- 1Department of Emergency, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2School of Basic Medical Sciences, Wenzhou Medical University, Wenzhou, China
Background: The neutrophil-lymphocyte ratio (NLR) is a simple marker of systemic inflammatory responses. The present study aims to evaluate the prognostic significance of the NLR on admission day in predicting outcomes for patients with upper gastrointestinal bleeding (UGIB), which is a prevalent medical emergency.
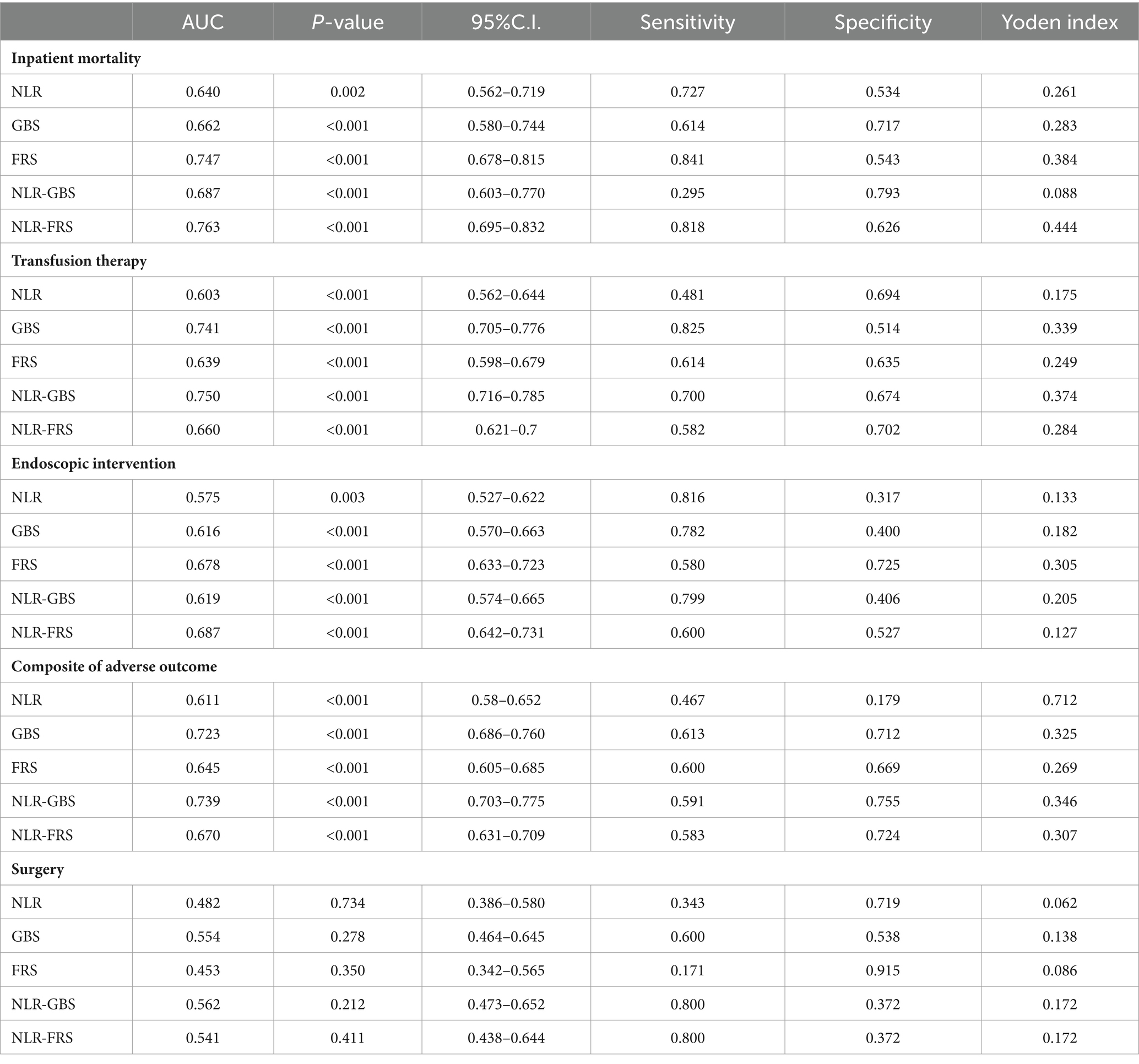
Methods: 726 patients who were admitted to our clinic between January 2019 and December 2022 diagnosed with UGIB, and who underwent necessary examinations, were included in the study. The patients’ Glasgow-Blatchford Score (GBS), Full Rockall Score (FRS), and NLR levels were calculated at the first admission. Outcomes were defined as in-hospital mortality, need for blood transfusion, surgical treatment and endoscopic therapy. Patients were categorized into four groups using NLR quartile levels to compare their clinical characteristics, Glasgow Blatchford Score, Full Rockall Score levels, and prognosis. Secondary, we modified FRS and GBS by adding NLR, respectively. We used area under the receiver operating characteristic curve (AUROC) to assess the accuracy of risk prediction for NLR, NLR-GBS, and NLR-FRS improved models.
