- 1Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Chonnam National University Hwasun Hospital, Gwangju, Republic of Korea
- 2Chonnam National University Hospital, Gwangju, Republic of Korea
Background: After the COVID-19 outbreak, interest in airborne virus infections has increased. We considered ways to reduce the risk of infection to other people by inactivating the virus before it is inhaled into the heating, ventilation, and air conditioning (HVAC) systems. We installed a recently developed air sterilizer in the newly remodeled outpatient clinic of a tertiary university hospital and confirmed its effectiveness.
Methods: After remodeling the ENT outpatient clinic at Chonnam National University Hospital, 15 KOKKOS air sterilizers (Bentech Frontier Co., Ltd., Gwangju, Korea) were installed. Total culturable microorganisms (TCMs) and volatile organic compounds (VOCs) were measured in five separate inspection areas three days before installation, 2 weeks after installation, and 4 weeks after installation.
Results: After measurement of TCMs, improvement in air quality occurred 2 weeks after air sterilizer instatement at all timepoints except inspection area 5, and further improvement was achieved after 4 weeks (p < 0.05). After assessment of VOCs, improvement occurred 4 weeks after air sterilizer connection at all points (p < 0.05).
Conclusion: KOKKOS air sterilizers are effective in improving air quality in an outpatient clinic at a tertiary university hospital.
Introduction
After the COVID-19 outbreak, interest in airborne virus infections has increased (1–8). Natural ventilation is difficult in hospitals due to patient overcrowding, and mechanical sources have been applied to assist in ventilation (1–8). Therefore, heating, ventilation, and air conditioning (HVAC) systems are beginning to be studied more deeply to help eliminate airborne viruses, fungi, and bacteria (1–3, 7, 8).
