- Department of Translational Biomedicine and Neuroscience, University of Bari Medical School, Bari, Italy
Mast cells release different anti-and pro-inflammatory agents changing their role from protective to pro-inflammatory cells involved in the progression of different pathological conditions, including autoimmune diseases and tumors. Different mediators released by mast cells are involved in their biological activities which may be anti-tumorigenic and/or pro-tumorigenic. For these reasons, tumor mast cells have been considered a novel therapeutic target to prevent tumor progression and metastatic process. Many different agents have been suggested and used in the past pre-clinical and clinical settings. Among the novel immunotherapeutic approaches to cancer treatment, different immune checkpoint inhibitors targeting PD-1/PDL-1 have been used in the treatment of many human tumors improving overall survival. In this context, inhibition of mast cell activity may be considered a novel strategy to improve the efficacy of anti-PD-1/PDL-1 therapy. The blockade of the PD-1/PD-L1 interaction may be suggested as a useful and novel therapeutic approach in the treatment of tumors in which mast cells are involved.
Tumor mast cells and related therapeutic approaches
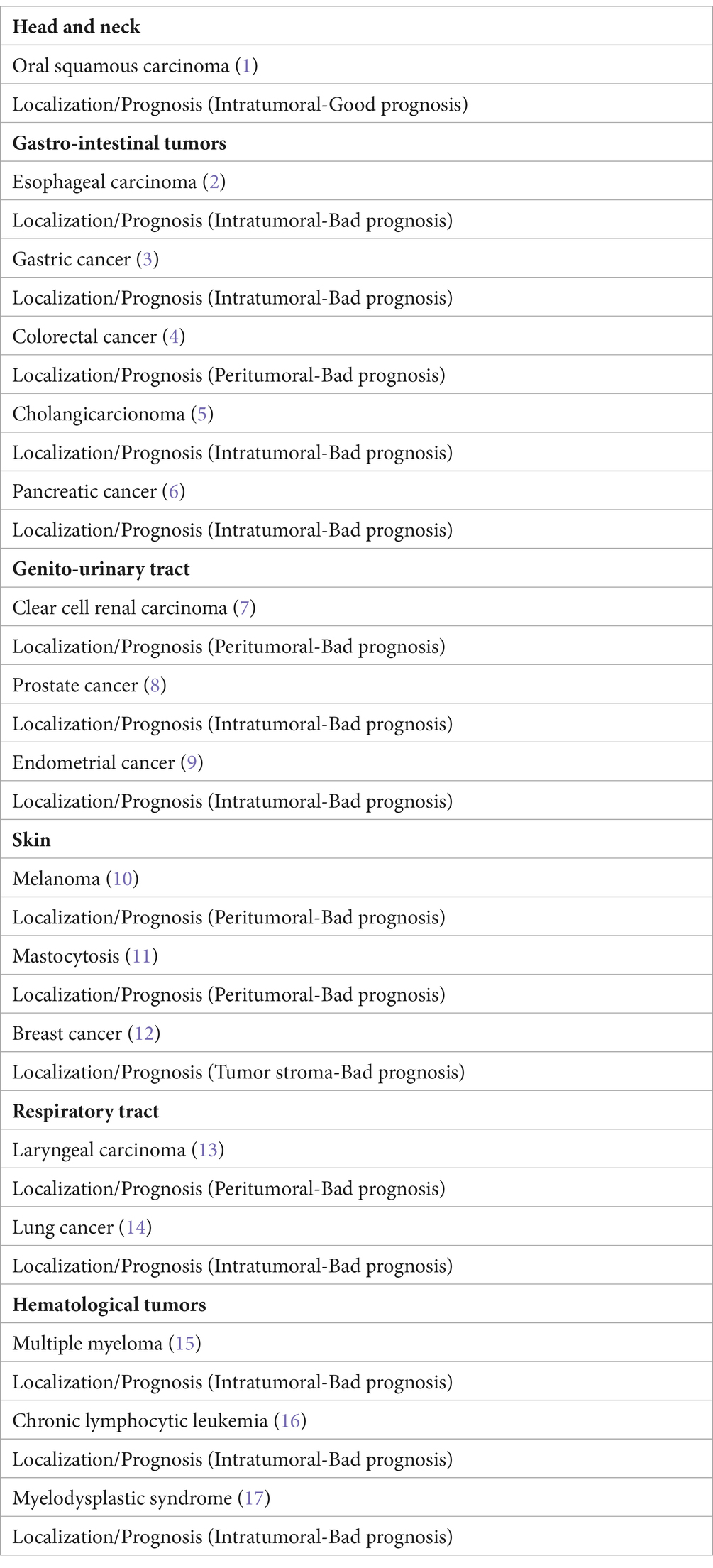
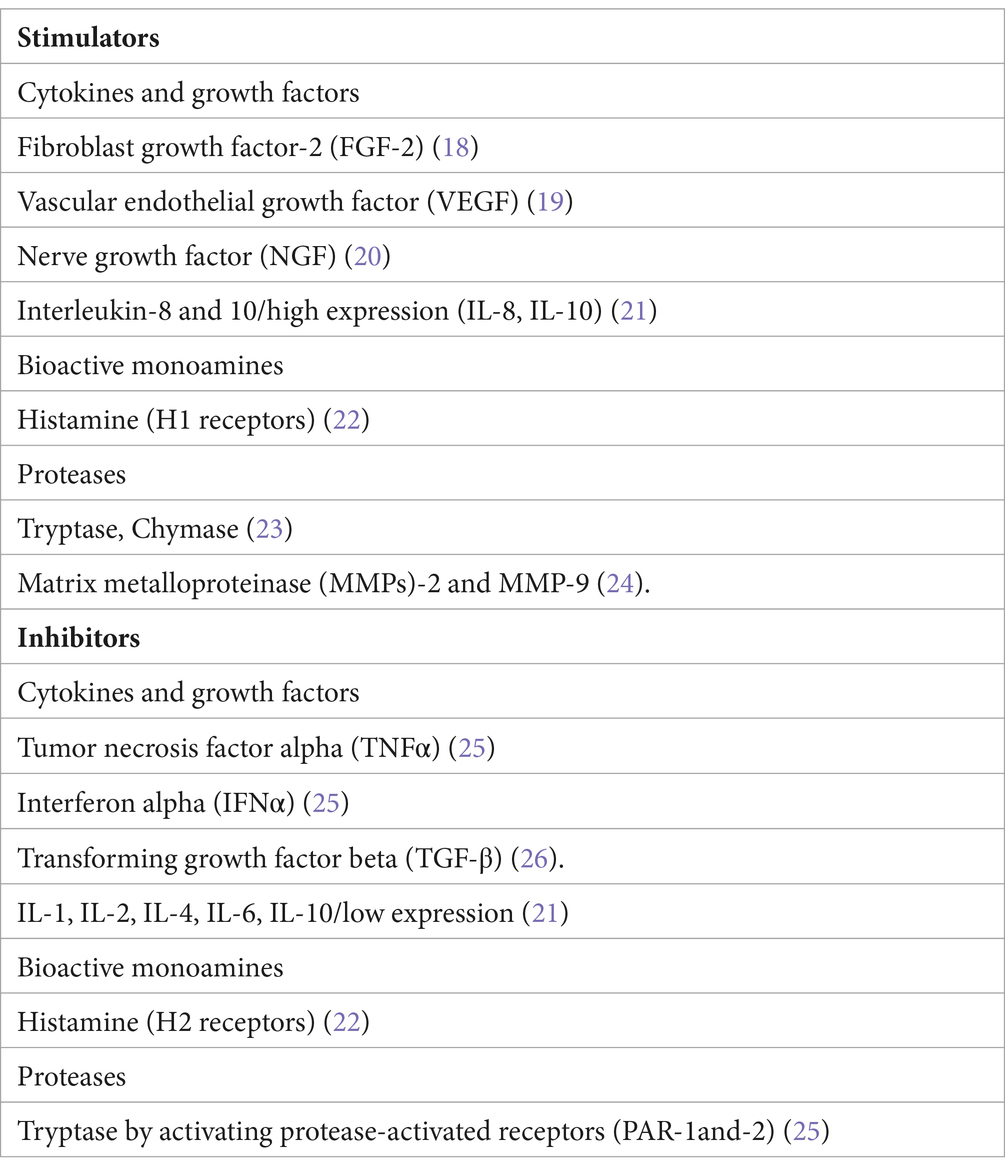
Mast cells have multiple roles extending beyond their classical role in Ig-E-mediated allergic reactions. Mast cells release different anti-and pro-inflammatory agents changing their role from protective to pro-inflammatory cells involved in the progression of different pathological conditions, including autoimmune diseases and tumors (Table 1). Mast cells can be recruited into the tumor microenvironment by different chemotactic molecules