- 1Department of Critical Care Medicine, Southern University of Science and Technology Yantian Hospital, Shenzhen, Guangzhou Province, China
- 2Department of Critical Care Medicine, Changshu Hospital Affiliated to Nanjing University of Traditional Chinese Medicine, Changshu, Jiangsu, China
- 3Department of Cardiovascular Medicine, The Third Xiangya Hospital of Central South University, Changsha, Hunan, China
- 4Department of Emergency Center, Affiliated Huaian Hospital of Xuzhou Medical University, Huaian, China
- 5Center for Tuberculosis Control of Guangdong Province, Guangzhou, China
- 6Department of Traditional Chinese Medicine, Changshu Hospital Affiliated to Nanjing University of Traditional Chinese Medicine, Changshu, Jiangsu, China
Background: The purpose of this network meta-analysis (NMA) was to evaluate the efficacy of intravenous opioid μ-receptor analgesics in shortening the duration of mechanical ventilation (MV) in ICU patients.
Methods: Randomized controlled trials comparing the efficacy of remifentanil, sufentanil, morphine, and fentanyl on the duration of MV in ICU patients were searched in Embase, Cochrane, Pubmed, and Web of Science electronic databases. The primary outcome was MV duration. The Bayesian random-effects framework was used to evaluate relative efficacy.
Results: In total 20 studies were included in this NMA involving 3,442 patients. Remifentanil was not associated with a reduction in the duration of MV compared with fentanyl (mean difference (MD) -0.16; 95% credible interval (CrI): −4.75 ~ 5.63) and morphine (MD 3.84; 95% CrI: −0.29 ~ 10.68). The secondary outcomes showed that, compared with remifentanil, sufentanil can prolong the duration of extubation. No regimen significantly shortened the ICU length of stay and improved the ICU mortality, efficacy, safety, and drug-related adverse events.
Conclusion: Among these analgesics, remifentanil did not appear to be associated with a reduction in MV duration. Clinicians should carefully titrate the analgesia of MV patients to prevent a potentially prolonged duration of MV due to excessive or inadequate analgesic therapy.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, CRD42021232604.
Highlights
• Question: Is remifentanil more effective than other intravenous opioid analgesics at reducing mechanical ventilation duration in ICU patients?
• Findings: Compared to other intravenous analgesics that target the μ-receptor opioid, Remifentanil did not show any decrease in the length of mechanical ventilation.
• Meaning: To prevent excessive or inadequate analgesia prolonging the duration of mechanical ventilation, clinicians are advised to carefully titrate analgesia.
Introduction
Description of the intervention
Intensive care unit (ICU) patients on invasive mechanical ventilation (MV) experience pain, especially in patients requiring long-term MV (1–4). These unpleasant sensory experiences may prevent MV weaning (5). Therefore, preemptive analgesic therapy should be administered to ICU patients on MV to alleviate pain (5–7).
Intravenous (IV) opioid μ-receptor analgesics, such as morphine, fentanyl, and sufentanil, are considered first-line drugs for the treatment of nonneuropathic pain (5, 7, 8). However, they have long half-lives and are easily redistributed and accumulated. Even when administered at doses normally used for several days, they are associated with increased respiratory depression and prolonged duration of MV (5, 9–17). Hence, the use of fentanyl, sufentanil, and morphine should be restricted to mechanically ventilated patients requiring long-term analgesia (4, 7, 18).
Remifentanil is a potent selective μ-opioid receptor that is rapidly metabolized by non-specific esterases into inactive metabolites (19, 20). As a result, regardless of the dose and duration of infusion, its onset and offset are very rapid and its context-sensitive half-life is extremely short (19–21). Therefore, remifentanil can be easily titrated and administered for prolonged periods, with a lower risk of respiratory depression (5, 10, 22). It seems to make remifentanil more ideal for ventilated ICU patients.
Controversy of the intervention
The advantages of remifentanil in reducing the duration of MV in ICU patients have been debated. Randomized controlled trials (RCTs) have examined that the MV duration for remifentanil-based analgesia was significantly shorter than that for morphine-based, fentanyl-based, and sufentanil-based analgesia in postsurgical patients and patients undergoing MV for up to 10 days (23–26). Similarly, remifentanil reduced MV duration in these patients when compared with other opioid μ-receptor analgesics, according to two meta-analyses (27, 28). Even so, opioids administered intravenously at similar pain intensity endpoints seem to exhibit similar MV durations (5). Analgesia with remifentanil had a similar duration of MV as that with fentanyl or morphine when used in postsurgical and non-surgical mechanically ventilated patients and NICU patients undergoing MV for up to 5 days (29–31). In addition, a meta-analysis including 1,067 critically ill patients showed that remifentanil was not associated with a significantly shorter duration of MV than other opioids (32). Moreover, the majority of RCTs have even shown an increased risk of hypotension and bradycardia (25, 33).
Importance of study
No network meta-analysis (NMA) has evaluated the efficacy of intravenous opioid μ-receptor analgesics in shortening the duration of MV in ICU patients. In view of the uncertainty surrounding sufentanil, fentanyl, morphine, and remifentanil’s efficacy in shortening the duration of MV, we designed this systematic review and NMA to evaluate and rank their effectiveness in reducing MV duration among ICU patients. In addition, the efficacy of these drugs on clinically important outcomes and drug-related adverse events (AEs) was also investigated.
Methods
Approval
This article complies with the PRISMA statement (34). The registration number of PROSPERO was CRD 42021232604.
Eligibility criteria
Types of studies, participants, and interventions
In this NMA, we included only full-text published RCTs that involved 16-year-old ICU patients undergoing invasive MV via endotracheal intubation. Studies comparing two or more of the four therapies were included (remifentanil, sufentanil, fentanyl, and morphine).
Types of outcome measures
As a primary outcome, the duration of MV was evaluated. Secondary outcomes included extubation duration, ICU mortality, ICU length of stay (LOS), safety, drug-related bradycardia, drug-related hypotension, and drug-related bradycardia.
Exclusion criteria
Studies with controlled before-and-after comparisons, interrupted time series studies, and controlled clinical trials were excluded from our analysis. A study without reporting outcome variables, or a study with duplicate publications, was excluded from the study.
Search strategy
Electronic searches
Electronic medical databases including PubMed, Embase, Web of Science, and Cochrane were systematically searched for clinical trials published from 1 January 1991 to 31 December 2023. No language restrictions were applied. Each database used specific search terms, and the search strategy details (Supplementary File 1) were developed as proposed by Cochrane (35). We searched relevant literature using the following MeSH terms and their entry terms: ‘Critical Care’ OR ‘Critical Illness’ OR ‘Intensive Care Units’ OR ‘Coronary Care Units’ OR ‘Respiratory Care Units’ OR ‘Postoperative Care’ OR ‘Burn Units’ AND ‘Respiration, Artificial’ OR ‘Ventilators, Mechanical’ OR ‘Liquid Ventilation’ OR ‘active Ventilatory Support’ OR ‘Continuous Positive Airway Pressure’ OR ‘Intermittent Positive-Pressure Breathing’ OR ‘Positive-Pressure Respiration’ OR ‘High-Frequency Ventilation’ OR ‘Airway Extubation’ OR ‘Intubation, Intratracheal’ AND ‘Analgesics, Opioid’ OR ‘Analgesics’ OR ‘Remifentanil’ OR ‘Sufentanil’ OR ‘Fentanyl’ OR ‘Morphine’.
Searching other resources
Our search for relevant gray literature was conducted via Google Scholar. We also searched the following registers for complete trials (latest search 31 December 2023): ISRCTN,1 World Health Organization International Clinical Trials Registry Platform (ICTRP),2 Chinese Clinical Trial Register,3 and ClinicalTrials.gov.
Data collection and analysis
Study selection
Abstracts and titles of selected articles were independently reviewed by four reviewers. Thereafter, they carefully read the full text and decided to include studies. When there were any discrepancies between the reviewers, it was necessary to discuss them with the fifth reviewer and make a decision after consensus.
Definition of interventions and outcomes
All study drugs included in this study were IV opioid μ-receptor analgesics. The duration of MV was defined as the time from administration of the study drug after the patients were randomized into groups until the time of actual extubation. The extubation duration was defined as the time from the patient meeting the extubation criteria to the actual extubation. Safety was defined as the occurrence of drug-related AE. Drug-related AE included drug-related hypotension, drug-related bradycardia, and drug-related bradypnea. If AE was not specified as drug-related, it was presumed to be related. In the definition of drug-related hypotension, mean arterial pressure was multiplied by 50 millimeters of mercury. In the definition of drug-related bradycardia, the heart rate was multiplied by 50 beats per minute. In the definition of drug-related bradypnea, the respiratory rate was multiplied by 12 breaths per minute. The criteria for the MV model, weaning from MV, and extubation are shown in Supplementary File 2.
