
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 07 June 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1379369
Background: Preoxygenation before endotracheal intubation (ETI) maintains asphyxiated oxygenation and reduces the risk of hypoxia-induced adverse events. Previous studies have compared various preoxygenation methods. However, network meta-analyses (NMAs) of the combined comparison of preoxygenation methods is still lacking.
Methods: We searched for studies published in PubMed, Embase, Web of Science, Scopus, and the Cochrane Library. Review Manager version 5.3 was used to evaluate the risk of bias. The primary outcome of this meta-analysis was low oxygen saturation (SpO2) during ETI. The secondary outcomes included SpO2 <80%, SpO2 <90%, and apnea time during ETI. NMA was performed using R 4.1.2 software gemtc packages in RStudio.
Results: A total of 15 randomized controlled trials were included in this study. Regarding the lowest SpO2, the noninvasive ventilation (NIV) with high-flow nasal cannula (HFNC) group performed better than the other groups. For SpO2 <80%, the NIV group (0.8603467) performed better than the HFNC (0.1373533) and conventional oxygen therapy (COT, 0.0023) groups, according to the surface under the cumulative ranking curve results. For SpO2 <90%, the NIV group (0.60932667) performed better than the HFNC (0.37888667) and COT (0.01178667) groups. With regard to apnea time, the HFNC group was superior to the COT group (mean difference: −50.05; 95% confidence interval: −90.01, −10.09; P = 0.01).
Conclusion: Network analysis revealed that NIV for preoxygenation achieved higher SpO2 levels than HFNC and COT and offered a more significant advantage in maintaining patient oxygenation during ETI. Patients experienced a longer apnea time after HFNC preoxygenation. The combination of NIV with HFNC proved to be significantly superior to other methods. Given the scarcity of such studies, further research is needed to evaluate its effectiveness.
Systematic review registration: identifier CRD42022346013
Invasive mechanical ventilation is a crucial measure to safeguard patient safety during surgical procedures conducted under general anesthesia, typically necessitating tracheal intubation. Prior to endotracheal intubation (ETI), the induction of anesthesia renders the patient unconscious, and neuromuscular blockade ensues, leading to hypopnea and apnea. This subsequent apnea period poses a heightened risk of hypoxia for the patient, particularly if tracheal intubation poses difficulties, further compounding existing risks (1, 2). Preoxygenation refers to the process of enhancing oxygen concentration and reserves by saturating the patient's body with oxygen prior to surgery, ensuring that the patient maintains a safe oxygen saturation (SpO2) level during apnea. Administering preoxygenation before ETI can sustain oxygenation during asphyxia and mitigate the hazards associated with hypoxia-induced adverse events. Consequently, it is highly advisable to routinely recommend preoxygenation as standard practice prior to ETI (3–5).
The most common form of preoxygenation is mask ventilation with 100% oxygen for 3–5 min, also known as conventional oxygen therapy (COT) (6). The simplicity of mask ventilation lies in its ease of operation; however, prolonged ventilation can compromise patient comfort. In addition to non-invasive ventilation (NIV), a novel approach, the high-flow nasal cannula (HFNC), has been increasingly employed in the preoxygenation process for patients in the operating room (7). HFNC administers heated and humidified gases through a nasal catheter, maintaining a specified fraction of inspired oxygen (FiO2) at a maximum flow rate exceeding 60 L/min. HFNC demonstrates satisfactory oxygenation effects, improves patient comfort, and ensures a more tolerable preoperative experience. Furthermore, mask ventilation obstructs the oral airway, necessitating the removal of the mask during laryngoscopy. This underscores the dual functionality of HFNC as a preoxygenation device capable of maintaining oxygenation during asphyxia. (8).
Previous studies have compared various preoxygenation methods, and some have suggested that HFNC is a more effective preoxygenation device (9, 10). In addition, according to the studies on NIV (11) and HFNC (12), preoxygenation is more effective than COT. Based on published studies, several meta-analyses have compared the effects of different preoxygenation modalities (13–15). A meta-analysis by Kuo et al. (15) reported that high-flow nasal oxygenation can enhance PaO2 and prolong safe apnea time. Li et al. (13) pointed out that transnasal humidified rapid-insufflation ventilatory exchange did not have a significant advantage over the use of facemasks, but it could effectively improve PaO2. According to Chiang et al. (14), NIV is more effective than conventional preoxygenation methods. Nonetheless, network meta-analyses (NMAs) comprehensively comparing different preoxygenation methods are scarce. Consequently, a systematic review of published studies along with an NMA assessing various preoxygenation modalities is necessary to provide a holistic understanding of their relative effectiveness and safety.
