
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 10 May 2024
Sec. Ophthalmology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1377186
This article is part of the Research TopicWomen in Science: Ophthalmology 2023View all 8 articles
 Yvonne Nguyen1†
Yvonne Nguyen1† Josephine Rudd Zhong Manis2†
Josephine Rudd Zhong Manis2† Nicole Marie Ronczkowski2
Nicole Marie Ronczkowski2 Tommy Bui2
Tommy Bui2 Allston Oxenrider2
Allston Oxenrider2 Ravirajsinh N. Jadeja3
Ravirajsinh N. Jadeja3 Menaka C. Thounaojam2*
Menaka C. Thounaojam2*The intricate interplay between the gut microbiota and ocular health has surpassed conventional medical beliefs, fundamentally reshaping our understanding of organ interconnectivity. This review investigates into the intricate relationship between gut microbiota-derived metabolites and their consequential impact on ocular health and disease pathogenesis. By examining the role of specific metabolites, such as short-chain fatty acids (SCFAs) like butyrate and bile acids (BAs), herein we elucidate their significant contributions to ocular pathologies, thought-provoking the traditional belief of organ sterility, particularly in the field of ophthalmology. Highlighting the dynamic nature of the gut microbiota and its profound influence on ocular health, this review underlines the necessity of comprehending the complex workings of the gut-eye axis, an emerging field of science ready for further exploration and scrutiny. While acknowledging the therapeutic promise in manipulating the gut microbiome and its metabolites, the available literature advocates for a targeted, precise approach. Instead of broad interventions, it emphasizes the potential of exploiting specific microbiome-related metabolites as a focused strategy. This targeted approach compared to a precision tool rather than a broad-spectrum solution, aims to explore the therapeutic applications of microbiome-related metabolites in the context of various retinal diseases. By proposing a nuanced strategy targeted at specific microbial metabolites, this review suggests that addressing specific deficiencies or imbalances through microbiome-related metabolites might yield expedited and pronounced outcomes in systemic health, extending to the eye. This focused strategy holds the potential in bypassing the irregularity associated with manipulating microbes themselves, paving a more efficient pathway toward desired outcomes in optimizing gut health and its implications for retinal diseases.
The multifaceted roles of gut microbiota (GM) extend beyond conventional understanding, encompassing a spectrum of physiological and pathological functions (1–4). Beyond their role in digestion, these microorganisms communicate with various organ systems, prompting a paradigm shift in comprehending the interconnectedness of bodily functions (1–4). This review investigates into the evolving role of GM’s influence on ocular health, challenging historical presumptions of organ isolation and unveiling a profound interplay between microbiota-derived compounds and ocular pathogenesis. Recent scientific advancements have reshaped our perception of organ functionality, emphasizing the substantial impact of GM and their metabolites, notably short-chain fatty acids (SCFAs) like butyrate (5–7) and bile acids (BAs) (8–10), on various diseases including ocular pathologies. These observations contradict traditional beliefs of organ sterility, marking the GM as a key influencer in ocular pathology. This paradigm shift necessitates reevaluating therapeutic approaches within ophthalmology, highlighting the pivotal role of the GM in ocular diseases. The dynamic nature of the GM, thriving with diverse microbial communities and their metabolites, coordinates a complex interplay within the gut and extends its influence to ocular well-being (11). Understanding this bidirectional communication between the GM and ocular health propels exploration into potential therapeutic avenues embedded within this nexus. Acknowledging the promising therapeutic potential in manipulating the GM and its metabolites, a transformative shift advocates for a precise, tailored approach over broad interventions. This exemplary change comprises the utilization of specific microbiome-related metabolites as targeted therapeutic strategies, diverging from the historical blanket solutions (12–14). This nuanced precision aims to harness the potential of microbiome-related compounds, particularly within the context of retinal diseases, catering to individual microbiome intricacies. By advocating for a focused strategy tailored to the intricacies of individual microbiomes, this review aims to expedite and magnify outcomes in optimizing gut health and its implications for ocular pathologies. This precision-driven approach offers a more efficient pathway toward desired therapeutic outcomes, steering away from the uncertainties associated with altering the entire microbiome landscape.
The intestinal tract is inhabited by a diverse array of microorganisms, commonly referred to as the GM. The GM has recently come to light as a major topic of investigation in a multitude of research fields. Though it is debated when gut microbial colonization occurs (15, 16), it is widely accepted that there is a vast variety of microbial species within and on the surface of the body. These microorganisms include viruses and bacteria, among other microbes, which have physiologic and pathologic functions that affect many organ systems. Once present in the gut, these bacteria communicate with the rest of the body via various mechanisms, and their symbiosis and possible dysbiosis have been correlated with health and disease states in many different organ systems, ranging from the skin to the liver to the eye (17–19). This dynamic community has a wide range of impacts on our lives and is a major contender in altering health outcomes. The role of the GM should be considered in all fields of study, even in Ophthalmology, where the paradigm of organ sterility is constantly challenged (20).
Shortly after delivery, the gastrointestinal tract of a newborn is colonized by bacteria (21). Throughout this process, there are many factors that can alter the neonatal microbial community, including birth mode, environment, timing, diet, and gestational complications. In the neonatal gut, bacteria are less abundant but more diverse than those within the mature gut, most likely due to lower biomass and decreased species competition. The lower biomass complicates sampling of the neonatal gut, and there is a lack of consensus on the species present. However, Proteobacteria and Actinobacteria seem to dominate (22, 23), though Lactobacillus species (phylum Firmicutes) are commonly present as well (24). By the age of three, the gut bacterial population stabilizes and resembles that of adulthood (25). The adult gut consists of a highly individualized community that is impacted by extrinsic and intrinsic factors, including diet, environment, hygiene, host genetics and epigenetics, immune changes, metabolic status, and medications. In adulthood, the major phyla that colonize the gut are Bacteroides and Firmicutes, with anaerobes being present in the greatest quantities due to the gut’s limited oxygen availability (26, 27).
The prenatal period and the baby’s delivery mode, i.e., cesarean or vaginal, shape the neonatal microbiota as it is the baby’s first exposure to the world full of microbes (Figure 1). During the prenatal period, the baby is susceptible to microbiota changes, specifically due to pregnancy complications (28). It has been shown that the neonate takes on more of the maternal skin microbiome (e.g., Staphylococcus, Corynebacterium, and Propionibacterium) in cesarean delivery, and vaginally delivered babies take on the maternal vaginal flora (e.g., Lactobacillus, Prevotella, and Sneathia) (29). Other groups have found different changes in the microbiota based on birth mode (30, 31), but it is important to note the potential for microbiota alteration through factors outside of the delivery mode itself, such as antibiotics delivered to moms undergoing C-section, vaginal swab from mom given to C-section baby, or birth environment. For example, Selma-Royo in 2020 found that place of birth (i.e., home vs. hospital) was the most significant driver of the neonatal microbiota when compared with other birth alterations (32). Lastly, younger gestational age at the time of birth has been correlated with an immature GM and can even alter the composition of bacterial metabolites, such as SCFA present (25).
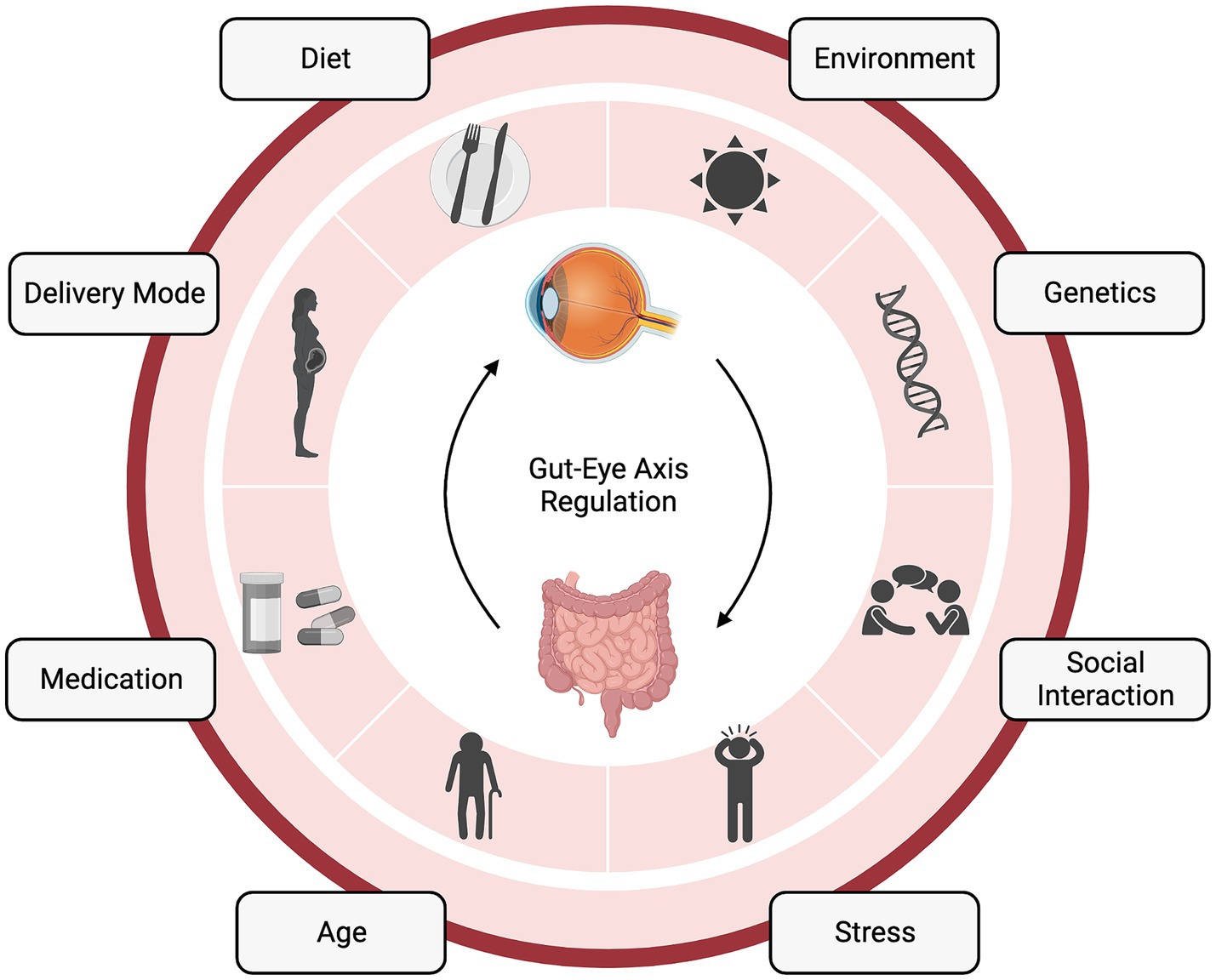
Figure 1. The gut-eye axis in retinal health and disease. The complex interplay between the gut microbiome (GM) and retinal health. This diagram illustrates the numerous factors influencing the gut-eye axis and its potential role in various retinal diseases. “Created with BioRender.com”.
From the immediate neonatal period and throughout infancy, the diet of both mom and baby can impact the bacterial community. Neonatal diet, i.e., breastfeeding or formula feeding, and feeding frequency and duration, can alter the availability of breast milk components to the baby (Figure 1). Breast milk contains not only nutrients for the baby but also antimicrobial peptides, human milk oligosaccharides, and potentially bacteria (33). Importantly, maternal fat and fiber consumption alter the breast milk and, therefore, the baby’s microbiota (25). Breastfeeding also provides an opportunity for the neonate to interact with the maternal skin microbiome and ingest these bacteria. Upon feeding, the baby is inoculated with factors that shape their intestinal microbial community and the metabolites it is able to produce (34). The baby’s transition to solid food also induces a microbiota shift those further shapes the microbiota and prepares the baby for adult immune insults (35) (Figure 1).
The ocular surface, comprising the conjunctiva, cornea, and eyelids, hosts a diverse array of microorganisms. Recent investigations have revealed the intricate microbial communities present in this environment, shaped by constant exposure to the external world. Among the prominent bacterial genera are Staphylococcus, Corynebacterium, Streptococcus, Propionibacterium, and Micrococcus (36). These species have evolved mechanisms to evade tear film defenses and interact with the local immune system. Dysbiosis of the ocular microbiota can occur due to factors like antibiotic use, disease states, and gut microbiome alterations. Notably, these microbiotas play a role in immunity, inflammation, and various eye conditions, including conjunctivitis, blepharitis, keratitis, and dry eye syndrome (37). Understanding their impact is crucial for maintaining ocular health and guiding therapeutic strategies.
Factors both intrinsic and extrinsic to the host shape the GM throughout our lifetimes (Figure 1) (38). Certain gene loci have been attributed to microbiota heritability (39), and the microbiota can even alter host epigenetics via metabolites (40), suggesting a bidirectional host-symbiont genetic relationship. Another bidirectional relationship that is intrinsic to the host is immunity, which is both shaped by gut bacteria and can shape the bacteria’s survivability in the gut (41). Additionally, the metabolic status of the host can be shaped by bacteria (42), which, can also be considered a symbiotic relationship between the host and gut community. One extrinsic factor that can shape the GM is antibiotics. In terms of the GM, antibiotics, by nature, kill beneficial microbes. However, there are still studies claiming their utility, specifically in terms of the gut-eye axis. Oral antibiotics have been shown to reduce the severity of experimental autoimmune uveitis in mice by altering the composition of the GM, raising the frequency of regulatory T cells (Treg) in the intestine, and decreasing inflammatory cytokines (43).
