- 1Department of Joint Surgery and Sports Medicine, The People's Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
- 2Department of Pharmacy, The People's Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
Objective: This study aimed to evaluate the efficacy of cyclic cryotherapy and vitamin D administration on early rehabilitation after total knee arthroplasty (TKA), as its efficacy remains unclear.
Methods: We divided 150 patients (three groups) who underwent TKA into those treated with or without cyclic cryotherapy and vitamin D.
Results: Compared with patients who did not receive cyclic cryotherapy, those who received postoperative cyclic cryotherapy and vitamin D supplementation had significantly higher American Knee Society Scores (AKSS) on postoperative day (POD) 7 and at 1 month postoperatively; higher visual analogue scale (VAS) values on POD1–3 and POD7; reduced thigh swelling on POD3 and POD7; increased range of motion (ROM) on POD3, POD7, and at 1 month postoperatively; and reduced postoperative length of stay (PLOS). However, no significant difference in patient satisfaction was observed between the patient groups. At 1 and 3 months postoperatively, patients administered cyclic cryotherapy and vitamin D had significantly higher AKSS, ROM, and vitamin D levels than those who did not receive vitamin D. No perioperative complications such as surgical site infection, skin frostbite, or vitamin D intoxication were observed.
Conclusion: Cyclic cryotherapy post-TKA had short-term advantages in terms of AKSS, VAS, thigh swelling, ROM, PLOS, and accelerated rehabilitation, but did not improve patient satisfaction. Cyclic cryotherapy combined with vitamin D improved AKSS and ROM at 1 and 3 months postoperatively.
1 Introduction
Knee osteoarthritis (KOA) is a common disease, which is often with local pain, joint deformity, swelling and motor dysfunction. In the late stage of KOA, it would greatly reduce the quality of life. Total knee arthroplasty (TKA) is an effective method for treating end-stage knee joint disease. With considerable progress in medical technology, TKA is widely used (1, 2). Owing to extensive soft tissue dissection, osteotomy, and other surgical interventions during TKA, blood loss, pain, swelling, and poor functional rehabilitation remain important factors hindering efficient rehabilitation in TKA (3, 4).
Some studies have reported that cryotherapy applied to soft tissue injuries successfully reduces early local limb pain, swelling, and the inflammatory response, and promotes early rehabilitation during TKA (5, 6). However, few studies have investigated the efficacy of cyclic cryotherapy post-TKA and its clinical efficacy remains unclear (7–9). Vitamin D (Vit D) has previously been shown to affect bone, muscle, and immunological function through a variety of mechanisms. Vit D deficiency is a common nutritional deficiency, which increases the risk of hip and non-vertebral fractures, muscle weakness, and the risk of falls (10, 11). Recently, the role of Vit D has been examined in relation to improving osteoarthritis (OA), perioperative pain, and muscle rehabilitation (12, 13). However, its role in improving functional rehabilitation post-TKA, which has been rarely reported, has not been established (14, 15). The effects of cold therapy are early, whereas the effects of vitamin D are slow and long-lasting, and they have different mechanisms. Whether there is a synergistic effect between a combination of cryotherapy and Vit D administration post-TKA to further accelerate postoperative rehabilitation and improve function is yet to be elucidated. Therefore, we aimed to evaluate the efficacy of applying cyclic cryotherapy and administering Vit D on early rehabilitation post-TKA.
2 Methods
2.1 Patients and design
This study was undertaken at the People’s Hospital of Guangxi Zhuang Autonomous Region (Guangxi Academy of Medical Sciences) from December 2017 to March 2022, and was approved by the Hospital Ethics Committee. The study was designed as a single-blind, prospective randomized controlled trial (Chinese Clinical Trial Registry No.: ChiCTR2300071161; registration date, 06/05/2023).
The inclusion criteria comprised patients who had undergone unilateral TKA for KOA with a pre-operative Vit D level < 75 nmol/L and who had provided their written informed consent prior to participation in the study. Exclusion criteria comprised patients with an allergy to Vit D and taking Vit D before surgery, or those unable to tolerate cryotherapy; and those with a history of severe heart disease (NYHA >2), liver or kidney failure, haematological diseases, diabetes mellitus, tumour, or systemic rheumatic diseases (rheumatoid arthritis, ankylosing spondylitis, or systemic lupus erythematosus). We also excluded patients with a prior history of knee surgery, preoperative venous thrombosis or vasculitis, severe osteoporosis; those with a lack of cognitive function and normal sensation, and those lost to follow-up.
The enrolled study patients were randomized into one of three groups (A, B, or C). The sequence to which they were randomly assigned was concealed in opaque sealed envelopes and opened only on the day before surgery.
During TKA, all patients were administered infused tranexamic acid (TXA, 1 g) 30 min prior to tourniquet deflation. TXA (1 g) was dissolved in normal saline (10 mL) and injected into the joint cavity prior to closing the incision. Patients in group A did not receive cryotherapy or Vit D. Patients in group B were treated with intermittent compression cyclic cryotherapy postoperatively for 30 min. Intermittent compression cyclic cryotherapy was administered four times daily (30 min each time) and continued until postoperative day (POD)7. In addition to the same treatment provided to group B, patients in group C were administered Vit D (intramuscular injection, 1 mL: 5 mg, 200,000 U, Haixin, Gannan, Jiangxi) on POD1, and a daily oral administration of 800 U (Xingsha, Xiamen, China) was continued for a further 3 months.
2.2 Surgery management
All TKAs were performed by senior physicians (the same team) in a 100-level laminar flow operating room. All patients were evaluated by an anaesthesiologist and were administered intravertebral anaesthesia in the supine position. All patients underwent a median incision approach and received a posterior stabilised prosthesis fixed with bone cement, and the patella was not replaced, and the infrapatellar fat was preserved in all patients. All patients were treated with the same prosthesis supplied by the same company and manufactured by Johnson & Johnson, United States. Following prosthesis implantation, the tourniquet was routinely released for wound hemostasis.
2.3 Postoperative care protocol
Ankle dorsi- and plantarflexion and quadricep strength exercises were started in the recovery bay. All patients received oral rivaroxaban (10 mg) once drainage was removed at 48 h postoperatively. Patients received standard supervised physiotherapy daily, including continuous passive motion, strength training, and walking. All patients received the same analgesia (Parecoxib and celecoxib, Pfizer Inc.). After returning to the ward, pain was assessed using a visual analogue scale (VAS) with scores ranging from 0 (no pain) to 10 (worst pain imaginable). Evaluations for detecting deep vein thrombosis were undertaken using Doppler ultrasonography pre-discharge and at 1 month postoperatively.
2.4 Outcome assessment
We used the American Knee Society Score (AKSS) (16) on POD7, and at 1 and 3 months postoperatively. The VAS was used on POD1–POD3 and on POD7. We evaluated the difference in thigh circumference on POD3 and POD7. We measured knee joint range of motion (ROM) on POD3, POD7, and at 1 and 3 months postoperatively. Postoperative length of stay (PLOS), the degree of patient satisfaction, and the level of Vit D (Roche cobas e 411/e 601 automatic electrochemiluminescence immunoassay) were determined on POD1, POD7, and at 1 and 3 months postoperatively.