This systematic review and NMA has been registered with PROSPERO (registration number is CRD42022346013), and we performed it according to Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) guidelines. The researchers searched for studies from PubMed, Embase, Web of science, Scopus, and Cochrane library. The search terms are as follow: (“High-flow Nasal Cannula”[Title/Abstract] OR “HFNC”[Title/Abstract] OR “High flow nasal cannula therapy”[Title/Abstract] OR “nasal high flow”[Title/Abstract] OR “high flow nasal therapy”[Title/Abstract] OR “high flow oxygen therapy”[Title/Abstract] OR “high flow therapy”[Title/Abstract] OR “HFNO”[Title/Abstract] OR “high flow nasal oxygen”[Title/Abstract] OR “Non-invasive ventilation”[Title/Abstract] OR “NIV”[Title/Abstract] OR “Noninvasive Ventilation”[Title/Abstract] OR “helmet”[Title/Abstract] OR “face mask”[Title/Abstract] OR “Bag-valve mask”[Title/Abstract] OR “mask”[Title/Abstract] OR “conventional oxygen therapy”[Title/Abstract] OR “COT”[Title/Abstract] OR “facemask”[Title/Abstract] OR “nasal interface”[Title/Abstract] OR “bilevel positive airway pressure”[Title/Abstract] OR “BiPAP”[Title/Abstract] OR “continuous positive airway pressure”[Title/Abstract] OR “CPAP”[Title/Abstract] OR “low flow oxygen”[Title/Abstract] OR “standard nasal cannula”[Title/Abstract]) AND (“preoxygenation”[Title/Abstract] OR “apneic oxygenation”[Title/Abstract]). The searched literatures are managed with EndNote X9 (Thomson Reuters, NY, USA). Two investigators screened all studies, the flow diagram is shown in Figure 1. All disputes are resolved by AY.
We included RCTs involving adult patients who underwent preoxygenation prior to ETI. The preoxygenation devices included COT, NIV, and HFNC. Studies with the following characteristics were excluded: non-intubation; focus on only apneic oxygenation or ventilation; animal studies; protocols, reviews, guidelines, or conference abstracts; lack of control; and including healthy volunteers.
Review Manager version 5.3 (RevMan 5.3) was used to evaluate the risk of bias in the included studies according to the Cochrane Collaboration tool. A summary of the risk of bias is shown in Figure 2. Three researchers (MZ, RX, and JZ) completed the risk of bias assessment, whereas the other researchers were responsible for deciding on a different opinion.

Figure 2. Risk of bias summary review authors' judgements about each risk of bias item for included RCTs.
Five investigators (MZ, RX, JZ, JZ, and XY) independently extracted data. MZ, RX, and JYZ. reviewed all the studies and excluded duplicates, registered studies, and nonclinical studies. Additionally, we reviewed the titles, abstracts, and full texts of the RCTs that were determined to be included in the NMA. XY summarized the characteristics of the 15 included studies in Table 1. AY were responsible for resolving disputes in the data extraction process.
The primary outcome of this meta-analysis was low SpO2 during ETI. The secondary outcomes included SpO2 <80%, SpO2 <90%, and apnea time during ETI. We reported the odds ratios (ORs), mean differences (MDs), and 95% confidence intervals (CI) in a pairwise meta-analysis. The log-OR, MD, and 95% CI were reported for the NMA.
Five investigators (MZ, RX, JZ, JZ, and XY) used statistical methodology. First, RevMan 5.3 was used for pairwise meta-analysis. For the heterogeneity test, when P < 0.05 or I2 > 50%, we chose the random-effects model. When the heterogeneity test yielded P > 0.05 or I2 < 50%, the fixed effects model was often selected.
Second, STATA (version 17.0) was used to generate network plots for the different groups, to visualize the relationships between various interventions. The size of the node in the network plot represents the sample size of the group, and the edge width represents the number of studies.