In contrast to antibiotics, probiotics or fecal transplants represent methods of introducing microbiota to a host GI tract that have been the subject of great investigation. These methods function by directly inoculating the GI tract with exogenous microbes. Probiotics, by definition, are a strain of bacteria that have been shown to have at least one clinical benefit in a peer-reviewed study (44). However, their lack of regulation in production and sale led to mixed reviews. On one hand, many studies claim their benefit in many dysbiotic states or for general preventative medicine (45, 46), however, without proper education and regulation, their benefits are quite limited in practice. For example, there are no guarantees the bacteria strain in the probiotic is alive at the time of reaching the consumer, much less reaching the GI tract where it should be exerting its benefit [reviewed by Ayichew et al. (47)]. Fecal transplant, however, is a regulated process that has shown very promising results, for example, in the treatment of C. difficile infection (48). Though due to its risk for infection or contamination, this is a very costly and scarce procedure (49).
Another extrinsic factor of great interest for microbiota is the diet. Generally, the regional basis of diet (i.e., Mediterranean, Western, etc.) and these tendencies can greatly influence the overall profile of bacteria in the gut via their macronutrient composition. Carbohydrates, for example, can alter the SCFA production of the bacteria, while fats and proteins can foster the growth of specific bacterial species. Dietary fiber, which is non-absorbable to the host, can feed the gut bacteria and push contents through the GI tract, facilitating movement and a dynamic gut community (50). Outside of nutrition, factors such as host hygiene and social isolation can alter the GM (51). Even urbanization and changing host environments, such as living in a rural or urban area, can alter microbe exposure and determine which species are present in the host diet and community (52). Several studies have found reduced gut microbial diversity and altered microbiota composition in adults with obesity compared to normal-weight adults (53). In one study, weight loss in 33 adults with obesity was associated with an increase in gut microbial diversity and in butyrate producing GM (54). Similarly, levels of Bacteroides fragilis and Lactobacillus were higher in adolescents who lost more weight while on the same calorie-restricted diet than those who lost less weight (54). In addition to the overall dietary profile, adding so-called prebiotics (i.e., those fermented foods that might feed microbes) has been shown to be associated with improvements in metabolic outcomes and gut barrier against pathogens (46). Overall, diet plays a major role in shaping gut outcomes and could very well play a role in systemic and eye pathologies through the axes described in this review. Lastly, medications, in addition to the diseases they are being used to treat, are an important mix of iatrogenic and intrinsic sources of GM variability (55).
Although the precise mechanisms of communication between the GM and the eye are still being studied, it is becoming increasingly evident that GM and its products can impact ocular health, showed by its numerous systemic functions (Figure 1). In this review, we aim to uncover potential ways the GM may be in communication with the eye and specifically highlight how this might be altered in dysbiosis in various retinal ailments. As discussed above, the GM communicates with the rest of the body in a multitude of ways. Additionally, it is apparent there are opportunities for the so-called gut-eye axis to come about, whether that be through immune modulation, metabolite communication, or direct connections, as happens in the gut-brain axis. Furthermore, the GM may modulate immune responses that play a role in age-related macular degeneration (AMD) pathogenesis, including inflammation and oxidative stress. Taken together, this highlights the importance of GM in bidirectional immune modulation and the potential for its role in ocular disease. Emerging research suggests that GM and its metabolites may have implications for ocular health, and we will highlight this intricate relationship below.
Systemically, inflammation can result from alterations in the GM and its metabolites. While eye diseases are usually associated with an infectious component, eye inflammation due to GM dysbiosis is another way to develop eye disease (56). This process can be transient until the leaky gut tight junctions are healed, or more permanent, as seen in IBD, which presents with extraintestinal manifestations including ocular may be due to gut bacteria triggering a systemic, adaptive immune response (57). Thus, leaky gut, caused by diseases that breach the intestinal barrier, such as Crohn’s, as well as lifestyle risk factors inducing inflammation, such as smoking or alcohol consumption, plays a key role in establishing the gut-eye axis through systemic spread of bacteria and digestive products (58). Other methods of communication have been shown to play an important role in the gut-eye axis and ocular disease development, including metabolites and immune factors. Inflammation caused by activation of T-cells and breach of the blood-retina barrier is seen in autoimmune uveitis (AU), while immune activation due to gut bacterial irregularities due to high-fat diet is also seen in AMD in mice (59). The blood-ocular barrier consists of both the blood-aqueous barrier and the blood-retinal barrier (BRB) (60). The BRB is tight and restrictive and has inner and outer components. The outer component consists of tight junctions between retinal pigment epithelial cells, and the inner component consists of tight junctions between retinal capillary endothelial cells (61, 62). The BRB acts as a physiologic barrier from the systemic circulation that regulates ion, protein, and water flux into and out of the retina and is vital to maintaining the eye as a privileged site within the body (60). The microenvironment of the retina must be tightly regulated because the retina is susceptible to oxidative stress, which would cause damage to the central vision. The tight junctions of the blood-aqueous barrier are leaky, whereas the tight junctions of the BRB are nonleaky (63). The BRB has properties similar to the BBB, but small protein tracers like microperoxidase (19 kDa) are capable of entering the BBB but not the retina (63). This indicates the intercellular junctions of the retinal endothelium are sealed more tightly, most likely due to a large amount of zonulae occludent that forms the bulk of the BRB (63). Along with the physical barrier that the BRB provides, it also is an environment with active mechanisms of immunoregulation and immunosuppression (60). Neuropeptides in the aqueous humor assist in suppressing induction of delayed-type hypersensitivity and induce regulatory immunity. Collectively, the neuropeptides suppress the activation of Th1 cells while promoting the induction of CD25+ and CD4+ regulatory T cells (64). Inflammation can lead to breakdown of the BRB, leading to autoimmunity and inflammation and would also allow systemic drugs to penetrate into the eye (60).
Aging is another key factor in creating the gut-eye axis by weakening physical barriers and immune regulatory signals and increasing permeability, both in the retina through weakening of the aforementioned three tissue layers that give the eye immune privilege and in the intestines (56). Other factors that can play a role in gut-eye axis development include epigenetics, dietary influences, and GM metabolites (65). Dietary influences on the gut-eye axis have been subject to much investigation. Intermittent fasting has been shown to both restructure the GM as in the gut-brain axis and reduce risk factors for certain ocular diseases like blood pressure and heart rate (66, 67).
Bacteria themselves also influence susceptibility to ocular disease, as occurs in bacterial keratitis, where altered species in the GM increase corneal inflammation (68). Recently, new evidence through genome-wide association studies has emerged that metabolites of the GM, such as SCFAs, can further modify the epigenome and trigger intraocular inflammation. Kim et al. investigated the modulating effects of IRT-5, a cocktail of five probiotic strains, and discovered the treatment prevented the development of experimental autoimmune uveitis (EAU) and attenuated clinical manifestations of autoimmune dry eye models (69). In glaucoma, an imbalanced gut marked by high Firmicutes/Bacteroidetes ratio and pro-inflammatory bacteria like Prevotella worsens neurodegeneration, as evidenced by studies in germ-free mice (70). Higher levels of SCFAs, such as propionate, acetate, and butyrate, were further associated with glaucoma patients with a GM high in Dysgonamonadaceae species (71). AMD presents no single microbial signature, but specific shifts like increased Prevotella and Holdemanella point toward potential risk factors (72). High-fat and high-glycemic diets exacerbate AMD by altering the microbiome and promoting inflammatory markers (72). Uveitis patients exhibit a gut lacking diversity and are enriched in pro-inflammatory bacteria, with depleted butyrate-producing bacteria crucial for gut barrier function and anti-inflammatory response (73). Studies employing antibiotics and experimental models suggest the GM directly influences the severity of uveitis (73). Interestingly, mouse models of inherited retinal degenerations like retinitis pigmentosa (RP) and Batten disease exhibit altered gut bacterial profiles compared to healthy controls. In these models high-fat diets worsen retinal degeneration by further altering microbial diversity and inflammatory processes (74), suggesting potential avenues for managing these devastating conditions through microbiome modulation. Retinal vascular diseases like diabetic retinopathy (DR) and retinal artery occlusion show lower bacterial diversity and specific microbial shifts linked to inflammation and cholesterol metabolism. Microbial metabolites like lipopolysaccharides and trimethylamine N-oxide may play a role in disease development through vascular risk factors (75). Preterm infants with retinopathy of prematurity (ROP) often exhibit decreased Firmicutes and Lactobacillus with increased Enterobacteriaceae, potentially impacting vascularization and contributing to oxidative stress (76). An analysis of GM in premature infants by Skondra et al. in 2020 showed changes in early GM composition associated with severe ROP and proposed metabolic pathways as potential therapeutic targets (77). Understanding the microbiome-ROP relationship could advance screening and intervention strategies. Similar to the gut, a diverse and balanced ocular surface microbiome is key to a healthy eye. Various eye diseases, from dry eye to Sjögren’s syndrome, are characterized by a depletion of beneficial bacteria and an overgrowth of harmful ones, disrupting the delicate balance and contributing to disease development (78) (Table 1).
In conclusion, the intricate interplay between the GM and eye health unfolds through diverse communication channels, highlighting the potential of targeting the gut for novel therapeutic approaches in retinal diseases. From immune modulation and metabolite exchange to direct bacterial influences, the gut-eye axis presents a captivating avenue for future research and clinical applications. Understanding and harnessing this potent connection holds immense promise for revolutionizing the way we prevent and manage a spectrum of retinal ailments, offering hope for improved vision and well-being. Further investigation of the gut-eye axis will be critical in understanding how diet and metabolites play a role in disease. Metabolites of the greatest interest include SCFAs, butyrate, and BAs because of their implications in dysbiosis and inflammation.
Metabolites are produced via various methods, including catabolism of food products in the gut (Figure 2). SCFAs are key metabolites produced by gut bacteria through the fermentation of dietary fiber. Specific bacteria, such as Faecalibacterium prausnitzii and Roseburia spp., are known to produce significant amounts of butyrate, a type of SCFA (81, 82). Butyrate has been extensively studied for its beneficial effects on gut health, including its anti-inflammatory properties and its role in maintaining the integrity of the intestinal barrier. Another important metabolite involved in GM communication are various BAs. Bile acids, produced by the liver and modified by the GM, not only aid in the digestion and absorption of dietary fats but also act as signaling molecules. They can influence various physiological functions, including lipid metabolism and glucose homeostasis (83–85). In states of dysbiosis, alterations in the production and metabolism of SCFAs and BAs can occur. This dysregulation may contribute to the development of certain pathological conditions, such as IBD and metabolic disorders.
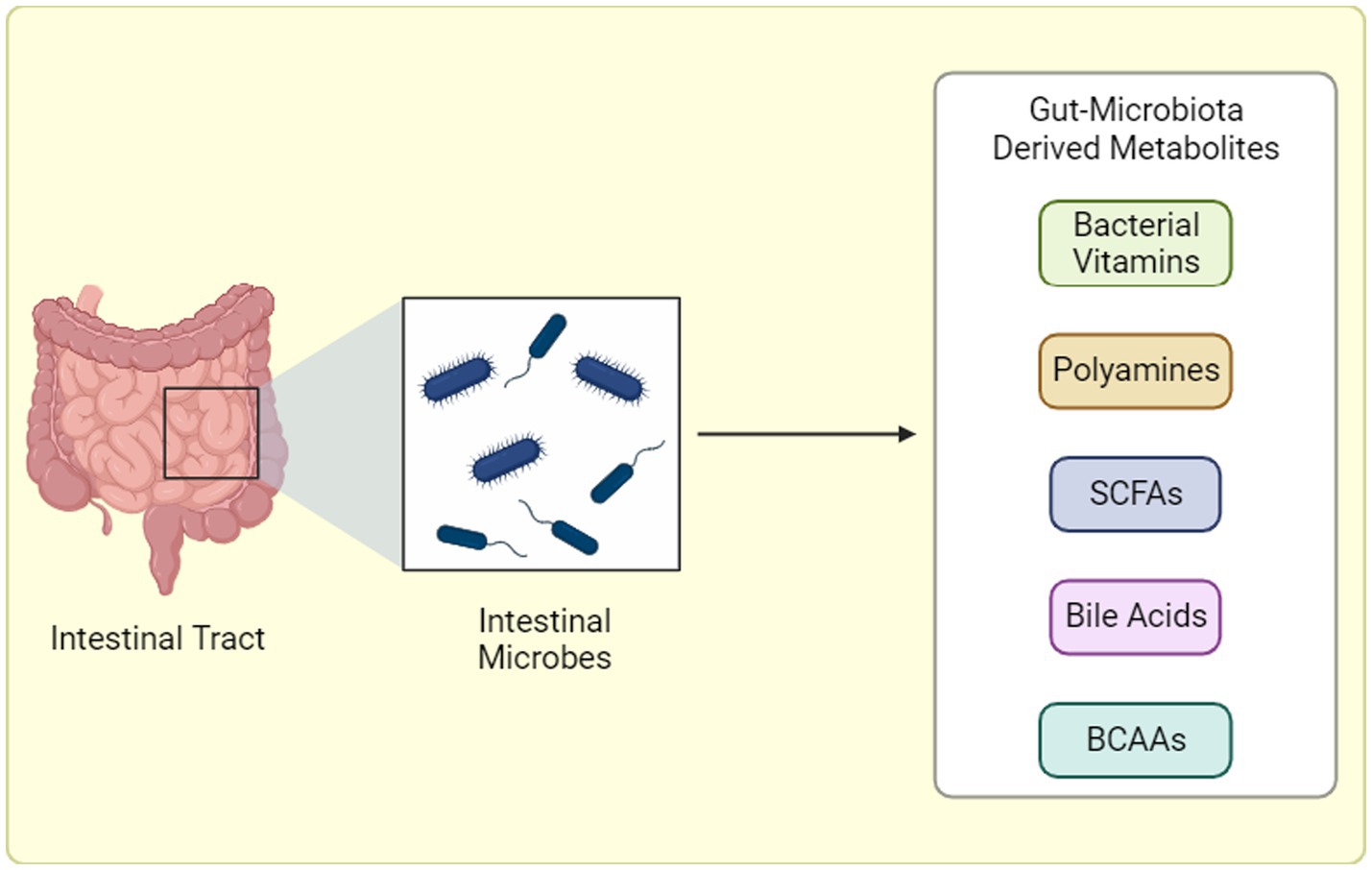
Figure 2. Gut Microbiota-Derived Metabolite Production. Gut microbiota generates a diverse array of metabolites through distinct pathways: (1) Dietary Fermentation: Here, bacteria “transform” ingested food components, like fibers, into bioactive molecules like short-chain fatty acids (SCFAs) or branched-chain amino acids (BCAAs). (2) Host-Microbial Collaboration: In this interplay, the host produces primary metabolites that gut bacteria refine into potent compounds. (3) Microbial Independence: Some bacteria possess the remarkable ability to “craft” specific metabolites, like trimethylamine-N-oxide (TMAO), entirely from scratch, independent of diet or host precursors. BCAAs: Branched-chain amino acids, SCFAs: Short-chain fatty acids, TMAO: Trimethylamine-N-oxide. “Created with BioRender.com”.