The AKSS includes knee joint activity and function scores, with a maximum score of 200. The higher the score, the better the knee function and joint activities.
2.5 Patient satisfaction
A Quality of Recovery-40 questionnaire (QoR-40), which has been externally validated, was used to assess patient satisfaction following major surgery. It has five recovery criteria, namely, physical comfort, emotional state, physical independence, psychological support, and pain, and is graded from 40 to 200 (40 indicates poor patient satisfaction, 41–119 indicates minimal patient satisfaction, 120–159 indicates good satisfaction, and ≥ 160 indicates excellent patient satisfaction) (17).
2.6 Postoperative complications
Patients were evaluated for complications such as surgical site infections, skin frostbite, deep vein thrombosis, or Vit D intoxication. Surgical site infections were mainly assessed in relation to the condition of the incision and inflammatory indicators. Frostbite mainly manifested as cold, pale, and hard skin tissue, and numbness or loss of sensation in the affected area. The clinical manifestations of deep vein thrombosis mainly included pain and swelling in the calf region, and all patients were re-examined using B-mode ultrasound imaging of the lower limbs postoperatively. The main clinical manifestations of Vit D poisoning are loss of appetite, anorexia, irritability, emotional distress, lethargy, and low-grade fever. We monitored each patient’s Vit D levels during the follow-up period. We planned to stop Vit D supplementation immediately if a patient was observed to have a Vit D level above the normal level.
2.7 Statistical analyses
All statistical analyses were performed using SPSS.24 (IBM Corp., Armonk, NY, United States) software. Results are presented as mean ± standard deviation (for continuous variables) and number (for qualitative variables). One-way ANOVA and Tukey’s post-hoc tests were used to evaluate parametric data, and a Mann–Whitney U-test was used for nonparametric data. Pearson’s chi-square or Fisher’s exact tests were used to analyse qualitative comparative data. A p-value <0.05 was considered statistically significant. Based on a power of 0.90 and a significance level of 0.05, 43 patients per treatment group were required for this study. Considering the dropout rate, the sample size was increased from 10 to 15%. Therefore, a sample size of 50 patients in each group was required, with a total sample size of 150 patients (17).
3 Results
3.1 Patient demographics
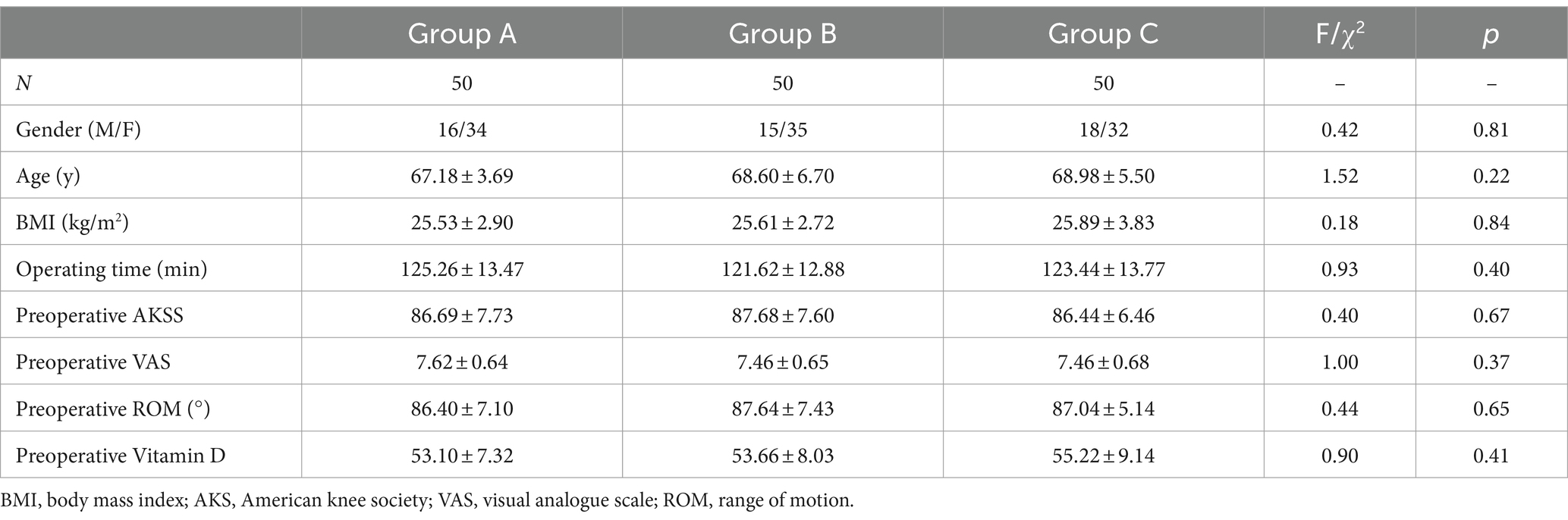
A total of 176 patients were recruited between December 2017 and March 2022. All of the recruited patients were scheduled to undergo primary unilateral TKA at our medical institution. Of these, 12 patients did not meet the inclusion criteria, and 14 patients could not complete follow-up. The remaining 150 eligible patients were included in the intervention as part of three randomized groups (groups A-C, 50 participants per group) (Figure 1). Baseline characteristics and preoperative variables were comparable among the three groups (Table 1).