However, when the Middle East Respiratory Syndrome (MERS) spread, infection within buildings through HVAC systems was confirmed (9). Therefore, the authors considered ways to reduce the risk of infection to other people by inactivating the virus before it is inhaled into the HVAC system (2, 3). We installed a recently developed air sterilizer
in the newly remodeled outpatient clinic of a tertiary university hospital and confirmed its effectiveness.
Materials and methods
This study was conducted with the approval of the Institutional Review Board of Chonnam National University Hwasun Hospital. After remodeling the ENT outpatient clinic at Chonnam National University Hospital, 15 KOKKOS air sterilizers (Bentech Frontier Co., Ltd., Gwangju, Korea) were installed based on the clean air delivery rate (CADR) value (Figure 1). KOKKOS air sterilizers is equipped with a VORTEX function on the front air intake part to enhance indoor air intake with a suction flow speed of up to 4 m/s or more. It was independently fixed to a wall 1.5 m above the ground in order to absorb the air released when a person breathes indoors as quickly as possible.
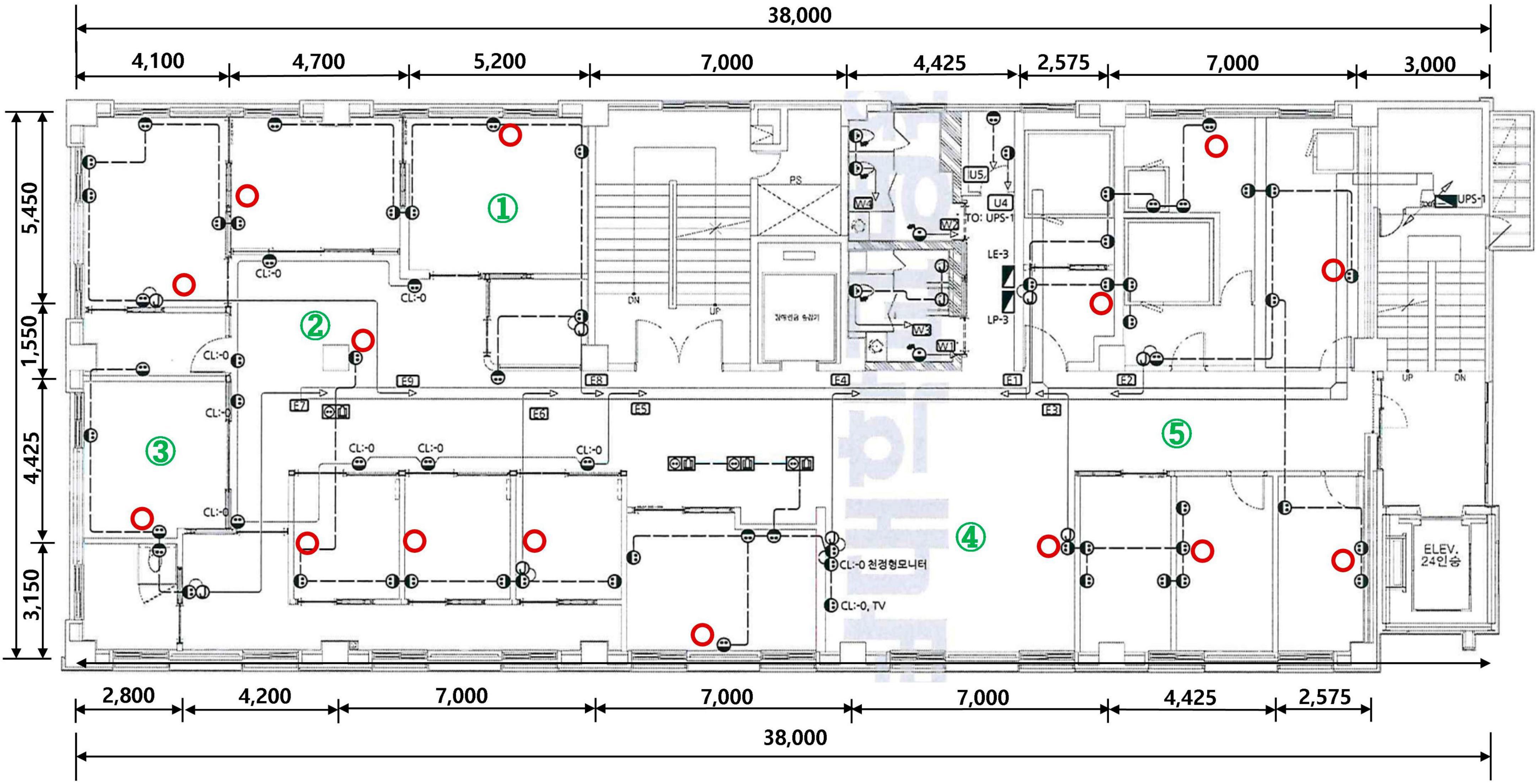
Figure 1. KOKKOS (Bentech Frontier Co., Ltd., Gwangju, Korea) air sterilizer installation location (red circle) and air quality measurement stations (numbers in green circle).
KOKKOS air sterilizers contains two filters, and the HEPA Filter at the front primarily filters PM1.0-size fine dust, bacteria, and viruses, and the sterilization filter at the rear plays a major role in inactivating viruses by generating OH-radicals. KOKKOS sterilization filters were also applied to rapidly and completely eliminate harmful substances such as volatile organic compounds (VOCs), bad smells, harmful gases, bacteria, and viruses without discharging them. Additionally, the KOKKOS filter also included a HEPA filter to remove fine particulate matter (Figure 2). In recognition of its excellent infection prevention, KOKKOS was designated as one of Top 100 National R&D Technologies with Outstanding Performance in 2020.