SCFAs are organic acids produced within the intestinal lumen. They are primarily produced by bacterial fermentation of undigested dietary carbohydrates (starches, celluloses, fibers, sugars) but also by dietary and endogenous proteins (86). There are three primary SCFAs: acetate, propionate, and butyrate. Butyrate is the primary source of energy for the epithelial cells within the colon (82). Acetate serves as a cofactor for enhancing the growth of other bacteria, and propionate can be converted to glucose in the intestine (87). SCFA absorption occurs through passive diffusion, as well as active transport by intestinal epithelial cells via sodium-coupled monocarboxylate transporter 1 (SMCT1) and proton-coupled monocarboxylate transporter 1 (MCT1) (88). SCFAs are downstream mediators of the GM anti-inflammatory activity and are fundamental to preserving immune homeostasis and functionality of the host immune system (89). SCFAs have anti-inflammatory properties through a variety of mechanisms. Microbial flora associated with the production of SCFA, such as Bifidobacterium spp., promote the production of anti-inflammatory cytokines, such as tumor necrosis factor (TNF) and IL-β. They also assist with the maturation of immune cells, promote IgA secretion, and possess antioxidant properties. SCFAs also reduce the production of cytokines by neutrophils and reduce macrophagic NF-κB signaling. The SCFA receptors within the colon are free fatty acid receptors and G protein-coupled receptors.
SCFAs have been shown to increase colonic Treg frequencies in the gut in mice, which are critical to regulating intestinal inflammation (90). In cases of intestinal breach, such as leaky gut or chronic inflammatory gut diseases, these SCFAs may travel in the bloodstream to other organs, including the eye. SCFAs can ameliorate immune-mediated ocular conditions partially by altering the migration of lymphocytes from the intestines, but once the SCFAs reach the eyes, they can also inhibit lipopolysaccharide (LPS)-induced intraocular inflammation (91, 92). Most often, metabolites and inflammation go hand in hand, as with DR, in which it is hypothesized that hyperglycemia and products of gut dysbiosis, such as uremic toxins, promote chronic low-grade inflammation, which impacts neovascularization (93).
Butyrate is a four-carbon SCFA that serves as the primary energy source for colonocytes and has many cellular functions impacting colonic health (82). Although, as discussed previously, the main three SCFAs are butyrate, propionate, and acetate, butyrate has the most anti-inflammatory properties and is the most studied within the literature as well (94). Butyrate has been implicated in a variety of health conditions, including ocular pathology. Once absorbed, 95% of butyrate is oxidized into ketone bodies for ATP synthesis (95). The 5% of butyrate not metabolized in the colon is transported to the liver and used as an energy substrate for hepatocytes, leaving little butyrate in systemic circulation (95). Butyrate specifically has been implicated in dysbiosis states of the GM and in several immune disorders, such as IBD, colorectal cancer, and type II diabetes (81). Recent studies, as detailed in Table 2. have also reported the efficacy of SCFAs in various retinal ailments. Similar decreases in butyrate due to the GM have been implicated as part of the possible pathogenesis of autoimmune Vogt-Koyanagi-Harada (VKH) disease (80). Transplantation of feces from patients with active VKH to mice undergoing EAU resulted in more severe manifestations of intraocular inflammation (80). Although both Behcet Syndrome and VKH disease cause uveitis involving GM dysbiosis, one study in China found specific changes in the GM for each etiology (80). Through metagenomic analysis, patients with VKH were found to be enriched in gram-negative bacteria in their gut; however, they were depleted with butyrate-producing bacteria, lactate-producing bacteria, and methanogens (80).
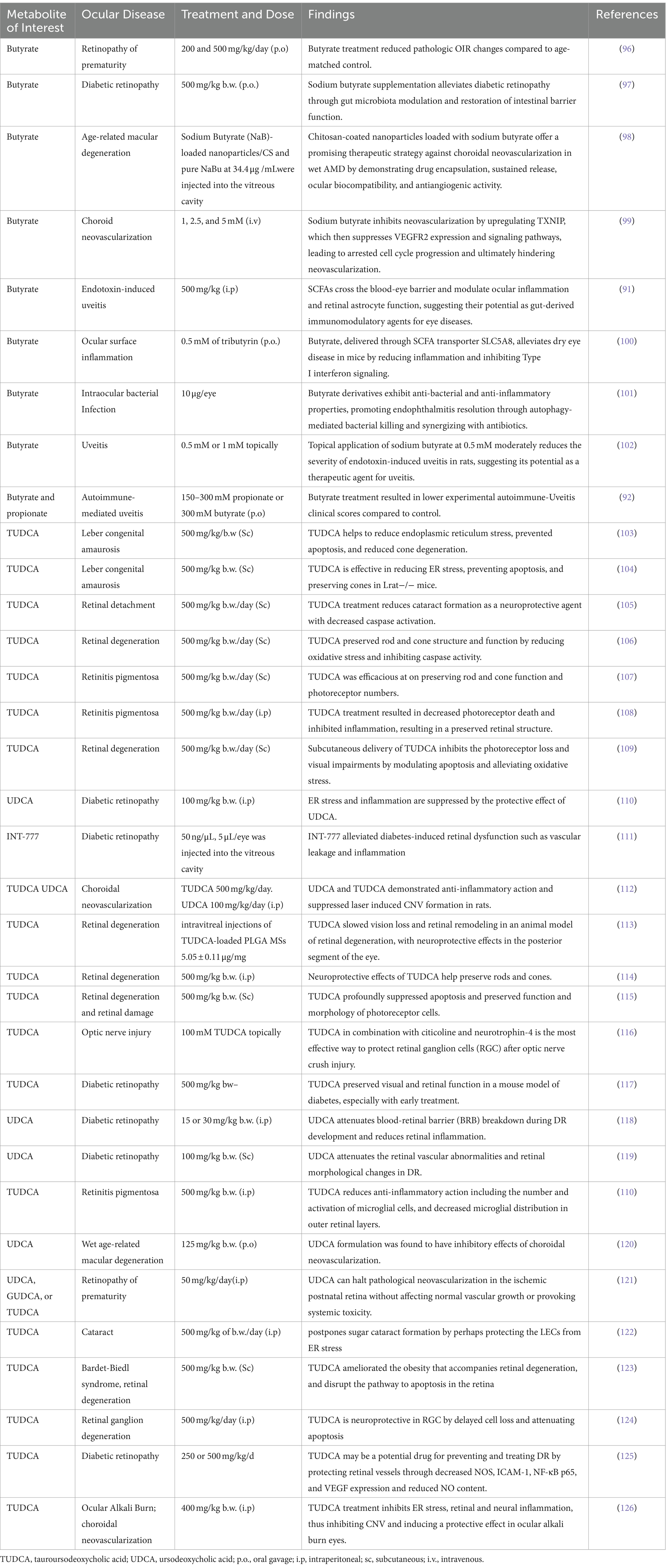
Table 2. Pharmacological effects of microbiome-related metabolites treatment on various ocular diseases.
In a study by Chen et al., mice were injected with butyrate prior to intravitreal injection of LPS to study the effect of butyrate on intraocular inflammation (91). After the eyes were evaluated for 18 h, the authors noted that clinical signs of ocular inflammation in butyrate-treated mice were significantly milder than in control mice. The number of infiltrating cells (CD45+), a subset of monocytes/macrophages (CD45 + CD11b + Ly6chi), and pro-inflammatory cytokines, such as IL-6 and CCL2, were also significantly reduced in the treated mice (91). Other SCFAs were studied, and they all inhibited IL-6 production in a dose-dependent manner, with butyrate being the most potent and acetate the least (91). The results of the study also suggest the existence of gut-eye cross talk and that SCFAs can cross the blood-eye barrier via systemic circulation. These findings also demonstrate the inhibitory roles of SCFAs on innate immunity, and their levels may inhibit or enhance intraocular inflammation (91). Oral propionate effectively reduced uveitis severity by boosting Treg and dampening effector T cells in the gut and lymph nodes. Notably, it hindered the migration of immune cells between the gut and the eye, suggesting a novel therapeutic approach. Further research on SCFA’s specific mechanisms and optimal doses could open the door to improved treatments for uveitis and potentially other inflammatory diseases (92). Oral supplementation with butyrate in diabetic mice also showed improved visual function compared to control diabetic mice (97). Oral butyrate also resulted in increased expression of Zonula occludens (ZO)-1and occludin proteins in the gut, representing increased gut barrier integrity. Furthermore, decreases in butyric acid, 4-methyl-valeric acid, and caproic acid associated with microbiota changes suggest a metabolic change exerted through the GM following oral butyrate supplementation. Another study sheds light on the potential of butyrate, in combatting intraocular bacterial infections like endophthalmitis. Butyrate derivatives injected into infected eyes effectively diminished bacterial growth and inflammatory responses, preserving retinal structure and function. The mechanism appears multi-pronged, involving both NLRP3-independent anti-inflammatory effects and enhanced bacterial killing through autophagy and antimicrobial peptides. Notably, butyrate synergized with antibiotics, suggesting its promise as an immunomodulatory and antibacterial co-therapy for improved visual outcomes in ocular bacterial infections (101). On a similar line, another study tested the potential of topical sodium butyrate as an alternative to corticosteroids in treating uveitis, an inflammatory eye disease. While not reaching the effectiveness of dexamethasone, butyrate at 0.5 mM dose showed a moderate but clinically relevant reduction in inflammation compared to untreated rats. Interestingly, its anti-inflammatory effect did not involve changes in measured cytokine levels, suggesting a distinct mechanism from corticosteroids (102).
The VEGF receptor 2 (VEGFR2) pathway plays a central role in orchestrating blood vessel growth. A study by Xiao et al. reveals a novel regulator of VEGFR2: TXNIP, whose expression is significantly enhanced by sodium butyrate (NaBu). Interestingly, NaBu potently inhibited neovascularization in various models, suggesting its anti-angiogenic properties may work through the TXNIP-VEGFR2 axis. This opens exciting avenues for developing new therapies targeting this critical pathway in diseases characterized by excessive blood vessel growth (99).
It is known that a function of intestinal epithelium derived butyrate is to help maintain the intestinal barrier by modulating goblet cell expression of mucins and goblet cell differentiation (127). One study hypothesized butyrate produced in the gut also has a direct effect on the differentiation or maintenance of conjunctival goblet cells, lending to its role in dry eye disease (128). In a pharmacologic study, butyrate was shown to modulate the inflammatory responses at the ocular surface and, when delivered intragastrically, attenuated ocular surface disease in a dry eye disease mouse model through SCFA transporter SLC5A8 (100). In-vitro experiments showed the reduction of Tumor Necrosis Factor alpha (TNF alpha) expression in corneal epithelial cultures when pre-treated with phenylbutyrate. Cultures from Slc5a8 knockout mice showed no response to pre-treatment with phenylbutyrate. Furthermore, the anti-inflammatory effect of butyrate in vivo was shown to partially require SLC5A8 (100).
The role of the GM in the gut and retinal brain barrier has been shown through experiments elucidating the improved integrity in both organs and after probiotic use in mice, potentially playing a role in the severity of DR (129). Alongside butyrate, other compounds and medications have been shown to impact the microbiome via modulation of SCFAs. Fenofibrate is an FDA-approved lipid-lowering medication used to treat hypertriglyceridemia, primary hypercholesterolemia, and mixed dyslipidemia (130). High-fat diets (HFD) significantly decrease levels of SCFA in the serum and retina, which fenofibrate supplementation mitigates. Fenofibrate can also decrease inflammation within the retina by reducing activation of microglia and Muller cells, inhibiting toll-like receptor 4 expression (TLR-4), and decreasing the expression of inflammatory cytokines in the eye, such as TNF-alpha, IL1beta (Interleukin-1 beta), and IL-6 (Interleukin 6) (131). One study speculated the potential for fenofibrate to attenuate HFD-induced inflammation in the retina through suppressing lipopolysaccharide (LPS)-TLR4/inflammatory cells-activation of the NF-kB and JNK signaling pathways-inflammatory cytokines (131). Thus, fenofibrate’s role in modulating levels of SCFAs can be a candidate for future pharmacologic investigation, as well as an avenue to better understand SCFAs’ inflammatory effects. Increases in butyrate-producing bacteria, such as Lachnospiraceae, after oral omega-3 fatty acid supplementation support potential gut-microbiome-focused approaches to disease. Coupled with epidemiological studies that show potential protective effects of consumption of omega-3 fatty-acid-rich fish against AMD, the potential for butyrate as a pharmacologic target in AMD is tantalizing (94, 132). Of interest, Reis et al. designed sodium butyrate-loaded nanoparticles coated with chitosan for enhanced ocular biocompatibility. In AMD models, these nanoparticles effectively inhibited neovascularization, highlighting their potential as a targeted therapeutic strategy against abnormal blood vessel growth (98). Our research group recently reported on the pharmacologic application with butyrate in the oxygen-induced retinopathy (OIR) model, which replicates pathologic neovascularization in mice similar to ROP (96). Mice pups subjected to OIR had significantly decreased butyrate-producing bacteria in cecal samples compared to mice pups on room air (RA). Furthermore, OIR pups treated with butyrate via oral gavage had significantly decreased neovascular tufts and areas of avascular retina, compared to age-matched control mice. Butyrate demonstrated significantly decreased extravasation on leakage assays and restoration of ZO-1 levels, supporting the role of butyrate in preserving the blood-retina barrier.