Results: Of 726 patients, 6% died in hospital, 23.9% received endoscopic interventon, 4.8% received surgical treatment, and 46.4% received transfusion therapy. Multifactorial logistic regression showed that a high level of NLR was a risk factor for death in patients with UGIB (p = 0.028). NLR, GBS, FRS, NLR-GBS, and NLR-FRS have sufficient accuracy in predicting inpatient mortality, endoscopic treatment, and transfusion treatment, and the differences are statistically significant (p < 0.05). In the comprehensive prediction of adverse outcomes, NLR-GBS has the highest AUROC, and in predicting inpatient mortality, NLR-FRS has the highest AUROC.
Conclusion: For UGIB patients, a high NLR was strongly associated with high risk UGIB. Combined testing with the GBS and FRS can achieve good predictive results, which is valuable in guiding the pre-screening and triage of emergency nursing care and clinical treatment to ensure that patients receive rapid and effective treatment and improve the quality of care.
1 Introduction
Upper gastrointestinal bleeding (UGIB) is a commonly encountered emergency in clinical practice, with a mortality rate ranging from 2 to 15% (1, 2). Despite the advances in the management and endoscopic therapy strategies, the rate of mortality has not significantly improved (3, 4). Therefore, it is imperative to evaluate risk factors so that patients can be accurately stratified and disease activity and mortality can be assessed (5). Multiple systems have been designed and recommended by international guidelines. The most well-known systems are FRS, GBS, and AIMS65 (6, 7). However, certain limitations, such as complex parameters and the need for endoscopic findings, existed against the prevailing management of these scoring systems. Therefore, discovering simple, readily available markers for evaluating the prognosis of UGIB will be a challenging topic in UGIB practice.
When patients bleed, the body’s inflammatory factors are altered, and an inflammatory response occurs, affecting immune function (8). Peripheral blood inflammation index can be used to assist in the diagnosis of UGIB and reflect Outcomes (9). Most studies have shown that hematological indicators such as hemoglobin and RDW are associated with the prognosis of patients with upper gastrointestinal bleeding (10). The neutrophil-lymphocyte ratio (NLR) is a rapid and simple parameter of systemic inflammation and stress, which expresses the severity of the disease in the patients. In recent years, NLR has become new markers for predicting systemic inflammatory status due to their potential to evaluate the prognosis in critically ill patients (11), sepsis (12), hepatocellular carcinoma (13), gastric cancer (14), gastrointestinal bleeding in Henoch-Schonlein purpura (15), and so on. Furthermore, few studies have analyzed the usefulness of NLR in predicting the prognosis in UGIB patients.
In this study, we analyzed the expression level of NLR in patients with UGIB to clarify the value of NLR in prognosis and provide a new serological indicator for early risk detection. Exploring whether adding NLR to GBS and FRS can improve predictive efficacy. To investigate whether the addition of NLR to GBS and FRS can improve the predictive efficacy, aiming to help healthcare workers to be able to make a quick and simple supplementary comprehensive assessment of the severity and prognosis prediction of patients.