3.2 Functional rehabilitation
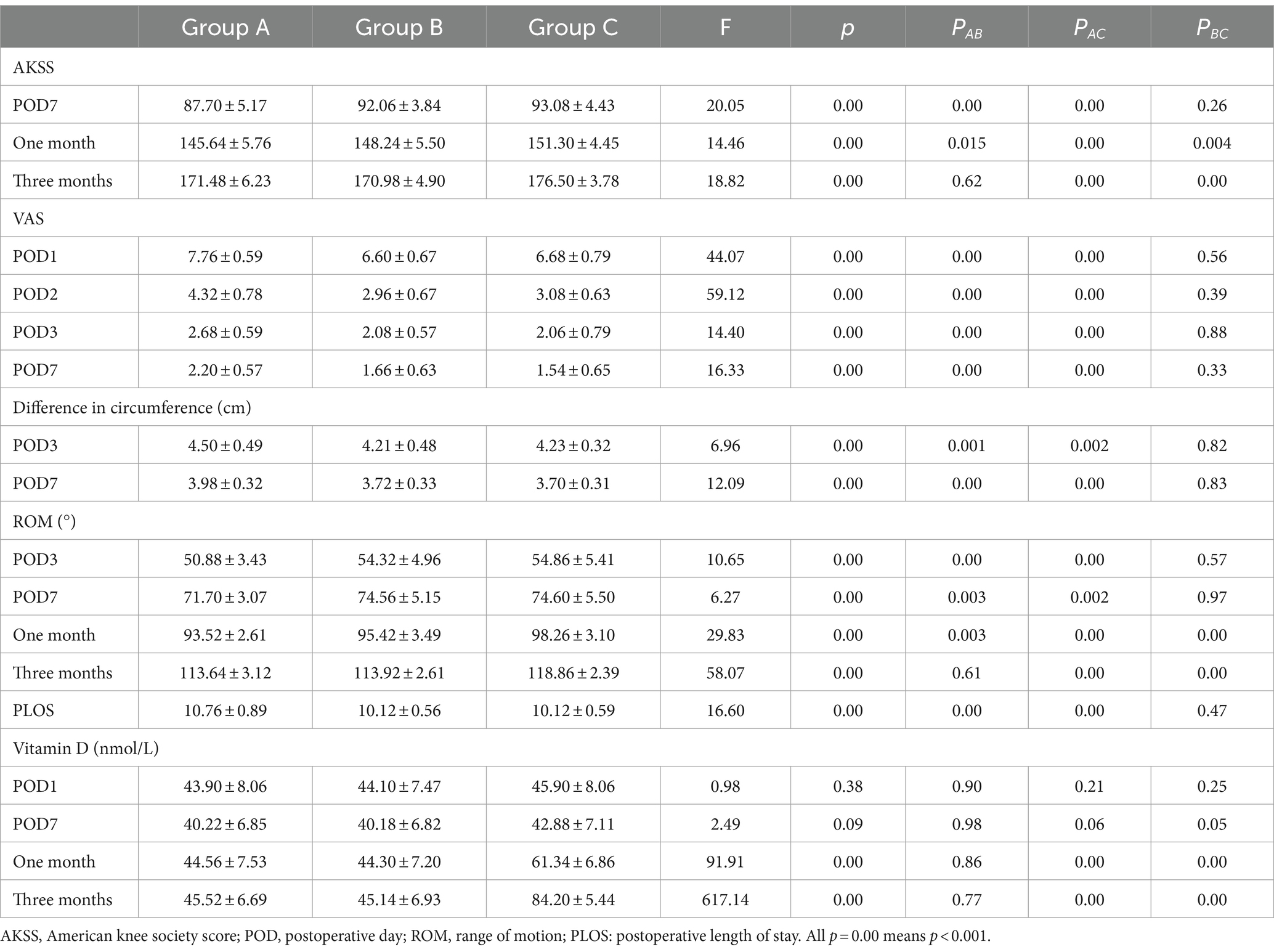
No significant difference in AKSS among the three groups was observed prior to TKA (F = 0.38, p = 0.69). Compared with the AKSS in group A on POD7 and at 1 month postoperatively (87.70 ± 5.17 and 145.64 ± 5.76, respectively), the AKSS in groups B (92.06 ± 3.84 and 148.24 ± 5.50, respectively) and C (93.08 ± 4.43 and 151.30 ± 4.45, respectively) were higher, and the difference was statistically significant (all p < 0.001). The AKSS in group C at 1 and 3 months postoperatively (151.30 ± 4.45 and 176.50 ± 3.78, respectively) was higher than that in groups A (145.64 ± 5.76 and 171.48 ± 6.23, respectively) and B (148.24 ± 5.50 and 170.98 ± 4.90, respectively), and the difference was statistically significant (all p < 0.05). However, no statistically significant difference was observed between groups A and B at 3 months postoperatively (p = 0.62) (Table 2; Figure 2).
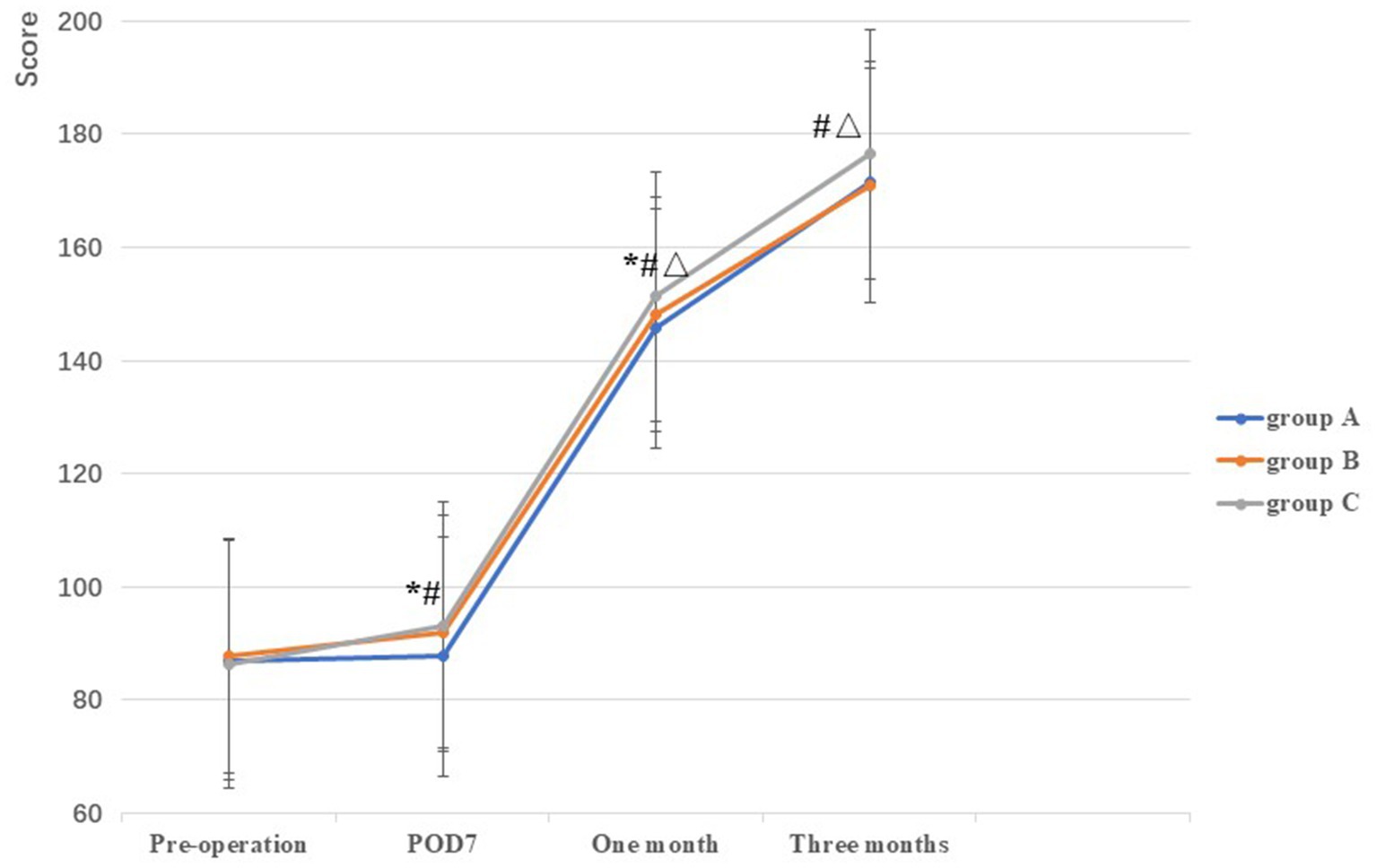
Figure 2. Comparison of AKSS among the three groups at different stages. *: A vs B, p<0.05; #: A vs C, p<0.05, Δ: B vs C, p<0.05.