released by tumor cells. One of the main chemoattractant factors produced by tumor cells is stem cell factor (SCF), which recruits mast cells expressing its tyrosine kinase receptor c-kit (CD117). Mast cells can exert both anti-tumorigenic and/or pro-tumorigenic roles (Table 2). Mast cells may exert detrimental effects on the host by releasing cytokines and growth factors, such as fibroblast growth factor2- (FGF-2), vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and interleukin-8 (IL-8), which stimulate tumor cell expansion. Mast cells are a major source of histamine, which can induce tumor cell proliferation through H1 receptors while suppressing the immune system through H2 receptors. Mast cells produce several angiogenic factors, as well as proteases, which promote tumor vascularization and tumor invasiveness, respectively. By contrast, mast cells may promote the inhibition of tumor cell growth, tumor cell apoptosis, and inflammation.
by releasing cytokines such as inreleukin-1 (IL-1), IL-4, IL-6, and tumor necrosis factor alpha (TNF-α). Chondroitin sulfate may inhibit tumor cell diffusion and tryptase causes both tumor cell disruption and inflammation through activation of protease-activated receptors (PAR-1 and -2). Two mast cell phenotypes have been described called mast cell 1 and 2 (MC1 and MC2), related to pro-inflammatory and anti-inflammatory profiles, respectively. Mast cells promote tumor development by alterations in stroma-epithelial interactions, by inducing tumor angiogenesis and lymphangiogenesis, and by releasing different cytokines and growth factors. In solid and hematologic tumors, mast cells may be localized in intra-tumoral or peri-tumoral areas, with expression of favorable/unfavorable and, respectively, bed prognosis (25).