2 Materials and methods
2.1 Population and study design
This single-center retrospective observational study was administered in upper gastrointestinal bleeding patients who were admitted to the emergency department of the First Affiliated Hospital of Wenzhou Medical University between January 2019 and December 2022; 726 adult patients (aged ≥18 years) who conformed to the diagnostic standard of the expert consensus on emergency diagnosis and treatment of acute upper gastrointestinal bleeding were enrolled in this study. We excluded patients with a reference standard that included (1) Bleeding caused by surgical factors such as trauma, (2) missing baseline data. The enrolled participant was undergone endoscopy. The research was conducted in accordance with the principle of the Declaration of Helsinki and approved by the Medical Ethics Committee established in the First Affiliated Hospital of Wenzhou Medical University, China (KY2021-R063).
2.2 Data collection
Based on the existing work on this subject, a data questionnaire was designed to retrospectively collect general clinical information and laboratory data of patients at the time of admission from the electronic medical record system, including age, gender, previous medical history, heart rate, systolic blood pressure, hemoglobin, initial symptoms, related medication history (non-steroidal anti-inflammatory drugs), blood transfusion needs, endoscopic treatment, and in-hospital death, etc. NLR is defined as neutrophil count (109/L) /lymphocyte count (109/L), which is collected when the patient is first admitted to the hospital and has not received any treatment. Outcomes were defined as in-hospital mortality, need for blood transfusion, surgical treatment and endoscopic therapy. Endoscopic therapy was defined as embolization, ligation, sclerotherapy and application of hemostatic clips within 24 h after admission. GBS, FRS, and NLR levels were calculated at first admission, and GBS and NLR were calculated by the investigator.
2.3 Statistical analysis