Data extraction
The Cochrane Handbook was used to collect all the data. Using the data from the study, five investigators extracted details of the study (language, published year, author, institutions, and funding), participant information (gender and age range), intervention information (drug, duration, and route of administration), results (MV duration and secondary outcomes), and methodological design (randomization, blinding, and allocation concealment) from each study. When there were any discrepancies between the reviewers, it was necessary to discuss and make a decision after consensus with the sixth reviewer.
Risk of bias assessment
Using the Cochrane Collaboration ROB (risk of bias) tool, we assessed the methodological quality of the study (35). Every study evaluated ROB in seven domains, categorizing it as high, unclear, or low. Low ROB studies were defined as three or less as unclear risk and none as high risk. Moderate ROB studies were defined as none rated as high risk but four or more were rated as unclear risk, or one was rated as high risk. All other studies have identified higher ROB studies.
Measures of treatment effect
Data synthesis
Continuous and dichotomous variables were analyzed using mean difference (MD) and odds ratio (OR), respectively. An NMA with random effects was used to estimate effect sizes using MDs or ORs with a 95% credible interval (CrI). Continuous and dichotomous outcomes were used for normal and binomial likelihoods, respectively. Model convergence was satisfactory when the potential scale reduction factor approached 1.0 (36). The treatments were evaluated and ranked according to the surface area under the cumulative ranking curve (SUCRA) (37).
Assessment of heterogeneity
Statistically significant heterogeneity was I2 greater than 50%, and we discussed the sources of heterogeneity (38–40).
Assessment of inconsistency
Node splitting and design-by-treatment tests were used to assess inconsistencies (39, 41). A p-value less than 0.05 was considered an inconsistency between the indirect and direct comparisons.
Assessment of transitivity
In order to test the transitivity assumption of NMA, the distribution of clinical variables was compared (37, 42).
Subgroup analysis
Subgroup analyses for the primary outcome were evaluated using population, duration of analgesia, and quality of the study. The patients were divided into postoperative critical and general critical groups. The duration of analgesia was divided into the short-term (≤72 h) and long-term (>72 h).
Sensitivity analysis
Sensitivity analysis was evaluated through studies quality and studies without publication bias datasets.
Quality assessment
GRADE was used to assess the certainty of evidence contributing to the network estimates (high, moderate, low, or very low) (43). Additionally, the comparison-adjusted funnel plots were used to assess publication bias (44, 45).
Statistical software
R software, Stata, and Review Manager were used for analysis.
Results
Results of the search
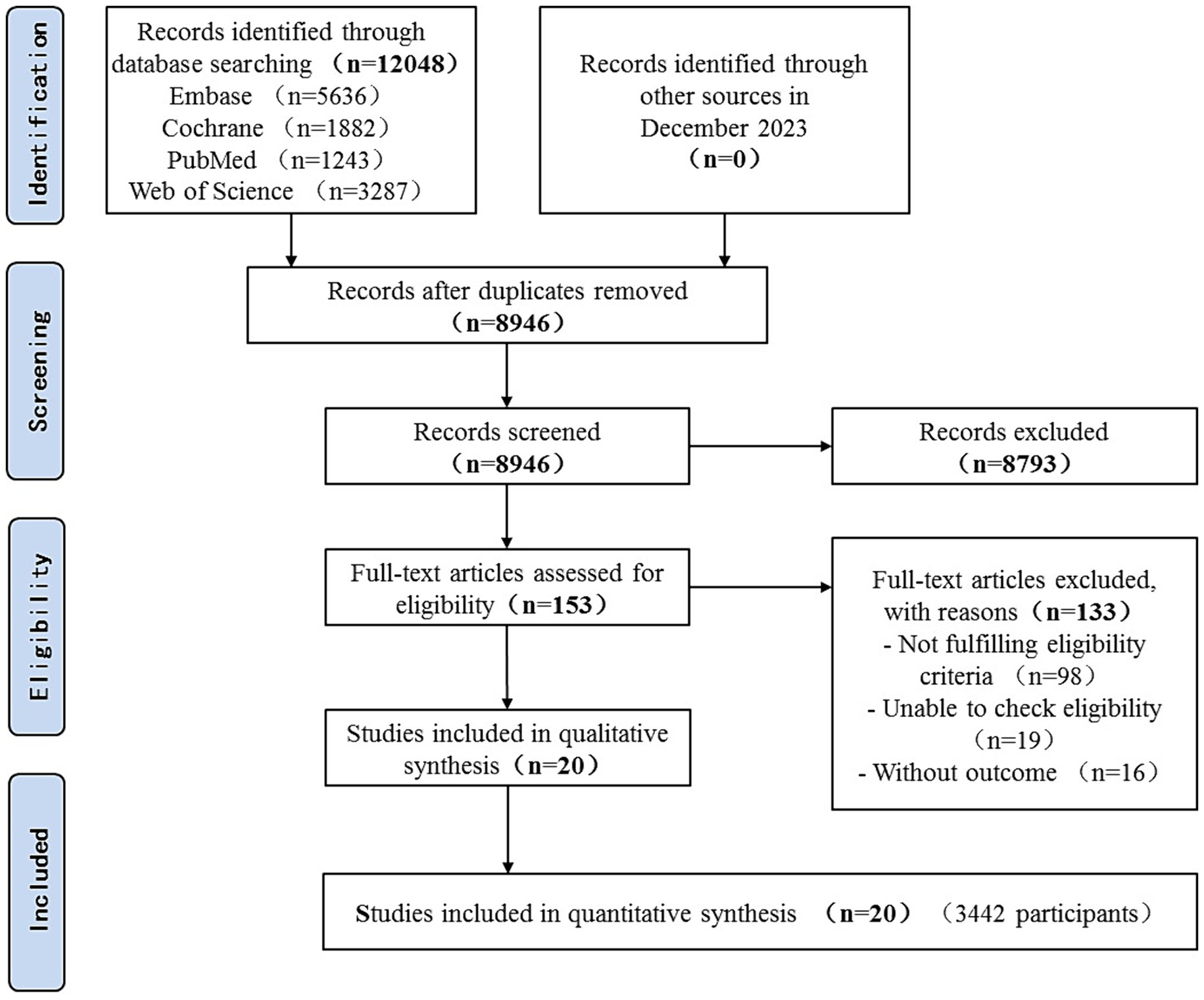
Over 12,048 articles were identified, of which 153 in full-text were potentially eligible for inclusion. In total, 20 RCTs involving 3,442 patients were identified (Figure 1).
