- 1Department of Biomedical Sciences, School of Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 2Department of Biomedical Sciences, School of Medicine, Jimma University, Jimma, Ethiopia
- 3Department of Pharmaceutical Chemistry, School of Pharmacy, Hawassa University, Hawassa, Ethiopia
- 4Department of Biomedical Sciences, School of Medicine, Debre Markos University, Debre Markos, Ethiopia
Background: After the introduction of antiretroviral therapy, the care given to people living with HIV has become complicated by the appearance of comorbidities as a result of HIV and HAART toxicities, in which cardiovascular disease got the most attention. So, this study aimed to assess serum uric acid and high-sensitivity C-reactive protein levels among people living with HIV on dolutegravir (DTG) and ritonavir-boosted atazanavir (ATV/r)-based therapy.
Methods: An institutional-based comparative cross-sectional study was conducted from November 4, 2021, to January 4, 2022. An equal number of dolutegravir- and ritonavir-boosted atazanavir-treated patients (n = 86 each) were enrolled. A consecutive sampling method was used to select participants. Data were entered into Epidata version 4.6, exported to SPSS version 25.0, and analyzed using Chi-square, Student’s t-test, Mann–Whitney U-test, and logistic regression. Statistical significance was set at p < 0.05.
Results: The prevalence of hyperuricemia and high-sensitivity C-reactive protein levels ≥2 mg/L were 46.5% (40/86) and 24.4% (21/86) in the DTG group, and 30.2% (26/86) and 44.2 (38/86) in the ATV/r group, respectively. When compared to ATV/r, a higher mean level of uric acid was found among DTG-based regimens (5.38 mg/dL). Duration of ART (AOR = 2, 95% CI: 1.2, 4.4) and DTG-based regimen (AOR = 1.9, 95% CI: 1.04, 3.8) were significant predictors of developing hyperuricemia. ATV/r-based regimen (AOR = 3, 95% CI: 1.5, 8.3) and high waist circumference (AOR = 2.5, 95% CI: 1, 3.5) were significantly associated with increased high-sensitivity C-reactive protein levels.
Conclusion: It is observed that DTG-based and ATV/r-based ART are associated with hyperuricemia and increased high-sensitivity C-reactive protein levels, respectively. Therefore, it is important to consider and evaluate serum uric acid and high-sensitivity C-reactive protein levels in patients taking DTG and ATV/r-based ART, as well as among those on HAART for years and with a higher waist circumference, so as to detect and prevent early the risk of having CVD.
Background
After the introduction of antiretroviral therapy (ART), the world successfully achieved higher viral suppression and a lower viral load burden, followed by a reduction in mortality and morbidity associated with HIV and an improvement in the quality of life of people living with HIV (1, 2). But, now a days, the care given to this population is complicated by the emergency of other comorbidities introduced by HIV itself and as a result of ART toxicities (3, 4). Exposure to ART for a longer duration of time enhances the susceptibility of these patients to developing these comorbidities, in which atherosclerotic cardiovascular disease (ASCVD) gains the most attention due to its association with the morbidity and mortality of people living with HIV (5–8).
Several studies have revealed that hyperuricemia and chronic inflammation have been proposed as potential mechanisms through which HIV and ART cause ASCVD (9, 10). Uric acid, the end product of purine metabolism, has been associated with metabolic syndrome, and it induces endothelial dysfunction, both of which are the main risk factors for ASCVD in general as well as in people living with HIV (9–16). Increased the activity of the pentose phosphate pathway (PPP) and increase the production of ribose 5-phosphate which stimulate purine nucleotide phosphorylase for nucleotide synthesis, that in turn increased the production of uric acid as well as ART-induced mitochondrial toxicity subsequently increased lactate which compete with uric to be excreted were the possible ways for hyperuricemia seen in treated HIV patients (17–19).
Hyperuricemia was increasingly seen among people living with HIV on ART than in the general population, which indicates people living with HIV are at greater risk of cardiovascular disease (CVD) than HIV-negative people, which means attention must be given to these people to prevent CVD (20). An increased risk of having hyperuricemia was seen among treatment-naive compared to treatment experienced people living with HIV (21, 22). It is observed that hyperuricemia was also detected in HIV patients taking HAART (23, 24). Accordingly, a study from Italy reported that 25.2% of people living with HIV on HAART developed elevated serum uric acid levels (12). The potential to cause an altered level of uric acid was different for different ART classes. Patients treated with stavudine, didanosine, and protease inhibitors had significantly increased levels of uric acid. However, tenofovir is associated with reduced levels of uric acid, and abacavir has a neutral effect on uric acid concentration (25).
Studies have reported that patients treated with dolutegravir (DTG) have elevated levels of uric acid (26, 27). Another study also showed people living with HIV on ritonavir-boosted protease inhibitors, especially ritonavir-boosted atazanavir (ATV/r), were observed to be hyperuricemic (28, 29).
Research into CVD risk factors has identified a number of inflammatory biomarkers, such as interleukin 6 (IL-6) and high-sensitivity C-reactive protein (hsCRP) (30–32). A study from Italy showed an increased hsCRP level was detected in people living with HIV on combination antiretroviral therapy (cART), which makes hsCRP a useful additional biomarker to predict the risk of HIV patients developing CVD (33, 34). The risk of elevated hsCRP levels was higher among people living with HIV than HIV-negative populations, but the risk was higher for treatment-naïve patients (35, 36). Another study showed similar hsCRP levels were observed during pre-ART and 36 weeks after commencing ART (37). However, one study from Ghana reported that 32.5% of patients on ART have increased hsCRP levels greater than or equal to 3 mg/L (31). A study from France complains that the use of ATV/r containing ART enhances inflammation due to its effect on activation of NF-kB and increased levels of different inflammatory biomarkers (38). It is speculated that the use of integrase strand transfer inhibitors (INSTI) is associated with reduced inflammation, which is determined by elevated levels of different inflammatory biomarkers including hsCRP levels (39). Inflammation as measured by hsCRP levels is reduced after patients switch to INSTI from boosted-protease inhibitors (40, 41). However, a conflicting result was posted, which indicates the association of INSTI including DTG with increased inflammation (42–44).
Several factors contributed to hyperuricemia and increased inflammation measured by hsCRP, such as duration of HIV and HAART, age, obesity, alcohol intake, smoking, region, ethnicity, and low CD4 cell count (45–47).
The DTG is a new integrase inhibitor that has been recommended by the World Health Organization (WHO) as the preferred first-line regimen for people living with HIV (48). ATV/r is widely used as a second-line therapy in Ethiopia (49). DTG is effective, has high antiviral potency, and can be used without pharmacological enhancements (50, 51). As a result of this, most of the patients are on a regimen containing these ARTs.
Despite this, there is no well-documented evidence that shows the level of uric acid and hsCRP among patients taking DTG and ATV/r-based regimens in sub-Saharan Africa, including Ethiopia. The current WHO guideline does not include uric acid monitoring in patients taking these regimens, but they are useful biomarkers to detect and prevent the early occurrence of CVD. Therefore, the present study is designed to assess the serum level of uric acid, hsCRP, and their associated factors among adult HIV patients taking DTG and ATV/r-based antiretroviral therapy at Jimma University Medical Center, Southwest Ethiopia.