The quantitative variables that conformed to normal distribution were expressed as means ± standard deviation ( ) and the skewed distribution data were expressed as [M*(P25, P75)], and comparisons were made using a t-test and rank sum test, respectively. Data analyses were performed using SPSS 22.0. The chi-square test was used to compare two groups, and the Kruskal-Wallis test was used to compare multiple groups. Univariate and multivariate logistic regression analyses were performed on the influencing factors. The receiver–operator curve (ROC) was plotted to assess the predictive value of variables for outcomes. The regression model was used to establish the NLR-GBS combined model and the NLR-FRS combined model, and the area under the ROC curve compared the prediction accuracy of each scoring system and NLR for in-hospital mortality, Endoscopic interventon, surgery, transfusion therapy and composite of adverse outcome, p < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics and clinical outcomes
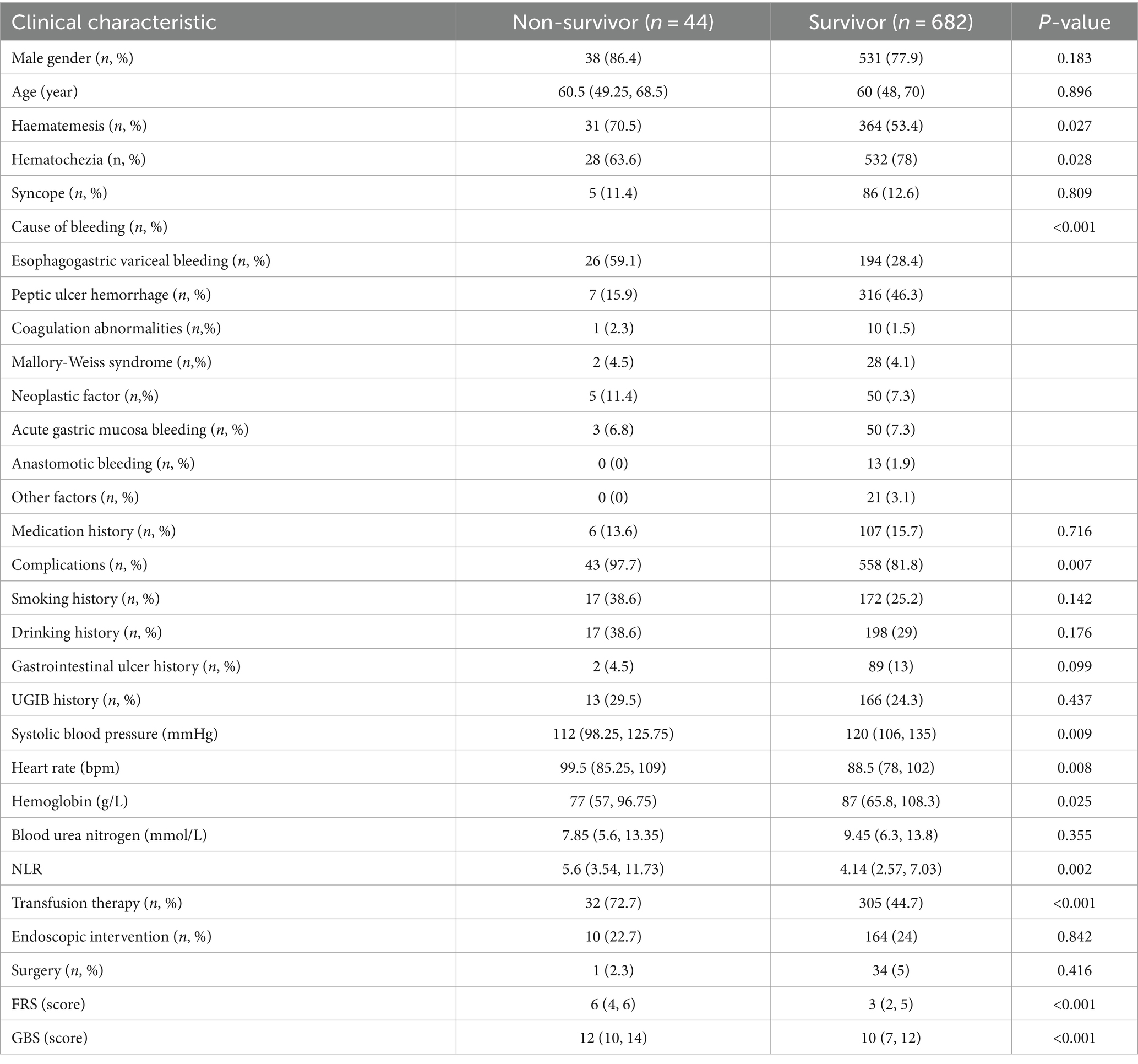
A total of 726 eligible patients admitted to the emergency department during the study period were included and categorized into survivor (n = 682) and non-survivor groups (n = 44), based on their eventual outcome. The differences in demographic characteristics between these groups and the comparative results are presented in Table 1.
The age of the patients in the survival and non-survivor groups was 60 (48, 70) and 60.5 (49.25, 68.5), respectively, and the difference was not statistically significant (p ≥ 0.05). Concerning the laboratory data, the levels of Systolic blood pressure [112 (98.25, 125.75) vs. 120 (106, 135); p = 0.009] and hemoglobin [77 (57, 96.75) vs. 87 (65.8, 108.3); p = 0.025] in the non-survivor group were significantly lower, whereas the levels of heart rate [99.5 (85.25, 109) vs. 88.5 (78, 102); p = 0.008] was markedly higher than those of the survivor group. For bioindicators, the levels of NLR [5.6 (3.54, 11.73) vs. 4.14 (2.57, 7.03); p = 0.002] in the non-survivor group were markedly elevated than those in the survivor group. Otherwise, there was no significant difference in blood urea nitrogen levels between the survivor and non-survivor groups. Patients in the non-survivor group presented poorer prognoses with the outcomes of full Rockall scores [6 (4, 6) vs. 3 (2, 5); P<0.001] and Glasgow Blatchford scores [12 (10, 14) vs. 10 (7, 12); P <0.001]. Nevertheless, we failed to observe significant differences in terms of gender, smoking, and alcohol history.