Based on the involvement of mast cells in tumor growth, these cells have been recently considered a novel therapeutic target in the control of tumor progression and metastatic capability. Many different agents have been suggested and used in pre-clinical and clinical settings. These therapeutic agents include inhibitors of c-kit (imatinib mesylate, mastinib, nilotinib, dasatinib, sunitinib, midostaurin, and ibrutinib). In this context, imatinib mesylate (Gleevec), which exerts inhibitory activity against the signaling cascade activated by CD117 (27), has been used against gastrointestinal stromal tumors (GIST) and metastatic melanoma with c-Kit mutations (28, 29). Masitinib has been used in the treatment of mastocytosis, GIST, colon cancer, prostate cancer, and pancreatic cancer (30). Sunitinib has been used in patients with imatinib-resistant GIST (31). Gabexate mesylate an inhibitor of tryptase has been used as an inhibitor of colon cancer growth with an anti-angiogenic effect (32). Cromolyn sodium, a mast cell stabilizing agent (33) that prevent cell degranulation (34), has been used in a xenograft mouse model of thyroid cancer (35). Obatoclax, which binds and blocks the anti-apoptotic activity of members of the Bcl-2 family, induces growth arrest in human neoplastic mast cells, and different mast cell lines (36), and exerts synergistic antineoplastic effects when combined with dasatinib (36).
H1 receptor antagonists reduced tumor growth, mast cell infiltration, and VEGF levels through the inhibition of hypoxia-inducible factor-1alpha (HIF-1α) in melanoma-bearing mice (37). Moreover, treatment with cimetidine, an H2 receptor antagonist, slows the growth of tumors in mice (38, 39). Chondroitin sulfate may inhibit tumor growth cell diffusion through activation of PAR-1 and-2 (40).