There was no significant differences in ROM among the three groups prior to TKA (p = 0.65). Compared with group A on POD3, POD7, and at 1 month postoperatively (50.88 ± 3.43, 71.70 ± 3.07, and 93.52 ± 2.61, respectively), the ROM in groups B (54.32 ± 4.96, 74.56 ± 5.15, and 95.42 ± 3.49, respectively) and C (54.86 ± 5.41, 74.60 ± 5.50, and 98.26 ± 3.10, respectively) were higher, and the difference was statistically significant (all p < 0.05). However, no statistically significant difference was observed between groups B and C on POD3 and POD7 (p > 0.05). At 1 and 3 months postoperatively, the ROM in group C (98.26 ± 3.10 and 118.86 ± 2.39, respectively) was higher than that in the groups A (93.52 ± 2.61 and 113.64 ± 3.12, respectively) and B (95.42 ± 3.49 and 113.92 ± 2.61, respectively), and the difference was statistically significant (all p < 0.05) (Table 2; Figure 3).
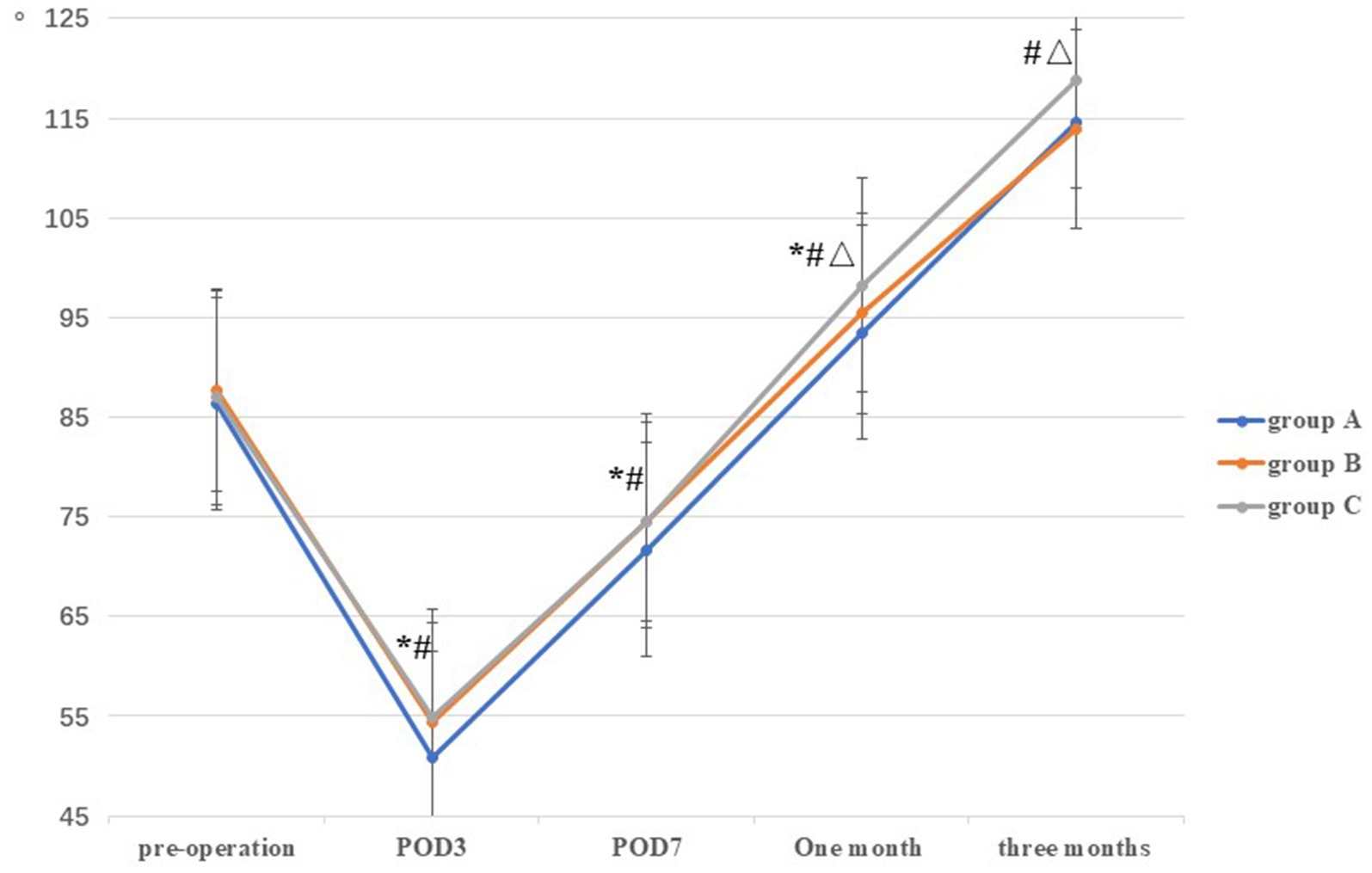
Figure 3. Comparison of ROM among the three groups at different stages. *: A vs B, p<0.05; #: A vs C, p<0.05, Δ: B vs C, p<0.05.
3.3 Pain level
There was no significant difference in VAS among the three groups prior to TKA (F = 1.00, p = 0.37). Compared with group A on POD1, POD2, POD3, and POD7 (7.76 ± 0.59, 4.32 ± 0.78, 2.68 ± 0.59, and 2.20 ± 0.57, respectively), VAS scores in groups B (6.60 ± 0.67, 2.96 ± 0.67, 2.08 ± 0.57, and 1.66 ± 0.63, respectively) and C (6.68 ± 0.79, 3.08 ± 0.63, 2.06 ± 0.79, and 1.54 ± 0.65, respectively) were lower, and the difference was statistically significant (all p < 0.001). However, no statistically significant difference was observed between groups B and C on POD1–POD3 and POD7 (all p > 0.05) (Table 2; Figure 4).