Third, NMA was performed using R 4.1.2 software gemtc packages in RStudio, based on a Bayesian framework with Markov Chain Monte Carlo (MCMC) simulation. We ran the estimation with a burn-in of 25,000 iterations and sampling of 50,000 iterations from the three chains of initial values. The selection between models was based on the Deviance Information Criteria (DIC). A DIC difference in the consistency test results >5 was considered significant. The fluctuation process of the MCMC chain is represented by a trace plot and the convergence degree of the model is diagnosed together with the density and Brooks-Gelman-Rubin diagnosis plots with the potential scale reduction factor (PSRF). If the degree of model convergence is poor or 1 < PSRF ≤ 1.05, the frequency of pre-iteration and iterations need to be changed.
Fourth, we used R 4.1.2 software to calculate the surface under the cumulative ranking (SUCRA) to rank the interventions. A heatmap was used to visualize the SUCRA results. Results of two-tailed tests with P < 0.05 were considered statistically significant.
We searched five databases for 2,622 studies (Databases: 2,204; Registers: 418). After a review by two investigators, 15 RCTs were included in the systematic review and NMA. The search details are represented in a flow diagram in Figure 1.
The characteristics of the 15 included RCTs, including the study and publication year, participants, and intervention characteristics, are summarized in Table 1. All the studies were published after 2000 and the sample size for each group was at least 20. In addition, the preoxygenation maintenance time was 3–5 min.
The risk of bias assessment is shown in Figure 2. All the studies reported random sequence generation and allocation concealment. Blinding was defined as impossible in the included studies; therefore, performance bias for 14 RCTs was defined as high risk, and outcome assessments were defined as unclear owing to a lack of reporting. However, blinding was achievable in a study by Jaber et al. (20); hence, the risk of bias for blinding was defined as low.
Network maps of the outcomes are shown in Figure 3. The outcomes include SpO2 <80% during ETI (Figure 3A), SpO2 <90% during ETI (Figure 3B), and the lowest SpO2 during ETI (Figure 3C).
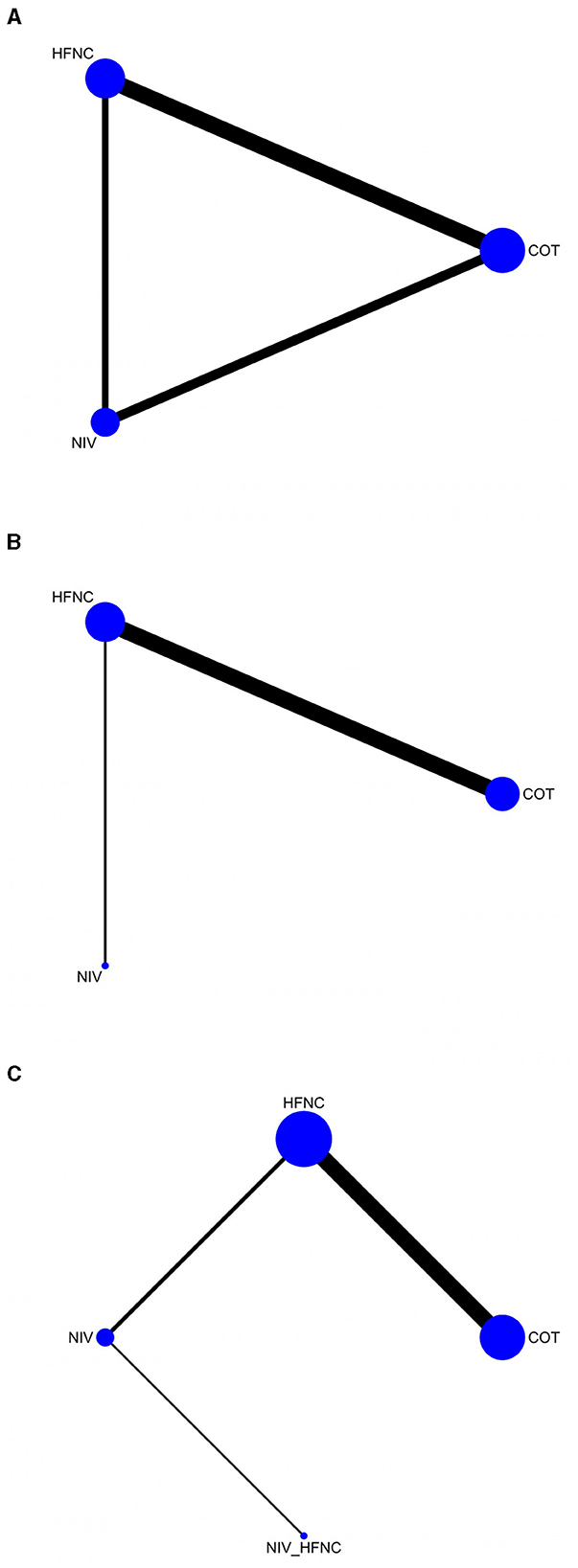
Figure 3. The network geometry of SpO2 <80% during ETI procedure (A); SpO2 <90% during ETI procedure (B); Lowest SpO2 during ETI procedure (C).