Immune checkpoint inhibitors
Different studies have highlighted the importance of the programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway controlling inflammation degree to prevent an exacerbated immune response in tumor growth, in which PD-L1 expressed on tumor cells can inhibit the effector functions of CD8+ T cells, leading to the progression of tumors (41). Different immune checkpoint inhibitors targeting PD-1/PDL-1 have been used in the treatment of many human tumors, such as melanoma, non-small-cell lung cancer, and renal cancer, improving overall survival (42, 43). However, this therapeutic approach may be ineffective, because of the development of resistance mechanisms mediated by inflammatory cells present in the tumor microenvironment, including mast cells.
Relationship between tumor-infiltrating mast cells and response to anti-PD1/PD-L1 blockade
Human mast cells express several co-stimulatory and co-inhibitory molecules, including PDL-1 and PD-L2 (44). In the skin, mast cells express high levels of PD-L1, and in contact hypersensitivity mast cell absence abolished the PD-L1 blockade effect on CD8+T-cell activation (45). According, high levels of PDL-1 in mast cells promotes T cell immunosuppression and tumor growth in gastric cancer (46).
In high-grade serous ovarian cancer, infiltration of mast cells is associated with a decreased response to anti-PD1 blockade (47). Similarly, in a melanoma experimental model of resistance to anti-PD-1 therapy, high infiltration of mast cells predicted poor response to anti-PD1 blockade (48). An increased number of mast cells was detectable in melanoma patients after anti-PD1 therapy (49). In tumor histological sections, a co-localization of mast cells and forkhead box P3 (FOXP3)-positive Treg cells have been recognizable and associated with a down-modulation of HLA class I on tumor cells and correlated with resistance to anti-PD-1 therapy. Melanoma cells secrete chemokine (C-X-C motif) ligand 10 (CXCL10) that binds CXC motif chemokine receptor 3 (CXCR3) expressed by mast cells, favoring the recruitment of mast cells (49). Anti-PD1 treatment activates and induces expression on mast cells leading to therapeutic resistance through stimulation of angiogenesis and tumor growth (50).
Otherwise, the reduction of mast cells is associated with an improvement in the efficacy of anti-PD-1/anti-PD-L1 blockade. Combining anti-PD-1 with sunitinib or imatinib, but not PD-1 blockade alone, resulted in the depletion of mast cells and tumor regression (48). Cromolyn sodium decreases mast cell infiltration, the release of inflammatory cytokines, and improves the efficacy of anti-PD1 therapy (50). Targeting mast cells with ketotifen enhances T cells’ infiltration and cytotoxic capacity and sensitizes sarcoma cells to anti-PDL-1 therapy (51).
Concluding remarks
Mast cells play a crucial role in the control of tumor immunity and tumor growth. They can modulate the biological activity of immune and non-immune components of the tumor microenvironment through the release of a plethora of mediators, leading to different cancer-promoting and cancer-suppressive activities. The reduction of mast cell infiltration may be considered a novel therapeutic approach to cancer treatment. Mast cells can be therapeutically targeted by decreasing their number through c-Kit inhibitors; modulating mast cell activation and phenotype, and altering secreted mast cell mediators. In this context, inhibition of mast cell activity may be considered a novel strategy to improve the efficacy of anti-PD-1/PDL-1 therapy. The blockade of the PD-1/PD-L1 interaction may be suggested as a useful and novel therapeutic approach in the treatment of tumors in which mast cells are involved.