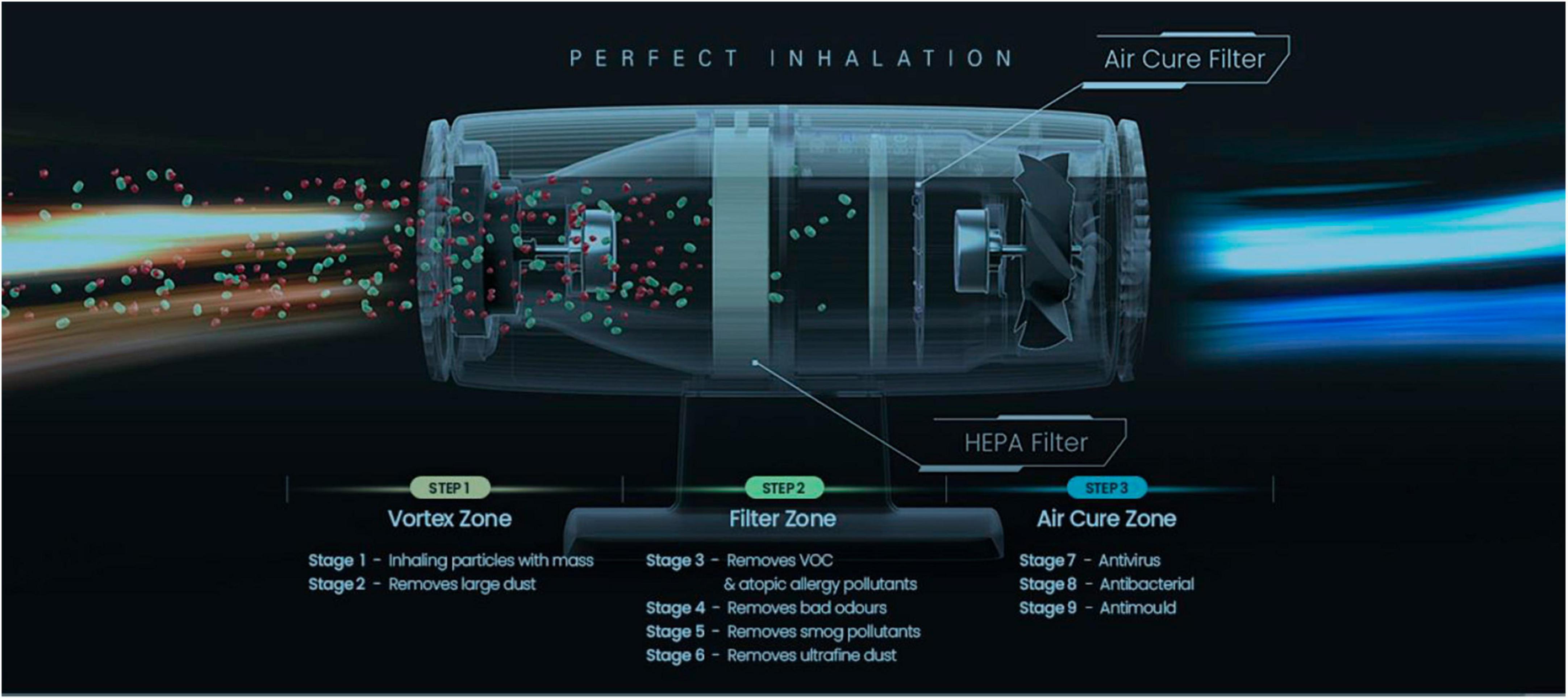
Figure 2. Description of internal functionality of KOKKOS (Bentech Frontier Co., Ltd., Gwangju, Korea).
To analyze the impact of KOKKOS on the indoor air quality of the newly remodeled ENT outpatient clinic, total culturable microorganisms (TCMs) and VOCs were measured three days before installation, 2 weeks after installation, and 4 weeks after installation of the air sterilizers. Because the ENT outpatient clinic is divided into several structures, including a treatment room, waiting room, and examination room, we conducted air quality tests in five different locations to ensure the accuracy of the test (Figure 1). TCMs was measured with KAS-110 (1CFM, 28.3 L/m) equipment using the “Indoor Air Quality Test Method” (10). After installing the bacteria and fungi culture medium on the equipment, TCMs was measured three times at each of five designated locations. VOCs were measured with GC Mass equipment using the “Indoor Air Quality Test Method” (10). VOCs were measured three times at each of the five designated locations.
