.083
16. Pérez-Tarazona, S, Rueda Esteban, S, García-García, ML, Arroyas Sanchez, M, de Mir, MI, Acevedo Valarezo, T, et al. Respiratory outcomes of «new» bronchopulmonary dysplasia in adolescents: a multicenter study. Pediatr Pulmonol. (2021) 56:1205–14. doi: 10.1002/ppul.25226
17. Martínez-Nadal, S, and Bosch, L. Cognitive and learning outcomes in late preterm infants at school age: a systematic review. Int J Environ Res Public Health. (2020) 18:E74. doi: 10.3390/ijerph18010074
18. Palumbi, R, Peschechera, A, Margari, M, Craig, F, Cristella, A, Petruzzelli, MG, et al. Neurodevelopmental and emotional-behavioral outcomes in late-preterm infants: an observational descriptive case study. BMC Pediatr. (2018) 18:318. doi: 10.1186/s12887-018-1293-6
19. Fenton, TR, and Kim, JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
20. Asher, MI, Keil, U, Anderson, HR, Beasley, R, Crane, J, Martinez, F, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. (1995) 8:483–91. doi: 10.1183/09031936.95.08030483
21. Carvajal-Urueña, I, García-Marcos, L, Busquets-Monge, R, Morales Suárez-Varela, M, García de Andoin, N, Batlles-Garrido, J, et al. Geographic variation in the prevalence of asthma symptoms in Spanish children and adolescents. International study of asthma and allergies in childhood (ISAAC) phase 3, Spain. Arch Bronconeumol. (2005) 41:659–66. doi: 10.1016/S0300-2896(05)70721-3
22. Bousquet, J, Heinzerling, L, Bachert, C, Papadopoulos, NG, Bousquet, PJ, Burney, PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. (2012) 67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x
23. García-Río, F, Calle, M, Burgos, F, Casan, P, Del Campo, F, Galdiz, JB, et al. Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol. (2013) 49:388–401. doi: 10.1016/j.arbres.2013.04.001
24. Zapletal, A, Paul, T, and Samánek, M. Significance of contemporary methods of lung function testing for the detection of airway obstruction in children and adolescents (author’s transl). Z Erkr Atmungsorgane. (1977) 149:343–71.
25. Quanjer, PH, Stanojevic, S, Cole, TJ, Baur, X, Hall, GL, Culver, BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. (2012) 40:1324–43. doi: 10.1183/09031936.00080312
26. Global initiative for Asthma. Global strategy for asthma management and prevention (2021) Available online at: https://www.ginasthma.org (Accessed March 8, 2023).
27. Miller, MR, Hankinson, J, Brusasco, V, Burgos, F, Casaburi, R, Coates, A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
28. National High Blood Pressure Education Program Working Group on high blood pressure in children and adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114:555–76. doi: 10.1542/peds.114.S2.555
29. Lurbe, E, Agabiti-Rosei, E, Cruickshank, JK, Dominiczak, A, Erdine, S, Hirth, A, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. doi: 10.1097/HJH.0000000000001039
30. Pettersen, MD, Du, W, Skeens, ME, and Humes, RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children and adolescents: an echocardiographic study. J Am Soc Echocardiogr. (2008) 21:922–34. doi: 10.1016/j.echo.2008.02.006
31. Eidem, BW, McMahon, CJ, Cohen, RR, Wu, J, Finkelshteyn, I, Kovalchin, JP, et al. Impact of cardiac growth on doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. (2004) 17:212–21. doi: 10.1016/j.echo.2003.12.005
32. Cui, W, Roberson, DA, Chen, Z, Madronero, LF, and Cuneo, BF. Systolic and diastolic time intervals measured from doppler tissue imaging: normal values and Z-score tables, and effects of age, heart rate and body surface area. J Am Soc Echocardiogr. (2008) 21:361–70. doi: 10.1016/j.echo.2007.05.034
33. Sánchez-González, E, Carrascosa-Lezcano, A, Fernández-García, JM, Ferrández-Longás, A, López-de-Lara, D, and López-Siguero, JP. Spanish growth studies: the current situation, their effectiveness and recommendations for their use. An Pediatr. (2011) 74:193.e1–193.e16. doi: 10.1016/j.anpedi.2010.10.005
34. Marugán, JM, Torres, MC, Alonso, C, and Redondo, MP. Valoración del estado nutricional. Pediatr Integral. (2015) 19:e1–6.