Author contributions
DR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Associazione Italiana contro le Leucemie, Linfomi, e Mieloma (AIL), Bari, Italy.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tamma, R, Limongelli, L, Maiorano, E, Pastore, D, Cascardi, E, Tempesta, A, et al. Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2019) 98:979–86. doi: 10.1007/s00277-018-3575-3
2. Elpek, GO, Gelen, T, Aksoy, NH, Erdoğan, A, Dertsiz, L, Demircan, A, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. (2001) 54:940–4. doi: 10.1136/jcp.54.12.940
3. Guidolin, D, Ruggieri, S, Annese, T, Tortorella, C, Marzullo, A, and Ribatti, D. Spatial distribution of mast cells around vessels and glands in human gastric carcinoma. Clin Exp Med. (2017) 17:531–9. doi: 10.1007/s10238-017-0452-7
4. Ammendola, M, Patruno, R, Sacco, R, Marech, I, Sammarco, G, Zuccalà, V, et al. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin Ther Targets. (2016) 20:533–40. doi: 10.1517/14728222.2016.1158811
5. Tamma, R, Annese, T, Ruggieri, S, Brunetti, O, Longo, V, Cascardi, E, et al. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest. (2019) 49:e13087. doi: 10.1111/eci.13087
6. Longo, V, Tamma, R, Brunetti, O, Pisconti, S, Argentiero, A, Silvestris, N, et al. Mast cells and angiogenesis in pancreatic ductal adenocarcinoma. Clin Exp Med. (2018) 18:319–23. doi: 10.1007/s10238-018-0493-6
7. Tamma, R, Rutigliano, M, Lucarelli, G, Annese, T, Ruggieri, S, Cascardi, E, et al. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol Oncol. (2019) 37:355.e11–9. doi: 10.1016/j.urolonc.2019.01.025
8. Ronca, R, Tamma, R, Coltrini, D, Ruggieri, S, Presta, M, and Ribatti, D. Fibroblast growth factor modulates mast cell recruitment in a murine model of prostate cancer. Oncotarget. (2017) 8:82583–92. doi: 10.18632/oncotarget.19773
9. Ribatti, D, Finato, N, Crivellato, E, Marzullo, A, Mangieri, D, Nico, B, et al. Neovascularization and mast cells with tryptase activity increase simultaneously with pathological progression in human endometrial cancer. Am J Obstet Gynecol. (2005) 193:1961–5. doi: 10.1016/j.ajog.2005.04.055
10. Ribatti, D, Ennas, MG, Vacca, A, Ferreli, F, Nico, B, Orru, S, et al. Tumor vascularity and tryptase positive-mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest. (2003) 33:420–5. doi: 10.1046/j.1365-2362.2003.01152.x
11. Ribatti, D, Nico, B, Finato, N, Crivellato, E, and Beltrami, CA. Co-localization of tryptase and cathepsin-G in mast cells in cutaneous mastocytosis. Cancer Lett. (2009) 279:209–12. doi: 10.1016/j.canlet.2009.01.039
12. Ribatti, D, Annese, T, and Tamma, R. Controversial role of mast cells in breast cancer tumor progression and angiogenesis. Clin Breast Cancer. (2021) 21:486–91. doi: 10.1016/j.clbc.2021.08.010
13. Sawatsubashi, M, Yamada, T, Fukushima, N, Mizokami, H, Tokunaga, O, and Shin, T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma Virchows arch B cell Pathol. Mol Pathol. (2000) 436:243–8. doi: 10.1007/s004280050037
14. Longo, V, Catino, A, Montrone, M, Galetta, D, and Ribatti, D. Controversial role of mast cells in NSCLC tumor progression and angiogenesis. Thorac Cancer. (2022) 13:2929–34. doi: 10.1111/1759-7714.14654
15. Ribatti, D, Vacca, A, Nico, B, Quondamatteo, F, Ria, R, Minischetti, M, et al. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. (1999) 79:451–5. doi: 10.1038/sj.bjc.6690070
16. Molica, S, Vacca, A, Crivellato, E, Cuneo, A, and Ribatti, D. Tryptase-positive mast cells predict clinical outcome of patients with B-cell chronic lymphocytic leukemia. Eur J Haematol. (2003) 71:137–9. doi: 10.1034/j.1600-0609.2003.00110.x
17. Ribatti, D, Polimeno, G, Vacca, A, Marzullo, A, Crivellato, E, Nico, B, et al. Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes. Leukemia. (2002) 16:1680–4. doi: 10.1038/sj.leu.2402586
18. Qu, Z, Kayton, RJ, Ahmadi, P, Liebler, GM, Powers, MR, Planck, SR, et al. Ultrastructural immunolocalization of basic fibroblast growth factor in mast cell secretory granules: morphological evidence for bFGF release through degranulation. J Histochem Cytochem. (1998) 46:1119–28. doi: 10.1177/002215549804601004
19. Grutzkau, A, Kruger-Krasagakes, S, Baumeister, H, Schwarz, C, Kogel, H, Welker, P, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF 206. Mol Biol Cell. (1998) 9:875–84. doi: 10.1091/mbc.9.4.875
20. Cantarella, G, Lempereur, L, Presta, M, Ribatti, D, Lombardo, G, Lazarovici, P, et al. Nerve growth factor-endothelial cell interactions lead to angiogenesis in vitro and in vivo. FASEB J. (2002) 16:1307–9. doi: 10.1096/fj.01-1000fje
21. Solimando, AG, Desantis, V, and Ribatti, D. Mast cells and interelukins. Int J Mol Sci. (2022) 23:14004. doi: 10.3390/ijms232214004
22. Sorbo, J, Jakobson, A, and Norrby, K. Mast cell histamine is angiogenic through receptors for histamine 1 and histamine 2. Int J Exp Pathol. (1994) 75:43–50.