All statistical calculations were performed using SPSS version 28.0. Concentration differences and measurement timing as dependent variables were examined by the Friedman test and the Wilcoxon signed rank test. In all analyses, p < 0.05 was considered statistically significant.
Results
The results of TCMs and VOCs conducted three days before installation, 2 weeks after installation, and 4 weeks after installation are summarized in Tables 1, 2.
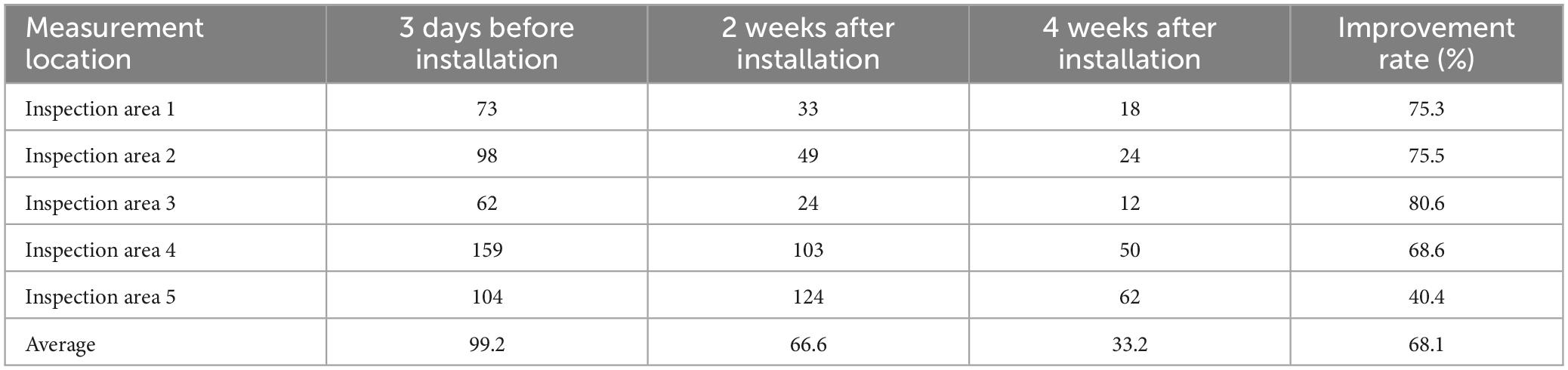
Table 1. Total culturable microorganism concentrations (CFU/m3) before and after air sterilizer installation.
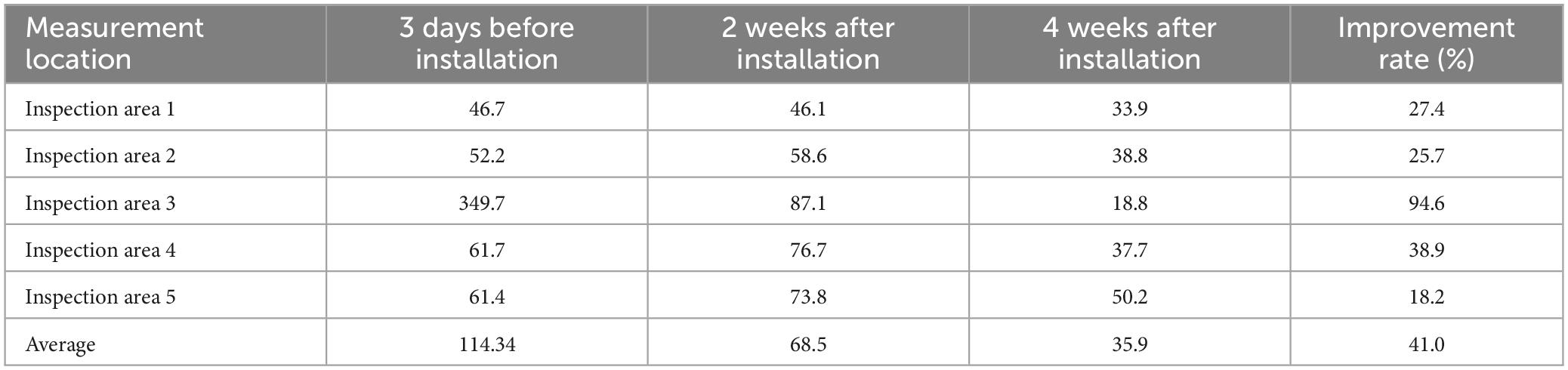
Table 2. Total volatile organic compound concentrations (μg/m3) before and after air sterilizer installation.