3.2 Analysis of UGIB patients grouped by NLR quartiles
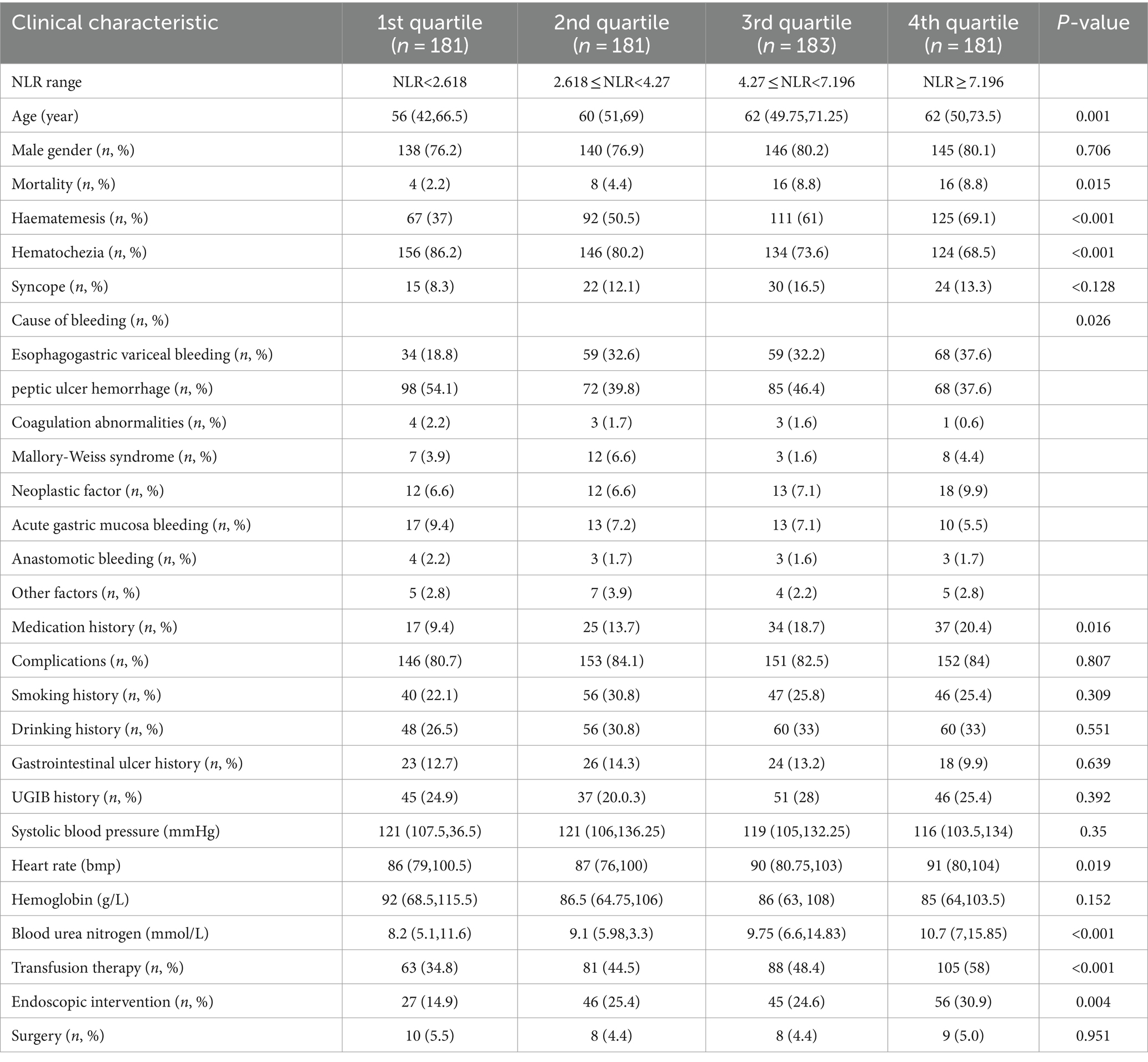
According to the NLR quartile level, they were divided into G1 group (NLR <2.618, 181 cases), G2 group (2.618 ≤ NLR <4.27, 181 cases), G3 group (4.27 ≤ NLR <7.196, 183 cases), and G4 group (NLR ≥7.196, 181 cases), and the differences of death, age, heart rate, and urinary urea were statistically significant among the groups. All patients were categorized into the high NLR group (n = 363) and low NLR group (n = 363) based on the median NLR of 4.27 as the cut-off value. Relatively more patients died, received endoscopic treatment, and were treated with blood transfusion within the high NLR group compared to the low NLR group, and the difference was statistically significant (p < 0.01). As the NLR values increased, the values of GBS and FRS also gradually increased, and the difference was statistically significant (p < 0.05) (Table 2).