23. Ribatti, D, Ranieri, G, Nico, B, Benagiano, V, and Crivellato, E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int J Dev Biol. (2011) 55:99–102. doi: 10.1387/ijdb.103138dr
24. Galli, SJ, Tsai, M, Marichal, T, Tchougounova, E, Reber, LL, and Pejler, G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. (2015) 126:45–127. doi: 10.1016/bs.ai.2014.11.002
25. Ribatti, D, and Crivellato, E. The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. (2009) 275:89–131. doi: 10.1016/S1937-6448(09)75004-X
26. Kelly, A, Houston, SA, Sherwood, E, Casulli, J, and Travis, MA. Regulation of innate and adaptive immunity by TGFbeta. Adv Immunol. (2017) 134:137–233. doi: 10.1016/bs.ai.2017.01.001
27. Heinrich, MC, Griffith, DJ, Druker, BJ, Wait, CL, Ott, KA, and Zigler, AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571 a selective tyrosine kinase inhibitor. Blood. (2000) 96:925–32. doi: 10.1182/blood.V96.3.925
28. Hodi, FS, Corless, CL, Giobbie-Hurder, A, Fletcher, JA, Zhu, M, Marino-Enriquez, A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. (2013) 31:3182–90. doi: 10.1200/JCO.2012.47.7836
29. Kitamura, Y, and Hirotab, S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci. (2004) 61:2924–31. doi: 10.1007/s00018-004-4273-y
30. Ribatti, D. Mast cells as therapeutic target in cancer. Eur J Pharmacol. (2016) 778:152–7. doi: 10.1016/j.ejphar.2015.02.056
31. Demetri, GD, van Oosterom, AT, Garrett, CR, Blackstein, ME, Shah, MH, Verweij, J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. (2006) 368:1329–38. doi: 10.1016/S0140-6736(06)69446-4
32. Yoon, WH, Jung, YJ, Kim, TD, Li, G, Park, BJ, Kim, JY, et al. Gabexate Mesilate inhibits Colon Cancer growth, invasion, and metastasis by reducing matrix metalloproteinases and angiogenesis. Clin Cancer Res. (2004) 10:4517–26. doi: 10.1158/1078-0432.CCR-04-0084
33. Cimpean, AM, and Raica, M. The hidden side of disodium Cromolyn: from mast cell stabilizer to an Angiogenic factor and antitumor agent. Arch Immunol Ther Ex. (2016) 64:515–22. doi: 10.1007/s00005-016-0408-8
34. Soucek, L, Lawlor, ER, Soto, D, Shchors, K, Swigart, LB, and Evan, GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med.. (2007) 13:1211–1218.