Description of included studies
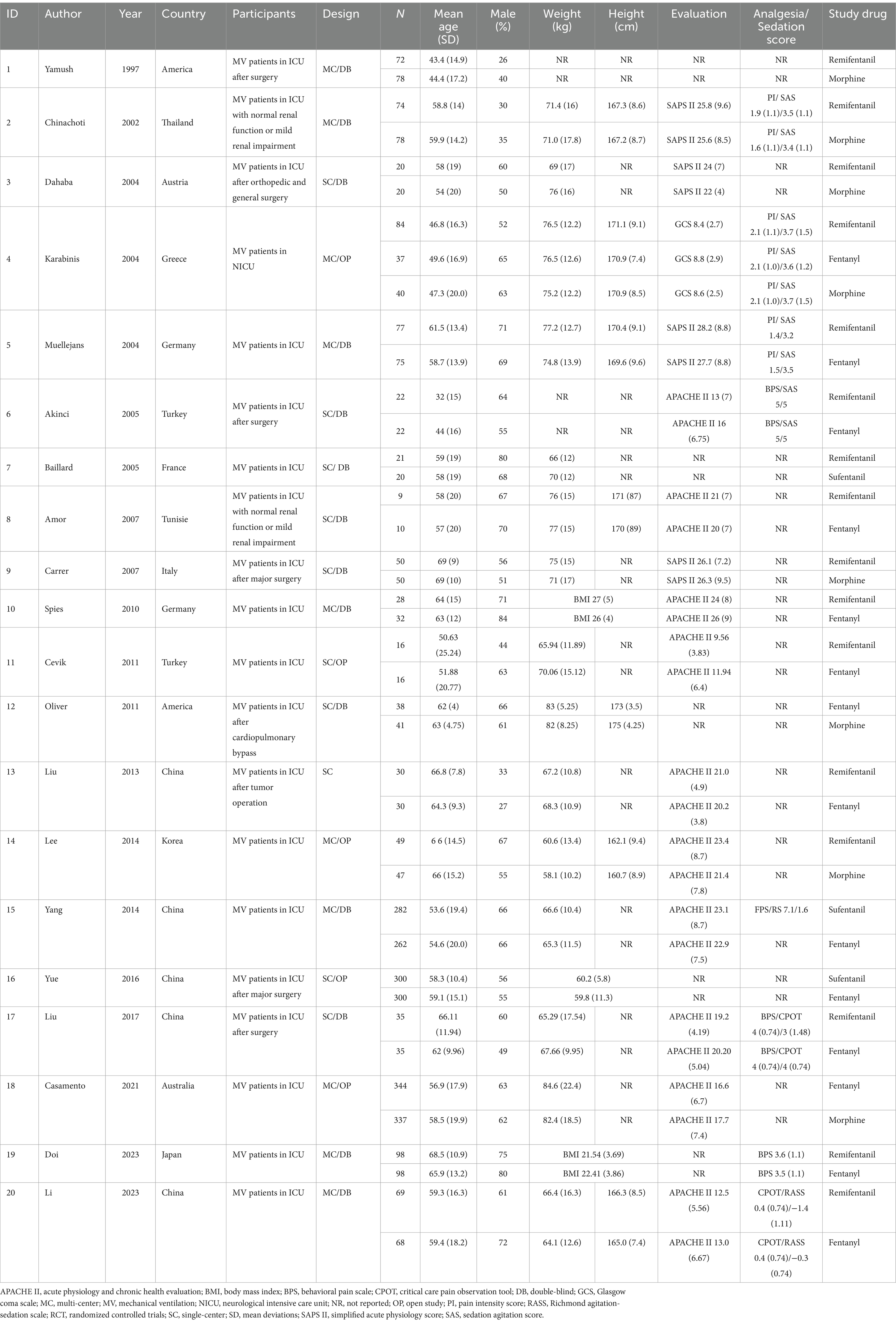
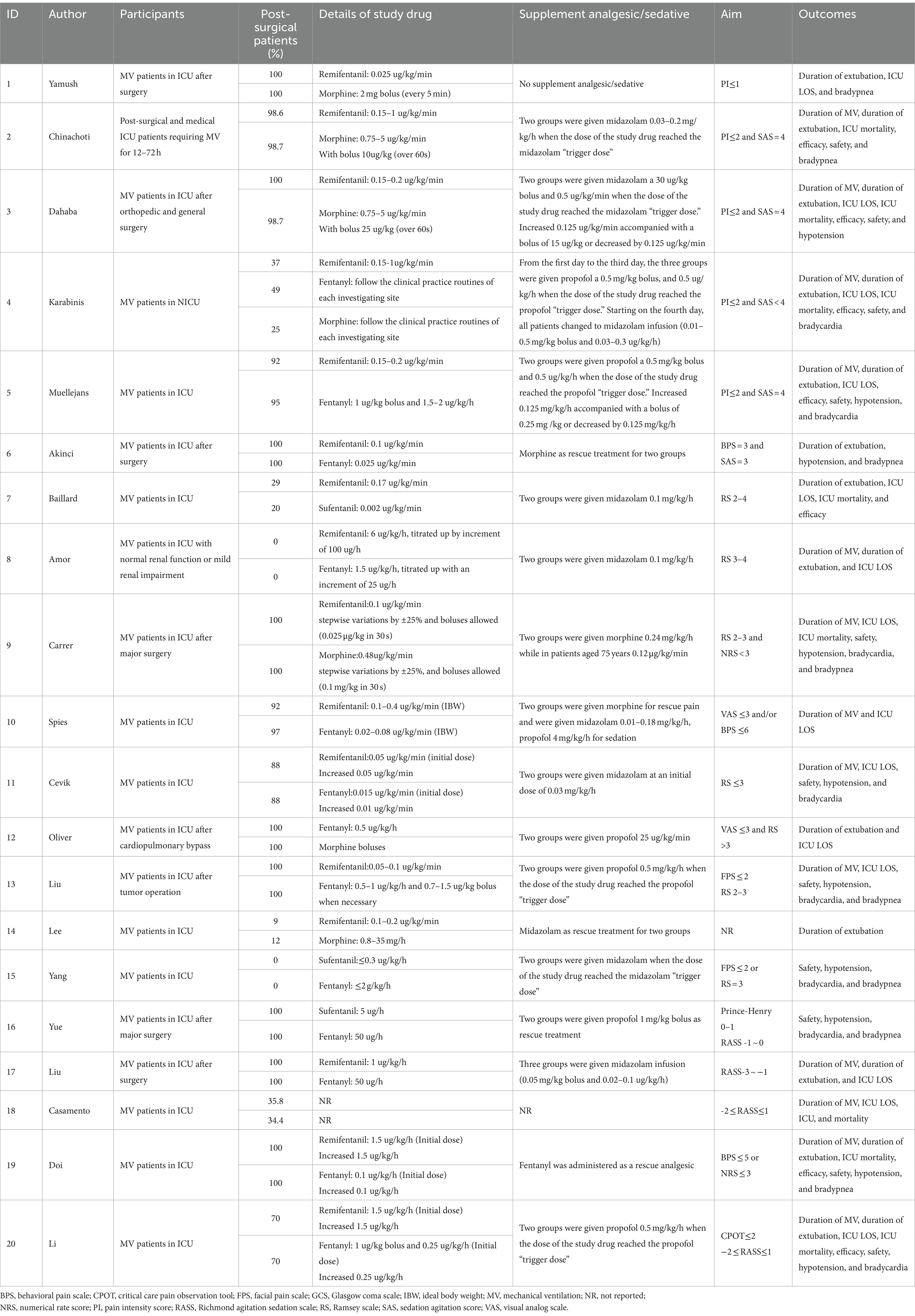
A total of 20 studies have been published in 12 countries between 1997 and 2023 (25, 29–31, 33, 46–60). There were 14 English articles, 3 Chinese articles, and 1 each in Turkish, French, and Tunisian. A total of nine (45%) trials recruited patients from Asia, seven (35%) trials recruited patients from Europe, two (10%) trials recruited patients from America, and each (5%) trial recruited patients from Oceania and Africa. Study samples ranged from 19 to 681 participants, with an average of 69 (standard deviation [SD] = 84). Participants were 55 years old (SD = 17 years), and 59% were men. Participants in one study (5%) were randomly assigned to three groups, and six (30%) were conducted at different research centers. In total, 14 (70%) studies were double-blind. The most critical patients were postoperative in the ICU, followed by those with brain trauma alone, severe multiple traumas, sepsis, and septic shock. Ten studies involved remifentanil versus fentanyl, 6 studies involved remifentanil versus morphine, and 1 study involved remifentanil versus sufentanil. There were three other studies involving fentanyl and morphine and two studies involving sufentanil versus morphine. Despite this, there have been no studies examining the interactions between sufentanil and morphine. The dose of opioids varied among studies; remifentanil, 0.05–1.0 ug/kg•min; fentanyl, 0.015–2.0 ug/kg•min; morphine, 0.75–2 ug/kg•min; sufentanil, 0.002–0.005 ug/kg•min (Tables 1, 2).