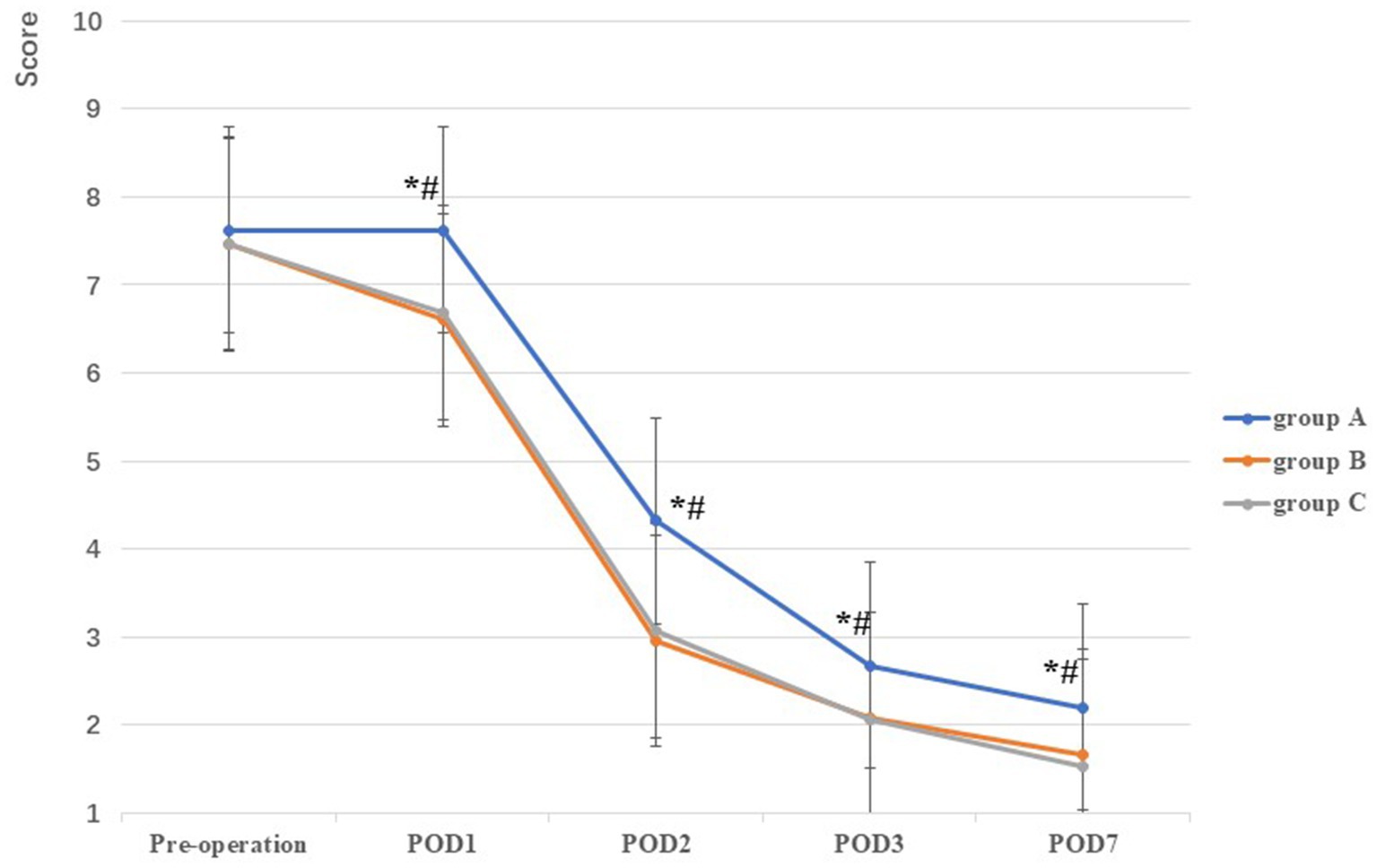
Figure 4. Comparison of VAS among the three groups at different stages. *: A vs B, p<0.05; #: A vs C, p<0.05, Δ: B vs C, p<0.05.
3.4 Joint swelling
Circumference variations on POD3 and POD7 were significantly lower for groups B (4.21 ± 0.48 and 3.72 ± 0.33, respectively) and C (4.23 ± 0.32 and 3.70 ± 0.31, respectively) than for group A (4.50 ± 0.49 and 3.98 ± 0.32, respectively) (all p < 0.001). No statistically significant difference was observed between groups B and C (p > 0.05) on POD3 and POD7 (Table 2).
3.5 PLOS
While PLOS was significantly shorter for groups B (10.12 ± 0.56) and C (10.12 ± 0.59) compared with group A (10.76 ± 0.89) (p < 0.001), no statistically significant difference was observed between groups B and C (p = 0.47).
3.6 Vit D levels
No significant difference in Vit D levels were observed among the three groups prior to TKA, or on POD1 and POD7 (p > 0.05). At 1 and 3 months postoperatively, the levels of Vit D in group C (61.34 ± 6.86 and 84.20 ± 5.44, respectively) were higher than those in groups A (44.56 ± 7.53 and 45.52 ± 6.69, respectively) and B (44.30 ± 7.20 and 45.14 ± 6.93, respectively), and the difference was statistically significant (p < 0.05). However, no statistically significant difference was observed between groups A and B (p > 0.05) (Table 2; Figure 5).
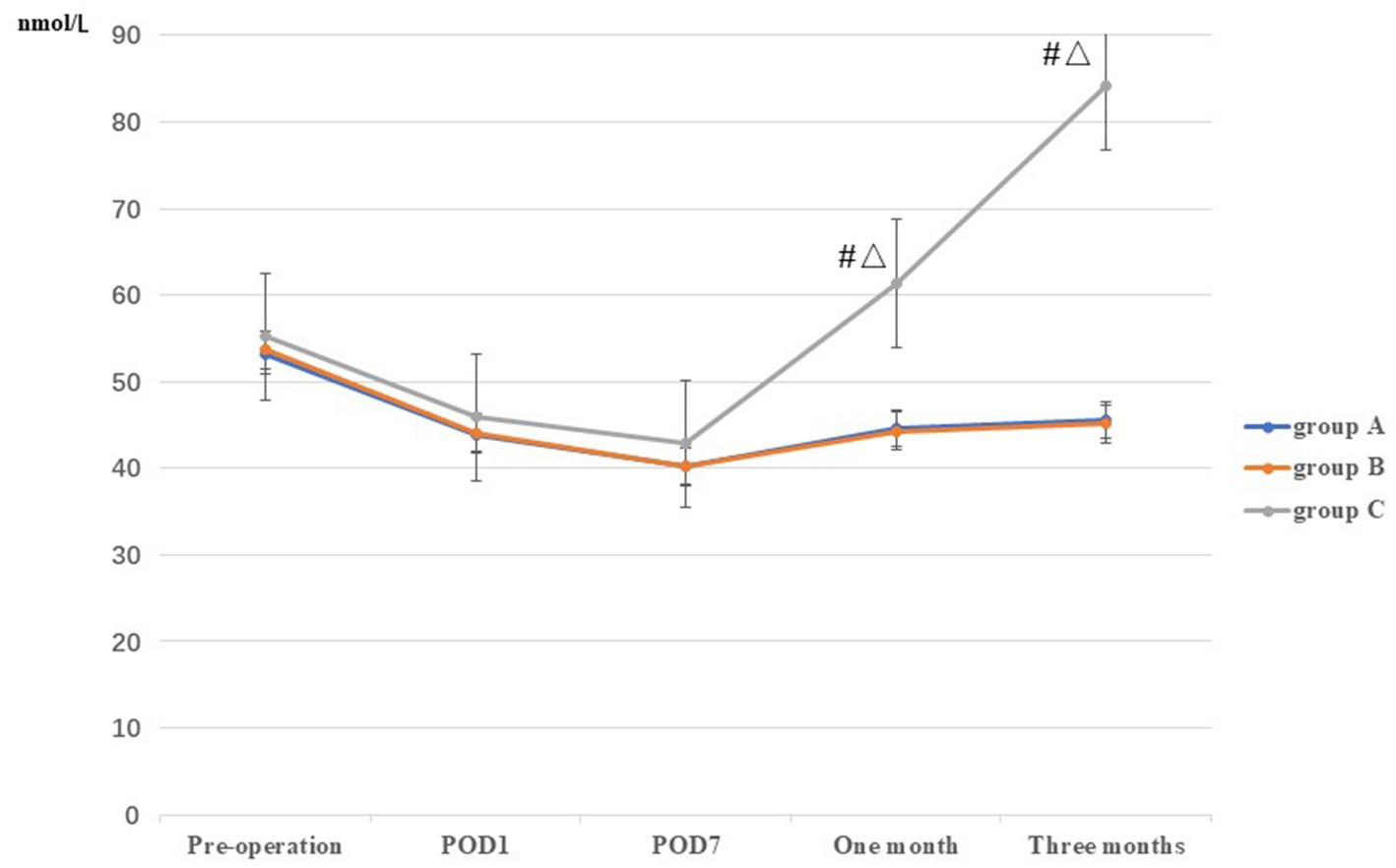
Figure 5. Comparison of Vit D among the three groups at different stages. *: A vs B, p<0.05; #: A vs C, p<0.05, Δ: B vs C, p<0.05.