In conclusion, SCFAs, particularly butyrate, emerge as potent regulators of ocular health through diverse mechanisms (Figure 3). From dampening inflammation and modulating barrier integrity to influencing angiogenesis and bacterial killing, SCFAs hold immense promise for novel therapeutic approaches in various retinal diseases. Research exploring their gut-eye communication pathways, optimal delivery methods, and synergistic potential with other interventions paves the way for revolutionizing our understanding and treatment of these debilitating conditions. As we delve deeper into the intricate world of the GM and its influence on the eye, the therapeutic horizon continues to expand, offering hope for improved vision and well-being for individuals suffering from retinal diseases.
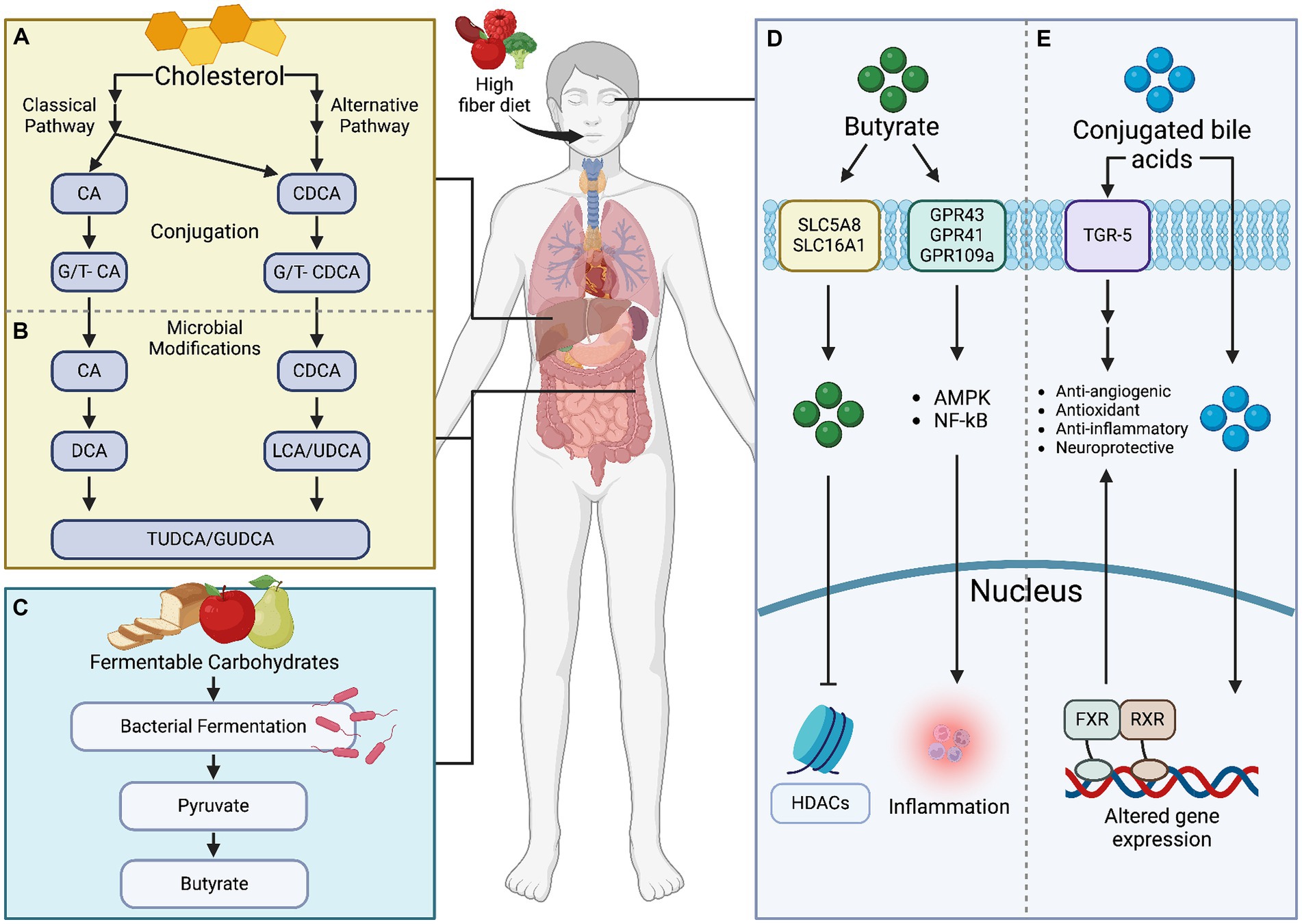
Figure 3. Microbial metabolites, specifically bile acids and butyrate, exert significant influence on retinal health. (A) Bile acids, produced through cholesterol metabolism, impact retinal inflammation, vascular function, and oxidative stress. Meanwhile, (B) butyrate, a short-chain fatty acid, modulates (C–E) immune responses and neuronal survival within the retina. Understanding these metabolites’ roles is crucial for developing targeted therapies to mitigate retinal pathologies. Created with BioRender.com.
BAs are produced in hepatocytes and transported to the gallbladder through bile canaliculi. They are further modified in the intestinal lumen by the GM through hydroxylation, deconjugation, oxidation, and epimerization (133, 134). 95% of the total BAs (unconjugated and conjugated) are reabsorbed and returned to the liver via enterohepatic circulation (135, 136). Of the 95% reabsorbed, only 10% of BAs reach systemic circulation, therefore, BA synthesis in the liver is crucial (135). The 5% that is not reabsorbed is excreted in the feces (135, 136). BAs are hormones with unique chemical compositions that contribute to their anti-inflammatory and antioxidant effects. They serve many different roles in the body’s digestive and metabolic systems, which has long been appreciated.
BAs maintain liver cell viability, assist with cholesterol catabolism, lipid digestion, and absorption, and stimulate bile flow and biliary phospholipid secretion (83–85). They are produced in hepatocytes through two major pathways: the classical and alternative pathways (83). In the classic pathway, cholesterol is converted to 7α-hydroxycholesterol by the rate-limiting enzyme CYP7A1 (cholesterol 7 alpha-hydroxylase A1) (135, 137). 7α-hydroxycholesterol is ultimately converted to cholic acid (CA) by a sterol 12α-hydroxylase (CYP8B1) or converted to chenodeoxycholic acid (CDCA) spontaneously (135, 137). In the alternate pathway, cholesterol is first converted to 27-hydroxycholesterol by CYP27A1 (cytochrome P450 Family 27 Subfamily A Member 1) and then converted to CDCA (135, 138, 139).
BAs are utilized in traditional Chinese medicine (TCM) (140–142), as bear bile is used to remove toxins from the body, stop convulsions, and improve vision (140–142). BAs have also been used therapeutically for liver disease and biliary cirrhosis in both TCM and modern Western medicine (141–143). The discovery of their signaling properties and ability to regulate the activity of genes through the orphan nuclear receptor, farnesoid X receptor (FXR), has generated more interest in their function in homeostasis and metabolism (144). Most of the earlier studies on the pharmacologic use of BAs have focused on the liver because it is the primary site of BA production, but recent studies have also identified synthesis within extrahepatic sites, such as the brain and retina (135, 145). Production in these sites relies on the alternate synthesis pathway, which plays an important role in retinal cholesterol and BA metabolism (135, 146).
In the retina, BAs act as signaling molecules and can activate the FXR and TGR5 receptors; however, the literature is limited, and TGR5 expression has only been reported in the adult retina (147). Interestingly, studies with mice have shown the protective effects against DR through intermittent fasting, which exerts its effects through microbiome restructuring and TGR5 activation (148). Through activation of the TGR5 receptor, BAs like ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) can also reduce acellular capillaries, inflammation, and the number of macrophages, leukocytes, and activated microglia (148). In the last decade, studies have proposed to prove the usefulness of BAs to treat ocular diseases (146, 149). Recent studies have shown BAs are protective in neurodegenerative diseases, including several ocular afflictions, including Leber’s congenital amaurosis (103, 104), retinal detachment, cataracts, retinitis pigmentosa, diabetic retinopathy, choroidal neovascularization, retinopathy of prematurity (ROP) (Table 1). The mechanism of action of UDCA and TUDCA is unclear; however, it is hypothesized to involve the suppression of caspase-dependent and caspase-independent apoptosis by inhibiting the release of apoptosis-inducing factor (AIF) from the retinal pigment epithelium (RPE), photoreceptors, and RGC (145, 150). Paradoxical effects and an incomplete understanding on cancer cell proliferation from BAs have added notable caution to approaching these metabolites as therapeutic targets (151). Nevertheless, the potential for ocular applications remains promising.
Several preclinical studies suggest promising roles for TUDCA in protecting and preserving vision in retinal degeneration models. Boatright et al. observed significant preservation of photoreceptor cells, function, and morphology in mice with light-induced retinal degeneration after systemic injections of TUDCA (500 mg/kg) (115). Similarly, another study showed that both bilirubin and TUDCA reduced oxidative stress and protected photoreceptors in mice exposed to bright light or carrying a retinal degeneration mutation (106). Focusing on specific retinal cell types, Tao et al. found that subcutaneous TUDCA delivery effectively preserved cone photoreceptors and visual function in a chemically induced rodent model of retinopathy (109). Fernández-Sánchez et al. demonstrated that weekly TUDCA injections preserved photoreceptor structure and function in another RP model, highlighting its potential as a long-term neuroprotective therapy (114). Additionally, in a model of retinitis pigmentosa, Fernández-Sánchez et al. reported that slow-release TUDCA microspheres injected into the eye provided sustained protection for photoreceptors, synaptic connections, and retinal function (113). However, some studies indicate strain-specific effects and varying degrees of efficacy depending on the delivery method and treatment duration. Another study observed partial preservation of function and structure in one strain of rd1 mice with rapid retinal degeneration, but only maintained structure in the other (124). This suggests further research is needed to optimize TUDCA treatment for different forms of retinal degeneration. Overall, these preclinical studies paint a promising picture for TUDCA’s potential in protecting vision against retinal degeneration.
Several studies highlight the promising potential of TUDCA in protecting vision and delaying retinal degeneration in animal models of RP. Phillips et al. observed significant preservation of photoreceptor structure and function in rd10 mice treated with TUDCA (500 mg/kg every 3 days) from postnatal day 6 to 30, demonstrating its efficacy in early stages of the disease (107). Similar effects were seen in Rpgr knockout mice, where TUDCA treatment prevented photoreceptor degeneration and suppressed microglial activation, suggesting its potential against specific RP mutations (108). Noailles et al. further explored the neuroprotective mechanisms, finding that TUDCA reduced microglial activation in a P23H rat model of RP (500 mg/kg weekly injections), offering an additional therapeutic avenue (152). These studies, with their different treatment durations and RP models, converge in suggesting TUDCA as a promising candidate for future RP therapies, warranting further research to optimize its application and efficacy in humans.
Growing evidence suggests potential for UDCA and its derivatives in mitigating DR progression. Studies exploring diverse mechanisms highlight UDCA’s ability to protect retinal microvasculature and preserve visual function. Chung et al. demonstrated UDCA’s effectiveness in reducing pericyte loss in diabetic mice (500 mg/kg), potentially delaying BRB breakdown (110). Zhu et al. identified TGR5 receptor activation as a promising therapeutic target, with its agonist attenuating DR via RhoA/ROCK signaling suppression (111). Another study observed early TUDCA treatment (50 mg/kg) preserving visual function and retinal structure in a mouse model, emphasizing the importance of timely intervention (117). Ouyang et al. revealed UDCA’s anti-inflammatory properties in DR, reducing retinal inflammation and reversing BRB breakdown (15 mg/kg and 30 mg/kg) (118). Additionally, Shiraya et al. demonstrated UDCA’s ability to rescue retinal vasculature and reduce edema in an antibody-induced pericyte depletion model (25 mg/kg) (119). Finally, Wang et al. explored TUDCA’s protective effects on human retinal microvascular endothelial cells and DR rats, suggesting its potential in preventing and treating DR by downregulating inflammatory mediators (5.0 μM to 125.0 μM in vitro and 250 mg/kg and 500 mg/kg in vivo) (153). These preclinical findings paint a promising picture for UDCA and its derivatives as potential therapeutic options for DR, warranting further clinical investigation to translate these benefits into effective patient care. Specific BAs, Taurolithocholic acid (TLCA) and TUDCA, have also been shown as independent predictors for DR in metabolic profiles of patients with Type 2 diabetes mellitus (T2DM) (154).
Oral delivery of UDCA has shown promise in treating choroidal neovascularization (CNV) associated with wet AMD. Maharjan et al. developed an aqueous oral UDCA formulation with improved bioavailability and demonstrated its efficacy in a mouse model. This formulation effectively suppressed CNV growth and improved retinal function after oral administration (120). Another study provided further evidence for UDCA’s potential, showing that systemic administration of UDCA (500 mg/kg) and its taurine conjugate TUDCA (100 mg/kg) significantly reduced CNV size and leakage in a laser-induced rat model (112). These studies suggest that oral UDCA formulations warrant further investigation as a non-invasive and potentially cost-effective alternative to current intravitreal injections for managing CNV and WAMD.