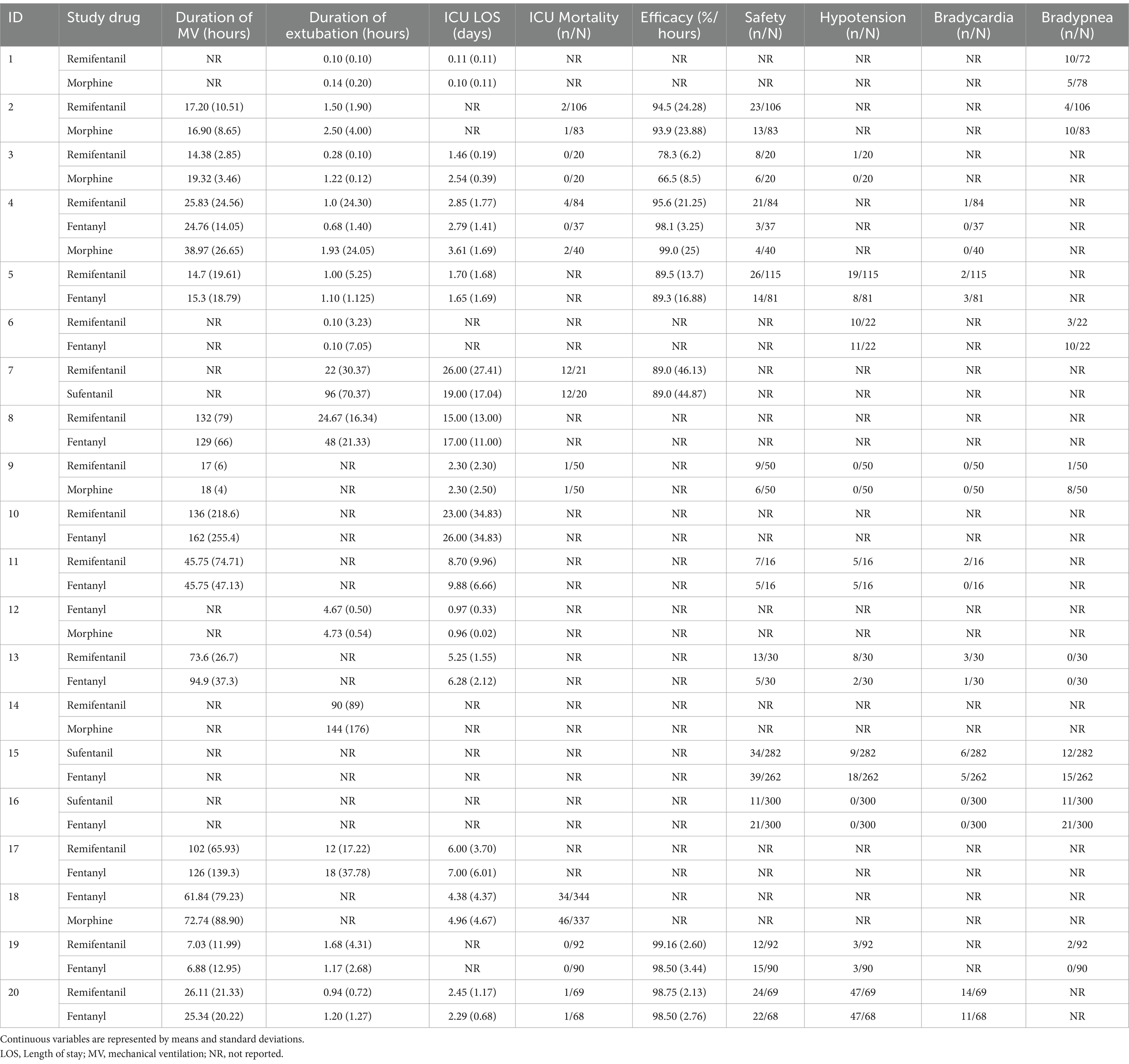
A total of 13 studies reported the duration of MV, 13 studies reported the duration of extubation, 14 reported ICU LOS, and 8 reported ICU mortality. In total, 7 studies reported efficacy, 11 reported safety, 10 reported drug-related hypotension, 8 reported drug-related bradycardia, and 8 reported drug-related bradypnea (Table 3).
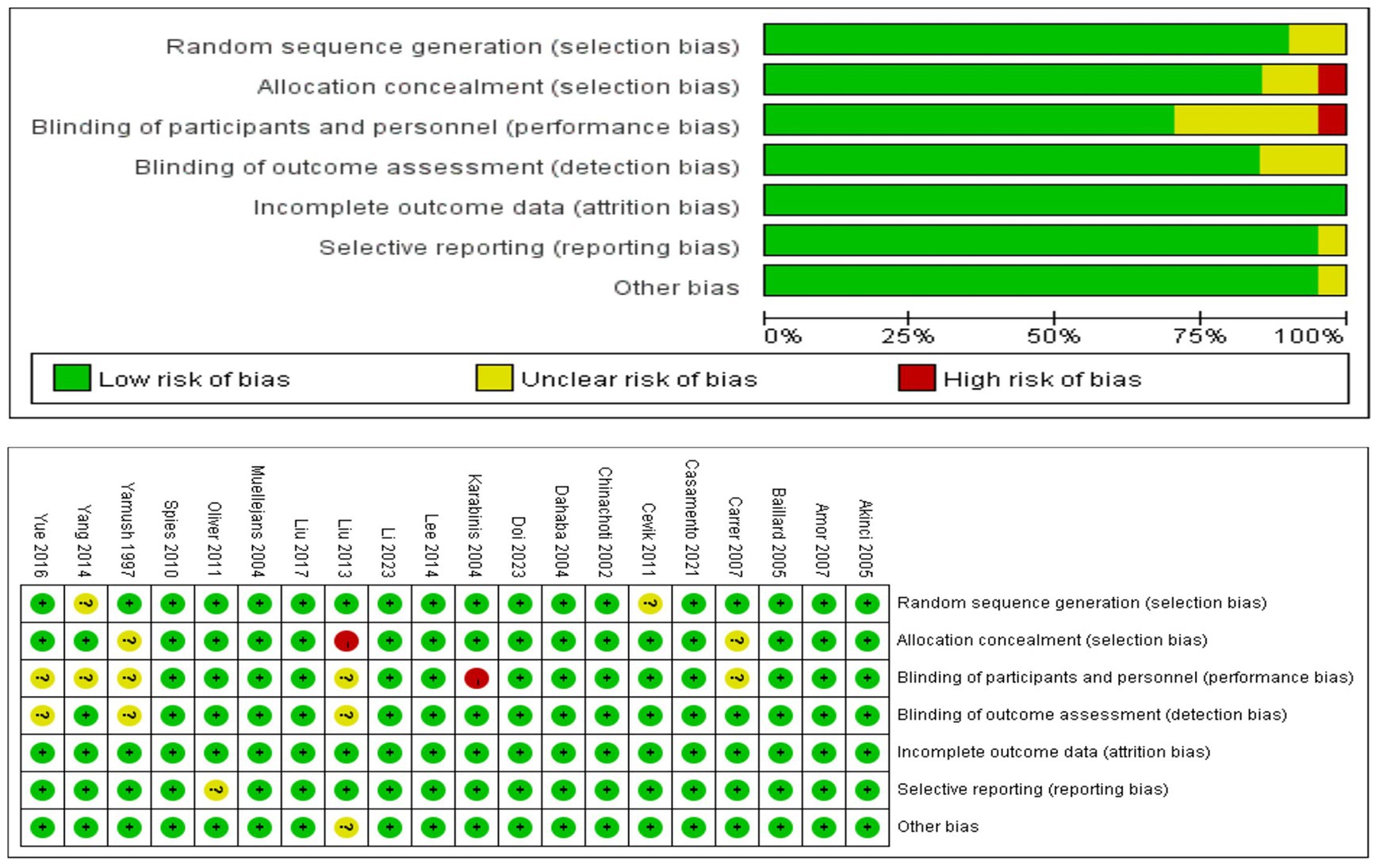
ROB in included studies
In summary (Figure 2), 17 (85%) of the 20 trials were rated as having low ROB, and 3 (20%) as having moderate ROB.
Effects of interventions
Primary outcome (duration of MV)
An analysis of 13 studies, including 1860 patients, was conducted to determine the duration of MV. There were 9, 4, and 2 trial arms involving direct comparisons of remifentanil and fentanyl, remifentanil and morphine, and morphine and fentanyl, respectively. None of the studies on sufentanil were included. All the Bayesian parameters converged well. Figure 3 displays a network of eligible comparisons for the MV duration.