3.7 Degree of satisfaction
As shown in Table 3, four patient satisfaction grades were reported: excellent, good, minimal, and poor, with grades of excellent and good considered to indicate patient satisfaction with treatment. The degrees of satisfaction in groups A, B, and C were 70, 80, and 78%, respectively. No significant differences were observed among the three groups (Table 3).
3.8 Postoperative complications
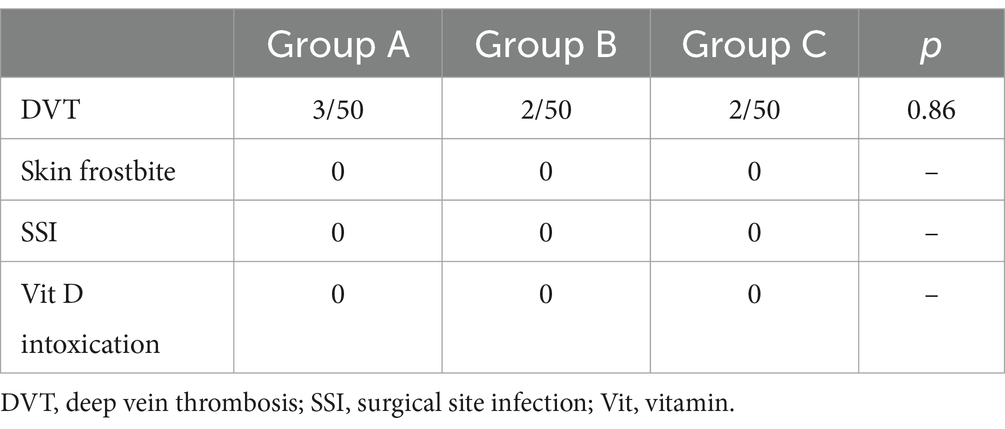
In our study, the incidence of deep vein thrombosis in groups A, B, and C were 3/50 (6.0%), 2/50 (4.0%), and 2/50 (4.0%), respectively, and the difference was not statistically significant. No patients had any complications in relation to frostbite, surgical site infection, or Vit D intoxication (Table 4).
4 Discussion
This study showed the AKSS on POD7 and at 1 month; the VAS on POD1, POD2, POD3, and POD7; thigh swelling on POD3 and POD7; knee joint ROM on POD3, POD7, and at 1 month; and POLS in group B were significantly higher than those in group A (p < 0.05). We concluded that cyclic cryotherapy could reduce postoperative pain and improve function post-TKA, and promote early rehabilitation. AKSS and ROM values at 1 and 3 months postoperatively in group C were significantly higher to those in group B (p < 0.05). We concluded that cyclic cryotherapy combined with Vit D could facilitate early rehabilitation post-TKA at 1 and 3 months. However, patient satisfaction scores did not improve significantly.
TKA is an effective surgical intervention for end-stage KOA, and shows long-lasting clinical and structural improvement for the management of severe OA, providing better overall improvements in function, mobility, pain, and health-related quality of life (18). However, patients in the immediate postoperative period frequently experience acute pain, severe oedema, and a reduced ROM, and attention should be paid to these issues. While patient satisfaction scores have improved (19), our findings indicate that further efforts are needed to reduce postoperative pain, improve knee function, and promote early rehabilitation post-TKA (20).
Cryotherapy was commonly first used for soft tissue injuries in sports medicine, and it has subsequently been widely used to facilitate good detumescence and for its analgesic effect (21). TKA requires extensive dissection of soft tissue and synovectomy, which inevitably result in extensive soft tissue injury; therefore, investigating the role of cryotherapy post-TKA is likely to be of value. We used a novel cryotherapy technique to apply intermittent cold to the knee. Some studies have shown that cryotherapy can appreciably reduce intraarticular temperature, especially in the knee, and reduce blood flow through vasoconstriction, the local inflammatory reaction, postoperative blood loss, thigh swelling, pain transmission, and the length of hospital stay (7, 22). Compared with standard cryotherapy (ice/gel pack application), cyclic cryotherapy devices have been developed that are more efficient as they maintain a steady low temperature for an extended period of time (23, 24). Thus, cyclic cryotherapy could accelerate rehabilitation time post-TKA through reducing inflammation, pain, and swelling.
We used the cyclic cryotherapy technique, which involved intermittent pressure cold compression on patients within 1 week postoperatively. Our findings indicated that the AKSS, pain VAS, degree of thigh swelling, ROM, and PLOS in group B were significantly better than those in group A in the early postoperative period, and the difference was statistically significant (p < 0.05). Moreover, cyclic cryotherapy did not increase the incidence of deep vein thrombosis post-TKA during the perioperative period. This suggests that the cyclic cryotherapy technique is helpful in controlling early postoperative pain and swelling, improving joint function, and in significantly promoting accelerated recovery post-TKA. In terms of its mechanism of action, cyclic cryotherapy reduces the temperature of surrounding tissues and the cell metabolic rate, thus reducing tissue hypoxia. At the same time, this local cooling also contracts capillaries, as well as reducing exudation, leukocyte adhesion, and the production and release of inflammatory factors; thus, relieving pain (21). In addition, cyclic cryotherapy has been found to increase intraarticular pressure, contract capillaries, reduce the flow of fluid in the tissue and reduce exudation to reduce edema (21, 25).