35. Moreno, LA, Mesana, MI, González-Gross, M, Gil, CM, Ortega, FB, Fleta, J, et al. Body fat distribution reference standards in Spanish adolescents: the AVENA study. Int J Obes. (2007) 31:1798–805. doi: 10.1038/sj.ijo.0803670
36. Pérez-Herrera, A, and Cruz-López, M. Childhood obesity: current situation in Mexico. Nutr Hosp. (2019) 36:463–9. doi: 10.20960/nh.2116
37. Murillo-Valles, M, and Bel-Comós, M. Obesidad y síndrome metabólico. Protoc Diagn Pediatr. (2019) 1:285–94.
38. Arroyo, FJ, Romero, JA, and López, GN. Dislipemias en edad pediátrica. Protoc Diagn Pediatr. (2019) 1:125–40.
39. Ehlers, S, Gillberg, C, and Wing, L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. (1999) 29:129–41. doi: 10.1023/A:1023040610384
40. Farré-Riba, A, and Narbona, J. Conners’ rating scales in the assessment of attention deficit disorder with hyperactivity (ADHD). A new validation and factor analysis in Spanish children. Rev Neurol. (1997) 25:200–4.
41. Sánchez, CR, Ramos, C, Díaz, F, and Simón, M. Validation of the attention deficit hyperactivity disorder adult assessment scale (EDAH) in a teenage population. Rev Neurol. (2010) 50:283–90.
42. Farré-Riba, A, and Narbona-García, J. EDAH: escalas para la evaluación del trastorno por déficit de atención con hiperactividad, 7th. Madrid: Tea (2013).
43. Morata-Alba, J, Romero-Rubio, MT, Castillo-Corullón, S, and Escribano-Montaner, A. Respiratory morbidity, atopy and asthma at school age in preterm infants aged 32-35 weeks. Eur J Pediatr. (2019) 178:973–82. doi: 10.1007/s00431-019-03372-1
44. Trigueros, JA, Plaza, V, Domínguez-Ortega, J, Serrano, J, Cisneros, C, Padilla, A, et al. Asthma, comorbidities and aggravating circumstances: the GEMA-FORUM II task force. J Investig Allergol Clin Immunol. (2020) 30:140–3. doi: 10.18176/jiaci.0460
45. Haataja, P, Korhonen, P, Ojala, R, Hirvonen, M, Paassilta, M, Gissler, M, et al. Asthma and atopic dermatitis in children born moderately and late preterm. Eur J Pediatr. (2016) 175:799–808. doi: 10.1007/s00431-016-2708-8
46. Narang, I, Rosenthal, M, Cremonesini, D, Silverman, M, and Bush, A. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med. (2008) 178:74–80. doi: 10.1164/rccm.200705-701OC
47. Leps, C, Carson, C, and Quigley, MA. Gestational age at birth and wheezing trajectories at 3-11 years. Arch Dis Child. (2018) 103:1138–44. doi: 10.1136/archdischild-2017-314541
48. Kotecha, SJ, Watkins, WJ, Paranjothy, S, Dunstan, FD, Henderson, AJ, and Kotecha, S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax. (2012) 67:54–61. doi: 10.1136/thoraxjnl-2011-200329
49. Thunqvist, P, Gustafsson, PM, Schultz, ES, Bellander, T, Berggren-Broström, E, Norman, M, et al. Lung function at 8 and 16 years after moderate-to-late preterm birth: a prospective cohort study. Pediatrics. (2016) 137:e20152056. doi: 10.1542/peds.2015-2056
50. Been, JV, Lugtenberg, MJ, Smets, E, van Schayck, CP, Kramer, BW, Mommers, M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. (2014) 11:e1001596. doi: 10.1371/journal.pmed.1001596
51. Ruggeri, P, and Caramori, G. Functional role of Inflammasome activation in a subset of obese nonsmoking patients with severe asthma. Am J Respir Crit Care Med. (2019) 199:1045–7. doi: 10.1164/rccm.201903-0667ED
52. Greenberg, D, Dagan, R, Shany, E, Ben-Shimol, S, and Givon-Lavi, N. Incidence of respiratory syncytial virus bronchiolitis in hospitalized infants born at 33-36 weeks of gestational age compared with those born at term: a retrospective cohort study. Clin Microbiol Infect. (2020) 26:256.e1–5. doi: 10.1016/j.cmi.2019.05.025
53. Sánchez-Luna, M, Pérez-Muñuzuri, A, Leante-Castellanos, JL, Ruiz-Campillo, CW, Sanz-López, E, Benavente-Fernández, I, et al. An update of the recommendations of the Spanish neonatology society for the use of palivizumab as prophylaxis for severe infections due to syncytial respiratory virus in high risk infants. An Pediatr. (2019) 91:348–50. doi: 10.1016/j.anpedi.2019.08.003