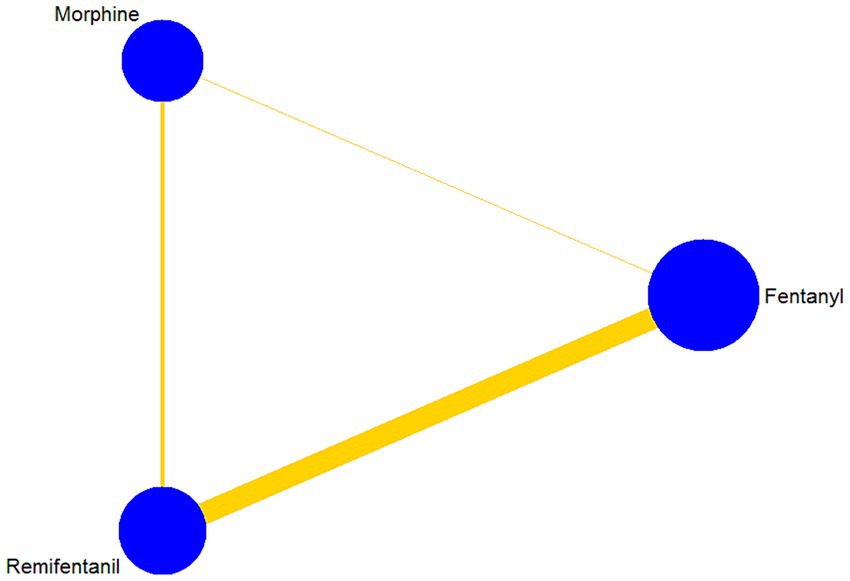
Figure 3. Network plot of all intervention comparisons for the duration of mechanical ventilation. The node size corresponds to the total number of participants in this study’s treatments. The comparable treatments are linked with a line. The colors and thickness of the line correspond to the quality and standard error of trials that study this comparison, respectively. Low risk of bias is green, moderate risk of bias is yellow.
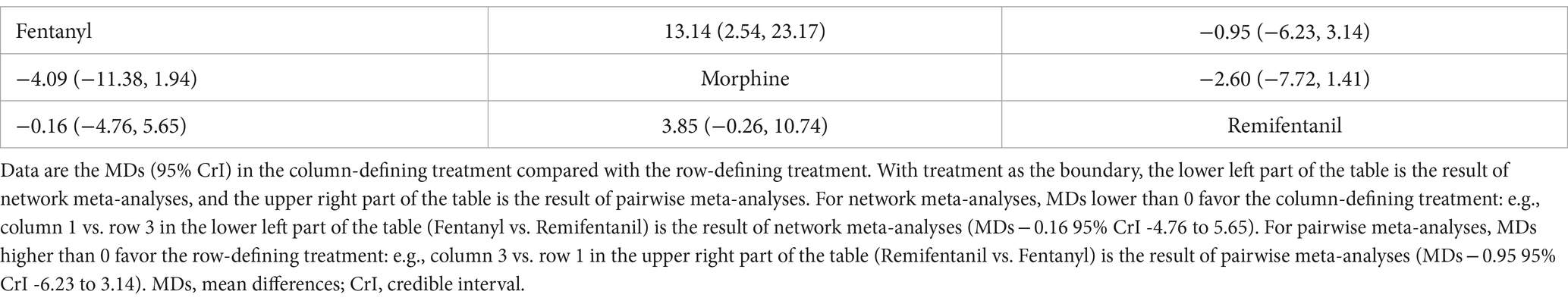
The results of the NMA are shown in Table 4 for the duration of MV. Compared with remifentanil, when fentanyl and morphine were administered to analgesia, the duration of MV was not significantly prolonged (MD -0.16; 95% CrI: −4.75 to 5.63) and (MD 3.84; −0.29 to 10.68), respectively. The differences between the three opioids were not significant. The SUCRA results showed that the best possible interventions for achieving the shortest duration of MV were remifentanil (46.0%), fentanyl (52.2%), and morphine (1.8%) (Supplementary Figure S8.1). However, we cannot conclude from the above results that fentanyl is the best regimen to shorten the duration of MV among the three opioids.
Secondary outcomes
Figure 4 presents the results of secondary outcomes. Compared with remifentanil, sufentanil can prolong the duration of extubation (MD 80.42; 95% CrI 18.31–127.36). No regimen significantly improved ICU LOS, efficacy, safety, and other secondary outcomes. The SUCRA ranking curve showed that remifentanil ranked first for shortening the extubation duration and reducing the occurrence of drug-related bradypnea. Fentanyl ranked first for ICU mortality. Moreover, morphine ranked first for efficacy, reducing the occurrence of drug-related hypotension and bradycardia. Furthermore, sufentanil ranked first for ICU-LOS and safety (Supplementary File 8).
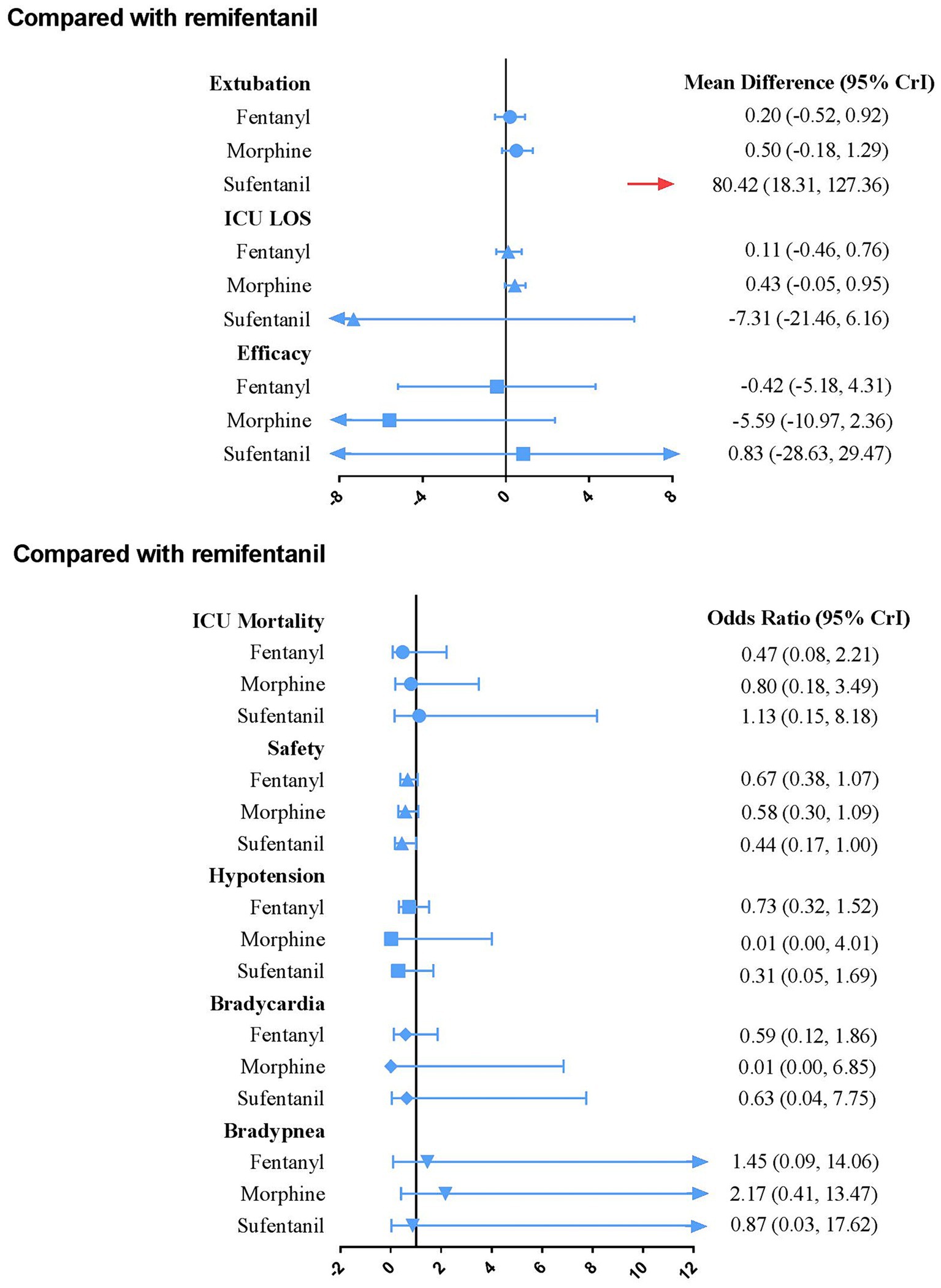
Figure 4. Forest plot for each active intervention versus remifentanil on secondary outcomes estimates are presented as MD (mean difference) or odds ratios (OR) and 95% CrI. OR < 1 favor the treatment. MD < 0 favor the treatment. CrI, credible interval; LOS, Length of stay.
Direct meta-analysis
A pairwise analysis of the duration of MV is presented in Table 4.
Heterogeneity, inconsistency, and transitivity
In terms of MV duration (I2 = 68.70%) and ICU LOS (I2 = 99.87%), there was moderate-to-high global heterogeneity (Supplementary File 4).