Materials and methods
Study design and setting
An institutional-based comparative cross-sectional study was carried out at the ART clinic of Jimma University Medical Center (JUMC). The medical center has active 3,500 people living with HIV (until October 15, 2021); among these, 1841 were on ART containing DTG + 3TC + TDF, and 167 were on ART containing ATV/r + 3TC + TDF. The center provides services to the surrounding villages and nearby towns. Currently, the JUMC ART clinic provides voluntary counseling and testing (VCT), prevention of mother-to-child transmission of HIV, follow-up services for HIV-infected patients on ART therapy, and treatment of opportunistic infections.
Study participant and study processes
The study was conducted from November 4, 2021, up to January 4, 2022. All people living with HIV ≥18 years who had received DTG-based and ATV/r-based regimens for at least 6 months (52) and who volunteered to participate in the study were included. Whereas, patients taking DTG-based and ATV/r-based ART regimens for less than 6 months, pre-existing liver and renal problems, or active cancer, and those who did not volunteer to participate in the study were excluded.
Sample size determinations and sampling procedure
The number of participants included in the study was calculated using the G* power statistical power analysis version 3.1 software. The sample size was calculated by considering α = 0.05, power (1–β) = 90%, with a DTG to ATV/r ratio of 1:1, two tail t-tests, and an effect size of 0.5. The computed sample size was 172. Of these, 86 were in the DTG-based group and 86 were in the ATV/r-based group. A consecutive sampling technique was applied. All participants who fulfilled the inclusion criteria and were ready to participate were included in the study until the required sample size of 172 was achieved.
Data collection procedures
Before data collection, training was provided to the data collectors for 1 day by the principal investigator. Data collection was controlled by the principal investigator, and the collected data were checked for completeness, consistency, and clarity. Trained nurses collected sociodemographic and related clinical data using a structured questionnaire. The time of initiation of HAART, medical history, WHO clinical stage, and viral load values were obtained from the medical record card. A sample of venous blood was collected from each study participant for laboratory testing by laboratory technologists. Anthropometric measurements such as height, weight, waist circumference, and hip circumference were collected by trained nurses using a standard balance and a SECA meter. BMI was calculated by dividing the individual’s weight in kilograms by the square of their height in meters (kg/m2). BMI was categorized as obese if BMI ≥25 kg/m2 and normal if BMI <25 kg/m2. The range of abnormal waist circumference for males and females was >102 and >88 cm, respectively. The cut-off for the waist-hip ratio was ≥0.9 for males and ≥0.85 for females, according to the criteria of the WHO guidelines (53, 54).
Blood sample collection
To determine the serum UA, hsCRP, and CD4 cell count, 2 mL of blood was collected. Before centrifugation, whole blood was used to measure the CD4 cell count. The remaining blood was centrifuged at 3,000 rpm for 5 min. The serum obtained was stored at 4°C until the analysis of UA and hsCRP levels. UA and hsCRP levels were determined using a Roche Cobas 6,000 analyzer. The CD4 cell count was determined using a near-patient CD4 count system, which consisted of a FACSPresto counter machine and a BD FACSPresto™ Cartridge kit.
Definitions
Hyperuricemia: patients having serum uric acid levels >6.3 mg/dL for males and >4.9 mg/dL for females (55).
Increased serum hsCRP levels: patients having serum hsCRP levels ≥2 mg/dL for both genders. It was categorized based on its risk enhancing factor for ASCVD (56).
Viral load (VL): ≤1,000 copies/ml was categorized as suppressed and >1,000 copies/ml as non-suppressed VL (57).
Patient on DTG-based regimen: patient taking DTG + 3TC + TDF for at least 6 months.
Patient on ATV/r-based regimen: patient taking ATV/r + 3TC + TDF for at least 6 months.
CD4 cell count: ≥500 cells/mm3 as normal range and competent immune system and <500 cells/mm3 as compromised immune system (58).
Statistical methods
The data were checked, cleaned, entered into Epi-data software version 3.1, and exported to SPSS version 25 for further analysis. Simple descriptive statistics were used to present the participants’ sociodemographic characteristics. Categorical variables were presented as numbers and percentages, computed using the chi-square test to detect differences between the groups, while continuous variables were presented as mean ± standard deviation. A student’s t-test was used to detect differences between the groups. Factors with a p-value less than or equal to 0.25 in bivariate analysis were considered candidates for multivariate modeling. Multivariable logistic regression was performed to identify factors associated with hyperuricemia and increased hsCRP levels. Hosmer Lemeshow and pseudo-R square tests were checked, and no problems were found. The level of significance for statistical analysis was set at a p-value less than 0.05.
Ethical consideration
Before data collection, ethical clearance was obtained from the Institutional Review Board of Jimma University with reference number of IHRPG1/5/2021. A permission letter had been obtained from the hospital, and data collection had started. Written informed consent was obtained from all patients before the interviews after providing information about the purpose and method of the study. Participants were coded to ensure confidentiality, and their information was kept confidential during data analysis. COVID-19 infection prevention measures recommended by the WHO were strictly followed during data collection to reduce the. The Declaration of Helsinki was strictly followed during the data collection.