A total of 10 RCTs (10–12, 16, 18, 19, 21–24) (1,544 patients) reported SpO2 <80% during the ETI procedure. According to the pairwise meta-analysis results shown in Table 2, the NIV group was superior to the COT group (OR: 4.46; 95% CI: 1.29; 15.43, P = 0.02).
The NMA results (Figure 4A) showed that the NIV group was superior to the COT group (Log OR 1.23; 95% CI: 0.31, 2.42). For the SUCRA results shown in Table 3, the NIV group (0.8603467) performed better than the HFNC (0.1373533) and COT (0.0023) groups.
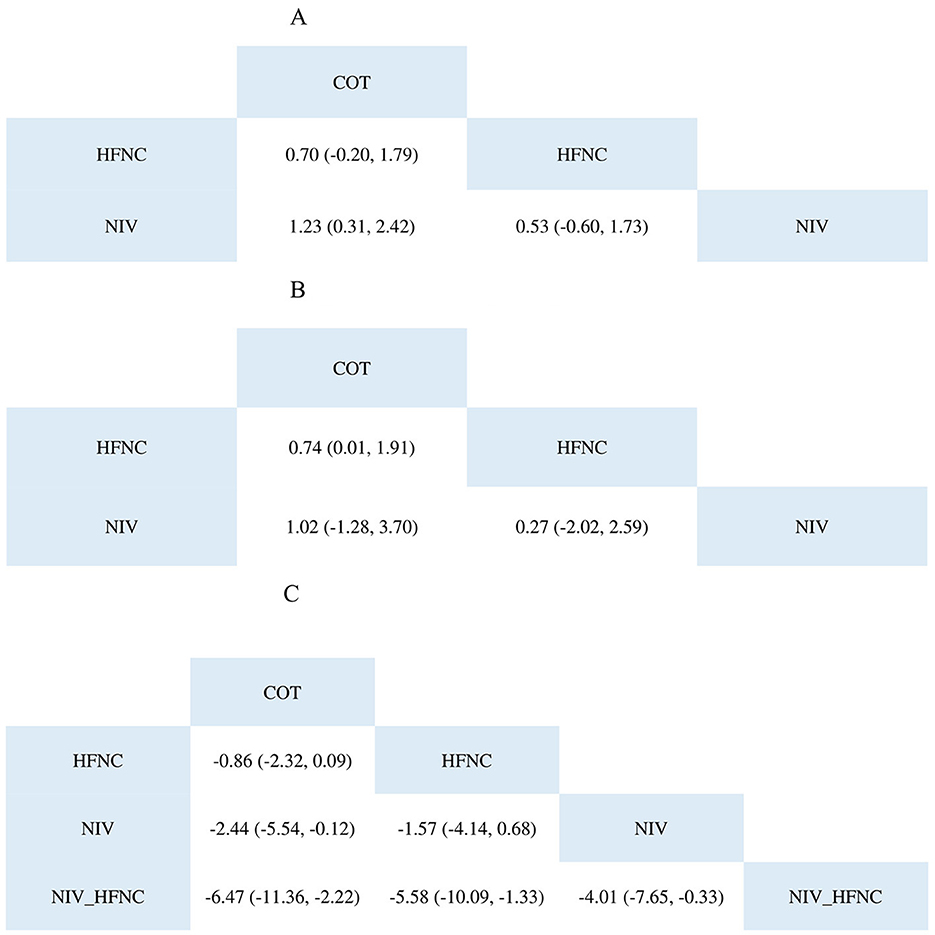
Figure 4. The league table of SpO2 <80% during ETI procedure (A); SpO2 <90% during ETI procedure (B); Lowest SpO2 during ETI procedure (C).