No global inconsistency was observed in any of the outcomes (Supplementary Table S4.2). When the node-splitting model was compared indirectly and directly, there was no evidence of inconsistency.
Most comparisons had similar mean ages in the assessment of transitivity (Supplementary File 5).
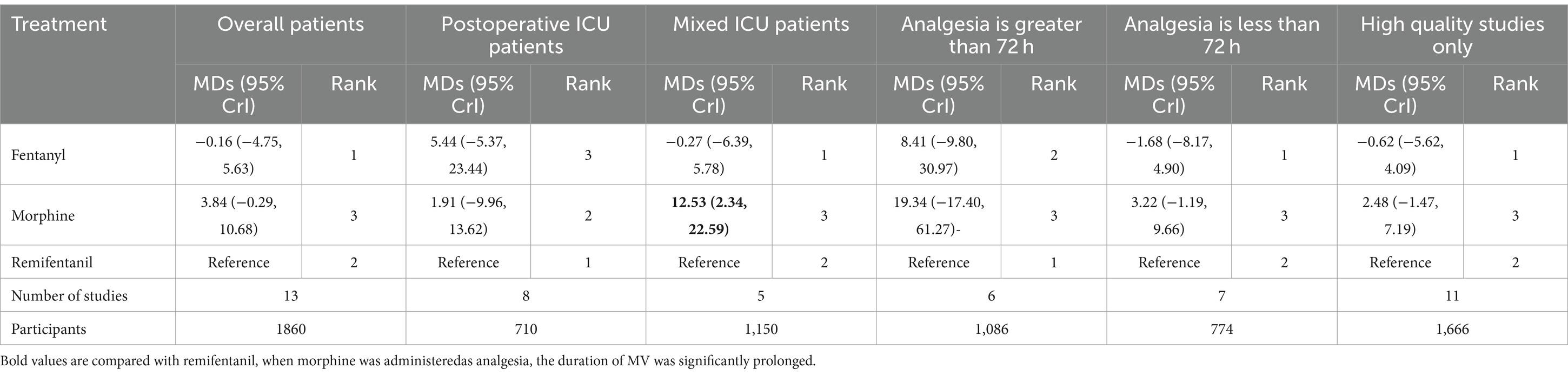
Subgroup analyses and sensitivity analyses for the duration of MV
Compared with remifentanil, when morphine was administered as analgesia, the duration of MV was significantly prolonged (MD 12.53; 95% CrI: 2.34 to 22.59). The three opioids had similar effects on shortening the duration of MV in each subgroup of patients, regardless of their patient population, duration of analgesia, and study quality (Table 5). In addition, heterogeneity and consistency were not statistically significant among the subgroups.
The sensitivity analysis did not change substantially (Supplementary File 9).
GRADE assessments
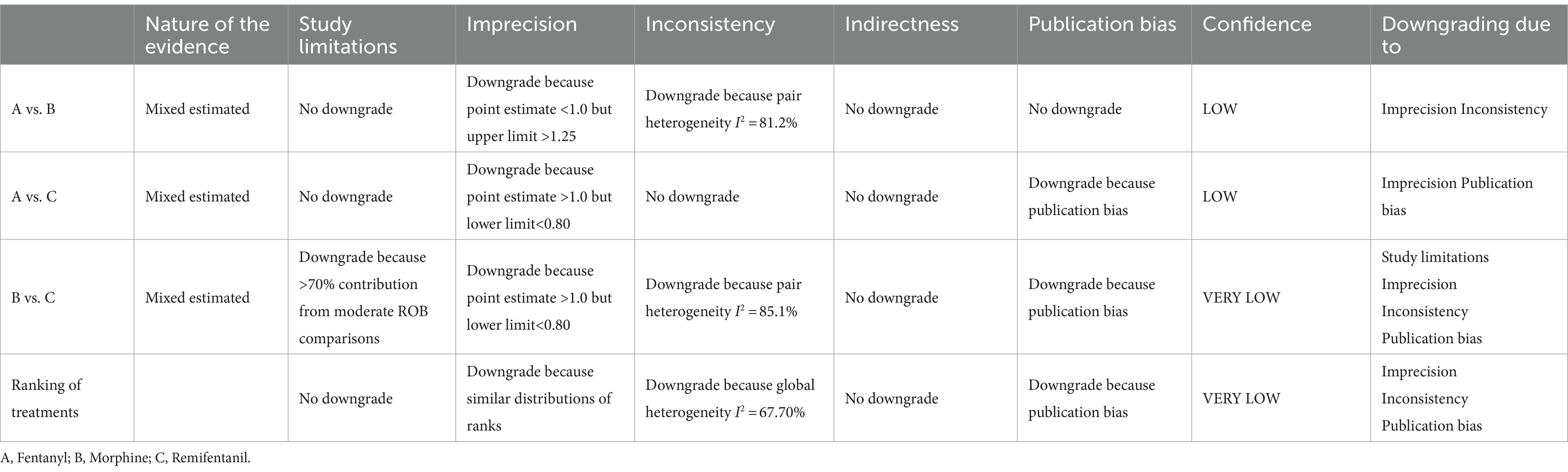
Except for the extubation duration, no publication bias was found (Supplementary File 6). The degree of certainty about shortening MV time was variable (Supplementary Table S7.1). For comparisons involving fentanyl, morphine, and remifentanil, it was low, whereas, for comparisons involving morphine and remifentanil, it was very low. The GRADE of ranking of treatment was very low. The GRADE was raised to at least moderate when subgroup analysis was performed. Table 6 and Supplementary File 7 presents details of GRADE.
Discussion
Main results summary
This study was conducted to investigate the effect of analgesic regimens using remifentanil, morphine, and fentanyl on the duration of MV. It was concluded that remifentanil did not significantly shorten the duration of MV in mechanically ventilated patients compared to morphine or fentanyl. This finding was supported by sensitivity and subgroup analyses. In addition, the SUCRA ranking curve indicated that fentanyl ranked first among the three opioids for shortening the duration of MV, but the difference was not statistically significant.
Applicability of evidence
Remifentanil did not reduce the duration of MV, which is consistent with the previous conclusion that all opioids administered intravenously appear to exhibit a similar duration of MV when titrated to similar pain intensity endpoints (5). However, the pharmacokinetics of remifentanil is not similar to those of morphine and fentanyl. The results were interpreted carefully for the following reasons: First, elimination independent of renal function seems to make remifentanil more effective in patients with renal impairment (20). Amor and Chinachoti’s study focused on patients with mild renal impairment, although not suggested remifentanil can shorten the duration of MV, they indicated remifentanil was associated with shorter the duration of weaning (47, 50). In Chinachoti et al.’s study, it should be noted that twice the amount of midazolam in the morphine group may have reduced morphine-related side effects (47). Second, a prolonged infusion did little to affect the context-sensitive half-life of remifentanil. Remifentanil shortened the duration of MV by at least 24 h when analgesia was > 5 days (29, 33, 57). Although the difference was not statistically significant, it is important to avoid ventilator-associated pneumonia, improve ICU outcomes, and reduce costs (23, 61). This suggests that remifentanil is the most suitable treatment for mechanically ventilated patients undergoing long-term analgesia (28). Third, as a result of remifentanil’s rapid onset and offset action, it permitted a significantly quicker and more predictable awakening when it came to performing neurological assessment (31). Thus, although the reduced duration between remifentanil and either of the comparator opioids was less than 1 h, remifentanil may be more meaningful for these patients (31, 62). Fourth, the agents and sedation protocols used differed between studies. Seven studies used midazolam as an adjuvant sedative, and the other three used propofol as an adjuvant sedative. It was more difficult to estimate the effect of opioids when sedatives and analgesics were combined. Finally, heterogeneity and publication bias were the main reasons for the reduction in the GRADE scores. Therefore, these factors weaken the inference drawn from the current findings. Larger, well-powered, and more definitive clinical trials based on different populations are urgently needed to avoid such biases.
Analysis of secondary outcomes
In terms of extubation duration, sufentanil showed a prolonged effect compared with remifentanil. However, these findings were inconclusive. We need to note that the CrI was too wide because this result was only determined in one study that enrolled 41 patients on MV and was stopped after an interim analysis (48). Therefore, caution should be exercised when interpreting the impact of sufentanil, and it is imperative to conduct future large RCTs to validate these clinical results. Neither of the four opioid medications significantly differed in ICU-LOS, ICU mortality, efficacy, safety, or drug-related adverse events. It can be interpreted for two reasons. First, all available IV opioids were equally effective when titrated to similar pain intensity end points (5). Second, the frequent reassessment of pain and careful titration of analgesic interventions were helpful in preventing negative sequelae due to excessive or inadequate analgesic therapy (63).