54. Yaacoby-Bianu, K, Plonsky, MT, Gur, M, Bar-Yoseph, R, Kugelman, A, and Bentur, L. Effect of late preterm birth on lung clearance index and respiratory physiology in school-age children. Pediatr Pulmonol. (2019) 54:1250–6. doi: 10.1002/ppul.24357
55. Er, İ, Günlemez, A, Uyan, ZS, Aydoğan, M, Oruç, M, Işık, O, et al. Evaluation of pulmonary functions in preschool children born late-preterm. Turk Pediatri Ars. (2017) 52:72–8. doi: 10.5152/TurkPediatriArs.2017.4187
56. Dantas, FMNA, Magalhães, PAF, Hora, ECN, Andrade, LB, Rizzo, JÂ, Peixoto, DM, et al. Lung mechanics and respiratory morbidities in school-age children born moderate-to-late preterm. Pediatr Res. (2022) 91:1136–40. doi: 10.1038/s41390-021-01538-y
57. Arroyas, M, Calvo, C, Rueda, S, Esquivias, M, González-Merchen, C, González-Carrasco, E, et al. Asthma prevalence, lung and cardiovascular function in adolescents born preterm. Sci Rep. (2020) 10:19616. doi: 10.1038/s41598-020-76614-0
58. Näsänen-Gilmore, P, Sipola-Leppänen, M, Tikanmäki, M, Matinolli, HM, Eriksson, JG, Järvelin, MR, et al. Lung function in adults born preterm. PLoS One. (2018) 13:e0205979. doi: 10.1371/journal.pone.0205979
59. Corbellini, C, Tavella, S, Gugliotta, E, Zampese, S, Sanchez-Romero, EA, and Villafane, J. Hypercapnia and functional improvements during pulmonary rehabilitation. Eur Respir J. (2021) 58:PA1827. doi: 10.1183/13993003.congress-2021.PA1827
60. Corbellini, C, Rossino, E, Massaccesi, R, Battaglino, A, Pedersini, P, Sánchez Romero, EA, et al. Improvements in perimeter thoracic mobility on patients with COPD after pulmonary rehabilitation: a case series. Electron J Gen Med. (2022) 19:em361. doi: 10.29333/ejgm/11671
61. Manti, S, Galdo, F, Parisi, GF, Napolitano, M, Decimo, F, Leonardi, S, et al. Long-term effects of bronchopulmonary dysplasia on lung function: a pilot study in preschool children's cohort. J Asthma. (2021) 58:1186–93. doi: 10.1080/02770903.2020.1779289
62. Alves, PJS, Araujo Júnior, E, Henriques, ACPT, and Carvalho, FHC. Preterm at birth is not associated with greater cardiovascular risk in adolescence. J Matern Fetal Neonatal Med. (2016) 29:3351–7. doi: 10.3109/14767058.2015.1126577
63. Dalziel, SR, Parag, V, Rodgers, A, and Harding, JE. Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol. (2007) 36:907–15. doi: 10.1093/ije/dym067
64. Andraweera, PH, Condon, B, Collett, G, Gentilcore, S, and Lassi, ZS. Cardiovascular risk factors in those born preterm—systematic review and meta-analysis. J Dev Orig Health Dis. (2020) 12:539–54. doi: 10.1017/S2040174420000914
65. Mathai, S, Derraik, JGB, Cutfield, WS, Dalziel, SR, Harding, JE, Biggs, JB, et al. Blood pressure abnormalities in adults born moderately preterm and their children. Int J Cardiol. (2015) 181:152–4. doi: 10.1016/j.ijcard.2014.11.162
66. Lewandowski, AJ, Augustine, D, Lamata, P, Davis, EF, Lazdam, M, Francis, J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry and function. Circulation. (2013) 127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920
67. Lewandowski, AJ, Levy, PT, Bates, ML, McNamara, PJ, Nuyt, AM, and Goss, KN. Impact of the vulnerable preterm heart and circulation on adult cardiovascular disease risk. Hypertension. (2020) 76:1028–37. doi: 10.1161/HYPERTENSIONAHA.120.15574
68. Dissanayake, HU, McMullan, RL, Kong, Y, Caterson, ID, Celermajer, DS, Phang, M, et al. Cardiac and vascular health in late preterm infants. J Dev Orig Health Dis. (2022) 13:128–34. doi: 10.1017/S204017442100009X
69. Kowalski, RR, Beare, R, Doyle, LW, Smolich, JJ, and Cheung, MMH. Victorian infant collaborative study group. Elevated blood pressure with reduced left ventricular and aortic dimensions in adolescents born extremely preterm. J Pediatr. (2016) 172:75–80.e2. doi: 10.1016/j.jpeds.2016.01.020
70. Rallis, D, Balomenou, F, Tzoufi, M, and Giapros, V. A systematic review indicates an association between birth weight and body fat in childhood. Acta Paediatr. (2021) 110:2023–39. doi: 10.1111/apa.15834