Seven RCTs (10, 17, 19, 21, 23, 24, 26) (1,101 patients) reported SpO2 <90% during the ETI procedure. According to the pairwise meta-analysis results shown in Table 2, the HFNC group was superior to the COT group (OR: 1.87; 95% CI: 1.23, 2.82; P = 0.003).
The NMA results (Figure 4B) showed that the HFNC group was superior to the COT group (Log OR 0.74; 95% CI: 0.01, 1.91). Regarding the SUCRA results, as shown in Table 3, the NIV group (0.60932667) performed better than the HFNC (0.37888667) and COT (0.01178667) groups.
Eleven RCTs (10, 12, 17–21, 23–26) (1,543 patients) reported the lowest SpO2 during the ETI procedure. Table 2 presents the results of the pairwise meta-analysis. The HFNC group was superior to the COT group (MD: −0.77; 95% CI: −1.49, −0.05; P = 0.04), the NIV group was superior to the HFNC group (MD: 1.31; 95% CI: 0.05, 2.57; P = 0.04), and the NIV with HFNC group was better than the NIV group (MD: −4.00; 95% CI: −6.53, −1.47; P = 0.002).
According to the NMA results (Figure 4C), the NIV group was superior to the COT group (MD: −2.44; 95% CI: −5.54, −0.12), whereas the NIV with HFNC group was better than the other groups. The SUCRA results are presented in a heatmap (Table 3). The NIV with HFNC group (0.9797) was better than the NIV (0.01541), HFNC (0.004345), and COT (0.000545) groups.
Four RCTs (9, 10, 21, 25) (508 patients) reported apnea time during the ETI procedure. According to the pairwise meta-analysis results, the HFNC group had a longer apnea time (MD: −50.05; 95% CI: −90.01, −10.09; P = 0.01) than the other groups.
This study marks the first comparison of multiple preoxygenation techniques using NMA. The findings revealed that NIV coupled with HFNC effectively maintained high SpO2 levels during ETI. Both NIV and HFNC significantly reduced the risk of SpO2 dipping to <80% and <90%, respectively, with NIV demonstrating superiority over both HFNC and COT. Furthermore, the use of HFNC for preoxygenation extended the duration of apnea.
Preoxygenation is a crucial measure for safeguarding patient oxygenation throughout the intubation process and ensuring surgical safety, particularly in individuals anticipated to encounter airway challenges. Both the 2015 Difficult Airway Society guidelines and 2022 American Society of Anesthesiologists recommendations emphasize the significance of this practice (6, 27). Among them, the 2022 guidelines recommend 3 min of preoxygenation to reach an end-tidal oxygen concentration of 0.90 or higher (EtO2 ≥ 0.9) and use of various NIV devices, such as nasal catheters and masks. As a modification of conventional nasal catheters, HFNC can deliver high-flow oxygen, generate low levels of positive end-expiratory pressure (PEEP), and allow asphyxiation and oxygenation, making it useful for preoxygenation or oxygen therapy (28). Despite the obvious benefits of HFNC in patients with acute hypoxic respiratory failure and after scheduled extubation (10, 23), its effectiveness is still controversial compared with other preoxygenation methods (29, 30). Guitton et al. (19) reported that HFNC preoxygenation reduced tracheal intubation-related adverse events but did not improve lowest SpO2. In addition, Vourc'h et al. (23) compared preoxygenation methods in obese patients and found that the HFNC group had a significant advantage in the lowest SpO2 compared with the NIV group. We included the study by Jaber et al. (20) on NIV combined with HFNC for preintubation oxygenation in critically ill patients with severe hypoxemia and acute respiratory failure. The results of our study indicate that when used in combination with preoxygenation, NIV and HFNC were associated with the lowest SpO2 levels. Paradoxically, our findings suggest that the combined use of NIV and HFNC could potentially improve the lowest SpO2 recorded. However, because of the scarcity of relevant studies, our analysis was limited to only one study, thus precluding a direct comparison. Consequently, further studies are required to comprehensively evaluate the effects of this combined approach on preoxygenation. In addition, owing to the small sample size, treatment effects and publication bias need to be considered; therefore, caution must be exercised when interpreting the results of HFNC and NIV studies. Additionally, the best method for combining HFNC and NIV to reduce air leakage has not been well described. Whether continuous nasal positive airway pressure masks play a special role in preoxygenation is worth exploring.