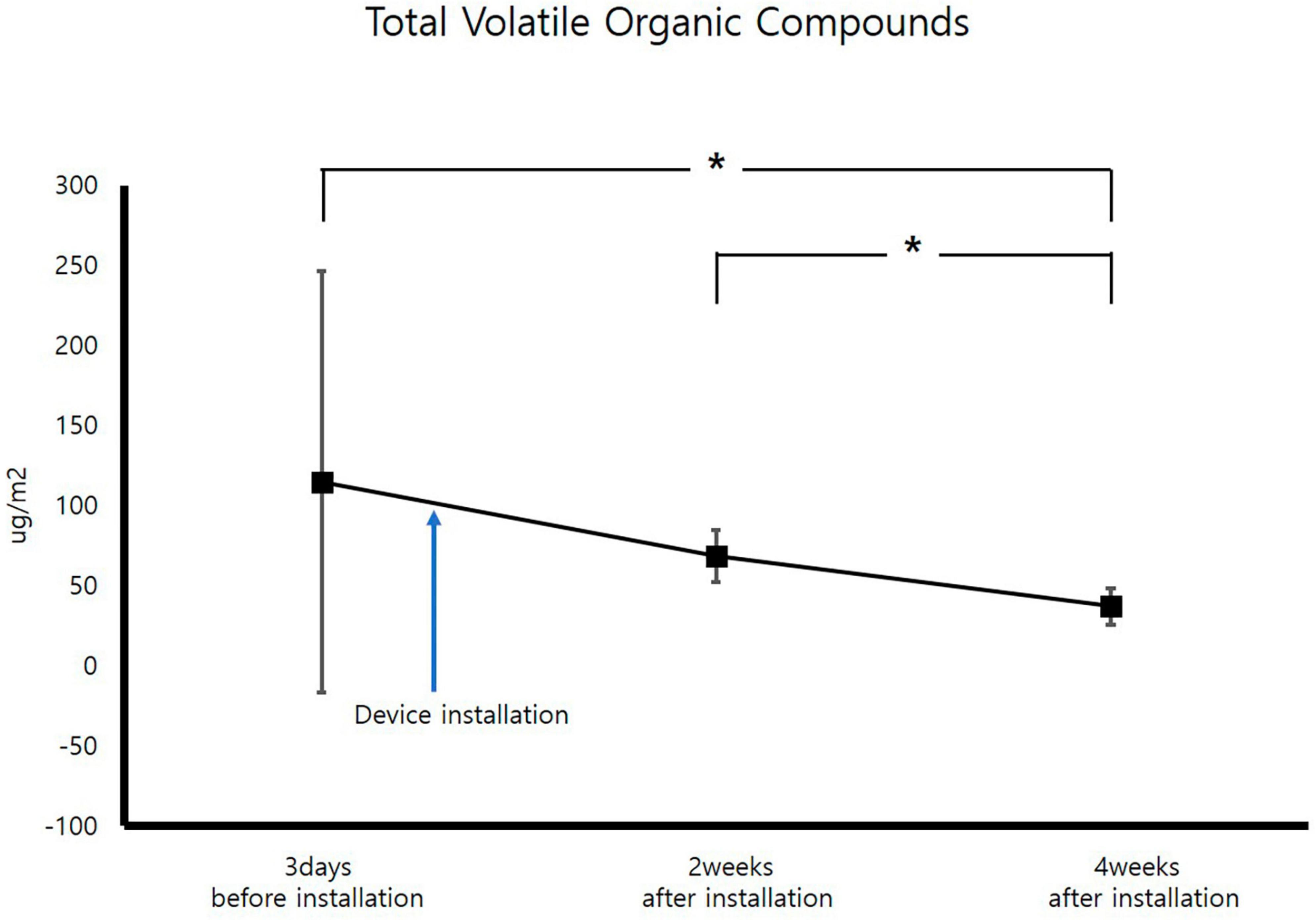
For measurement of TCMs, improvement occurred 2 weeks after air sterilizer installation at all points except inspection area 5, and further improvement was achieved after 4 weeks (p < 0.05). The area with the highest TCMs improvement rate was inspection area 3, which displayed an enhancement rate of 80.6%. The area with the lowest improvement rate was inspection area 5 (40.4%). The average improvement rate for removal of TCM for the entire space was 68.1%, confirming that the air sterilizer was effective (Figure 3).