Strengths of this NMA
This study has several strengths. First, this is the first NMA to assess the effectiveness of IV opioid μ-receptor analgesics to shorten the duration of MV in mechanically ventilated patients. Second, it was the most updated evaluation of IV opioid μ-receptor analgesics for patients on MV. A structured search strategy retrieved all identified studies. Third, several relevant clinical outcomes were examined in a heterogeneous population. Fourth, we focused on the co-interventions of sedatives and included only studies that employed the same strategies for sedation. Finally, this study focused on a wide range of clinical outcomes.
Limitations of this NMA
There are still several limitations in drawing strong treatment inferences. First, several studies did not provide accurate study criteria, such as mode of MV, weaning, and extubation. It is difficult to make these definitions consistent. In addition, the varying opioid doses, sedative types, length of administration, and consumption in different studies weakened any possible recommendations and conclusions. Second, because of the inconsistency in adjuvant sedatives, fewer eligible studies were included and subgroup analyses could not be performed. Therefore, we downgraded the GRADE score. Third, many comparisons had low-level evidence. Mainly because of a wide 95% CrI, possibly implying a small number of studies. Finally, European and Asian countries accounted for 80% of all studies.
Conclusion
This study provides evidence that remifentanil, compared with fentanyl and morphine, does not shorten the duration of MV in ICU patients. Clinicians should carefully titrate the analgesia of mechanically ventilated patients to prevent a potentially prolonged duration of MV. As such, based on current data, no final recommendations or conclusions can be made. Further large-scale multicenter RCTs according to the characteristics of different populations, especially organ failure patients and long-term analgesic patients, are needed to clarify the most appropriate analgesics, dosages, duration of infusion, and strategies of analgesia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
FL: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. SQ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. ChL: Data curation, Formal analysis, Investigation, Writing – review & editing. XC: Data curation, Investigation, Project administration, Writing – review & editing. ZD: Data curation, Formal analysis, Investigation, Project administration, Writing – review & editing. CoL: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Key Research and Development project of Xuzhou (KC22238). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1370481/full#supplementary-material
Footnotes
References
1. Chanques, G, Sebbane, M, Barbotte, E, Viel, E, Eledjam, JJ, and Jaber, S. A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology. (2007) 107:858–60. doi: 10.1097/01.anes.0000287211.98642.51
2. Stanik-Hutt, JA, Soeken, KL, Belcher, AE, Fontaine, DK, and Gift, AG. Pain experiences of traumatically injured patients in a critical care setting. Am J Crit Care. (2001) 10:252–9. doi: 10.4037/ajcc2001.10.4.252
3. Rotondi, AJ, Chelluri, L, Sirio, C, Mendelsohn, A, Schulz, R, Belle, S, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. (2002) 30:746–52. doi: 10.1097/00003246-200204000-00004
4. Payen, JF, Chanques, G, Mantz, J, Hercule, C, Auriant, I, Leguillou, JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. (2007) 106:687–95. doi: 10.1097/01.anes.0000264747.09017.da
5. Barr, J, Fraser, GL, Puntillo, K, Ely, EW, Gélinas, C, Dasta, JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182783b72
6. Pun, BT, Balas, MC, Barnes-Daly, MA, Thompson, JL, Aldrich, JM, Barr, J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. (2019) 47:3–14. doi: 10.1097/CCM.0000000000003482
7. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
8. Chinese Society of Critical Care Medicine . Chinese adult guidelines for analgesia and sedation. Chin Crit Care Intensive Care Med. (2018) 4:90–113.
9. Egan, TD, Lemmens, HJ, Fiset, P, Hermann, DJ, Muir, KT, Stanski, DR, et al. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology. (1993) 79:881–92. doi: 10.1097/00000542-199311000-00004
10. Baldo, BA . Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. (2021) 95:2627–42. doi: 10.1007/s00204-021-03068-2
11. Robleda, G, Roche-Campo, F, Sendra, MÀ, Navarro, M, Castillo, A, Rodríguez-Arias, A, et al. Fentanyl as pre-emptive treatment of pain associated with turning mechanically ventilated patients: a randomized controlled feasibility study. Intensive Care Med. (2016) 42:183–91. doi: 10.1007/s00134-015-4112-7
12. Dres, M, Dubé, BP, Mayaux, J, Delemazure, J, Reuter, D, Brochard, L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. (2017) 195:57–66. doi: 10.1164/rccm.201602-0367OC
13. Demoule, A, Molinari, N, Jung, B, Prodanovic, H, Chanques, G, Matecki, S, et al. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care. (2016) 6:75. doi: 10.1186/s13613-016-0179-8
14. Trescot, AM, Datta, S, Lee, M, and Hansen, H. Opioid pharmacology. Pain Physician. (2008) 11:S133–53. doi: 10.36076/ppj.2008/11/S133
15. Rozendaal, FW, Spronk, PE, Snellen, FF, Schoen, A, van Zanten, AR, Foudraine, NA, et al. Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: a Centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med. (2009) 35:291–8. doi: 10.1007/s00134-008-1328-9
16. Dres, M, Jung, B, Molinari, N, Manna, F, Dubé, BP, Chanques, G, et al. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts. Crit Care. (2019) 23:370. doi: 10.1186/s13054-019-2650-z
17. Goligher, EC, Dres, M, Fan, E, Rubenfeld, GD, Scales, DC, Herridge, MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. (2018) 197:204–13. doi: 10.1164/rccm.201703-0536OC
18. Nordness, MF, Hayhurst, CJ, and Pandharipande, P. Current perspectives on the assessment and management of pain in the intensive care unit. J Pain Res. (2021) 14:1733–44. doi: 10.2147/JPR.S256406
19. Westmoreland, CL, Hoke, JF, Sebel, PS, Hug, CC Jr, and Muir, KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. (1993) 79:893–903. doi: 10.1097/00000542-199311000-00005
20. Breen, D, Wilmer, A, Bodenham, A, Bach, V, Bonde, J, Kessler, P, et al. Offset of pharmacodynamic effects and safety of remifentanil in intensive care unit patients with various degrees of renal impairment. Crit Care. (2004) 8:R21–30. doi: 10.1186/cc2399
21. Kapila, A, Glass, PS, Jacobs, JR, Muir, KT, Hermann, DJ, Shiraishi, M, et al. Measured contextsensitive half-times of remifentanil and alfentanil. Anesthesiology. (1995) 83:968–75. doi: 10.1097/00000542-199511000-00009
22. Pitsiu, M, Wilmer, A, Bodenham, A, Breen, D, Bach, V, Bonde, J, et al. Pharmacokinetics of remifentanil and its major metabolite, remifentanil acid, in ICU patients with renal impairment. Br J Anaesth. (2004) 92:493–503. doi: 10.1093/bja/aeh086
23. Muellejans, B, Matthey, T, Scholpp, J, and Schill, M. Sedation in the intensive care unit with remifentanil/propofol versus midazolam/fentanyl: a randomised, open-label, pharmacoeconomic trial. Crit Care. (2006) 10:R91. doi: 10.1186/cc4939
24. Futier, E, Chanques, G, Cayot Constantin, S, Vernis, L, Barres, A, Guerin, R, et al. Influence of opioid choice on mechanical ventilation duration and ICU length of stay. Minerva Anestesiol. (2012) 78:46–53.