3.3 Boxplot analysis of NLR and severity scores
Scatterplots were distributed to describe the correlation between NLR and the severity of UGIB. Rockall score (16) and Glasgow-Blatchford score (17) were used to score the enrolled patients, respectively; Rockall score 0–2 was categorized as a low-risk group, 3–4 was categorized as an intermediate-risk group, and ≥ 5 was categorized as a high-risk group; GBS <6 was categorized as a low-risk group, and ≥ 6 was a categorized as an intermediate-high-risk group. In the GBS subgroup, the NLR of patients in the medium-high-risk group was significantly higher than that of the low-risk group (p = 0.043); in the FRS subgroup, there was statistical difference in NLR among the three groups (p = 0.043) (Figures 1, 2).
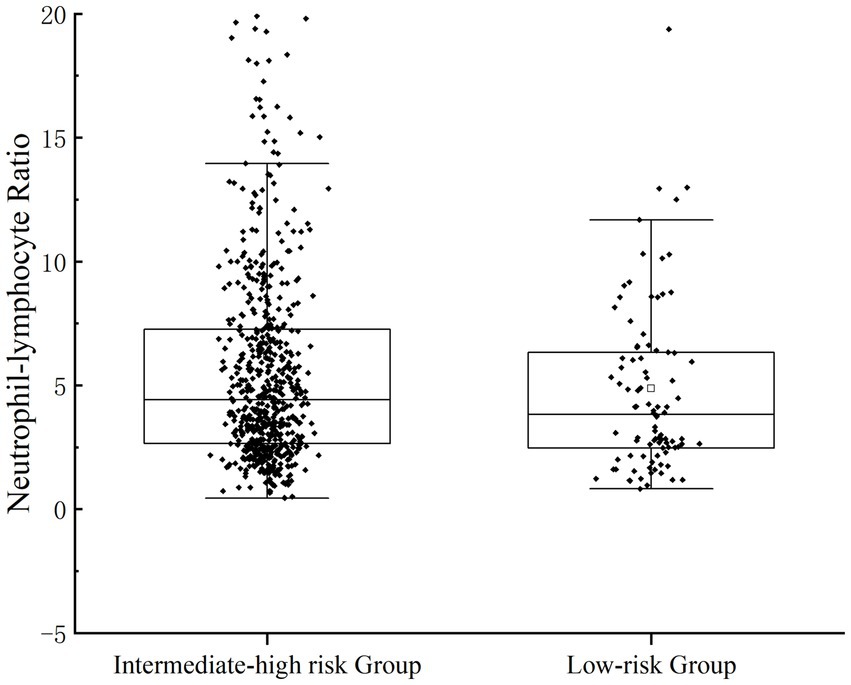
Figure 1. Patients’ NLR levels were categorized for severity. The correlation between NLR and the severity of UGIB was assessed by GBS.