35. Melillo, RM, Guarino, V, Avilla, E, Galdiero, MR, Liotti, F, Prevete, N, et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene. (2010) 29:6203–15. doi: 10.1038/onc.2010.348
36. Peter, B, Cerny-Reiterer, S, Hadzijusufovic, E, Schuch, K, Stefanzl, G, Eisenwort, G, et al. The pan-Bcl-2blocker obatoclax promotes the expression of Puma, Noxa, and Bimm RNA and induces apoptosis in neoplastic mast cells. J Leukoc Biol. (2014) 95:95–104. doi: 10.1189/jlb.1112609
37. Jeong, HJ, Oh, HA, Nam, SY, Han, NR, Kim, YS, Kim, JH, et al. Oh HAThe critical role of mast cell-derived hypoxia-inducible factor-1α in human and mice melanoma growth. Int J Cancer. (2013) 132:2492–501. doi: 10.1002/ijc.27937
38. Bowrey, PF, King, J, Magarey, C, Schwartz, P, Marr, P, Bolton, E, et al. Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect? Br J Cancer. (2000) 82:167–70. doi: 10.1054/bjoc.1999.0895
39. Nordlund, JJ, and Askenase, PW. The effect of histamine, antihistamines, and a mast cell stabilizer on the growth of cloudman melanoma cells in DBA/2mice. J Invest Dermatol. (1983) 81:28–31. doi: 10.1111/1523-1747.ep12538356
40. Ribatti, D, and Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. (2012) 1822:2–8. doi: 10.1016/j.bbadis.2010.11.010
41. Alsaab, HO, Sau, S, Alzhrani, R, Tatiparti, K, Bhise, K, Kashaw, SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. (2017) 8:561. doi: 10.3389/fphar.2017.00561
42. Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. (2020) 11:3801. doi: 10.1038/s41467-020-17670-y
43. Siu, LL, Ivy, SP, Dixon, EL, Gravell, AE, Reeves, SA, and Rosner, GL. Challenges and opportunities in adapting clinical trial design for immunotherapies. Clin Cancer Res. (2017) 23:4950–8. doi: 10.1158/1078-0432.CCR-16-3079
44. Nakae, S, Suto, H, Iikura, M, Kakurai, M, Sedgwick, JD, and Tsai, M. Galli SJ mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. (2006) 176:2238–48. doi: 10.4049/jimmunol.176.4.2238
45. Hirano, T, Honda, T, Kanameishi, S, Honda, Y, Egawa, G, Kitoh, A, et al. PD-L1 on mast cells suppresses effector CD81T-cell activation in the skin in murine contact hypersensitivity. J Allergy Clin Immunol. (2021) 148:563–573.e7. doi: 10.1016/j.jaci.2020.12.654
46. Lv, Y, Zhao, Y, Wang, X, Chen, N, Mao, F, Teng, Y, et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNFα-PD-L1 pathway. J Immunother Cancer. (2019) 7:54. doi: 10.1186/s40425-019-0530-3
47. Cao, K, Zhang, G, Zhang, X, Yang, M, Wang, Y, He, M, et al. Stromal infiltrating mast cells identify immunoevasive subtype high-grade serous ovarian cancer with poor prognosis and inferior immunotherapeutic response. Onco Targets Ther. (2021) 10:1969075. doi: 10.1080/2162402X.2021.1969075
48. Somasundaram, R, Connelly, T, Choi, R, Choi, H, Samarkina, A, Li, L, et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat Commun. (2021) 12:346. doi: 10.1038/s41467-020-20600-7
49. Kuo, PT, Zeng, Z, Salim, N, Mattarollo, S, Wells, JW, and Leggatt, GR. The role of CXCR3 and its chemokine ligand in skin disease and cancer. Front Med. (2018) 5:271. doi: 10.3389/fmed.2018.00271
50. Li, J, Peng, G, Zhu, K, Jie, X, Xu, Y, Rao, X, et al. PD-1+ mast cell enhanced by PD-1 blocking therapy associated with resistance to immunotherapy. Cancer Immunol Immunother. (2022) 72:633–45. doi: 10.1007/s00262-022-03282-6
Keywords: immune checkpoint inhibitors, PD-1/PDL-1, tumor growth, mast cells, tumor therapy
Citation: Ribatti D (2024) New insights into the role of mast cells as a therapeutic target in cancer through the blockade of immune checkpoint inhibitors. Front. Med. 11:1373230. doi: 10.3389/fmed.2024.1373230
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Alessandro Gambella, University of Pittsburgh, United StatesCopyright © 2024 Ribatti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Ribatti, ZG9tZW5pY28ucmliYXR0aUB1bmliYS5pdA==
 Domenico Ribatti
Domenico Ribatti