Photoreceptor cell death and retinal detachment are associated with numerous ocular diseases (155), and a study by Mantopoulos et al. demonstrated that TUDCA preserved photoreceptor survival by reducing ER and oxidative stress (105). TUDCA has also been shown to prevent cataract formation in galactosemic rats and slow the progression of retinal degeneration by inhibiting the unfolded protein response (UPR)-dependent pathway and reducing superoxide radicals, respectively (122). TUDCA has emerged as a promising neuroprotective agent against retinal ganglion cell (RGC) loss. Gómez-Vicente et al. demonstrated that systemic TUDCA administration (30 mg/kg) in NMDA-induced retinal injury in rats preserved RGC density and improved electroretinogram amplitudes, suggesting its potential for treating diseases involving RGC degeneration (125). Furthermore, TUDCA’s protective effects extend to cataract formation. Mulhern et al. reported that oral TUDCA treatment (300 mg/kg) in galactosemic rats effectively reduced lens epithelial cell death and delayed cataract development, possibly by mitigating endoplasmic reticulum stress and oxidative stress (122). These findings highlight the multifaceted neuroprotective and anti-cataract properties of TUDCA, warranting further investigation of its therapeutic potential in various ocular diseases.
Pharmacologic application has involved UDCA, that reduces retinal inflammation by reducing pericyte depletion, expression of inflammatory cytokines, such as IL-1β and IL-6, and expression of angiogenic factors and inflammatory mediators, such as ICAM-1 (118). In our previous study, OIR mouse pups showed attenuated pathologic neovascularization, decreased OIR-induced BRB dysfunction, and decreased levels of oxidative stress when treated with UDCA (121). In the OIR model, our research group demonstrated the protective role of BAs, such as UDCA, within the GM for the pathogenesis of ROP. Cecal samples from OIR mice compared to RA mice showed increased levels of unconjugated BAs. Furthermore, the microbiota in OIR mice consisted of lower levels of bile-salt hydrolase (BSH)-producing phyla in compared to control mice (156). Further studies in retinal BA signaling showed compromised FXR signaling in OIR mice, exacerbated OIR pathology in FXR knockout mice, and amelioration of OIR with OCA, an FXR-specific agonist (157).
Additionally, TUDCA has emerged as a promising therapeutic agent for various retinal diseases treating a wide range of retinal diseases, including ocular alkali burns, and glaucoma due to its anti-inflammatory, neuroprotective, and regenerative properties. In a mouse model of ocular alkali burn, Huang et al. demonstrated that systemic TUDCA administration (400 mg/kg) effectively suppressed corneal and retinal inflammation, protected RGC from apoptosis, and promoted corneal re-epithelization (126). Another study reported that TUDCA treatment (subcutaneous injections, twice a week) preserved photoreceptor function and structure in two different mouse models of retinitis pigmentosa, Bardet-Biedl syndrome mice, and rd10 mice (123). Kitamura et al. investigated the neuroprotective effects of TUDCA in a rat optic nerve crush model. They found that topical application of TUDCA, alone or in combination with other neurotrophic factors, significantly protected RGC from degeneration (116).
BAs have emerged as promising therapeutic agents for various ocular diseases due to their multifaceted roles in digestion, metabolism, and signaling. BAs exert neuroprotective and anti-inflammatory effects, making them suitable candidates for treating photoreceptor degeneration, diabetic retinopathy, and ROP (Figure 3). Despite the encouraging preclinical findings, further research is necessary to elucidate the precise mechanisms of BA action in the eye, optimize the route of administration and dosage, and ensure long-term safety and efficacy for clinical translation. Understanding the crosstalk between BAs, the GM, and retinal BA signaling pathways will be crucial for developing novel therapeutic strategies targeting ocular diseases.
While we summarize the crucial roles of SCFAs and BAs in the body and how they relate to ocular health, it should also be noted that the gut microbiota itself affects the metabolism of amino acids that in turn impact ocular health. Of note, tryptophan has also been linked to the gut-brain axis (79). In this study, mice fed a high-glycemic diet had low levels of the metabolite serotonin compared to mice fed a lower-glycemic diet. The high glycemic diet resulted in many AMD features, such as photoreceptor degeneration, hypopigmentation, and atrophy linked to the accumulation of advanced glycosylation end products. It was found that in mice with AMD-like changes, there was an inverse relationship between serotonin and frequency of AMD features. Since serotonin derives from tryptophan, an increased number of spores forming bacteria in the gut to create more serotonin may be protective in cases of AMD (79). The study also found that microbiota in the Clostridiales order were associated with AMD features, while the Bacteroidales order was associated with protection (79). Other metabolites investigated include branched-chain amino acids (BCAAs) and glutamate. BCAAs are key components in glucose and protein metabolism, and their decomposition can be altered through dysbiosis. This ultimately promotes oxidative stress responses, and raised serum levels of BCAAs may predict the development of T2DM. Specifically, levels of leucine, isoleucine, and valine in BCAA metabolism were found in studies to be increased in the serum of DR patients and in the diabetic rat retina (158). In one study, mice were fed a short-term diet with a reduction of BCAAs, and they had improved metabolism of white adipose tissue and intestinal microbiota composition with a lowered postprandial insulin secretion (159). Catabolism of BCAAs provides nitrogen for the synthesis of glutamate, and glutamate plays an important role in DR neurodegeneration as it is the major excitatory neurotransmitter in the CNS and retina. Branched chain amino acids (BCAAs) are associated with glutamate toxicity contributing to retinopathy (160), especially in DR and AMD. It has been found that BCAA supplements play a neuroprotective role by enhancing retinal ganglion cell survival, in addition to inflammatory and oxidative stress protective effects (160). BCAAs from protein diets are absorbed by the intestine, thus any dysregulation in absorption through altered gut microbiomes or intestinal malabsorption syndromes could be implicated in lower BCAA levels and susceptibility to retinopathy.
Within the retina, glutamatergic synapses connect its fundamental functional cells, such as photoreceptors, bipolar cells, and retinal ganglion cells. Studies found that increased glutamate in the retina activates ionotropic glutamate receptors in excess, mainly the NMDAR, resulting in uncontrolled intracellular calcium responses and cell death (161). Other studies have tried to confirm the correlation between intake of glutamate with the incidence of DR and have not found significant results, so more studies need to be done before glutamate can be used for screening of DR or used as a measure of treatment response. High levels of the glutamate, a neurotransmitter derived from the amino acid glutamic acid, triggers a neurotoxic cascade in the retina (162, 163). This can be explained by glutamate’s activation of NDMA (N-nitrosodimethylamine) receptors causing retinal ganglion cell death in DR, glaucoma, and retinal ischemia (162, 163). Other amino acids, such as arginine and lysine, have also been previously summarized for their impacts in DR, AMD, and ROP (164), though most notably it is glutamine and arginine that appear to be biomarkers of early retinopathy diagnosis (164). Glutamate enters the TCA cycle after being absorbed by the intestines and is converted to other amino acids such as arginine. Dietary glutamate is also used an energy source by the intestines (165). By decreasing glutamate levels through receptor inhibition, including NMDA receptor modulation, diseases like DR can improve (162, 164). Metabolism of amino acids due to gut microbiota, in addition to SCFAs and BAs, should be considered for their important effects on ocular health.
It has been long established that the microbiota has the ability to produce vitamins, notably vitamins B and K. Many species harbored in the gut microbiome have been shown to have potential in synthesizing such vitamins (166). However, more recently, there has come to light a role for the microbiota in regulating host metabolism of Vitamin A, and further, they have been able to directly metabolize vitamin A (167). Also described is a relationship between the gut microbiota and vitamin D, with the gut microbiota playing a role in vitamin D metabolism (168). Outside of gut microbe effect on metabolism of vitamins, there seems to be an emerging role for vitamins as modulators of the gut microbiota, which could help to play a role in the clinical applications of gut microbiota (169). For example, altering one’s intake of different vitamins could work to shape their gut microbial community.
Free fatty acids (FFA’s) represent another group of molecules that can be affected by GM in various manners. Some GM are capable of metabolizing dietary fats directly, ultimately converting them into FFAs (170). For this reason, GM dysregulation and its subsequent effect on FFA concentration is likely to play a role in various ocular pathologies. Although, the specific role of FFA’s in multiple ocular pathologies has recently become the subject of much speculation. Altered FFA levels are often seen in chronic diseases and ocular pathologies that present as comorbidities (171). T2DM, for example, is often associated with increased FFA levels, and it has been hypothesized that FFA’s may facilitate a hyperperfusion response that results in deteriorated microvascular function (172). Furthermore, low grade chronic inflammation (LGCI) is a pro-inflammatory state associated with many chronic conditions and ocular diseases that present as comorbidities, including diabetes mellitus linked retinopathy. LGCI, when left unchecked, can eventually lead to a depressed anti-inflammatory response. Long-term diabetes mellitus can also reduce blood-retinal barrier integrity, which leads to free radical and pro-inflammatory molecule release, polyunsaturated fatty acid pathway dysregulation, and increased VEGF levels (171).
With the shift toward an inflammatory and oxidative state, FFA’s have been investigated as a potential therapeutic agent in modulating inflammation within the context of ocular disease. Long term exposure to a hyperglycemic environment culminates in marked oxidative stress levels that can lead to free radical formation and apoptosis, deteriorating retinal microvasculature (173). Omega-3 polyunsaturated fatty acids (ω-3 PUFA’s) represent a FFA that has been found to elicit an anti-inflammatory and antioxidant response in multiple ocular pathologies, including diabetic retinopathy. Due to high photoreceptor demand for PUFA’s, it is recommended that fish oil supplements be taken daily as a valuable source of ω-3 PUFA’s (173).
Ultimately, the notion that the modulation of free fatty acids by the GM plays an important role in ocular health appears to be well substantiated based on currently available data. FFAs have been clearly linked to multiple forms of ocular pathogenesis, including but not limited to deteriorated microvascular function, a pro-inflammatory state, and oxidative stress (171–173). FFA’s, such as ω-3 PUFA, also represent a potential therapeutic agent in the treatment of oxidative stress in ocular disease (173). As both a direct and indirect modulator of FFA levels, the GM should be considered an important factor in FFA dysregulation. Although, more research is required to elucidate the complex nature of the relationship between the pair in the context of eye disease.
Bacteroidetes, a prominent phylum within the gut microbiota, harbors Gram-negative bacterial species that elicit immune-mediated responses via components of their cell wall, particularly lipopolysaccharide (LPS) and flagellin. These responses are initiated when pattern recognition receptors (PPRs) of the innate immune system detect these bacterial products. For instance, the sensing of flagellin by dendritic cells triggers the production of IL-22 by innate lymphoid cells (ILCs), as demonstrated in studies like (174). Additionally, variations in toll-like receptor (TLR) 4, responsible for recognizing LPS and potentially leading to changes in the trabecular meshwork, have been linked to primary open-angle glaucoma (POAG), as discussed in the review article by (175). Moreover, experimental studies utilizing animal models of glaucoma have revealed the detrimental impact of exogenously administered LPS on axons and neurons, as seen in investigations such as those by Napolitano et al. (18). Notably, these experiments also showed that exogenous LPS exacerbated photoreceptor loss and impaired retinal function in dystrophic P23H rats, as reported in studies like (176). While previous sections delve into metabolites that can positively modulate ocular health, the role of LPS underscores the potential for microbiota-derived substances to have adverse effects on ocular tissues.
There are countless animal studies looking at the role of the GM and its relation to certain diseases, and over the past decade, the number of human clinical trials has quickly grown as well. UDCA was one of the first BA’s to be widely utilized to treat primary biliary cirrhosis, and the research on the compound has continued. In the PEGASUS-D study in Korea, adults with a diagnosis of gastric cancer and who underwent gastrectomies were enrolled and randomly assigned to receive different dosages of UDCA or placebo. Patients were followed over a 12-month period, and it was found that administration of UDCA significantly reduced the incidence of gallstones after gastrectomy in patients with gastric cancer (177). Recently, studies have looked at SCFA’s role in neurological disorders as well. In one study on ALS, treatment with TUDCA was associated with a slower deterioration of function; TUDCA has no known symptomatic modulation on muscle strength or motor function, so this observation may potentially reflect a disease-modifying effect. Similarly, high-dose UDCA was given to patients with early Parkinson’s Disease (PD), and midbrain P-MRS demonstrated improved ATP hydrolysis with a possible improvement in cadence and other gait parameters as well (178). Incredible advancements have been made with GM research and its clinical applications, but there is still much to be studied. Despite the absence of clinical trials, substantial preclinical data support the exploration of microbiome-related metabolites as promising candidates for managing retinal diseases.
Bile acid derivatives, while therapeutically promising as our lab’s and previous work has shown, are not as well-researched as other gut-health enhancing domains like prebiotics or probiotics. This could be due to multiple reasons. One reason is that the function of bile acid derivatives in disease relevance is novel, with the gut microbiome and metabolomics only recently coming to interest in contributing to overall health. Another is that there must be substantial preclinical evidence before advancement to clinical trials. While SCFAs have been implicated in diseases like IBD, there has been much more volume of research in other domains not related to the specific organ system of the eye. Finally, clinical need is another factor contributing to lack of bile acid derivative clinical trials. We anticipate an increase in SCFA and BA clinical trials in the future as more research and animal trials support the need for clinical trials.
The GM plays a crucial role in human health and disease, and the gut-eye axis is a relatively novel avenue needing further research to understand functions and potential clinical applications, even in Ophthalmology, where the paradigm of organ sterility is constantly being challenged. The GM and its metabolites have emerged as a major player for many pathologies of the eye. This review highlights the importance of understanding the function of the GM and its metabolite production, particularly butyrate and BAs. The bidirectional relationship between the GM and its metabolites is useful in these therapeutic opportunities; however, it should be explored further in hopes to better understand the relationships between GM, metabolites, and the eye. Our ongoing studies are focused on exploring microbiome related metabolites as a potential therapeutic strategy in retinal disease. Hence, focusing on microbiome-related metabolites over the broader approach of fixing the entire microbiome offers a more targeted and precise intervention. This targeted strategy is expected to provide a more nuanced understanding of the microbiome ecosystem. We are hopeful that this focused strategy, will not only enhance precision but also offer a more efficient pathway toward achieving desired outcomes in optimizing gut health and its implications in retinal diseases.