Results
Sociodemographic characteristics
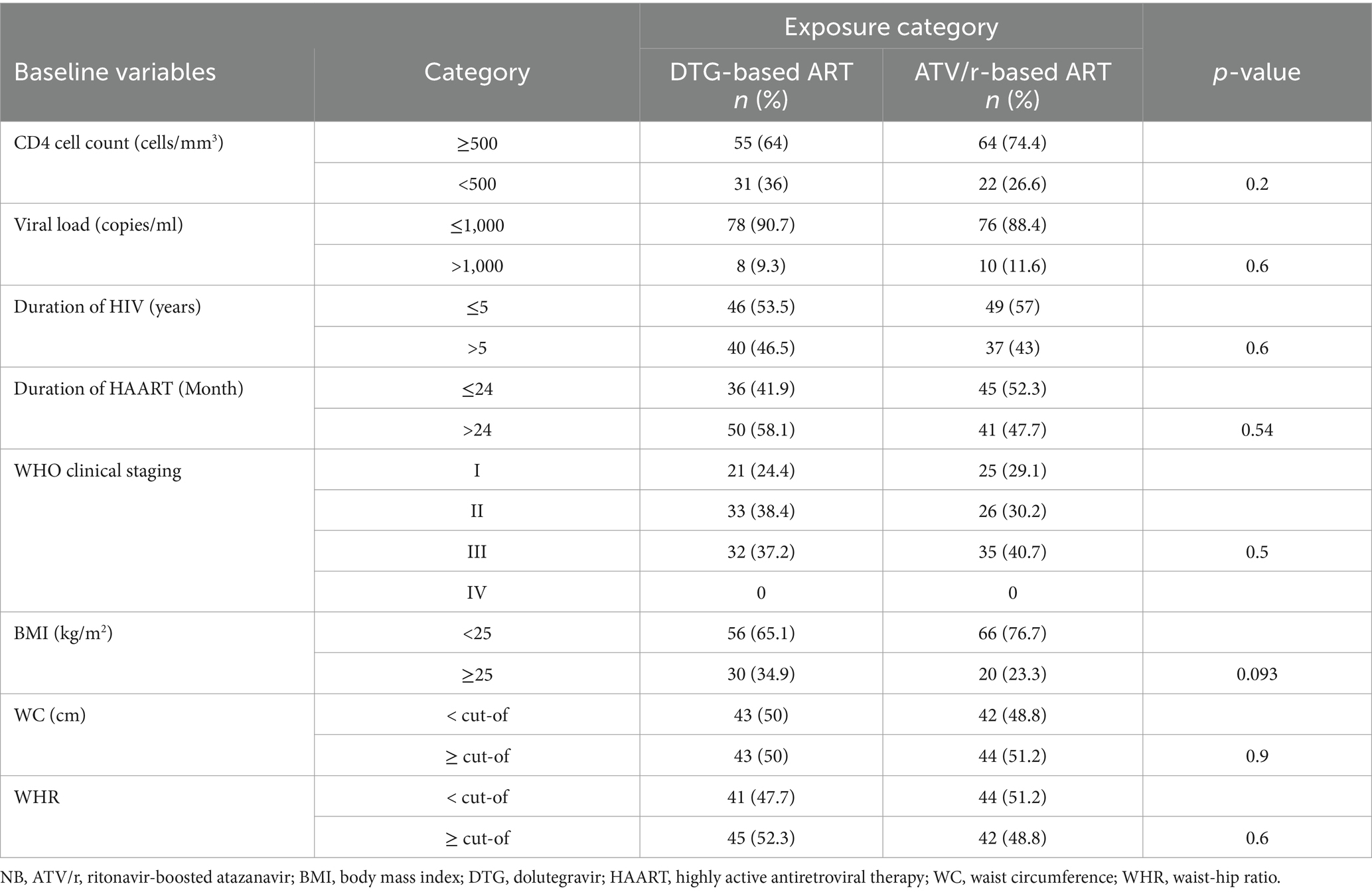
From a total of 172 participants, which is described in Table 1, 86 were DTG-treated and 86 were ATV/r-treated patients. The proportion of patients with an age greater than or equal to 40 was 52.3% (45/86) and 50% (43/86) among DTG and ATV/r-based regimens, respectively (p = 0.8). Regarding gender, 66.3% (57/86) and 69.8% (60/86) of them were female on DTG- and ATV/r-based regimens, respectively.
Clinical characteristics of the study participants
This study shows the proportion of patients with CD4 cell count <500 cells/mm3 was 36% (31/86) for DTG and 26.6% (22/86) for ATV/r-based regimen. Patients who had taken ART for more than 24 months were 58.1% (50/86) on DTG and 47.7% (41/86) on the ATV/r-based regimen. Furthermore, 34.9% (30/86) of DTG-treated and 23.3% (20/86) of ATV/r-treated patients had a BMI ≥ 25 kg/m2 (p = 0.093) (for more, see Table 2).
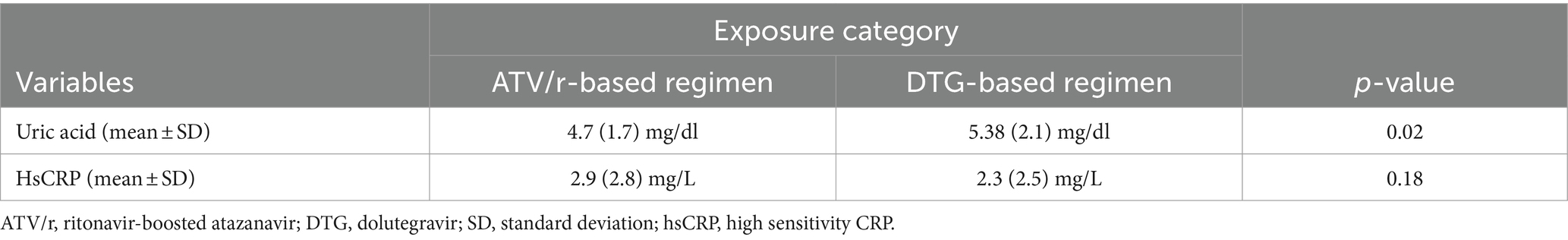
Mean difference among study participants
Table 3 shows higher mean levels of uric acid were observed among patients treated by DTG compared to ATV/r-based regimens (p = 0.02), but insignificant differences were observed regarding hsCRP levels (p = 0.18).
Prevalence of hyperuricemia among study participants
The prevalence of hyperuricemia shown in Figure 1 was 46.5% (40/86) and 30.2% (26/86) among patients treated by DTG and ATV/r-based regimens of ART, respectively.
The prevalence of hsCRP levels ≥2 mg/L
This study found (Figure 2) that 24.4% (21/86) of patients on the DTG-based regimen and 44.2% (38/86) on the ATV/r-based regimen have increased hsCRP levels ≥2 mg/L.

Figure 2. Prevalence of elevated hsCRP levels among people living with HIV taking DTG and ATV/r-based ART.
