- 1IncellDX Inc., San Carlos, CA, United States
- 2Department of Anesthesiology, Beth Israel Lahey Health, Burlington, MA, United States
- 3Centro de Investigación en Cirugía y Cáncer (CICICA), Universidad de Costa Rica, San Jose, Costa Rica
- 4Lab of Tumor Chemosensitivity, CIET/DC Lab, Faculty of Microbiology, Universidad de Costa Rica, San Jose, Costa Rica
- 5Department of Medicine, St. Francis Medical Center, Trenton, NJ, United States
- 6Department of Emergency Medicine, Novant Health Kernersville Medical Center, Kernersville, NC, United States
- 7Department of Allergy and Immunology, NYU Langone Tisch Hospital, New York, NY, United States
- 8Department of Emergency Medicine, New York Presbyterian Hospital, Brooklyn, NY, United States
- 9Department of Anesthesiology, Stamford Hospital, Stamford, CT, United States
- 10Rutland Medical Group, Newport Beach, CA, United States
- 11Department of Community and Family Medicine, Georgetown University Medical Center, Washington, DC, United States
- 12Department of Medicine, Creighton University School of Medicine, Phoenix, AZ, United States
A corrigendum on
Case Series: Maraviroc and pravastatin as a therapeutic option to treat long COVID/Post-acute sequalae of COVID (PASC)
by Patterson, B. K., Yogendra, R., Guevara-Coto, J., Mora-Rodriguez, R. A., Osgood, E., Bream, J., Parikh, P., Kreimer, M., Jeffers, D., Rutland, C., Kaplan, G., and Zgoda, M. (2023). Front. Med. 10:1122529. doi: 10.3389/fmed.2023.1122529
In the published article there was an error in the Methodology section.
The sentence previously said:
“The records and immunological lab reports from 18 adult PASC patients treated with maraviroc 300 mg per oral twice daily and pravastatin 10 mg per oral daily from our virtual medical clinic were collected and analyzed.
The 18 participants selected for this case series were from a pool of patients who reported symptom improvement while on maraviroc and pravastatin and who fit the inclusion and exclusion criteria we set below.”
The corrected sentence appears below:
“The medical records and immunological lab reports from 18 adult PASC patients treated with maraviroc 300 mg per oral twice daily and pravastatin 10 mg per oral daily by independent private practice physicians and clinics were collected and analyzed. The CCTC is a virtual consultation group that works in collaboration with these physicians to collect and analyze immunological data. The 18 patients selected for this case series were from a pool of patients who reported symptom improvement while on maraviroc and pravastatin and who fit the inclusion and exclusion criteria we set below:
5 patients were previously treated with ivermectin, 2 with fluvoxamine, and 1 with prednisone.”
In the original manuscript there was an error in the Results section, the Y axis in Figures 2, 3 lacked metric units and the data plots were too clustered for visibility.
In Figure 2, we created absolute difference for before and after cytokine measurements for each of the 14 cytokines since the original graph was too clustered and hard to interpret. In the corrected version, we also added the y-axis legend (in pg/ml) for clarity.
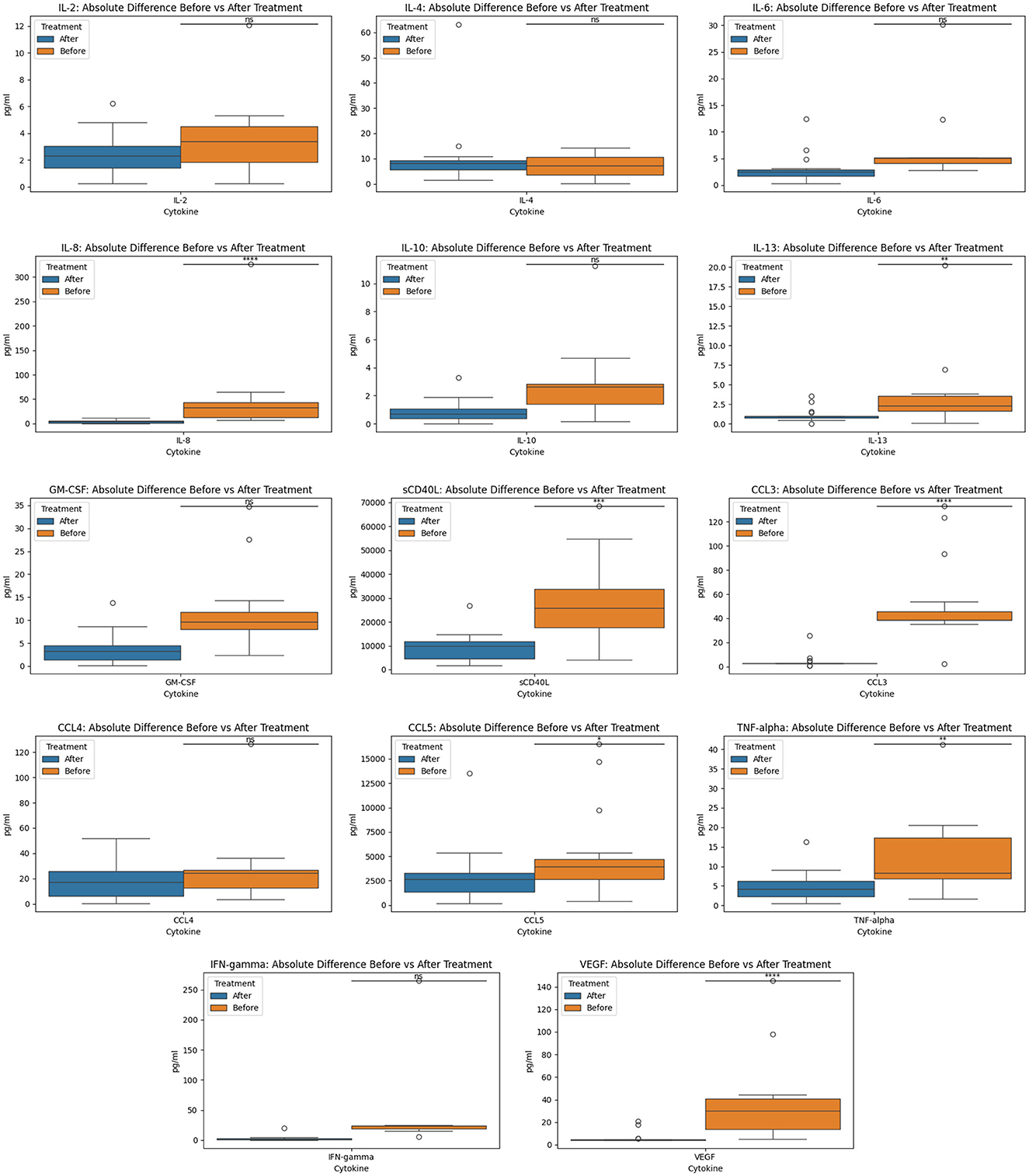
Figure 2. Before and after treatment individual cytokine measurement comparisons. The box plot represent the statistical comparison using the Wilcoxon paired test between the two treatment groups (before and after).
In Figure 3, we added box plots and added the y-axis legend for clarity. The following revised Figures are below. We renamed the Y-axis to “Subjective Score Values” and the X-axis to “Subjective Scores.”
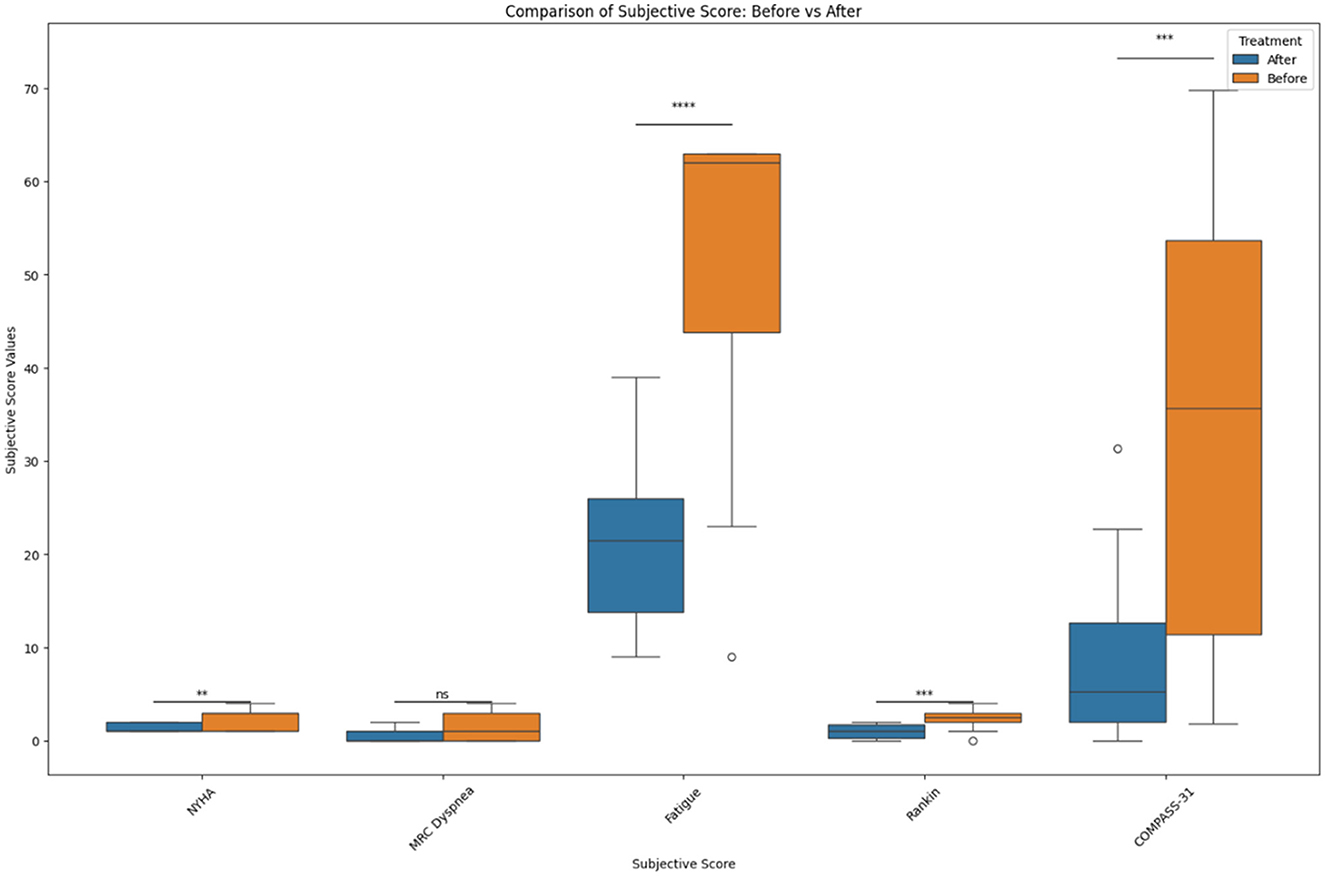
Figure 3. Before and after treatment individual subjective score comparisons. The box plot represents the statistical comparison using the Wilcoxon paired test between the two treatment groups (before and after). Statistical significance intervals are represented with asterisks (*), where ns indicates non-significant. *1.00e-02 < p ≤ 5.00e-02, **1.00e-03 < p ≤ 1.00e-02, ***1.00e-04 < p ≤ 1.00e-03, and ****p ≤ 1.00e-04.
In the published article, there was an error in the Ethics statement. The sentence previously stated:
“The studies involving human participants were reviewed and approved by CCTC Ethics and IRB Committee. The patients/participants provided their written informed consent to participate in this study.”
The corrected sentence appears below:
Ethics statement
The studies involving human participants were reviewed and approved by the CCTC IRB committee. The CCTC IRB is financially and operationally independent of the CCTC. No one from this IRB has any financial or research stake in IncellDX or CCTC. This IRB was chosen for cost reasons as this study was self-funded with limited resources. The study protocol was approved by this IRB because it was only designed to collect and analyze patient data from independent physician practices who were responsible for prescribing and monitoring the medications. The CCTC did not and does not prescribe or monitor any medications and has been set up only as a data analytics practice.