YN: Writing – original draft, Writing – review & editing. JRZM: Writing – original draft, Writing – review & editing. NMR: Writing – original draft, Writing – review & editing. TB: Writing – original draft, Writing – review & editing. AO: Writing – review & editing. RNJ: Conceptualization, Supervision, Writing – review & editing. MCT: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research work in MCT’s laboratory is supported by NIH EY34568 Grant.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hou, K, Wu, ZX, Chen, XY, Wang, JQ, Zhang, D, Xiao, C, et al. Microbiota in health and diseases. Signal Transduct Target Ther. (2022) 7:135. doi: 10.1038/s41392-022-00974-4
2. Senchukova, MA . Microbiota of the gastrointestinal tract: friend or foe? World J Gastroenterol. (2023) 29:19–42. doi: 10.3748/wjg.v29.i1.19
3. Debnath, N, Kumar, R, Kumar, A, Mehta, PK, and Yadav, AK. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol Genet Eng Rev. (2021) 37:105–53. doi: 10.1080/02648725.2021.1989847
4. Colella, M, Charitos, IA, Ballini, A, Cafiero, C, Topi, S, Palmirotta, R, et al. Microbiota revolution: how gut microbes regulate our lives. World J Gastroenterol. (2023) 29:4368–83. doi: 10.3748/wjg.v29.i28.4368
5. Zhang, D, Jian, YP, Zhang, YN, Li, Y, Gu, LT, Sun, HH, et al. Short-chain fatty acids in diseases. Cell Commun Signal. (2023) 21:212. doi: 10.1186/s12964-023-01219-9
6. Shin, Y, Han, S, Kwon, J, Ju, S, Choi, T, Kang, I, et al. Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients. (2023) 15:466. doi: 10.3390/nu15204466
7. Feng, Y, and Xu, D. Short-chain fatty acids are potential goalkeepers of atherosclerosis. Front Pharmacol. (2023) 14:1271001. doi: 10.3389/fphar.2023.1271001
8. Yin, C, Zhong, R, Zhang, W, Liu, L, Chen, L, and Zhang, H. The potential of bile acids as biomarkers for metabolic disorders. Int J Mol Sci. (2023) 24:2123. doi: 10.3390/ijms241512123
9. Shi, M, Wei, J, Yuan, H, Li, Y, and Guo, Z. The role of the gut microbiota and bile acids in heart failure: a review. Medicine (Baltimore). (2023) 102:e35795. doi: 10.1097/MD.0000000000035795
10. He, S, Li, L, Yao, Y, Su, J, Lei, S, Zhang, Y, et al. Bile acid and its bidirectional interactions with gut microbiota: a review. Crit Rev Microbiol. (2023) 2023:1–18. doi: 10.1080/1040841X.2023.2262020
11. Lozupone, CA, Stombaugh, JI, Gordon, JI, Jansson, JK, and Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. doi: 10.1038/nature11550
12. Xu, H, Xu, Z, Long, S, Li, Z, Jiang, J, Zhou, Q, et al. The role of the gut microbiome and its metabolites in cerebrovascular diseases. Front Microbiol. (2023) 14:1097148. doi: 10.3389/fmicb.2023.1097148
13. Li, L, Yang, J, Liu, T, and Shi, Y. Role of the gut-microbiota-metabolite-brain axis in the pathogenesis of preterm brain injury. Biomed Pharmacother. (2023) 165:115243. doi: 10.1016/j.biopha.2023.115243
14. Du, P, Jing, J, and He, X. Microbiota and their metabolites potentiate cancer immunotherapy: therapeutic target or resource for small molecule drug discovery? Front Pharmacol. (2022) 13:1091124. doi: 10.3389/fphar.2022.1091124
15. Hornef, M, and Penders, J. Does a prenatal bacterial microbiota exist? Mucosal Immunol. (2017) 10:598–601. doi: 10.1038/mi.2016.141
16. Walter, J, and Hornef, MW. A philosophical perspective on the prenatal in utero microbiome debate. Microbiome. (2021) 9:5. doi: 10.1186/s40168-020-00979-7
17. Schwenger, KJ, Clermont-Dejean, N, and Allard, JP. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep. (2019) 1:214–26. doi: 10.1016/j.jhepr.2019.04.004
18. Napolitano, P, Filippelli, M, Davinelli, S, Bartollino, S, dell’Omo, R, and Costagliola, C. Influence of gut microbiota on eye diseases: an overview. Ann Med. (2021) 53:750–61. doi: 10.1080/07853890.2021.1925150
19. Snyder, A, Abbott, J, Jensen, MK, and Secrest, AM. Fecal microbiota transplant and dermatologic disorders: a retrospective cohort study assessing the gut microbiome’s role in skin disease. World J Dermatol. (2021) 9:1–10. doi: 10.5314/wjd.v9.i1.1
20. Deng, Y, Ge, X, Li, Y, Zou, B, Wen, X, Chen, W, et al. Identification of an intraocular microbiota. Cell Discov. (2021) 7:13. doi: 10.1038/s41421-021-00245-6
21. Bajorek, S, Parker, L, Li, N, Winglee, K, Weaver, M, Johnson, J, et al. Initial microbial community of the neonatal stomach immediately after birth. Gut Microbes. (2019) 10:289–97. doi: 10.1080/19490976.2018.1520578
22. Qin, J, Li, R, Raes, J, Arumugam, M, Burgdorf, KS, Manichanh, C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
23. Eckburg, PB, Bik, EM, Bernstein, CN, Purdom, E, Dethlefsen, L, Sargent, M, et al. Diversity of the human intestinal microbial flora. Science. (2005) 308:1635–8. doi: 10.1126/science.1110591
24. Pantoja-Feliciano, IG, Karl, JP, Perisin, M, Doherty, LA, McClung, HL, Armstrong, NJ, et al. In vitro gut microbiome response to carbohydrate supplementation is acutely affected by a sudden change in diet. BMC Microbiol. (2023) 23:32. doi: 10.1186/s12866-023-02776-2
25. Yao, Y, Cai, X, Ye, Y, Wang, F, Chen, F, and Zheng, C. The role of microbiota in infant health: from early life to adulthood. Front Immunol. (2021) 12:708472. doi: 10.3389/fimmu.2021.708472
26. Jandhyala, SM, Talukdar, R, Subramanyam, C, Vuyyuru, H, Sasikala, M, and Nageshwar Reddy, D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
28. Yao, Y, Cai, X, Chen, C, Fang, H, Zhao, Y, Fei, W, et al. The role of microbiomes in pregnant women and offspring: research Progress of recent years. Front Pharmacol. (2020) 11:643. doi: 10.3389/fphar.2020.00643
29. Dominguez-Bello, MG, Costello, EK, Contreras, M, Magris, M, Hidalgo, G, Fierer, N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–5. doi: 10.1073/pnas.1002601107
30. Chu, DM, Ma, J, Prince, AL, Antony, KM, Seferovic, MD, and Aagaard, KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. (2017) 23:314–26. doi: 10.1038/nm.4272
31. Shao, Y, Forster, SC, Tsaliki, E, Vervier, K, Strang, A, Simpson, N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574:117–21. doi: 10.1038/s41586-019-1560-1
32. Selma-Royo, M, Calatayud Arroyo, M, García-Mantrana, I, Parra-Llorca, A, Escuriet, R, Martínez-Costa, C, et al. Perinatal environment shapes microbiota colonization and infant growth: impact on host response and intestinal function. Microbiome. (2020) 8:167. doi: 10.1186/s40168-020-00940-8
33. Lyons, KE, Ryan, CA, Dempsey, EM, Ross, RP, and Stanton, C. Breast Milk, a source of beneficial microbes and associated benefits for infant health. Nutrients. (2020) 12:1039. doi: 10.3390/nu12041039
34. Van den Elsen, LWJ, Garssen, J, Burcelin, R, and Verhasselt, V. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front Pediatr. (2019) 7:47. doi: 10.3389/fped.2019.00047
35. al Nabhani, Z, Dulauroy, S, Marques, R, Cousu, C, al Bounny, S, Déjardin, F, et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity. (2019) 50:1276–1288.e5. doi: 10.1016/j.immuni.2019.02.014
36. Miller, D, and Iovieno, A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. (2009) 9:466–70. doi: 10.1097/ACI.0b013e3283303e1b
37. Petrillo, F, Pignataro, D, Lavano, MA, Santella, B, Folliero, V, Zannella, C, et al. Current evidence on the ocular surface microbiota and related diseases. Microorganisms. (2020) 8:1033. doi: 10.3390/microorganisms8071033
38. Leeming, ER, Johnson, AJ, Spector, TD, and le Roy, CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. (2019) 11:2862. doi: 10.3390/nu11122862
39. Kurilshikov, A, Medina-Gomez, C, Bacigalupe, R, Radjabzadeh, D, Wang, J, Demirkan, A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. (2021) 53:156–65. doi: 10.1038/s41588-020-00763-1
40. Miro-Blanch, J, and Yanes, O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front Genet. (2019) 10:638. doi: 10.3389/fgene.2019.00638
41. Belkaid, Y, and Hand, TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
42. Ottman, N, Smidt, H, de Vos, WM, and Belzer, C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. (2012) 2:104. doi: 10.3389/fcimb.2012.00104
43. Nakamura, YK, Metea, C, Karstens, L, Asquith, M, Gruner, H, Moscibrocki, C, et al. Gut microbial alterations associated with protection from autoimmune uveitis. Invest Ophthalmol Vis Sci. (2016) 57:3747–58. doi: 10.1167/iovs.16-19733
44. Reid, G, Gadir, AA, and Dhir, R. Probiotics: reiterating what they are and what they are not. Front Microbiol. (2019) 10:424. doi: 10.3389/fmicb.2019.00424
45. Wang, ZB, Xin, SS, Ding, LN, Ding, WY, Hou, YL, Liu, CQ, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and Meta-analysis. Evid Based Complement Alternat Med. (2019) 2019:3862971. doi: 10.1155/2019/3862971
46. Beserra, BT, Fernandes, R, do Rosario, VA, Mocellin, MC, Kuntz, MGF, and Trindade, EBSM. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr. (2015) 34:845–58. doi: 10.1016/j.clnu.2014.10.004
47. Ayichew, T, Belete, A, Alebachew, T, Tsehaye, H, Berhanu, H, and Minwuyelet, A. Bacterial probiotics their importances and limitations: a review. J Nutr Health Sci. (2017) 4:202.
48. Hui, W, Li, T, Liu, W, Zhou, C, and Gao, F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: an updated randomized controlled trial meta-analysis. PLoS One. (2019) 14:e0210016. doi: 10.1371/journal.pone.0210016
49. Choi, HH, and Cho, YS. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. (2016) 49:257–65. doi: 10.5946/ce.2015.117
50. Riaz Rajoka M.S.Shi, J, Mehwish, HM, Zhu, J, Li, Q, Shao, D, et al. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci Human Wellness. (2017) 6:121–30. doi: 10.1016/j.fshw.2017.07.003
51. Finlay, BB, Amato, KR, Azad, M, Blaser, MJ, Bosch, TC, Chu, H, et al. Correction for Finlay et al., the hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc Natl Acad Sci U S A. (2021) 118:118. doi: 10.1073/pnas.2102333118
52. McCall, LI, Callewaert, C, Zhu, Q, Song, SJ, Bouslimani, A, Minich, JJ, et al. Home chemical and microbial transitions across urbanization. Nat Microbiol. (2020) 5:108–15. doi: 10.1038/s41564-019-0593-4
53. Ley, RE, Turnbaugh, PJ, Klein, S, and Gordon, JI. Human gut microbes associated with obesity. Nature. (2006) 444:1022–3. doi: 10.1038/4441022a
54. Remely, M, Tesar, I, Hippe, B, Gnauer, S, Rust, P, and Haslberger, AG. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes. (2015) 6:431–9. doi: 10.3920/BM2014.0104
55. Jackson, MA, Verdi, S, Maxan, ME, Shin, CM, Zierer, J, Bowyer, RCE, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. (2018) 9:2655. doi: 10.1038/s41467-018-05184-7
56. Scuderi, G, Troiani, E, and Minnella, AM. Gut microbiome in retina health: the crucial role of the gut-retina Axis. Front Microbiol. (2021) 12:726792. doi: 10.3389/fmicb.2021.726792
57. Vavricka, SR, Schoepfer, A, Scharl, M, Lakatos, PL, Navarini, A, and Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:1982–92. doi: 10.1097/MIB.0000000000000392
58. Camilleri, M . Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. (2019) 68:1516–26. doi: 10.1136/gutjnl-2019-318427
59. Hu, ML, Quinn, J, and Xue, K. Interactions between apolipoprotein E metabolism and retinal inflammation in age-related macular degeneration. Life (Basel). (2021) 11:635. doi: 10.3390/life11070635
60. Cunningham, MA, Tarantola, RM, Folk, JC, Sohn, EH, Boldt, HC, Graff, JA, et al. Proliferative vitreoretinopathy may be a risk factor in combined macular hole retinal detachment cases. Retina. (2013) 33:579–85. doi: 10.1097/IAE.0b013e31826b0c41
61. Yang, X, Yu, XW, Zhang, DD, and Fan, ZG. Blood-retinal barrier as a converging pivot in understanding the initiation and development of retinal diseases. Chin Med J. (2020) 133:2586–94. doi: 10.1097/CM9.0000000000001015