Vit D is a fat-soluble steroid hormone precursor, which functions to improve osteoblastic activity, increase bone mineral density, and may transform articular cartilage (11, 12). Currently, Vit D-related studies have focused mostly on KOA, with limited research on improving functional rehabilitation post-TKA, and with no uniform conclusions reported. Traven et al. (26) retrospectively analyzed 126 patients who had undergone hip and knee revision surgery. They reported no association between a low level of Vit D and the risk of 30-day readmission; however, an association between low levels of Vit D and an increased risk of complications and periprosthetic joint infection at 90 days was observed, as well as a lower joint function score (p < 0.05). Allain et al. (27) reported similar findings. Hwang et al. (15) reported that Vit D deficiency was very common in older adult women in their study. They found that preoperative Vit D deficiency had no significant effect on knee joint function in the short-term postoperatively (p > 0.05) based on a follow-up of 1,013 patients with TKA for >12 months, which was not consistent with our results. Some studies have reported that Vit D plays a role in improving muscle strength, while other studies have also shown that Vit D plays an important role in reducing chronic inflammation and relieving chronic pain (28–30). However, no studies have investigated the application of Vit D combined with cryotherapy in the TKA perioperative period. In group C, we administered cyclic cryotherapy in combination with Vit D. We intermittently applied a cold compress to the patients’ knees within 1 week postoperatively, and 200,000 units of Vit D were administered on POD1, followed by 800 units of Vit D as a daily supplement for 3 months. The results showed that the AKSS and the ROM in group C were significantly improved compared with those in group B at 1 and 3 months postoperatively, and the difference was statistically significant (p < 0.05). Our findings suggest that cyclic therapy combined with Vit D can help to improve joint function at 1 and 3 months and accelerate recovery post-TKA, and our findings are consistent with those in previous studies (31, 32). Notably, the levels of Vit D in groups A and B decreased briefly postoperatively and then slowly recovered, but still failed to recover to preoperative levels at 3 months postoperatively. This may be related to the loss of bone mass and activity resulting from surgery, and a decrease of Vit D supplementation and absorption owing to poor diet in the short-term postoperatively. In addition, no significant difference in the AKSS was observed on POD7, in the VAS on POD1– POD3 and POD7, in thigh swelling on POD3 and POD7, in ROM on POD3 and POD7, and in PLOS between groups B and C, which also indicates that the absorption and utilization of Vit D is a slow process. Moreover, no significant improvement in pain was noted in the acute phase, which was consistent with the level of Vit D. Based on these findings, we consider that cyclic cryotherapy combined with Vit D is beneficial in promoting continuity of rehabilitation after TKA at 1 and 3 months. Shin et al. (14) conducted a prospective study on 92 patients and followed up for 3 months, and also found that patients with Vit D deficiency had poor joint function recovery post-TKA, and the difference was statistically significant (p < 0.05). However, some studies have reported that the level of Vit D had no significant improvement effect on functional rehabilitation following joint replacement (15, 28, 33). Therefore, the effects of Vit D levels on postoperative rehabilitation in relation to TKA require further investigation.
No statistically significant difference in patient satisfaction was observed among the three groups; however, low satisfaction rates post-TKA have also been reported elsewhere (34, 35). One explanation for the patient satisfaction results is that we evaluated patient satisfaction prior to discharge. Another possible reason may be related to the ongoing effects of cyclic cryotherapy and Vit D. Further efforts are needed to enhance patient satisfaction rates post-TKA at our institution.
This study had some limitations. Only assessing patients up to 3 months of follow-up might have restricted our capacity to assess long-term safety profiles and the efficacy of cyclic cryotherapy and Vit D supplementation. Given this was a small-sample single-centre study, the reliability of our findings should be validated using larger samples in multicentre studies. Moreover, a single-blind randomized controlled trial may cause subjective bias in the results. We could not confirm whether cyclic cryotherapy was superior to standard cryotherapy. We evaluate satisfaction of the patients only at discharge and ignored that at 3 months, which may bring some bias in patient satisfaction. The effects of cryotherapy combined with Vit D on inflammation were not investigated; however, we intend to investigate this in future research.
5 Conclusion
Cyclic cryotherapy post-TKA showed short-term advantages in terms of the AKSS, VAS, thigh swelling, ROM, PLOS, and accelerated rehabilitation, but it did not improve patient satisfaction scores. Cyclic cryotherapy combined with Vit D also improved the AKSS and ROM at 1 and 3 months postoperatively.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the People's Hospital of Guangxi Zhuang Autonomous Region. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FL: Writing – original draft, Conceptualization, Data curation, Investigation, Software. YM: Conceptualization, Data curation, Investigation, Writing – original draft, Methodology. XH: Project administration, Resources, Writing – review & editing. KS: Formal analysis, Project administration, Resources, Conceptualization, Writing – original draft. BL: Resources, Methodology, Supervision, Writing – original draft. DY: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by self-funded research project of Guangxi Health Commission (Z20170346) and Guangxi Science and Technology Major Program (No. AA23023004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hawker, GA, Bohm, E, Dunbar, MJ, Jones, CA, Noseworthy, T, Marshall, DA, et al. The effect of patient age and surgical appropriateness and their influence on surgeon recommendations for primary TKA: a cross-sectional study of 2,037 patients. J Bone Joint Surg Am. (2022) 104:700–8. doi: 10.2106/JBJS.21.00597
2. Neuprez, A, Neuprez, AH, Kaux, JF, Kurth, W, Daniel, C, Thirion, T, et al. Early clinically relevant improvement in quality of life and clinical outcomes 1 year Postsurgery in patients with knee and hip joint arthroplasties. Cartilage. (2018) 9:127–39. doi: 10.1177/1947603517743000
3. Joo, YB, Kim, YM, An, BK, Lee, CW, Kwon, ST, and Song, JH. Topical tranexamic acid can be used safely even in high risk patients: deep vein thrombosis examination using routine ultrasonography of 510 patients. Medicina (Kaunas). (2022) 58:1750. doi: 10.3390/medicina58121750
4. Lavand’homme, PM, Kehlet, H, Rawal, N, and Joshi, GPon behalf of the PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy (ESRA). Pain management after total knee arthroplasty. Eur J Anaesthesiol. (2022) 39:743–57. doi: 10.1097/EJA.0000000000001691
5. Fortier, LM, Rockov, ZA, Chen, AF, and Rajaee, SS. Activity recommendations after Total hip and Total knee arthroplasty. J Bone Joint Surg Am. (2021) 103:446–55. doi: 10.2106/JBJS.20.00983
6. Liao, X, and Xu, X. The effect of cold therapy combined with ERAS in the postoperative care of patients undergoing total knee arthroplasty. Am J Transl Res. (2022) 14:3154–63.
7. Thacoor, A, and Sandiford, NA. Cryotherapy following total knee arthroplasty: what is the evidence? J Orthop Surg (Hong Kong). (2019) 27:230949901983275. doi: 10.1177/2309499019832752