When comparing SpO2 <90% and SpO2 <80%, we found no significant advantage of HFNC over NIV, although both were superior to COT. A multicenter, randomized, open-label trial reported the effect of preoxygenation in patients with acute respiratory failure, showing that neither NIV nor HFNC changed severe hypoxemia in these patients, with no significant difference between them (18). A study designed by Kuo et al. pointed out that HFNC can better enhance PaO2 and has an advantage over mask oxygenation in preventing ETI (15). The reasons for this difference in outcomes may be patient characteristics and oxygen flow settings. Some studies have pointed out that NIV may be a better method of preoxygenation for obese patients (31, 32), and HFNC preoxygenation patients have lower EtO2 and SpO2 levels than the NIV group. This result may be related to the limited supraglottic pressure produced by HFNC, the inability to repair airway obstruction after general anesthesia, and the difficulty in maintaining or restoring FRC damage in obese patients (33). However, considering that HFNC is better tolerated and has a median SpO2, it is an acceptable alternative for obese patients without NIV or with contraindications (24, 34). In addition, several studies have reported the comparison of preoxygenation methods in critically ill patients with hypoxemia or acute respiratory failure (11, 12, 16, 23). In 2019, Fong et al. (35) conducted an NMA of RCTs on the effects of preoxygenation in patients with acute hypoxic respiratory failure. Seven RCTs encompassing 959 patients were comprehensively analyzed in this study. These findings indicate that NIV is a safe and potentially the most effective preoxygenation technique. One explanation for the inferior performance of HFNC could be the loss of the PEEP effect in patients experiencing respiratory distress due to mouth opening (36). In these patients, the nasal and oral inhalation flows can be as high as 110 and 280 L/min, respectively, which are significantly better than those of HFNC (37). Another possible explanation is that NIV allows the delivery of high levels of FiO2 and positive intrathoracic pressure, promoting alveolar replenishment, which may improve the efficiency of gas exchange (38, 39).
This study has some limitations. Constrained by the paucity of RCTs reporting pertinent comparisons, only 15 studies were included in this analysis, potentially introducing a reporting bias. Furthermore, the combined use of NIV and HFNC for preoxygenation was reported in a single study. Although the initial results appear promising, the absence of a direct comparative evidence necessitates cautious interpretation of the conclusions.
The results of the network analysis showed that NIV for preoxygenation achieved a higher SpO2 than HFNC and COT and had a more significant benefit in maintaining patient oxygenation during ETI. Patients had a longer apnea time after HFNC preoxygenation. The effect of NIV combined with HFNC was significantly better than that of other methods. Owing to a lack of studies, further investigation is warranted to evaluate their effects in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
MZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. RX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing—original draft, Writing—review & editing. JZho: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing. JZha: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing—original draft, Writing—review & editing. XY: Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing. AY: Project administration, Supervision, Validation, Writing—review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Thanks to Wuhua Ma for his contribution in managing and supervising the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NMA, network meta-analysis; OR, Odd Ratio; CI, Confidence Interval; NE, Not Estimate; NIV, Noninvasive Ventilation; COT, conventional oxygen therapy; HFNC, High Flow Nasal Cannula; ETI, Endotracheal Intubation; FiO2, fraction of inspired oxygen; PSV, pressure support ventilation; RSI, rapid sequence intubation; NC, nasal cannula; NR, no record; RR, respiratory rate; PS, pressure support; BMI, body mass index; SUCRA, surface under the cumulative ranking curve; RCTs, Randomized Controlled Trials; MCMC, Markov Chain Monte Carlo; DIC, Deviance Information Criteria; PSRF, potential scale reduction factor; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; EtO2, end-tidal oxygen concentration; PEEP, Positive End-expiratory Pressure; THRIVE, Transnasal Humidified Rapid-Insufflation Ventilatory Exchange.