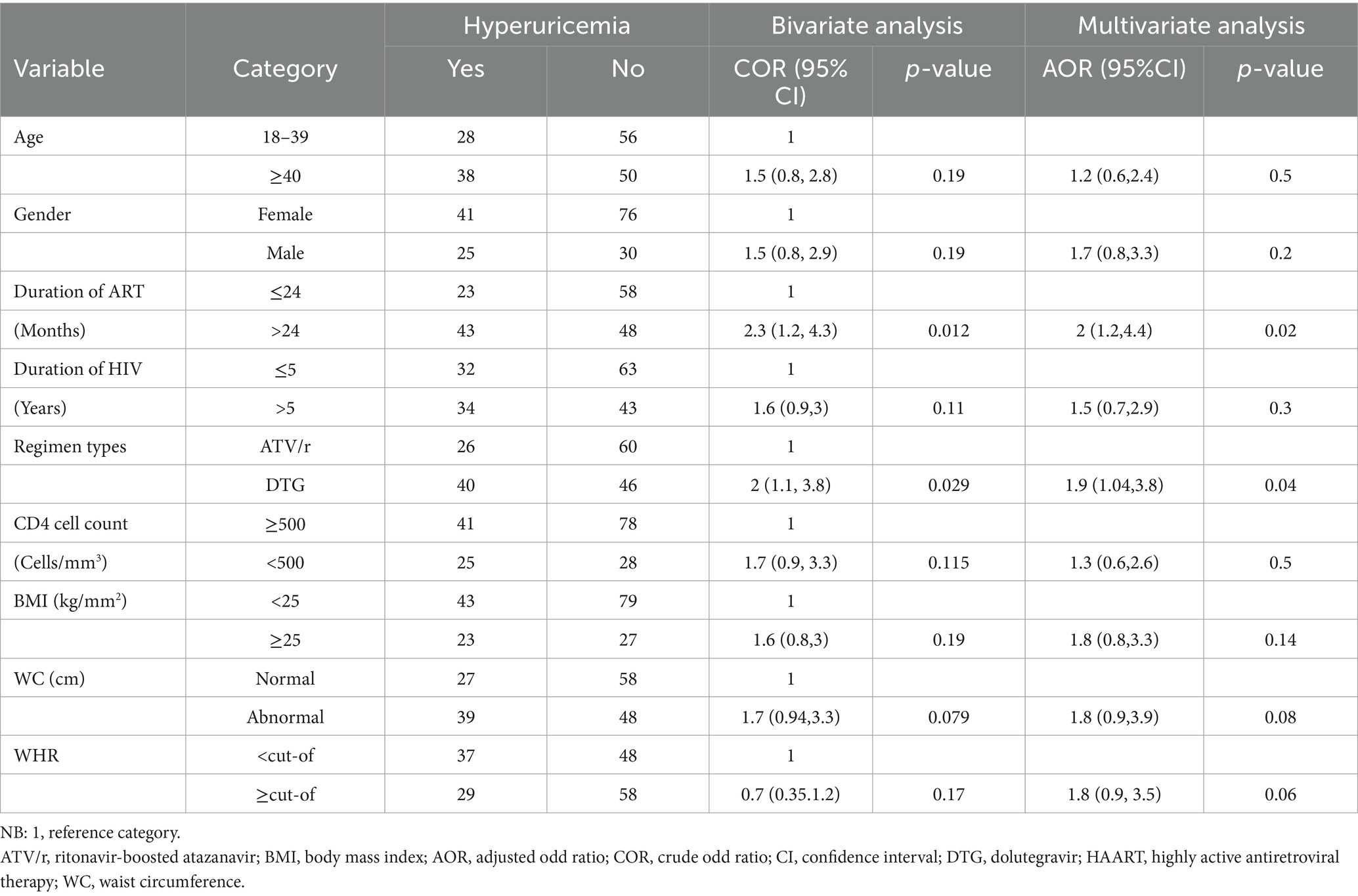
Factors associated with hyperuricemia in people living with HIV on DTG and ATV/r-based ART
Multivariable logistic regression was performed for all predictor variables with p < 0.25 in the bivariate analysis (Table 4). In the multivariate analyses, the duration of ART and DTG-based ART were identified as significant predictors of hyperuricemia. It was observed that the odds of developing hyperuricemia were two times (AOR = 1.9; 95% CI: 1.04, 3.8; p-values = 0.04) more likely for patients treated with DTG than ATV/r-based regimen. Our study also revealed that patients living with HIV for more than 5 years had two times (AOR = 2; 95% CI: 1.2, 4.4; p-value = 0.02) more chance of having hyperuricemia than those living with HIV for less than or equal to 5 years.
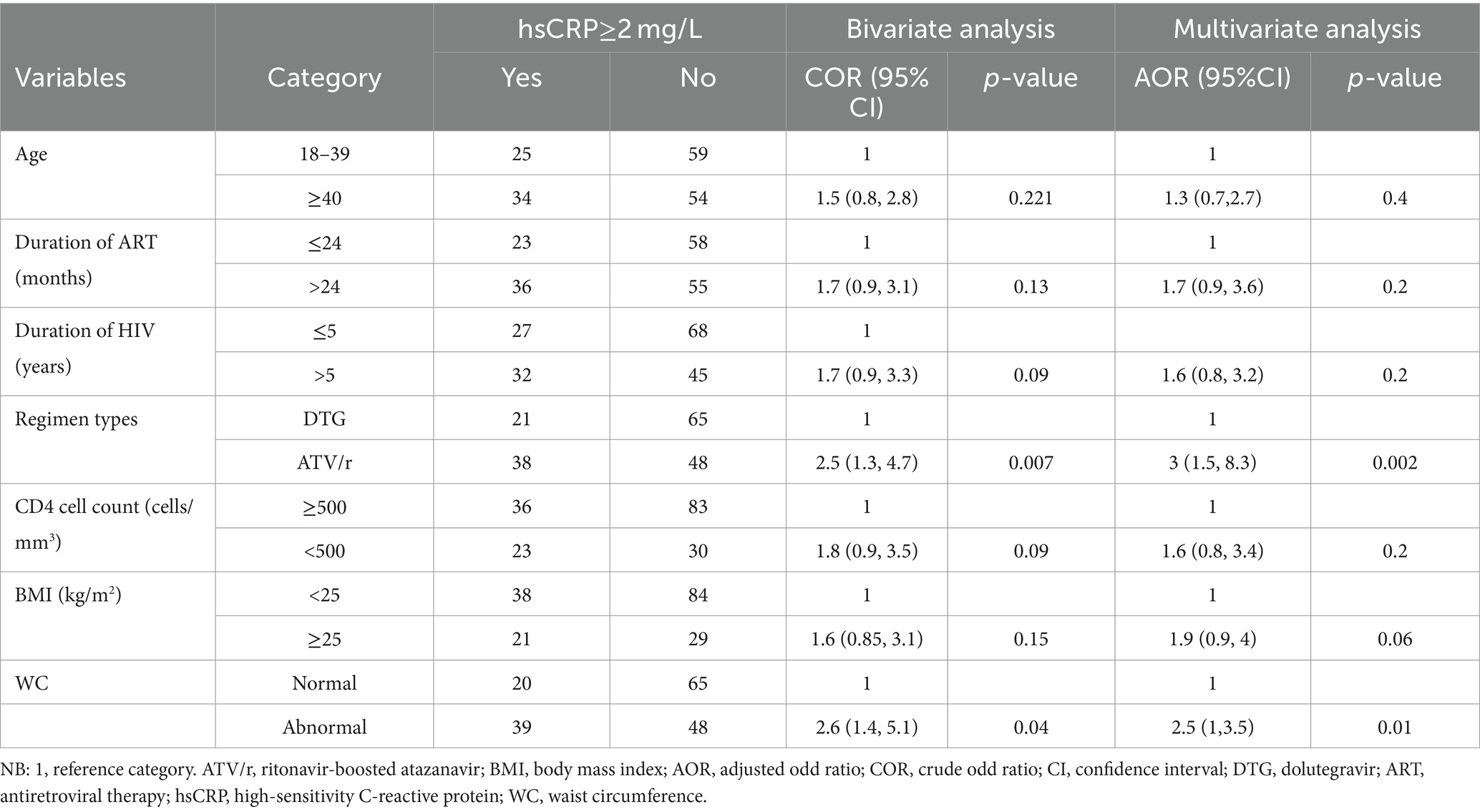
Factors associated with elevated hsCRP levels among people living with HIV on DTG-based and ATV/r-based ART
Bivariate and multivariate analyses of the factors associated with elevated hsCRP levels are presented by the following (Table 5). All variables with p < 0.25 (age, duration of ART, HIV, BMI, WC, regimen type, and CD4 cell count) were included in the multivariate analysis. After adjusting for all these variables in multiple logistic regressions, ATV/r-based ART (AOR = 3; 95% CI: 1.5, 8.3; p = 0.002) and waist circumference (AOR = 2.5; 95% CI: 1.3, 5; p = 0.01) were identified as significant predictors of elevated hsCRP levels.