25. Dahaba, AA, Grabner, T, Rehak, PH, List, WF, and Metzler, H. Remifentanil versus morphine analgesia and sedation for mechanically ventilated critically ill patients. Anesthesiology. (2004) 101:640–6. doi: 10.1097/00000542-200409000-00012
26. Breen, D, Karabinis, A, Malbrain, M, Morais, R, Albrecht, S, Jarnvig, IL, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]. Crit Care. (2005) 9:R200–10. doi: 10.1186/cc3495
27. Greco, M, Landoni, G, Biondi-Zoccai, G, Cabrini, L, Ruggeri, L, Pasculli, N, et al. Remifentanil in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. (2012) 26:110–6. doi: 10.1053/j.jvca.2011.05.007
28. Zhu, Y, Wang, Y, Du, B, and Xi, X. Could remifentanil reduce duration of mechanical ventilation in comparison with other opioids for mechanically ventilated patients? A systematic review and meta-analysis. Crit Care. (2017) 21:206. doi: 10.1186/s13054-017-1789-8
29. Spies, C, Macguill, M, Heymann, A, Ganea, C, Krahne, D, Assman, A, et al. A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med. (2011) 37:469–76. doi: 10.1007/s00134-010-2100-5
30. Muellejans, B, López, A, Cross, MH, Bonome, C, Morrison, L, and Kirkham, AJ. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713]. Crit Care. (2004) 8:R1–R11. doi: 10.1186/cc2398
31. Karabinis, A, Mandragos, K, Stergiopoulos, S, Komnos, A, Soukup, J, Speelberg, B, et al. Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial. Crit Care. (2004) 8:R268–80. doi: 10.1186/cc2896
32. Tan, JA, and Ho, KM. Use of remifentanil as a sedative agent in critically ill adult patients: a meta-analysis. Anaesthesia. (2009) 64:1342–52. doi: 10.1111/j.1365-2044.2009.06129.x
33. Liu, KB, Wang, DH, Ma, Y, and Xia, R. Remifentanil for analgesia and sedation in mechanically ventilated patients in intensive care unit. Chin Crit Care Med. (2013) 25:167–70.
34. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:4053. doi: 10.1186/2046-4053-4-1
35. Green, S, and Higgins, JPT (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: http://handbook.cochrane.org (Accessed December 15, 2012)
36. Gelman, A, and Rubin, DB. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res. (1996) 5:339–55. doi: 10.1177/096228029600500402
37. Salanti, G, Ades, AE, and Ioannidis, JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
38. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
39. Higgins, JP, Jackson, D, Barrett, JK, Lu, G, Ades, AE, and White, IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
40. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
41. Dias, S, Welton, NJ, Caldwell, DM, and Ades, AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
42. Furukawa, TA, Salanti, G, Atkinson, LZ, Leucht, S, Ruhe, HG, Turner, EH, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. (2016) 6:e010919. doi: 10.1136/bmjopen-2015-010919
43. Salanti, G, Del Giovane, C, Chaimani, A, Caldwell, DM, and Higgins, JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. (2014) 9:9682. doi: 10.1371/journal.pone.0099682
44. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
45. Chaimani, A, Higgins, JP, Mavridis, D, Spyridonos, P, and Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS One. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
46. Yarmush, J, D'Angelo, R, Kirkhart, B, O'Leary, C, Pitts, MC 2nd, Graf, G, et al. A comparison of remifentanil and morphine sulfate for acute postoperative analgesia after total intravenous anesthesia with remifentanil and propofol. Anesthesiology. (1997) 87:235–43. doi: 10.1097/00000542-199708000-00009
47. Chinachoti, T, Kessler, P, Kirkham, A, and Werawatganon, T. Remifentanil vs morphine for patients in intensive care unit who need short-term mechanical ventilation. J Med Assoc Thail. (2002) 85:S848–57.
48. Baillard, C, Cohen, Y, Le Toumelin, P, Karoubi, P, Hoang, P, Ait Kaci, F, et al. Remifentanil-midazolam compared to sufentanil-midazolam for ICU long-term sedation. Ann Fr Anesth Reanim. (2005) 24:480–6. doi: 10.1016/j.annfar.2005.02.027
49. Saricao, F, Akinci, LSB, Kanbak, M, and Aygün GÜler, Ülkü Aypar., Remifentanil versus fentanyl for analgesia based sedation in mechanically ventilated patients. Anestezi Dergisi. (2005) 13:182–8.
50. Belhadj Amor, M, Ouezini, R, Lamine, K, Barakette, M, Labbène, I, and Ferjani, M. Daily interruption of sedation in intensive care unit patients with renal impairment: remifentanil-midazolam compared to fentanyl-midazolam. Ann Fr Anesth Reanim. (2007) 26:1041–4. doi: 10.1016/j.annfar.2007.10.005
51. Carrer, S, Bocchi, A, Candini, M, Donegà, L, and Tartari, S. Short term analgesia based sedation in the intensive care unit: morphine vs remifentanil + morphine. Minerva Anestesiol. (2007) 73:327–32.
52. Cevik, F, Celik, M, Clark, PM, and Macit, C. Sedation and analgesia in intensive care: a comparison of fentanyl and remifentanil. Pain Res Treat. (2011) 2011:650320. doi: 10.1155/2011/650320
53. Oliver, WC Jr, Nuttall, GA, Murari, T, Bauer, LK, Johnsrud, KH, Hall Long, KJ, et al. A prospective, randomized, double-blind trial of 3 regimens for sedation and analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. (2011) 25:110–9. doi: 10.1053/j.jvca.2010.07.008
54. Lee, JM, Lee, SH, Kwak, SH, Kang, HH, Lee, SH, and Lim, JM. Comparison of morphine and remifentanil on the duration of weaning from mechanical ventilation. Korean J Crit Care Med. (2014) 29:281–7. doi: 10.4266/kjccm.2014.29.4.281
55. Yang, H, Sun, R, Chang, Y, Fu, Y, Li, B, Qin, B, et al. A multicenter randomized controlled trial of sufentanil for analgesia/sedation in patients in intensive care unit. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2014) 26:94–100. doi: 10.3760/cma.j.issn.2095-4352.2014.02.008
56. Yue, JX, Huang, QQ, Su, MX, Wan, LJ, Li, H, Liu, OY, et al. Effect of sufentanil on analgesia and sedation for ventilated critically ill patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2016) 28:563–6.
57. Liu, D, Lyu, J, Zhao, H, and An, Y. The influence of analgesic-based sedation protocols on delirium and outcomes in critically ill patients: a randomized controlled trial. PLoS One. (2017) 12:e0184310. doi: 10.1371/journal.pone.0184310
58. Casamento, AJ, Serpa Neto, A, Young, M, Lawrence, M, Taplin, C, Eastwood, GM, et al. A phase II cluster-crossover randomized trial of fentanyl versus morphine for Analgosedation in mechanically ventilated patients. Am J Respir Crit Care Med. (2021) 204:1286–94. doi: 10.1164/rccm.202106-1515OC
59. Doi, M, Takahashi, N, Nojiri, R, Hiraoka, T, Kishimoto, Y, Inoue, S, et al. Efficacy, safety, and pharmacokinetics of MR13A11A, a generic of remifentanil, for pain management of Japanese patients in the intensive care unit: a double-blinded, fentanyl-controlled, randomized, non-inferiority phase 3 study. J Intensive Care. (2023) 11:51. doi: 10.1186/s40560-023-00698-9
60. Li, C, Gao, ZW, Chen, H, Hu, LL, Ma, SL, Zhang, JW, et al. Remifentanil versus fentanyl for analgesia in mechanically ventilated patients. Altern Ther Health Med. (2023) 29:138–47.
61. Casamento, A, and Bellomo, R. Fentanyl versus morphine for analgo-sedation in mechanically ventilated adult ICU patients. Crit Care Resusc. (2019) 21:76–83. doi: 10.1016/S1441-2772(23)00666-X
62. Wilhelm, W, and Kreuer, S. The place for short-acting opioids: special emphasis on remifentanil. Crit Care. (2008) 12:S5. doi: 10.1186/cc6152
63. Erstad, BL, Puntillo, K, Gilbert, HC, Grap, MJ, Li, D, Medina, J, et al. Pain management principles in the critically ill. Chest. (2009) 135:1075–86. doi: 10.1378/chest.08-2264
Glossary
Keywords: critical illness, mechanical ventilation, analgesics, opioid, remifentanil, network meta-analysis
Citation: Lu F, Qin S, Liu C, Chen X, Dai Z and Li C (2024) ICU patients receiving remifentanil do not experience reduced duration of mechanical ventilation: a systematic review of randomized controlled trials and network meta-analyses based on Bayesian theories. Front. Med. 11:1370481. doi: 10.3389/fmed.2024.1370481
Edited by:
Abele Donati, Marche Polytechnic University, ItalyReviewed by:
Neha Gupta, University of Oklahoma Health Sciences Center, United StatesYiying Zhang, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2024 Lu, Qin, Liu, Chen, Dai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xunxun Chen, Z3JhY2VfY2hlbjUxNEAxNjMuY29t; Zhaoqiu Dai, NTE3NzExODI4QHFxLmNvbQ==; Cong Li, Y29uZ2NvbmdsaTIwMjBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Fangjie Lu
Fangjie Lu Sirun Qin
Sirun Qin Chang Liu4†
Chang Liu4† Cong Li
Cong Li