71. Aranceta-Bartrina, J, Gianzo-Citores, M, and Pérez-Rodrigo, C. Prevalence of overweight, obesity and abdominal obesity in the Spanish population aged 3 to 24 years. ENPE Study Rev Esp Cardiol. (2020) 73:290–9. doi: 10.1016/j.recesp.2019.07.011
72. Dicanio, D, Spoto, G, Alibrandi, A, Minutoli, R, Nicotera, AG, and Di Rosa, G. Long-term predictively of early neurological assessment and developmental trajectories in low-risk preterm infants. Front Neurol. (2022) 13:958682. doi: 10.3389/fneur.2022.958682
73. Woythaler, M. Neurodevelopmental outcomes of the late preterm infant. Semin Fetal Neonatal Med. (2019) 24:54–9. doi: 10.1016/j.siny.2018.10.002
74. Karnati, S, Kollikonda, S, and Abu-Shaweesh, J. Late preterm infants—changing trends and continuing challenges. Int J Pediatr Adolesc Med. (2020) 7:36–44. doi: 10.1016/j.ijpam.2020.02.006
75. Alterman, N, Johnson, S, Carson, C, Petrou, S, Kurinzcuk, JJ, Macfarlane, A, et al. Gestational age at birth and academic attainment in primary and secondary school in England: evidence from a national cohort study. PLoS One. (2022) 17:e0271952. doi: 10.1371/journal.pone.0271952
76. Johnson, S, Matthews, R, Draper, ES, Field, DJ, Manktelow, BN, Marlow, N, et al. Early emergence of delayed social competence in infants born late and moderately preterm. J Dev Behav Pediatr. (2015) 36:690–9. doi: 10.1097/DBP.0000000000000222
77. Polić, B, Bubić, A, Meštrović, J, Markić, J, Kovačević, T, Antončić Furlan, I, et al. Emotional and behavioral outcomes and quality of life in school-age children born as late preterm: retrospective cohort study. Croat Med J. (2017) 58:332–41. doi: 10.3325/cmj.2017.58.332
78. Sucksdorff, M, Lehtonen, L, Chudal, R, Suominen, A, Joelsson, P, Gissler, M, et al. Preterm birth and poor fetal growth as risk factors of attention-deficit/ hyperactivity disorder. Pediatrics. (2015) 136:e599–608. doi: 10.1542/peds.2015-1043
79. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Pub (2013).
80. Lindström, K, Lindblad, F, and Hjern, A. Psychiatric morbidity in adolescents and young adults born preterm: a Swedish national cohort study. Pediatrics. (2009) 123:e47–53. doi: 10.1542/peds.2008-1654
81. Muganthan, T, and Boyle, EM. Early childhood health and morbidity, including respiratory function in late preterm and early term births. Semin Fetal Neonatal Med. (2019) 24:48–53. doi: 10.1016/j.siny.2018.10.007
82. Ask, H, Gustavson, K, Ystrom, E, Havdahl, KA, Tesli, M, Askeland, RB, et al. Association of gestational age at birth with symptoms of attention-deficit/hyperactivity disorder in children. JAMA Pediatr. (2018) 172:749–56. doi: 10.1001/jamapediatrics.2018.1315
83. Bogičević, L, Verhoeven, M, and van Baar, AL. Distinct profiles of attention in children born moderate-to-late preterm at 6 years. J Pediatr Psychol. (2020) 45:685–94. doi: 10.1093/jpepsy/jsaa038
Keywords: moderate to late preterm, premature birth, asthma, lung function, cardiovascular risk, metabolic risk, developmental disabilities
Citation: Alonso-Lopez P, Arroyas M, Beato M, Ruiz-Gonzalez S, Olabarrieta I and Garcia-Garcia ML (2024) Respiratory, cardio-metabolic and neurodevelopmental long-term outcomes of moderate to late preterm birth: not just a near term-population. A follow-up study. Front. Med. 11:1381118. doi: 10.3389/fmed.2024.1381118
Edited by:
Sara Manti, University of Messina, ItalyReviewed by:
Antonio Gennaro Nicotera, University of Messina, ItalyPaolo Ruggeri, University of Messina, Italy
Eleuterio A. Sánchez Romero, European University of Madrid, Spain
Christopher Harris, King’s College London, United Kingdom
Copyright © 2024 Alonso-Lopez, Arroyas, Beato, Ruiz-Gonzalez, Olabarrieta and Garcia-Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Luz Garcia-Garcia, bWFyaWFsdXouaHNvQGdtYWlsLmNvbQ==
 Patricia Alonso-Lopez
Patricia Alonso-Lopez Maria Arroyas
Maria Arroyas Maite Beato
Maite Beato Sara Ruiz-Gonzalez
Sara Ruiz-Gonzalez Iciar Olabarrieta
Iciar Olabarrieta Maria Luz Garcia-Garcia
Maria Luz Garcia-Garcia