Discussion
This study explored the levels of uric acid and hsCRP as well as their associated factors among people living with HIV taking dolutegravir and ritonavir-boosted antiretroviral therapy. This is the first study in Ethiopia, given that the drug (DTG) is relatively new for HIV management in Ethiopia.
The prevalence of hyperuricemia among the whole participants was 76.7% (66/172), of which 46.5% (40/86) and 30.2% (26/86) were DTG-treated and ATV/r-treated patients, respectively (p = 0.028). It also found a higher mean level of uric acid among the patients treated by the DTG-based regimen than the ATV/r-based regimens. This observation is comparable to study findings from China (59), Portugal (27), and Japan (26) but is incongruent with another study finding reported from Japan (60). The proportion of treatment-naïve patients (71.6% in their study and with all were treatment-experienced in our study), male participants (91.9 and 51.1% in Japan and our study, respectively), in addition to study design, sociodemographic characteristics and differences in ethnicity might contribute to this incongruency of findings Although individuals of Africans origins had the lowest hyperuricemia and gout risk allele frequencies than Asian descent (61), they may respond differently to ART to result in hyperuricemia.
Consequently, according to our study findings, it was observed that the proportion of patients with increased hsCRP levels ≥2 mg/L was 24.4% (21/86) among patients with a DTG-based regimen and 44.2% (38/86) for patients treated with ATV/r-based regimen (p = 0.006), which was in line with San Francisco (52) and Boston (62). On the contrary, our finding is inconsistent with a study conducted in California (63). The possible reasons for this might be differences in sociodemographic, lifestyle, study design, ART combination, and ART exposure status. For instance, the study from California used TDF plus FTC as the backbone, which is TDF plus 3TC in our study. The participants were treatment-naïve in California and treatment-experienced in our study. In additions, the differences in race (which is whites in California and black Africans in our study) might contribute for this inconsistency of findings. For instance, the elevated levels of hsCRP were seen among African peoples compared to Europe and the United states (34). It is also essential to note that ART response of individuals might be varied due to variation in genetics that contributed for different plasma levels of hsCRP (64). Furthermore, our study was designed to assess factors associated with hyperuricemia and increased hsCRP levels. Accordingly, patients taking ART for more than 24 months were two times more likely to have hyperuricemia. This is similar to studies done in China and Nigeria (23, 65). Patients treated with a DTG-based regimen had a two-fold higher risk of developing hyperuricemia, which was in agreement with a study finding from China (59), which showed the number of patients with hyperuricemia increased with DTG-containing ART. ART-induced mitochondrial toxicity with increased lactate to compete with uric acid excretion by the renal tubules and increased cell turnover followed by increased degradation of nucleotides accompanied by elevated uric acid in the serum were some of the possibilities by which uric acid is elevated in people living with HIV on HAART (66–69). Patients with waist circumferences above the cutoff level had a two-fold increased chance of developing hsCRP levels ≥2 mg/L, which is in line with a study done in Brazil (70). This might be due to the fact that in obese patients, adipocytes produce powerful pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and IL-6, which in turn stimulate the secretion of CRP in the liver and endothelial cells (71). Elevated hsCRP levels increased the risk of HIV patients on HAART for ASCVD, independent of traditional risk factors (33).
The ATV/r was associated with elevated hsCRP levels. This is comparable to study findings from Spain (40) and Italy (72). Ubiquitin-specific protease 18 (USP18) inhibitions lead to activation of nuclear factor kB (NF-kB) signaling, and augmented expression of pro-inflammatory cytokines with elevated inflammatory biomarkers is the possible way by which ATV/r increases the serum level of hsCRP levels (38).
Limitations and strength
Concerning strength, this was the first study in Ethiopia that attempted to assess the effects of DTG and ATV/r-based regimens on serum uric acid and high-sensitivity C-reactive protein levels among people living with HIV, hence ultimately adding to the limited data. In spite of this strength, this study has several weaknesses. Since it is a cross-sectional study, we cannot associate causal relationships between the factors and outcomes under study. In addition, the study sample size was small; thus, it is difficult to generalize the findings to larger populations. Moreover, HIV-positive ART-naïve controls were not included, which may remove the effect of ART taken before starting DTG and ATV/r-based regimens of ART.
Conclusion and recommendations
In general, our study showed a higher mean as well as proportion of uric acid with the use of a DTG-based regimen, whereas a higher proportion of hsCRP levels was found among those treated by ATV/r-based regimens. Being on a DTG-based regimen and taking HAART for a long period of time was significantly associated with hyperuricemia. Increased waist circumference and ATV/r-based regimen were identified as significant predictors associated with increased hsCRP levels. Therefore, it is important to consider and evaluate hsCRP levels for patients with increased waist circumference and treated by ATV/r containing ART, along with uric acid levels for patients on HAART for a long duration and taking DTG containing ART, to prevent the risk of having ASCVD. For this, policymakers should take these factors into account when developing public health initiatives on ART side effects management and when strengthening on going non-communicable disease reduction programs. We propose that researchers conduct a prospective cohort study with a larger sample size to determine the exact effects of DTG and ATV/r on serum uric acid and hsCRP levels. Lastly, we suggest a comparative analysis of DTG and ATV/r containing ART in ART-naïve, HIV-positive patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Jimma University with reference number: IHRPG1/5/2021. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NW: Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Investigation, Supervision. SN: Conceptualization, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. RU: Formal analysis, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. MJ: Data curation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge the data collectors and all staff at the ART clinic of JUMC for their support during data collection, Jimma University Medical Center, Jimma University, and Wolaita Sodo University for their unreserved contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; ATV/r, ritonavir-boosted atazanavir; BMI, body mass index; CD4, cluster of differentiation 4; CVD, cardiovascular disease; DTG, dolutegravir; EFV, efavirenz; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; INSI, integrase strand transfer inhibitor; JUMC, Jimma University Medical Centre; NF-kB, nuclear factor kappa B; SPSS, statistical package for social sciences; TDF, tenofovir; UA, uric acid.
References
1. Kharsany, ABM, and Karim, QA. HIV infection and AIDS in sub-Saharan Africa: current status. Open AIDS. (2016) 10:34–48. doi: 10.2174/1874613601610010034
2. Ng, IYH, Shen, X, Sim, H, Sarri, RC, Stoffregen, E, and Shook, JJ. 基因的改变NIH public access. J Neurochem. (2015) 4:1–15.