1. Park S, Kim SY, Kim MS, Park WK, Byon HJ, Kim HJ. Comparison of preoxygenation efficiency measured by the oxygen reserve index between high-flow nasal oxygenation and facemask ventilation: a randomised controlled trial. BMC Anesthesiol. (2023) 23:159. doi: 10.1186/s12871-023-02126-9
2. Joffe AM, Aziz MF, Posner KL, Duggan LV, Mincer SL, Domino KB. Management of difficult tracheal intubation: a closed claims analysis. Anesthesiology. (2019) 131:818–29. doi: 10.1097/ALN.0000000000002815
3. De Jong A, Futier E, Millot A, Coisel Y, Jung B, Chanques G, et al. How to preoxygenate in operative room: healthy subjects and situations “at risk”. Annal Francaises d'anesthesie Reanim. (2014) 33:457–61. doi: 10.1016/j.annfar.2014.08.001
4. Baillard C, Boubaya M, Statescu E, Collet M, Solis A, Guezennec J, et al. Incidence and risk factors of hypoxaemia after preoxygenation at induction of anaesthesia. Br J Anaesth. (2019) 122:388–94. doi: 10.1016/j.bja.2018.11.022
5. Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. (2012) 59:165–75. doi: 10.1016/j.annemergmed.2011.10.002
6. Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American society of anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. (2022) 136:31–81. doi: 10.1097/ALN.0000000000004002
7. Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. (2015) 70:323–9. doi: 10.1111/anae.12923
8. Kim HJ, Asai T. High-flow nasal oxygenation for anesthetic management. Korean J Anesthesiol. (2019) 72:527–47. doi: 10.4097/kja.19174
9. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. (2017) 72:439–43. doi: 10.1111/anae.13799
10. Sjöblom A, Broms J, Hedberg M, Lodenius Å, Furubacke A, Henningsson R, et al. Pre-oxygenation using high-flow nasal oxygen vs. tight facemask during rapid sequence induction. Anaesthesia. (2021) 76:1176–83. doi: 10.1111/anae.15426
11. Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Resp Critic Care Med. (2006) 174: 171–7. doi: 10.1164/rccm.200509-1507OC
12. Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Resp Care. (2016) 61:1160–7. doi: 10.4187/respcare.04413
13. Li Y, Yang J. Comparison of transnasal humidified rapid-insufflation ventilatory exchange and facemasks in preoxygenation: a systematic review and meta-analysis. Biomed Res Int. (2022) 2022:9858820. doi: 10.1155/2022/9858820
14. Chiang TL, Tam KW, Chen JT, Wong CS, Yeh CT, Huang TY, et al. Non-invasive ventilation for preoxygenation before general anesthesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. (2022) 22:306. doi: 10.1186/s12871-022-01842-y
15. Kuo HC, Liu WC Li CC, Cherng YG, Chen JT, Wu HL, et al. A comparison of high-flow nasal cannula and standard facemask as pre-oxygenation technique for general anesthesia: a PRISMA-compliant systemic review and meta-analysis. Medicine. (2022) 101:e28903. doi: 10.1097/MD.0000000000028903
16. Baillard C, Prat G, Jung B, Futier E, Lefrant JY, Vincent F, et al. Effect of preoxygenation using non-invasive ventilation before intubation on subsequent organ failures in hypoxaemic patients: a randomised clinical trial. Br J Anaesthesia. (2018) 120:361–7. doi: 10.1016/j.bja.2017.11.067
17. Chua MT, Ng WM, Lu Q, Low MJW, Punyadasa A, Cove ME, et al. Pre- and apnoeic high-flow oxygenation for rapid sequence intubation in the emergency department (the Pre-AeRATE trial): a multicentre randomised controlled trial. Annal Acad Med. (2022) 51:149–60. doi: 10.47102/annals-acadmedsg.2021407
18. Frat JP, Ricard JD, Quenot JP, Pichon N, Demoule A, Forel JM, et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Resp Med. (2019) 7:303–12. doi: 10.1016/S2213-2600(19)30048-7
19. Guitton C, Ehrmann S, Volteau C, Colin G, Maamar A, Jean-Michel V, et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive care medicine. 2019; 45: 447-58. doi: 10.1007/s00134-019-05529-w
20. Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Int Care Med. (2016) 42:1877–87. doi: 10.1007/s00134-016-4588-9