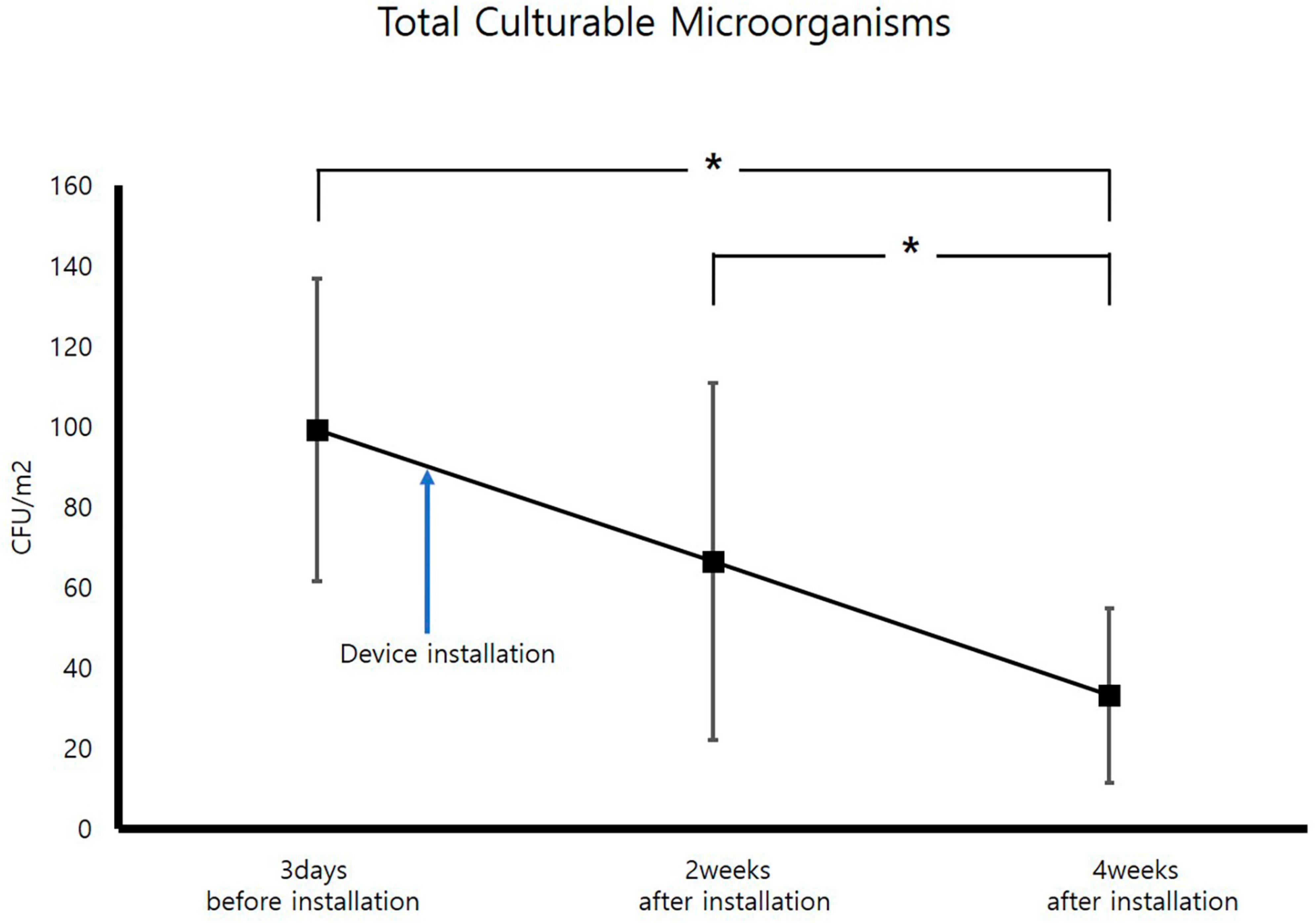
Figure 3. Change in average total culturable microorganism concentrations (CFU; Colony forming unit, *p < 0.05).
After VOCs assessment, improvement occurred 4 weeks after air sterilizer installation at all points (p < 0.05). The areas with the highest and lowest VOCs improvement rate were inspection area 3 and 5, which demonstrated improvement rates of 94.6 and 18.2%, respectively. The average improvement rate of VOCs for the entire space was 41.0%, verifying the efficacy of the air sterilizer (Figure 4).
Discussion
After comparing TCMs and VOCs before and after installation of the KOKKOS air sterilizers, air quality improvement was evident (p < 0.05). ENT outpatient clinics have many patients with colds accompanied by coughand aerosol generation, therefore, the risk of nosocomial infection is high (5, 7). In this situation, KOKKOS air sterilizers may play a significant role in prevention of hospital-acquired infections.
Following the measurement of TCM concentrations prior to installion of the air sterilizers, the maximum value was 159 CFU/m3, which is significantly lower than the air quality management standard of 800 CFU/m3 for multi-use facilities in South Korea, confirming that air quality is being managed well. TCMs concentrations exhibited a statistically significant improvement in air quality before and 4 weeks after installation of air sterilizers (p < 0.05). Additionally, TCMs concentrations revealed a statistically significant improvement in air quality 4 weeks after instatement of the air sterilizers compared to 2 weeks after installation (p < 0.05).
Assessment of VOCs concentrations before installation of the air sterilizer disclosed almost similar VOC concentrations across all areas except for inspection area 3. For inspection area 3, the concentration was judged to be high due to chemicals used inside. The improvement rate was considered low due to the low VOCs concentrations, but air quality showed a statistically significant improvement in VOCs concentrations before and 4 weeks after instatement of the air sterilizer (p < 0.05). Additionally, VOCs concentration showed a statistically significant improvement in air quality 4 weeks after installation of the air sterilizer as compared to 2 weeks after installation (p < 0.05).
Inspection area 3 exhibited the greatest improvement in both TCMs (80.6%) and VOCs (94.6%) after air sterilizer installation. This effect is believed to be because this is where most aerosol-generating procedures, such as local anesthesia surgery and endoscopic procedures, are performed among outpatient treatment spaces.
On the other hand, in inspection area 5, both TCMs (40.4%) and VOCs (18.2%) had the least improvement after air sterilizer installation, because this was a corridor space with a lot of movement and strong air flow. As mentioned above, TCMs and VOCs in an ENT outpatient clinic were statistically significantly improved after installing KOKKOS air sterilizers.