3. Getahun, Z, Azage, M, Abuhay, T, and Abebe, F. Comorbidity of HIV, hypertension, and diabetes and associated factors among people receiving antiretroviral therapy in Bahir Dar city, Ethiopia. J Comorbidity. (2020) 10:2235042X1989931. doi: 10.1177/2235042X19899319
4. Pelchen-Matthews, A, Ryom, L, Borges, AH, Edwards, S, Duvivier, C, Stephan, C, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. (2018) 32:2405–16. doi: 10.1097/QAD.0000000000001967
5. Osegbe, ID, Soriyan, OO, Ogbenna, AA, Okpara, HC, and Azinge, EC. Risk factors and assessment for cardiovascular disease among HIV-positive patients attending a Nigerian tertiary hospital. Pan Afr Med J. (2016) 23:1–9. doi: 10.11604/pamj.2016.23.206.7041
6. Sani, M, Okeahialam, B, and Muhammad, S. Cardiovascular disease risk factors among HIV-infected Nigerians receiving highly active antiretroviral therapy. Niger Med J. (2013) 54:185–90. doi: 10.4103/0300-1652.114591
7. Kaplan-Lewis, E, Aberg, JA, and Lee, M. Atherosclerotic cardiovascular disease and anti-retroviral therapy. Curr HIV/AIDS Rep. (2016) 13:297–308. doi: 10.1007/s11904-016-0331-y
8. Woldu, M, Minzi, O, Shibeshi, W, and Shewaamare, A. Predicting the risk of atherosclerotic cardiovascular disease among adults living with HIV/AIDS in Addis Ababa, Ethiopia. PLoS one. (2021) 16:1–19. doi: 10.1371/journal.pone.0260109
9. Nardi, V, Franchi, F, Prasad, M, Fatica, EM, Alexander, MP, Bois, MC, et al. Uric acid expression in carotid atherosclerotic plaque and serum uric acid are associated with. Hypertension. (2022) 79:1814–23. doi: 10.1161/HYPERTENSIONAHA.122.19247
10. Spiga, R, Marini, MA, Mancuso, E, Di, FC, Fuoco, A, Perticone, F, et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF- κ B signaling pathway in HepG2 cells. Arter Thromb Vasc Biol. (2017) 37:1241–9. doi: 10.1161/ATVBAHA.117.309128
11. Yao, S, Zhou, Y, Xu, L, Zhang, Q, and Bao, S. Association between hyperuricemia and metabolic syndrome: a cross-sectional study in Tibetan adults on the Tibetan plateau. Front Endocrinol. (2022) 13:964872–2. doi: 10.3389/fendo.2022.964872
12. Pirro, M, Bianconi, V, Schiaroli, E, Francisci, D, Mannarino, MR, Bagaglia, F, et al. Elevated serum uric acid levels are associated with endothelial dysfunction in HIV patients receiving highly-active antiretroviral therapy. Atherosclerosis. (2018) 272:101–7. doi: 10.1016/j.atherosclerosis.2018.03.031
13. Cammalleri, L, and Malaguarnera, M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. (2007) 4:83–93. doi: 10.7150/ijms.4.83
14. Lee, SJ, Oh, BK, and Sung, K. Uric acid and cardiometabolic diseases. Clin Hypertens. (2020) 26:1–7. doi: 10.1186/s40885-020-00146-y
15. Hu, Y, Zhao, H, Lu, J, Xie, D, Wang, Q, Huang, T, et al. High uric acid promotes dysfunction in pancreatic β cells by blocking IRS2/AKT signalling. Mol Cell Endocrinol [Internet]. (2021) 520:111070. doi: 10.1016/j.mce.2020.111070
16. Kimura, Y, Tsukui, D, and Kono, H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci. (2021) 22:12394. doi: 10.3390/ijms222212394
17. Chaparala, S, DA, SRC, and Papadopoulos, JP. Severe lactic acidosis due to acute intoxication by Emtricitabine/Tenofovir Alafenamide case presentation. Open Access Case Rep. (2021) 13:13–6. doi: 10.7759/cureus.19008
18. Hamed, MA, Aremu, AO, and Akhigbe, RE. Biomedicine and Pharmacotherapy Concomitant administration of HAART aggravates anti-Koch-induced oxidative hepatorenal damage via dysregulation of glutathione and elevation of uric acid production. Biomed Pharmacother. (2021) 137:111309. doi: 10.1016/j.biopha.2021.111309
19. Deme, P, Rubin, LH, Yu, D, Xu, Y, Nakigozi, G, Nakasujja, N, et al. Immunometabolic reprogramming in response to HIV infection is not fully normalized by suppressive antiretroviral therapy. Viruses. (2022) 14:313. doi: 10.3390/v14061313
20. Onwubuya, EI, Ukibe, NR, Kalu, OA, Nwabueze, S, and IMOJ, O. Assessment of kidney function, estimated glomerular filtration rate and body mass index in HIV seropositive subjects on antiretroviral therapy in Nnewi. Int J Pharm Pharm Sci. (2018) 10:291. doi: 10.22159/ijpps.2018v10i8.27291
21. Subjects, IV-I, Adeleke, TD, and Emokpae, MA. Assessment of some biomarkers of renal Function and myoglobin level in human immunodeficiency Virus-1 infected Subjects. J Med Lab Sci. (2019) 29:67–72.
22. Nicholson, P, Saunsbury, E, Angelo, SD, Churchill, D, and Walker-bone, K. Prevalence of and risk factors for gout in HIV-positive adults: a case – control study. Int J STD AIDS. (2019) 30:249–55. doi: 10.1177/0956462418799803
23. Priscilla, EI, Clement, A, Chukwuemeka, MS, Adamma, AR, Chinonso, NJ, Nwabunwanne, OV, et al. Evaluation of microalbumin, cystatin c, creatinine and uric acid levels in HIV patients in Nnamdi Azikiwe university teaching Hospital. Nnewi J Community Heal Manag. (2021) 8:132–42. doi: 10.18231/j.jchm.2021.030
24. Zhao, B, and Zhang, X. Gradually increased dyslipidemia in human immunodeficiency virus infected male patients with tenofovir plus lamivudine plus efavirenz primary treatment: a 3-year follow-up study. Res Sq. (2020):1–18.