62. Campbell, M, and Humphries, P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. (2012) 763:70–84.
63. Occhiutto, ML, Freitas, FR, Maranhao, RC, and Costa, VP. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics. (2012) 4:252–75. doi: 10.3390/pharmaceutics4020252
64. Taylor, AW . Ocular immunosuppressive microenvironment. Chem Immunol Allergy. (2007) 92:71–85. doi: 10.1159/000099255
65. Wen, X, Hu, X, Miao, L, Ge, X, Deng, Y, Bible, PW, et al. Epigenetics, microbiota, and intraocular inflammation: new paradigms of immune regulation in the eye. Prog Retin Eye Res. (2018) 64:84–95. doi: 10.1016/j.preteyeres.2018.01.001
66. Frank, J, Gupta, A, Osadchiy, V, and Mayer, EA. Brain-gut-microbiome interactions and intermittent fasting in obesity. Nutrients. (2021) 13:584. doi: 10.3390/nu13020584
67. Floyd, JL, and Grant, MB. The gut-eye Axis: lessons learned from murine models. Ophthalmol Ther. (2020) 9:499–513. doi: 10.1007/s40123-020-00278-2
68. Bu, Y, Chan, YK, Wong, HL, Poon, SHL, Lo, ACY, Shih, KC, et al. A review of the impact of alterations in gut microbiome on the Immunopathogenesis of ocular diseases. J Clin Med. (2021) 10:694. doi: 10.3390/jcm10204694
69. Kim, J, Choi, S, Kim, Y, Jeong, H, Ryu, J, Lee, H, et al. Clinical effect of IRT-5 probiotics on immune modulation of autoimmunity or Alloimmunity in the eye. Nutrients. (2017) 9:11166. doi: 10.3390/nu9111166
70. Chen, J, Chen, DF, and Cho, KS. The role of gut microbiota in Glaucoma progression and other retinal diseases. Am J Pathol. (2023) 193:1662–8. doi: 10.1016/j.ajpath.2023.06.015
71. Chen, S, Wang, Y, Liu, Y, Li, F, Chen, Y, Fang, X, et al. Dysbiosis of gut microbiome contributes to glaucoma pathogenesis. Med Comm Future Med. (2022) 1:e28. doi: 10.1002/mef2.28
72. Xiao, J, Zhang, JY, Luo, W, He, PC, and Skondra, D. The emerging role of gut microbiota in age-related macular degeneration. Am J Pathol. (2023) 193:1627–37. doi: 10.1016/j.ajpath.2023.04.006
73. Janetos, TM, Zakaria, N, and Goldstein, DA. The microbiome and uveitis: a narrative review. Am J Pathol. (2023) 193:1638–47. doi: 10.1016/j.ajpath.2023.03.004
74. Douglas, VP, Douglas, KAA, and Iannaccone, A. Microbiome and inherited retinal degenerations. Am J Pathol. (2023) 193:1669–74. doi: 10.1016/j.ajpath.2023.03.005
75. Lincke, JB, Christe, L, Unterlauft, JD, Zinkernagel, MS, and Zysset-Burri, DC. Microbiome and retinal vascular diseases. Am J Pathol. (2023) 193:1675–82. doi: 10.1016/j.ajpath.2023.02.017
76. Zhang, JY, Greenwald, MJ, and Rodriguez, SH. Gut microbiome and retinopathy of prematurity. Am J Pathol. (2023) 193:1683–90. doi: 10.1016/j.ajpath.2023.01.013
77. Skondra, D, Rodriguez, SH, Sharma, A, Gilbert, J, Andrews, B, and Claud, EC. The early gut microbiome could protect against severe retinopathy of prematurity. J AAPOS. (2020) 24:236–8. doi: 10.1016/j.jaapos.2020.03.010
78. Zilliox, MJ, and Bouchard, CS. The microbiome, ocular surface, and corneal disorders. Am J Pathol. (2023) 193:1648–61. doi: 10.1016/j.ajpath.2023.05.004
79. Rowan, S, Jiang, S, Korem, T, Szymanski, J, Chang, ML, Szelog, J, et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA. (2017) 114:E4472–81. doi: 10.1073/pnas.1702302114
80. Ye, Z, Wu, C, Zhang, N, Du, L, Cao, Q, Huang, X, et al. Altered gut microbiome composition in patients with Vogt-Koyanagi-Harada disease. Gut Microbes. (2020) 11:539–55. doi: 10.1080/19490976.2019.1700754
81. Consolandi, C, Turroni, S, Emmi, G, Severgnini, M, Fiori, J, Peano, C, et al. Behcet's syndrome patients exhibit specific microbiome signature. Autoimmun Rev. (2015) 14:269–76. doi: 10.1016/j.autrev.2014.11.009
82. Den Besten, G, Van Eunen, K, Groen, AK, Venema, K, Reijngoud, DJ, and Bakker, BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
83. Chiang, JY . Bile acid metabolism and signaling. Compr Physiol. (2013) 3:1191–212. doi: 10.1002/cphy.c120023
84. Shapiro, H, Kolodziejczyk, AA, Halstuch, D, and Elinav, E. Bile acids in glucose metabolism in health and disease. J Exp Med. (2018) 215:383–96. doi: 10.1084/jem.20171965
85. Insull, W . Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. (2006) 99:257–73. doi: 10.1097/01.smj.0000208120.73327.db
86. Visekruna, A, and Luu, M. The role of short-chain fatty acids and bile acids in intestinal and liver function, inflammation, and carcinogenesis. Front Cell Dev Biol. (2021) 9:703218. doi: 10.3389/fcell.2021.703218
87. Gill, PA, van Zelm, MC, Muir, JG, and Gibson, PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. (2018) 48:15–34. doi: 10.1111/apt.14689
88. Ruppin, H, Bar-Meir, S, Soergel, KH, Wood, CM, and Schmitt, MG Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology. (1980) 78:1500–7. doi: 10.1016/S0016-5085(19)30508-6
89. Atarashi, K, Tanoue, T, Oshima, K, Suda, W, Nagano, Y, Nishikawa, H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. (2013) 500:232–6. doi: 10.1038/nature12331
90. Smith, PM, Howitt, MR, Panikov, N, Michaud, M, Gallini, CA, Bohlooly-Y, M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. (2013) 341:569–73. doi: 10.1126/science.1241165
91. Chen, N, Wu, J, Wang, J, Piri, N, Chen, F, Xiao, T, et al. Short chain fatty acids inhibit endotoxin-induced uveitis and inflammatory responses of retinal astrocytes. Exp Eye Res. (2021) 206:108520. doi: 10.1016/j.exer.2021.108520
92. Nakamura, YK, Janowitz, C, Metea, C, Asquith, M, Karstens, L, Rosenbaum, JT, et al. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci Rep. (2017) 7:11745. doi: 10.1038/s41598-017-12163-3
93. Fernandes, RVS, Nunes, S, and Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol basis Dis. (2019) 1865:1876–97. doi: 10.1016/j.bbadis.2018.09.032
94. Grant, MB, Bernstein, PS, Boesze-Battaglia, K, Chew, E, Curcio, CA, Kenney, MC, et al. Inside out: relations between the microbiome, nutrition, and eye health. Exp Eye Res. (2022) 2022:216. doi: 10.1016/j.exer.2022.109216
95. Cook, SI, and Sellin, JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. (1998) 12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x
96. Bui, T, Oxenrider, A, Amaoo, R, Martin, PM, Thounaojam, M, and Jadeja, R. Short-chain fatty acid signaling in retinopathy of prematurity. Invest Ophthalmol Vis Sci. (2023) 64:575.
97. Huang, Y, Wang, Z, Ye, B, MA, JH, Ji, S, Sheng, W, et al. Sodium butyrate ameliorates diabetic retinopathy in mice via the regulation of gut microbiota and related short-chain fatty acids. J Transl Med. (2023) 21:451. doi: 10.1186/s12967-023-04259-4
98. Reis, JSD, Dos Reis Teixeira, A, De Vasconcelos Quaresma, A, Almeida, TC, Arribada, RG, Neto, JT, et al. Sodium butyrate-loaded nanoparticles coated with chitosan for the treatment of neovascularization in age-related macular degeneration: ocular biocompatibility and antiangiogenic activity. Eur J Pharm Biopharm. (2022) 179:26–36. doi: 10.1016/j.ejpb.2022.08.011
99. Xiao, X, Chen, M, Xu, Y, Huang, S, Liang, J, Cao, Y, et al. Sodium butyrate inhibits neovascularization partially via TNXIP/VEGFR2 pathway. Oxidative Med Cell Longev. (2020) 2020:6415671. doi: 10.1155/2020/6415671
100. Schaefer, L, Hernandez, H, Coats, RA, Yu, Z, Pflugfelder, SC, Britton, RA, et al. Author correction: gut-derived butyrate suppresses ocular surface inflammation. Sci Rep. (2022) 12:6581. doi: 10.1038/s41598-022-10856-y
101. Singh, S, Singh, PK, and Kumar, A. Butyrate ameliorates intraocular bacterial infection by promoting autophagy and attenuating the inflammatory response. Infect Immun. (2023) 91:e0025222. doi: 10.1128/iai.00252-22
102. Krix-Jachym, K, Onyszkiewicz, M, Swiatkiewicz, M, Fiedorowicz, M, Sapierzynski, R, Grieb, P, et al. Evaluation of butyric acid as a potential supportive treatment in anterior uveitis. Ophthalmol J. (2022) 7:117–26. doi: 10.5603/OJ.2022.0016
103. Zhang, T, Baehr, W, and Fu, Y. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. (2012) 53:3349–56. doi: 10.1167/iovs.12-9851
104. Fu, Y, and Zhang, T. Pathophysilogical mechanism and treatment strategies for Leber congenital amaurosis. Adv Exp Med Biol. (2014) 801:791–6. doi: 10.1007/978-1-4614-3209-8_99
105. Mantopoulos, D, Murakami, Y, Comander, J, Thanos, A, Roh, M, Miller, JW, et al. Tauroursodeoxycholic acid (TUDCA) protects photoreceptors from cell death after experimental retinal detachment. PLoS One. (2011) 6:e24245. doi: 10.1371/journal.pone.0024245
106. Oveson, BC, Iwase, T, Hackett, SF, Lee, SY, Usui, S, Sedlak, TW, et al. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J Neurochem. (2011) 116:144–53. doi: 10.1111/j.1471-4159.2010.07092.x
107. Phillips, MJ, Walker, TA, Choi, HY, Faulkner, AE, Kim, MK, Sidney, SS, et al. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol Vis Sci. (2008) 49:2148–55. doi: 10.1167/iovs.07-1012
108. Zhang, X, Shahani, U, Reilly, J, and Shu, X. Disease mechanisms and neuroprotection by tauroursodeoxycholic acid in Rpgr knockout mice. J Cell Physiol. (2019) 234:18801–12. doi: 10.1002/jcp.28519
109. Tao, Y, Dong, X, Lu, X, Qu, Y, Wang, C, Peng, G, et al. Subcutaneous delivery of tauroursodeoxycholic acid rescues the cone photoreceptors in degenerative retina: a promising therapeutic molecule for retinopathy. Biomed Pharmacother. (2019) 117:109021. doi: 10.1016/j.biopha.2019.109021
110. Chung, YR, Choi, JA, Koh, JY, and Yoon, YH. Ursodeoxycholic acid attenuates endoplasmic reticulum stress-related retinal Pericyte loss in Streptozotocin-induced diabetic mice. J Diabetes Res. (2017) 2017:1763292. doi: 10.1155/2017/1763292
111. Zhu, L, Wang, W, Xie, TH, Zou, J, Nie, X, Wang, X, et al. TGR5 receptor activation attenuates diabetic retinopathy through suppression of RhoA/ROCK signaling. FASEB J. (2020) 34:4189–203. doi: 10.1096/fj.201902496RR
112. Woo, SJ, Kim, JH, and Yu, HG. Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model. J Ocul Pharmacol Ther. (2010) 26:223–9. doi: 10.1089/jop.2010.0012
113. Fernández-Sánchez, L, Bravo-Osuna, I, Lax, P, Arranz-Romera, A, Maneu, V, Esteban-Pérez, S, et al. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS One. (2017) 12:e0177998. doi: 10.1371/journal.pone.0177998
114. Fernández-Sánchez, L, Lax, P, Pinilla, I, Martín-Nieto, J, and Cuenca, N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis Sci. (2011) 52:4998–5008. doi: 10.1167/iovs.11-7496
115. Boatright, JH, Moring, AG, McElroy, C, Phillips, MJ, Do, VT, Chang, B, et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. (2006) 12:1706–14.
116. Kitamura, Y, et al. In vivo effects of single or combined topical neuroprotective and regenerative agents on degeneration of retinal ganglion cells in rat optic nerve crush model. Sci Rep. (2019) 9:101. doi: 10.1038/s41598-018-36473-2
117. Fu, J, Aung, MH, Prunty, MC, Hanif, AM, Hutson, LM, Boatright, JH, et al. Tauroursodeoxycholic acid protects retinal and visual function in a mouse model of type 1 diabetes. Pharmaceutics. (2021) 13:1154. doi: 10.3390/pharmaceutics13081154
118. Ouyang, H, Mei, X, Zhang, T, Lu, B, and Ji, L. Ursodeoxycholic acid ameliorates diabetic retinopathy via reducing retinal inflammation and reversing the breakdown of blood-retinal barrier. Eur J Pharmacol. (2018) 840:20–7. doi: 10.1016/j.ejphar.2018.09.027
119. Shiraya, T, Araki, F, Ueta, T, Fukunaga, H, Totsuka, K, Arai, T, et al. Ursodeoxycholic acid attenuates the retinal vascular abnormalities in anti-PDGFR-β antibody-induced Pericyte depletion mouse models. Sci Rep. (2020) 10:977. doi: 10.1038/s41598-020-58039-x
120. Maharjan, P, Kim, D, Jin, M, Ko, HJ, Song, YH, Lee, Y, et al. Preclinical evaluation of UDCA-containing Oral formulation in mice for the treatment of wet age-related macular degeneration. Pharmaceutics. (2019) 11:561. doi: 10.3390/pharmaceutics11110561
121. Thounaojam, MC, Jadeja, RN, Rajpurohit, S, Gutsaeva, DR, Stansfield, BK, Martin, PM, et al. Ursodeoxycholic acid halts pathological neovascularization in a mouse model of oxygen-induced retinopathy. J Clin Med. (2020) 9:1921. doi: 10.3390/jcm9061921
122. Mulhern, ML, Madson, CJ, Kador, PF, Randazzo, J, and Shinohara, T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol Vis. (2007) 13:1397–405.