21. Lodenius Å, Piehl J, Östlund A, Ullman J, Jonsson Fagerlund M. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. (2018) 73:564–71. doi: 10.1111/anae.14215
22. Nong L, Liang W, Yu Y, Xi Y, Liu D, Zhang J, et al. Noninvasive ventilation support during fiberoptic bronchoscopy-guided nasotracheal intubation effectively prevents severe hypoxemia. J Critic Care. (2020) 56:12–7. doi: 10.1016/j.jcrc.2019.10.017
23. Vourc'h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Int Care Med. (2015) 41:1538–48. doi: 10.1007/s00134-015-3796-z
24. Vourc'h M, Baud G, Feuillet F, Blanchard C, Mirallie E, Guitton C, et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine. (2019) 13:112–9. doi: 10.1016/j.eclinm.2019.05.014
25. Wong DT, Dallaire A, Singh KP, Madhusudan P, Jackson T, Singh M, et al. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth Analg. (2019) 129:1130–6. doi: 10.1213/ANE.0000000000003966
26. Wu YM, Li CC, Huang SY, Su YH, Wang CW, Chen JT, et al. A comparison of oxygenation efficacy between high-flow nasal cannulas and standard facemasks during elective tracheal intubation for patients with obesity: a randomized controlled trial. J Clin Med. (2022) 11:700. doi: 10.3390/jcm11061700
27. Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. (2015) 115:827–48. doi: 10.1093/bja/aev371
28. Cortegiani A, Accurso G, Mercadante S, Giarratano A, Gregoretti C. High flow nasal therapy in perioperative medicine: from operating room to general ward. BMC Anesthesiol. (2018) 18:166. doi: 10.1186/s12871-018-0623-4
29. Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Int Care Med. (2019) 45:563–72. doi: 10.1007/s00134-019-05590-5
30. Zhu Y, Yin H, Zhang R, Ye X, Wei J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Critical Care. (2019) 23:180. doi: 10.1186/s13054-019-2465-y
31. Futier E, Constantin JM, Pelosi P, Chanques G, Massone A, Petit A, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. (2011) 114:1354–63. doi: 10.1097/ALN.0b013e31821811ba
32. Delay JM, Sebbane M, Jung B, Nocca D, Verzilli D, Pouzeratte Y, et al. The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: a randomized controlled study. Anesth Analg. (2008) 107:1707–13. doi: 10.1213/ane.0b013e318183909b
33. Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. (2009) 103:886–90. doi: 10.1093/bja/aep280
34. Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. (2011) 107:998–1004. doi: 10.1093/bja/aer265
35. Fong KM, Au SY, Ng GWY. Preoxygenation before intubation in adult patients with acute hypoxemic respiratory failure: a network meta-analysis of randomized trials. Critical Care. (2019) 23:319. doi: 10.1186/s13054-019-2596-1
36. Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. (2016) 61:529–41. doi: 10.4187/respcare.04577
37. Tsounis M, Swart KM, Georgalas C, Markou K, Menger DJ. The clinical value of peak nasal inspiratory flow, peak oral inspiratory flow, and the nasal patency index. Laryngoscope. (2014) 124:2665–9. doi: 10.1002/lary.24810
38. Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Int Care Med. (2017) 43:226–8. doi: 10.1007/s00134-016-4426-0
Keywords: preoxygenation, endotracheal intubation, network meta-analysis, ventilation, randomized clinical trial
Citation: Zhong M, Xia R, Zhou J, Zhang J, Yi X and Yang A (2024) The comparison of preoxygenation methods before endotracheal intubation: a network meta-analysis of randomized trials. Front. Med. 11:1379369. doi: 10.3389/fmed.2024.1379369
Received: 31 January 2024; Accepted: 27 May 2024;
Published: 07 June 2024.
Edited by:
Pavel Michalek, General University Hospital in Prague, CzechiaReviewed by:
Carmen Silvia Valente Barbas, University of São Paulo, BrazilCopyright © 2024 Zhong, Xia, Zhou, Zhang, Yi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anbo Yang, YW5ib3lhbmcyMDIwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.