8. Leutz, DW, and Harris, H. Continuous cold therapy in total knee arthroplasty. Am J Knee Surg. (1995) 8:121–3.
9. Morsi, E . Continuous-flow cold therapy after total knee arthroplasty. J Arthroplast. (2002) 17:718–22. doi: 10.1054/arth.2002.33562
10. Tan, ML, Abrams, SA, and Osborn, DA. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst Rev. (2020) 2020:D13046. doi: 10.1002/14651858.CD013046.pub2
11. Agostini, D, Zeppa, DS, Lucertini, F, Annibalini, G, Gervasi, M, Marini, CF, et al. Muscle and bone health in postmenopausal women: role of protein and vitamin D supplementation combined with exercise training. Nutrients. (2018) 10:1103. doi: 10.3390/nu10081103
12. Amini Kadijani, A, Bagherifard, A, Mohammadi, F, Akbari, A, Zandrahimi, F, and Mirzaei, A. Association of Serum Vitamin D with serum cytokine profile in patients with knee osteoarthritis. Cartilage. (2021) 13:1610S–8S. doi: 10.1177/19476035211010309
13. Wei, N, and Dai, Z. The role of nutrition in osteoarthritis: a literature review. Clin Geriatr Med. (2022) 38:303–22. doi: 10.1016/j.cger.2021.11.006
14. Shin, KY, Park, KK, Moon, SH, Yang, IH, Choi, HJ, and Lee, WS. Vitamin D deficiency adversely affects early post-operative functional outcomes after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2017) 25:3424–30. doi: 10.1007/s00167-016-4209-8
15. Hwang, IY, Park, KB, Chang, SW, Cho, SD, and Youm, YS. Preoperative vitamin D level does not affect the short-term functional outcome after total knee arthroplasty in elderly women. Knee Surg Relat Res. (2020) 32:30. doi: 10.1186/s43019-020-00050-7
16. Machhindra, MV, Kang, JY, Kang, YG, Chowdhry, M, and Kim, TK. Functional outcomes of a new Mobile-bearing ultra-congruent TKA system: comparison with the posterior stabilized system. J Arthroplast. (2015) 30:2137–42. doi: 10.1016/j.arth.2015.06.011
17. Li, F, Huang, X, Liu, W, Huang, W, Cheng, J, and Yin, D. Dexamethasone with aggressive warming facilitates pain reduction, reduced blood loss, and quicker recovery after total hip arthroplasty. Sci Rep. (2023) 13:19582. doi: 10.1038/s41598-023-47050-7
18. Patel, A, Pavlou, G, Mújica-Mota, RE, and Toms, AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales. Bone Joint J. (2015) 97-B:1076–81. doi: 10.1302/0301-620X.97B8.35170
19. Klem, NR, Smith, A, O’Sullivan, P, Dowsey, MM, Schütze, R, Kent, P, et al. What influences patient satisfaction after TKA? A qualitative investigation. Clin Orthop Relat Res. (2020) 478:1850–66. doi: 10.1097/CORR.0000000000001284
20. Chen, X, Li, X, Zhu, Z, Wang, H, Yu, Z, and Bai, X. Effects of progressive resistance training for early postoperative fast-track total hip or knee arthroplasty: a systematic review and meta-analysis. Asian J Surg. (2021) 44:1245–53. doi: 10.1016/j.asjsur.2021.02.007
21. Chughtai, M, Sodhi, N, Jawad, M, Newman, JM, Khlopas, A, Bhave, A, et al. Cryotherapy treatment after unicompartmental and total knee arthroplasty: a review. J Arthroplast. (2017) 32:3822–32. doi: 10.1016/j.arth.2017.07.016
22. Ewell, M, Griffin, C, and Hull, J. The use of focal knee joint cryotherapy to improve functional outcomes after total knee arthroplasty: review article. PM R. (2014) 6:729–38. doi: 10.1016/j.pmrj.2014.02.004
23. Bech, M, Moorhen, J, Cho, M, Lavergne, MR, Stothers, K, and Hoens, AM. Device or ice: the effect of consistent cooling using a device compared with intermittent cooling using an ice bag after total knee arthroplasty. Physiother Can. (2015) 67:48–55. doi: 10.3138/ptc.2013-78
24. Thienpont, E . Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty? Clin Orthop Relat Res. (2014) 472:3417–23. doi: 10.1007/s11999-014-3810-8
25. Brouwers, H, de Vries, AJ, van Zuilen, M, van Kouswijk, HW, and Brouwer, RW. The role of computer-assisted cryotherapy in the postoperative treatment after total knee arthroplasty: positive effects on pain and opioid consumption. Knee Surg Sports Traumatol Arthrosc. (2022) 30:2698–706. doi: 10.1007/s00167-021-06568-x
26. Traven, SA, Chiaramonti, AM, Barfield, WR, Kirkland, PA, Demos, HA, Schutte, HD, et al. Fewer complications following revision hip and knee arthroplasty in patients with Normal vitamin D levels. J Arthroplast. (2017) 32:S193–6. doi: 10.1016/j.arth.2017.02.038
27. Allain, TJ, Kirthisingha, V, Beresford, PA, and Bell, NJ. Use of vitamin D supplements and vitamin D status in patients taking bisphosphonate drugs. Rheumatology (Oxford). (2006) 45:486–7. doi: 10.1093/rheumatology/kei273
28. Unnanuntana, A, Rebolledo, BJ, Gladnick, BP, Nguyen, JT, Sculco, TP, Cornell, CN, et al. Does vitamin D status affect the attainment of in-hospital functional milestones after total hip arthroplasty? J Arthroplast. (2012) 27:482–9. doi: 10.1016/j.arth.2011.05.023
29. Visser, E, de Roos, NM, Oosting, E, Endenburg, SC, and Dronkers, JJ. Association between preoperative vitamin D status and short-term physical performance after Total hip arthroplasty: a prospective study. Ann Nutr Metab. (2018) 73:252–60. doi: 10.1159/000492938
30. Shen, J, Lin, X, Lin, Y, Xiao, J, Wu, C, Zheng, F, et al. Supplementation of hyaluronic acid injections with vitamin D improve knee function by attenuating synovial fluid oxidative stress in osteoarthritis patients with vitamin D insufficiency. Front Nutr. (2023) 10:1026722. doi: 10.3389/fnut.2023.1026722
31. Mouli, VH, Schudrowitz, N, Carrera, CX, Uzosike, AC, Fitz, W, and Rajaee, SS. High-dose vitamin D supplementation can correct Hypovitaminosis D prior to Total knee arthroplasty. J Arthroplast. (2022) 37:274–8. doi: 10.1016/j.arth.2021.10.016
32. Kenanidis, E, Kakoulidis, P, Karponis, D, and Tsiridis, E. The effect of perioperative vitamin D levels on the functional, patient-related outcome measures and the risk of infection following hip and knee arthroplasty: a systematic review. Patient Relat Outcome Meas. (2020) 11:161–71. doi: 10.2147/PROM.S261251
33. Zajonz, D, Prager, F, Edel, M, Möbius, R, Daikos, A, Fakler, JK, et al. The significance of the vitamin D metabolism in the development of periprosthetic infections after THA and TKA: a prospective matched-pair analysis of 240 patients. Clin Interv Aging. (2018) 13:1429–35. doi: 10.2147/CIA.S171307
34. Blakeney, WG, and Vendittoli, PA. The Future of TKA. In: Rivière C and Vendittoli PA, editors. Personalized hip and knee joint replacement [Internet]. Cham (CH): Springer (2020) Chapter 15.
Keywords: vitamin D, cyclic cryotherapy, total knee arthroplasty, rehabilitation, satisfaction
Citation: Li F, Mo Y, Huang X, Sun K, Li B and Yin D (2024) Cyclic cryotherapy with vitamin D facilitates early rehabilitation after total knee arthroplasty. Front. Med. 11:1380128. doi: 10.3389/fmed.2024.1380128
Edited by:
Philip M. Gallagher, University of Kansas, United StatesReviewed by:
Elisa Belluzzi, University of Padua, ItalyJianlin Shen, Affiliated Hospital of Putian University, China
Copyright © 2024 Li, Mo, Huang, Sun, Li and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Yin, cXl5eWluZG9uZ0AxMjYuY29t
†These authors have contributed equally to this work
 Fulin Li1†
Fulin Li1† Dong Yin
Dong Yin