123. Drack, AV, Dumitrescu, AV, Bhattarai, S, Gratie, D, Stone, EM, Mullins, R, et al. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci. (2012) 53:100–6. doi: 10.1167/iovs.11-8544
124. Lawson, EC, Bhatia, SK, Han, MK, Aung, MH, Ciavatta, V, Boatright, JH, et al. Tauroursodeoxycholic acid protects retinal function and structure in rd1 mice. Adv Exp Med Biol. (2016) 854:431–6. doi: 10.1007/978-3-319-17121-0_57
125. Gómez-Vicente, V, Lax, P, Fernández-Sánchez, L, Rondón, N, Esquiva, G, Germain, F, et al. Neuroprotective effect of Tauroursodeoxycholic acid on N-methyl-D-aspartate-induced retinal ganglion cell degeneration. PLoS One. (2015) 10:e0137826. doi: 10.1371/journal.pone.0137826
126. Huang, Y, Lin, L, Yang, Y, Duan, F, Yuan, M, Lou, B, et al. Effect of Tauroursodeoxycholic acid on inflammation after ocular alkali burn. Int J Mol Sci. (2022) 23:1717. doi: 10.3390/ijms231911717
127. Gaudier, E, Jarry, A, Blottière, HM, de Coppet, P, Buisine, MP, Aubert, JP, et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. (2004) 287:G1168–74. doi: 10.1152/ajpgi.00219.2004
128. Furusawa, Y, Obata, Y, Fukuda, S, Endo, TA, Nakato, G, Takahashi, D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
129. Prasad, R, Adu-Agyeiwaah, Y, Floyd, JL, Asare-Bediako, B, Li Calzi, S, Chakraborty, D, et al. Sustained ACE2 expression by probiotic improves integrity of intestinal lymphatics and retinopathy in type 1 diabetic model. J Clin Med. (2023) 12:1771. doi: 10.3390/jcm12051771
130. Sidhu, G, and Tripp, J. Fenofibrate, in the comprehensive pharmacology reference. Treasure Island, FL: StatPearls Publishing (2023).
131. Wang, X, Yu, C, Liu, X, Yang, J, Feng, Y, Wu, Y, et al. Fenofibrate ameliorated systemic and retinal inflammation and modulated gut microbiota in high-fat diet-induced mice. Front Cell Infect Microbiol. (2022) 12:839592. doi: 10.3389/fcimb.2022.839592
132. Lin, P, McClintic, SM, Nadeem, U, and Skondra, D. A review of the role of the intestinal microbiota in age-related macular degeneration. J Clin Med. (2021) 10:2072. doi: 10.3390/jcm10102072
133. Priamukhina, NS, and Semina, NA. The differentiation of intestinal Escherichia coli infections. Zh Mikrobiol Epidemiol Immunobiol. (1991) 2:81–7.
134. Hofmann, AF . The enterohepatic circulation of bile acids in man. Clin. Gastroenterology. (1977) 6:3–24. doi: 10.1016/S0300-5089(21)00383-7
135. Mertens, KL, Kalsbeek, A, Soeters, MR, and Eggink, HM. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci. (2017) 11:617. doi: 10.3389/fnins.2017.00617
136. Voronova, V, Sokolov, V, al-Khaifi, A, Straniero, S, Kumar, C, Peskov, K, et al. A physiology-based model of bile acid distribution and metabolism under healthy and pathologic conditions in human beings. Cell Mol Gastroenterol Hepatol. (2020) 10:149–70. doi: 10.1016/j.jcmgh.2020.02.005
137. Li, T, and Chiang, JY. Bile acid signaling in liver metabolism and diseases. J Lipids. (2012) 2012:754067. doi: 10.1155/2012/754067
138. Myant, NB, and Mitropoulos, KA. Cholesterol 7 alpha-hydroxylase. J Lipid Res. (1977) 18:135–53. doi: 10.1016/S0022-2275(20)41693-1
139. Schwarz, M, Russell, DW, Dietschy, JM, and Turley, SD. Alternate pathways of bile acid synthesis in the cholesterol 7α-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res. (2001) 42:1594–603. doi: 10.1016/S0022-2275(20)32213-6
140. Wang, DQ, and Carey, MC. Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol. (2014) 20:9952–75. doi: 10.3748/wjg.v20.i29.9952
141. Li, S, Tan, HY, Wang, N, Hong, M, Li, L, Cheung, F, et al. Substitutes for bear bile for the treatment of liver diseases: research Progress and future perspective. Evid Based Complement Alternat Med. (2016) 2016:4305074. doi: 10.1155/2016/4305074
142. Feng, Y, Siu, K, Wang, N, Ng, KM, Tsao, SW, Nagamatsu, T, et al. Bear bile: dilemma of traditional medicinal use and animal protection. J Ethnobiol Ethnomed. (2009) 5:2. doi: 10.1186/1746-4269-5-2
143. Ðanić, M, Stanimirov, B, Pavlović, N, Goločorbin-Kon, S, Al-Salami, H, Stankov, K, et al. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front Pharmacol. (2018) 9:1382. doi: 10.3389/fphar.2018.01382
144. Hofmann, AF, and Hagey, LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. (2014) 55:1553–95. doi: 10.1194/jlr.R049437
145. Daruich, A, Picard, E, Boatright, JH, and Behar-Cohen, F. Review: the bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol Vis. (2019) 25:610–24.
146. Win, A, Delgado, A, Jadeja, RN, Martin, PM, Bartoli, M, and Thounaojam, MC. Pharmacological and metabolic significance of bile acids in retinal diseases. Biomol Ther. (2021) 11:292. doi: 10.3390/biom11020292
147. Lobysheva, E, Taylor, CM, Marshall, GR, and Kisselev, OG. Tauroursodeoxycholic acid binds to the G-protein site on light activated rhodopsin. Exp Eye Res. (2018) 170:51–7. doi: 10.1016/j.exer.2018.02.015
148. Beli, E, Yan, Y, Moldovan, L, Vieira, CP, Gao, R, Duan, Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. (2018) 67:1867–79. doi: 10.2337/db18-0158
149. Boatright, JH, Nickerson, JM, Moring, AG, and Pardue, MT. Bile acids in treatment of ocular disease. J Ocul Biol Dis Infor. (2009) 2:149–59. doi: 10.1007/s12177-009-9030-x
150. Gaspar, JM, Martins, A, Cruz, R, Rodrigues, CMP, Ambrósio, AF, and Santiago, AR. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience. (2013) 253:380–8. doi: 10.1016/j.neuroscience.2013.08.053
151. Fu, J, Yu, M, Xu, W, and Yu, S. Research Progress of bile acids in Cancer. Front Oncol. (2021) 11:778258. doi: 10.3389/fonc.2021.778258
152. Noailles, A, Fernández-Sánchez, L, Lax, P, and Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J Neuroinflammation. (2014) 11:186. doi: 10.1186/s12974-014-0186-3
153. Wang, CF, Yuan, JR, Qin, D, Gu, JF, Zhao, BJ, Zhang, L, et al. Protection of tauroursodeoxycholic acid on high glucose-induced human retinal microvascular endothelial cells dysfunction and streptozotocin-induced diabetic retinopathy rats. J Ethnopharmacol. (2016) 185:162–70. doi: 10.1016/j.jep.2016.03.026
154. Feng, S, Guo, L, Wang, S, Chen, L, Chang, H, Hang, B, et al. Association of Serum Bile Acid and Unsaturated Fatty Acid Profiles with the risk of diabetic retinopathy in type 2 diabetic patients. Diabetes Metab Syndr Obes. (2023) 16:2117–28. doi: 10.2147/DMSO.S411522
155. Murakami, Y, Notomi, S, Hisatomi, T, Nakazawa, T, Ishibashi, T, Miller, JW, et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res. (2013) 37:114–40. doi: 10.1016/j.preteyeres.2013.08.001
156. Thounaojam, M, Oxenrider, A, Bui, T, Jadeja, R, Martin, PM, and Bartoli, M. Altered bile acids-gut microbiome axis in oxygen-induced retinopathy: potential implications for retinopathy of prematurity. Invest Ophthalmol Vis Sci. (2022) 63:803.
157. Thounaojam, M, Oxenrider, A, Ganta, VC, Martin, PM, and Jadeja, R. Bile acid receptor signaling in oxygen oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. (2023) 64:587.
158. Xuan, Q, Ouyang, Y, Wang, Y, Wu, L, Li, H, Luo, Y, et al. Multiplatform metabolomics reveals novel serum metabolite biomarkers in diabetic retinopathy subjects. Adv Sci (Weinh). (2020) 7:2001714. doi: 10.1002/advs.202001714
159. Bloomgarden, Z . Diabetes and branched-chain amino acids: what is the link? J Diabetes. (2018) 10:350–2. doi: 10.1111/1753-0407.12645
160. Zhang, X, Xia, M, Wu, Y, and Zhang, F. Branched-chain amino acids metabolism and their roles in retinopathy: from relevance to mechanism. Nutrients. (2023) 15:161. doi: 10.3390/nu15092161
161. Bogdanov, P, Corraliza, L, Villena, JA, Carvalho, AR, Garcia-Arumí, J, Ramos, D, et al. The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One. (2014) 9:e97302. doi: 10.1371/journal.pone.0097302
162. Opere, CA, Heruye, S, Njie-Mbye, YF, Ohia, SE, and Sharif, NA. Regulation of excitatory amino acid transmission in the retina: studies on neuroprotection. J Ocul Pharmacol Ther. (2018) 34:107–18. doi: 10.1089/jop.2017.0085
163. Chen, Y, Coorey, NJ, Zhang, M, Zeng, S, Madigan, MC, Zhang, X, et al. Metabolism dysregulation in retinal diseases and related therapies. Antioxidants (Basel). (2022) 11:942. doi: 10.3390/antiox11050942
164. Xia, M, and Zhang, F. Amino acids metabolism in retinopathy: from clinical and basic research perspective. Meta. (2022) 12:244. doi: 10.3390/metabo12121244
165. Tome, D . The roles of dietary glutamate in the intestine. Ann Nutr Metab. (2018) 73:15–20. doi: 10.1159/000494777
166. Rowland, I, Gibson, G, Heinken, A, Scott, K, Swann, J, Thiele, I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. (2018) 57:1–24. doi: 10.1007/s00394-017-1445-8
167. Bang, YJ . Vitamin a: a key coordinator of host-microbe interactions in the intestine. BMB Rep. (2023) 56:133–9. doi: 10.5483/BMBRep.2023-0005
168. Yamamoto, EA, and Jorgensen, TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. (2019) 10:3141. doi: 10.3389/fimmu.2019.03141
169. Pham, VT, Dold, S, Rehman, A, Bird, JK, and Steinert, RE. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr Res. (2021) 95:35–53. doi: 10.1016/j.nutres.2021.09.001
170. Schoeler, M, Ellero-Simatos, S, Birkner, T, Mayneris-Perxachs, J, Olsson, L, Brolin, H, et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat Commun. (2023) 14:5329. doi: 10.1038/s41467-023-41074-3
171. Eynard, AR, and Repossi, G. Role of omega3 polyunsaturated fatty acids in diabetic retinopathy: a morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. (2019) 18:114. doi: 10.1186/s12944-019-1049-9
172. Bayerle-Eder, M, Polska, E, Kopf, A, Roden, M, Waldhäusl, W, Pleiner, H, et al. Free fatty acids exert a greater effect on ocular and skin blood flow than triglycerides in healthy subjects. Eur J Clin Investig. (2004) 34:519–26. doi: 10.1111/j.1365-2362.2004.01383.x
173. Georgiou, M, and Prokopiou, E. Diabetic retinopathy and the role of Omega-3 PUFAs: a narrative review. Exp Eye Res. (2023) 231:109494. doi: 10.1016/j.exer.2023.109494
174. Moon, J, Yoon, CH, Choi, SH, and Kim, MK. Can gut microbiota affect dry eye syndrome? Int J Mol Sci. (2020) 21:443. doi: 10.3390/ijms21228443
175. Mzyk, P, Hernandez, H, Le, T, Ramirez, JR, and McDowell, CM. Toll-like receptor 4 signaling in the trabecular meshwork. Front Cell Dev Biol. (2022) 10:936115. doi: 10.3389/fcell.2022.936115
176. Noailles, A, Maneu, V, Campello, L, Lax, P, and Cuenca, N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. (2018) 9:350. doi: 10.1038/s41419-018-0355-x
177. Lee, SH, Jang, DK, Yoo, MW, Hwang, SH, Ryu, SY, Kwon, OK, et al. Efficacy and safety of Ursodeoxycholic acid for the prevention of gallstone formation after gastrectomy in patients with gastric Cancer: the PEGASUS-D randomized clinical trial. JAMA Surg. (2020) 155:703–11. doi: 10.1001/jamasurg.2020.1501
Keywords: microbiome, bile acids, butyrate, ocular diseases, short chain fatty acids
Citation: Nguyen Y, Rudd Zhong Manis J, Ronczkowski NM, Bui T, Oxenrider A, Jadeja RN and Thounaojam MC (2024) Unveiling the gut-eye axis: how microbial metabolites influence ocular health and disease. Front. Med. 11:1377186. doi: 10.3389/fmed.2024.1377186
Received: 26 January 2024; Accepted: 19 April 2024;
Published: 10 May 2024.
Edited by:
Georgios D. Panos, Nottingham University Hospitals NHS Trust, United KingdomReviewed by:
Zhiyuan Pan, Grand Life Sciences Group Ltd., ChinaCopyright © 2024 Nguyen, Rudd Zhong Manis, Ronczkowski, Bui, Oxenrider, Jadeja and Thounaojam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Menaka C. Thounaojam, bXRob3VuYW9qYW1AYXVndXN0YS5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.