In the published article, there was an error in the Funding statement. We did not state that there was no funding received for the study. The correct Funding statement appears below.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
In the published article, there was an error in the Conflict of interest. The original conflict of interest statement said:
IncellDX holds the patent for the use of CCR5 antagonists (maraviroc) in COVID and long COVID. was employed by IncellDX Inc. BP, RY, PP, JB, EO, DJ, CR, and MK were independent contractors of the CCTC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The corrected sentence appears below:
Conflict of interest
IncellDX holds the patent for the use of CCR5 antagonists (maraviroc) in COVID and long COVID. BP and the founder and CEO of IncellDX and a physician partner of the Chronic COVID Treatment Center (CCTC). The CCTC is a data analytics consulting physician practice that is financially independent from IncellDX. Except for BP, no one from the CCTC has received nor holds any financial or equity compensation from IncellDX. The CCTC IRB is financially independent of IncellDX and CCTC and no one from the IRB has received any financial or equity compensation from IncellDX or CCTC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: long COVID, maraviroc, CCR5 antagonist, PASC, statins, fractalkine (CX3CR1)
Citation: Patterson BK, Yogendra R, Guevara-Coto J, Mora-Rodriguez RA, Osgood E, Bream J, Parikh P, Kreimer M, Jeffers D, Rutland C, Kaplan G and Zgoda M (2024) Corrigendum: Case Series: Maraviroc and pravastatin as a therapeutic option to treat long COVID/Post-acute sequalae of COVID (PASC). Front. Med. 11:1375607. doi: 10.3389/fmed.2024.1375607
Received: 24 January 2024; Accepted: 24 April 2024;
Published: 15 May 2024.
Edited and reviewed by: Marc Jean Struelens, Université libre de Bruxelles, Belgium
Copyright © 2024 Patterson, Yogendra, Guevara-Coto, Mora-Rodriguez, Osgood, Bream, Parikh, Kreimer, Jeffers, Rutland, Kaplan and Zgoda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ram Yogendra, cnlvZ2VuZHJhbWRAZ21haWwuY29t
†These authors share senior authorship
 Bruce K. Patterson
Bruce K. Patterson Ram Yogendra2*
Ram Yogendra2* Jose Guevara-Coto
Jose Guevara-Coto Rodrigo A. Mora-Rodriguez
Rodrigo A. Mora-Rodriguez Eric Osgood
Eric Osgood Mark Kreimer
Mark Kreimer