Conclusion
KOKKOS air sterilizers significantly reduce the occurrence of TCMs and VOCs in an ENT outpatient clinic. The installation of KOKKOS air sterilizers is expected to diminish the existence of TCMs and VOCs, to help prevent hospital-acquired infections, and to protect the health of medical staff and patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DL: Writing−original draft, Writing−review and editing. SY: Writing−review and editing. HL: Writing−review and editing. H-HC: Writing−original draft, Writing−review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stawicki S, Wolfe S, Brisendine C, Eid S, Zangari M, Ford F, et al. The impact of comprehensive air purification on patient duration of stay, discharge outcomes, and health care economics: A retrospective cohort study. Surgery. (2020) 168:968–74. doi: 10.1016/j.surg.2020.07.021
2. Park S, Lee K, Yook S, Koo H. Enhancement and homogenization of indoor air quality in a classroom using a vertical airflow ventilation scheme. Toxics. (2022) 10:545. doi: 10.3390/toxics10090545
3. Park S, Yook S, Koo H. Natural ventilation and air purification for effective removal of airborne virus in classrooms with heater operation. Toxics. (2022) 10:573. doi: 10.3390/toxics10100573
4. Bergeron V, Chalfine A, Misset B, Moules V, Laudinet N, Carlet J, et al. Supplemental treatment of air in airborne infection isolation rooms using high-throughput in-room air decontamination units. Am J Infect Control. (2011) 39:314–20. doi: 10.1016/j.ajic.2010.06.013
5. Phan L, Sweeney D, Maita D, Moritz D, Bleasdale S, Jones R, et al. Respiratory viruses in the patient environment. Infect Control Hosp Epidemiol. (2020) 41:259–66. doi: 10.1017/ice.2019.299
6. Chamseddine A, Soudani N, Kanafani Z, Alameddine I, Dbaibo G, Zaraket H, et al. Detection of influenza virus in air samples of patient rooms. J Hosp Infect. (2021) 108:33–42. doi: 10.1016/j.jhin.2020.10.020
7. Atiyani R, Mustafa S, Alsari S, Darwish A, Janahi E. Clearing the air about airborne transmission of SARS-CoV-2. Eur Rev Med Pharmacol Sci. (2021) 25:6745–66.
8. Zhou B, Liu T, Yi S, Huang Y, Guo Y, Huang S, et al. Reducing the effectiveness of ward particulate matter, bacteria and influenza virus by combining two complementary air purifiers. Int J Environ Res Public Health. (2022) 19:10446. doi: 10.3390/ijerph191610446
9. Chirico F, Sacco A, Bragazzi N, Magnavita N. Can air-conditioning systems contribute to the spread of SARS/MERS/COVID-19 infection? Insights from a rapid review of the literature. Int J Environ Res Public Health. (2020) 17:6052. doi: 10.3390/ijerph17176052
10. Lee H, Kwon B, Jeong H, Jo K, Yoon W. Analysis of the status of suspended fungal concentration in indoor air of national multi-use facilities and their correlation with characteristics and contributing factors according to regional and facility classifications. J Odor Indoor Environ. (2022) 21:170–80. doi: 10.15250/joie.2022.21.3.170
Keywords: air, air filters, microorganisms, volatile organic compounds, hospitals
Citation: Lee DH, Yeom S, Lee HS and Cho H-H (2024) Effect of air sterilizers in an outpatient clinic at a tertiary university hospital. Front. Med. 11:1375260. doi: 10.3389/fmed.2024.1375260
Received: 23 January 2024; Accepted: 14 March 2024;
Published: 04 April 2024.
Edited by:
Shiyu Yang, Oregon Institute of Technology, United StatesReviewed by:
Shirun Ding, National University of Singapore, SingaporeNancy Burton, Centers for Disease Control and Prevention (CDC), United States
Copyright © 2024 Lee, Yeom, Lee and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyong-Ho Cho, dmljdG9jaG9AaGFubWFpbC5uZXQ=
 Dong Hoon Lee
Dong Hoon Lee Sujung Yeom1
Sujung Yeom1 Hyong-Ho Cho
Hyong-Ho Cho