25. Alter, G, Suscovich, TJ, Kleyman, M, Teigen, N, Streeck, H, Tauheed, M, et al. Research letters. AIDS. (2006) 20:1549–51. doi: 10.1097/01.aids.0000237371.31315.48
26. Hongo, H, Nagao, T, Nakamura, K, Kitaichi, T, Maeno, Y, Tokunaga, T, et al. Safety and effectiveness analysis of Dolutegravir in patients with HIV-1: interim report of Post-marketing surveillance in Japan. Adv Ther. (2021) 38:4480–504. doi: 10.1007/s12325-021-01842-3
27. Fernandes, SR, Leite, AR, Lino, R, Guimarães, AR, Pineiro, C, Serrão, R, et al. The impact of integrase inhibitors on steatosis and fibrosis biomarkers in persons with HIV naïve to antiretroviral therapy. BMC Infect Dis. (2023) 23:1–11. doi: 10.1186/s12879-023-08530-3
28. Nishijima, T, Hamada, Y, Watanabe, K, Komatsu, H, Kinai, E, Tsukada, K, et al. Ritonavir-boosted Darunavir is rarely associated with nephrolithiasis compared with ritonavir-boosted Atazanavir in HIV-infected patients. PLoS One. (2013) 8:1–8. doi: 10.1371/journal.pone.0077268
29. Adenuga, MA, Gambo, PP, Yakunat, OE, Dus, CA, Dalyop, KA, Adeyanju, ON, et al. Assessment of uric acid level of HIV patients on protease inhibitors attending faith alive foundation, JOS. IOSR J Environ Sci Toxicol Food Technol. (2017) 11:37–42. doi: 10.9790/2402-1106023742
30. Koosha, P, Roohafza, H, and Sarrafzadegan, N. High sensitivity C-reactive protein predictive value for cardiovascular disease: a nested case control from Isfahan cohort study (ICS). Glob Heart. (2020) 15:3–13. doi: 10.5334/gh.367
31. Appiah, LT, Sarfo, FS, Nguah, SB, Mark, D, Stiles, JK, and Feinstein, MJ. Lipoprotein (a) and high sensitivity C-reactive protein among patients with HIV in Ghana: the study on cardiovascular risk profile of HIV-infected patients on HAART (SCRIPT). Glob Heart. (2020) 15:74. doi: 10.5334/gh.850
32. Ridker, PM, Macfadyen, JG, Glynn, RJ, Bradwin, G, Hasan, AA, and Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the cardiovascular inflammation reduction trial. Eur Heart J. (2020) 41:2952–61. doi: 10.1093/eurheartj/ehaa160
33. De Luca, A, de Gaetano, DK, Colafigli, M, Cozzi-Lepri, A, De Curtis, A, Gori, A, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case-control study. BMC Infect Dis. (2013) 13:414. doi: 10.1186/1471-2334-13-414
34. Mabhida, SE, Mchiza, ZJ, Mokgalaboni, K, Hanser, S, Choshi, J, and Mokoena, H. High – sensitivity C – reactive protein among people living with HIV on highly active antiretroviral therapy: a systemic review and meta – analysis. BMC Infect Dis. (2024) 24:1–17. doi: 10.1186/s12879-024-09050-4
35. Kwame, WO, Jonathan, EF, Twimasiwah, FM, Berko, SB, Maxwell, A, and Albert, D. GSC biological and pharmaceutical sciences levels of pro-inflammatory biomarkers among HIV patients on highly active antiretroviral therapy (HAART) and HAART naive patients. GSC Biol Pharm Sci. (2018) 4:017–23. doi: 10.30574/gscbps.2018.4.2.0056
36. Hanser, S, Mphekgwana, PM, Moraba, MM, Erasmus, L, and van Staden, M. Increased endothelial biomarkers are associated with HIV antiretroviral therapy and C-reactive protein among a African rural population in Limpopo Province, South Africa. Front Public Heal. (2022) 10:980784. doi: 10.3389/fpubh.2022.980754
37. MacAtangay, BJ, Yang, M, Sun, X, Morton, J, De Gruttola, V, Little, S, et al. Changes in levels of inflammation after antiretroviral treatment during early HIV infection in AIDS clinical trials group study a5217. J Acquir Immune Defic Syndr. (2017) 75:137–41. doi: 10.1097/QAI.0000000000001320
38. Auclair, M, Guénantin, AC, Fellahi, S, Garcia, M, and Capeau, J. HIV antiretroviral drugs, dolutegravir, maraviroc and ritonavir-boosted atazanavir use different pathways to affect inflammation, senescence and insulin sensitivity in human coronary endothelial cells. PLoS One. (2020) 15:1–23. doi: 10.1371/journal.pone.0226924
39. Waritu, NC, Nair, SKP, Birhan, B, Adugna, T, Awgichew, GB, and Jemal, M. Serum lipid profiles, blood glucose, and high-sensitivity C-reactive protein levels among people living with HIV taking Dolutegravir and ritonavir-boosted Atazanavir-based antiretroviral therapy at Jimma University medical center, Southwest Ethiopia, 2021. HIV/AIDS. (2024) 16:17–32. doi: 10.2147/HIV.S430310
40. Martínez, E, D’Albuquerque, PM, Llibre, JM, Gutierrez, F, Podzamczer, D, Antela, A, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. (2012) 26:2315–26. doi: 10.1097/QAD.0b013e328359f29c
41. Randell, P, Jackson, A, Milinkovic, A, Boffito, M, and Moyle, G. An open-label, randomized study of the impact insulin sensitivity, lipid profile and vascular by treatment with lopinavir/ritonavir raltegravir in HIV-negative male volunteers. Antivir Ther. (2017) 22:145–51. doi: 10.3851/IMP3098
42. Quiros-Roldan, E, Castelli, F, Bonito, A, Vezzoli, M, Calza, S, Biasiotto, G, et al. The impact of integrase inhibitor-based regimens on markers of inflammation among HIV naïve patients. Cytokine. (2020) 126:154884. doi: 10.1016/j.cyto.2019.154884
43. Llibre, JM, Buzón, MJ, Massanella, M, Esteve, A, Dahl, V, Puertas, MC, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther. (2012) 17:355–64. doi: 10.3851/IMP1917
44. Llibre, JM, Cahn, PE, Lo, J, Barber, TJ, Mussini, C, Van, WBJ, et al. Changes in inflammatory and Atherogenesis biomarkers with the 2-drug regimen Dolutegravir plus lamivudine in antiretroviral therapy – experienced, virologically suppressed people with HIV-1: a systematic literature review. Open Forum Infect Dis. (2022) 9:2–10. doi: 10.1093/ofid/ofac068
45. Gahlot, A, Gahlot, P, and Acharya, J. A cross sectional study on correlation of quantitative C – reactive protein with CD4 count in patients of HIV on art. Acad J Med ¦. (2019) 2:14–7. doi: 10.21276/ajm.2019.2.2.5
46. Patel, NJ, Sheth, HS, Rajan, R, and Espinoza, LR. Techniques in infectious diseases hyperuricemia, its prevalence and correlation with metabolic syndrome in anti-retroviral naive HIV cohort: review of the literature. J Immunol Tech Infect Dis. (2013) 2:10–3. doi: 10.4172/2329-9541.1000108
47. Wada, NI, Breen, EC, Post, WS, Stosor, V, MacAtangay, BJ, and Margolick, JB. Long-term trajectories of C-reactive protein among men living with and without HIV infection in the multicenter AIDS cohort study. J Gerontol Ser A Biol Sci Med Sci. (2022) 77:1382–8. doi: 10.1093/gerona/glab190
48. WHO. Update of FIRST- and second-line antiretroviral regimens. (2019). Available at: https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15 (Accessed September 25, 2023).
49. Health F Democratic Republic of EM. Implementation manual for DTG rollout and ART optimization in Ethiopia. (2019).
50. Shah, BM, Schafer, JJ, and Desimone, JA. Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy. (2014) 34:506–20. doi: 10.1002/phar.1386
51. Zamora, F, and Ogbuagu, O. Dolutegravir/rilpivirine for the treatment of HIV-1 infection. HIV/AIDS. (2018) 10:215–24. doi: 10.2147/HIV.S157855
52. McComseya, GA, Kitchb, D, Daarc, ES, and Camlin, TN. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir: ACTG A5224 s, A5202 substudy. AIDS. (2013) 26:1371–85. doi: 10.1097/QAD.0b013e328354f4fb
53. WHO. Waist circumference and waist-hip ratio report of a WHO expert Consultation. (2008). Available at: https://www.who.int/publications/i/item/9789241501491 (Accessed September 27, 2023).
54. Becker, Diane M., The Johns Hopkins University Claude Bouchard PDLUR. Guide identification, evaluation, and treatment of overweight and obesity in adults. (2000).
55. Zhang, M, Gao, Y, Wang, X, Chang, H, and Huang, G. Serum uric acid and appropriate cutoff value for prediction of metabolic syndrome among Chinese adults. J Clin Biochem Nutr. (2013) 52:38–42. doi: 10.3164/jcbn.12-65
56. Arnett, DK, Blumenthal, RS, Albert, MA, Buroker, AB, Goldberger, ZD, Hahn, EJ, et al. Circulation ACC/AHA CLINICAL PRACTICE GUIDELINE 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease association task force on clinical practice guidelines. (2019).
57. Federal Ministry of Health. Guidelines for management of opportunistic infections and anti-retroviral treatment in adolescents and adults in Ethiopia. (2008). Available at: https://hivpolicywatch.org/duremaps/data/guidelines-rename/EthiopiaAdultARTguidelines2008.pdf (Accessed September 27, 2023).
58. Moncivaiz, A, and Alexander, D. CD4 vs. Viral Load: What’s in a Number? 2019; 1–15. Available at: https://www.healthline.com/health/hiv-aids/cd4-viral-count#cd-count
59. Study O. Tolerability and effectiveness of albuvirtide combined with dolutegravir for hospitalized people living with HIV/AIDS. Medicine. (2023) 102:e35344. doi: 10.1097/MD.0000000000035344
60. Hamada, Y, Nishijima, T, Watanabe, K, Komatsu, H, Tsukada, K, Teruya, K, et al. High incidence of renal stones among HIV-infected patients on ritonavir-boosted atazanavir than in those receiving other protease inhibitor-containing antiretroviral therapy. Clin Infect Dis. (2012) 55:1262–9. doi: 10.1093/cid/cis621
61. Butler, F, Alghubayshi, A, and Roman, Y. The epidemiology and genetics of hyperuricemia and gout across major racial groups: a literature review and population genetics secondary database analysis. Per Med. (2021) 11:1–15. doi: 10.3390/jpm11030231
62. Aberg, JA, Tebas, P, Overton, ET, Gupta, SK, Sax, PE, Landay, A, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retrovir. (2012) 28:1184–95. doi: 10.1089/aid.2011.0327
63. Kelesidis, T, Tran, TTT, Stein, JH, Brown, TT, Moser, C, Ribaudo, HJ, et al. Changes in in flammation and immune activation with Atazanavir-, Raltegravir-, Darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis. (2015) 61:651–60. doi: 10.1093/cid/civ327
64. Lee, CC, You, NY, Angeles, L, Song, Y, Hospital, W, and Hsu, Y. Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women’s health initiative observational cohort. Clin Chem. (2009) 55:351–60. doi: 10.1373/clinchem.2008.117176
65. Wang, X, Xiao, J, Zhang, L, Liu, Y, Chen, N, Deng, M, et al. Longitudinal analysis of immune reconstitution and metabolic changes in women living with HIV: a real-world observational study. Chin Med J. (2023) 136:2168–77. doi: 10.1097/CM9.0000000000002756
66. Liu, G, Chen, X, Lu, X, Zhao, J, and Li, X. Sun flower head enzymatic hydrolysate relives hyperuricemia by inhibiting crucial proteins (xanthine oxidase, adenosine deaminase, uric acid transporter1) and restoring gut microbiota in mice. J Funct Foods. (2020) 72:104055. doi: 10.1016/j.jff.2020.104055
67. Pasupathi, P, Ramchandran, T, Sindhu, PJ, Saranavan, G, and Bakthavathsalam, G. Enhanced oxidative stress markers and antioxidant imbalance in HIV infection and AIDS patients. J Sci Res. (2009) 1:370–80. doi: 10.3329/jsr.v1i2.2295
68. Wester, CW, Eden, SK, Shepherd, BE, Bussmann, H, Novitsky, V, Samuels, DC, et al. Risk factors for symptomatic hyperlactatemia and lactic acidosis among combination antiretroviral therapy-treated adults in Botswana: results from a clinical trial. AIDS Res Hum Retrovir. (2012) 28:759–65. doi: 10.1089/aid.2011.0303
69. Patel, K. Lactic acidosis in HIV-1 infected patients receiving antiretroviral therapy (2004) 52:666–9.
70. Guimarães, MMM, Greco, DB, De, FSM, Fóscolo, RB, De, OAR, and De C, MLJ. High-sensitivity C-reactive protein levels in HIV-infected patients treated or not with antiretroviral drugs and their correlation with factors related to cardiovascular risk and HIV infection. Atherosclerosis. (2008) 201:434–9. doi: 10.1016/j.atherosclerosis.2008.02.003
71. Pearson, TA, Mensah, GA, Alexander, RW, Anderson, JL, Cannon, RO, Criqui, M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
72. De Lima, LRA, Petroski, EL, Moreno, YMF, Silva, DAS, De Moraes Santos Trindade, EB, De Carvalho, AP, et al. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: the PositHIVe health study. PLoS One. (2018) 13:1–17. doi: 10.1371/journal.pone.0190785
Keywords: hyperuricemia, high-sensitivity C-reactive protein, dolutegravir, ritonavir-boosted atazanavir, HIV
Citation: Waritu NC, Nair SKP, Usure RE and Jemal M (2024) Serum uric acid and high-sensitivity C-reactive protein levels among people living with HIV on dolutegravir and ritonavir-boosted atazanavir-based antiretroviral therapy: a comparative cross-sectional study. Front. Med. 11:1370725. doi: 10.3389/fmed.2024.1370725
Edited by:
Alexandre Morrot, Federal University of Rio de Janeiro, BrazilReviewed by:
Phiwayinkosi V. Dludla, South African Medical Research Council, South AfricaAlvina Widhani, University of Indonesia, Indonesia
Copyright © 2024 Waritu, Nair, Usure and Jemal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuredin Chura Waritu, bnVybWF5ZTEyQGdtYWlsLmNvbQ==; bnVyZWRpbi5jaHVyYUB3c3UuZWR1LmV0; Mohammed Jemal, bW9oYWplbTk4MDFAZ21haWwuY29t
 Nuredin Chura Waritu
Nuredin Chura Waritu Suresh Kumar P. Nair2
Suresh Kumar P. Nair2 Rashed Edris Usure
Rashed Edris Usure Mohammed Jemal
Mohammed Jemal