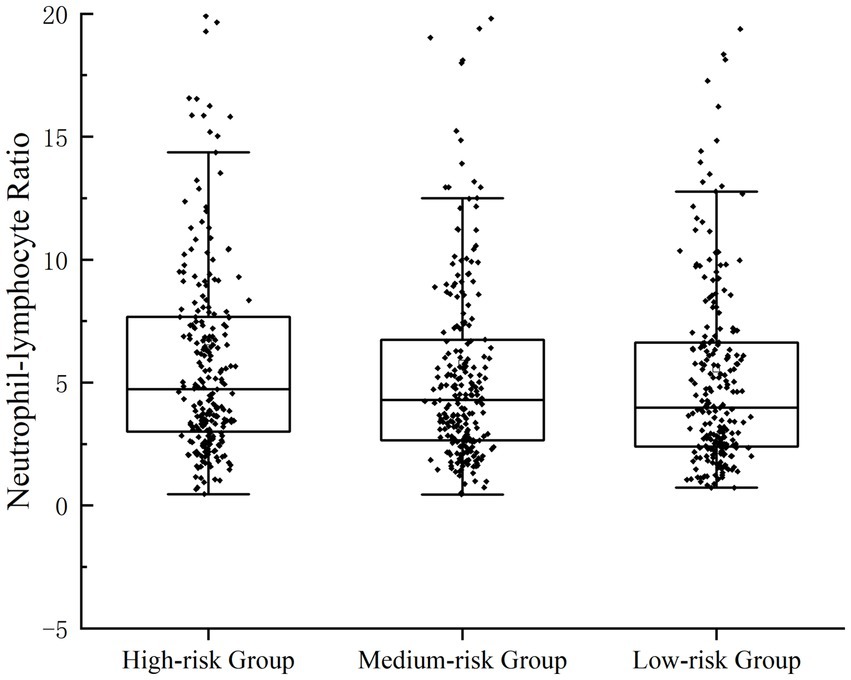
Figure 2. Patients’ NLR levels were categorized for severity. Correlation between NLR and the severity of UGIB, which was assessed by FRS.
3.4 Risk factors related to mortality
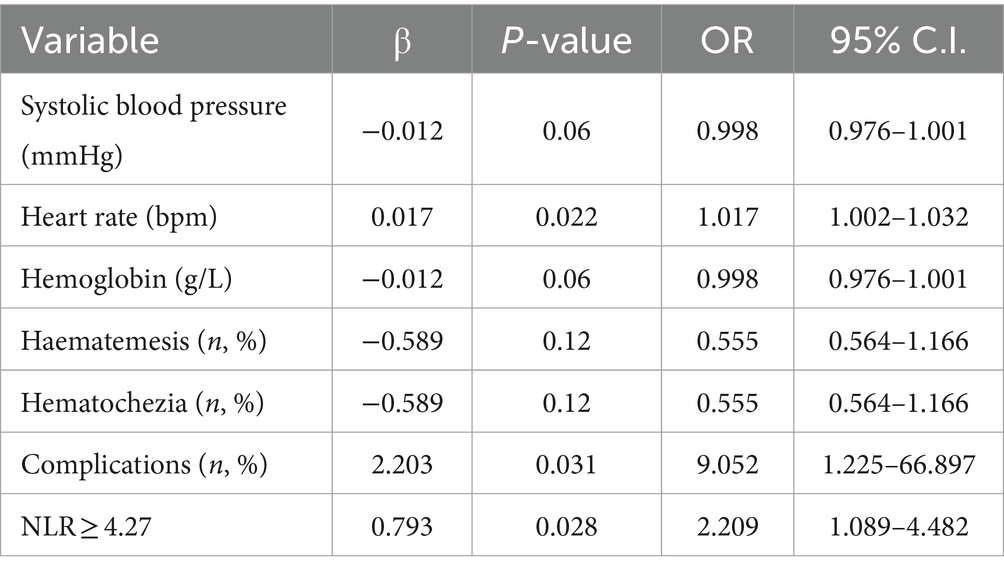
The univariate logistic regression analysis revealed that comorbidity, vomiting blood, blood in stool, heart rate, systolic blood pressure, hemoglobin levels, and NLR ≥ 4.27 were significantly associated with the occurrence of death in patients with upper gastrointestinal bleeding. These variables were subsequently included in a multifactorial logistic regression model to further analyze their impact on adverse outcomes in these patients (Tables 3, 4).
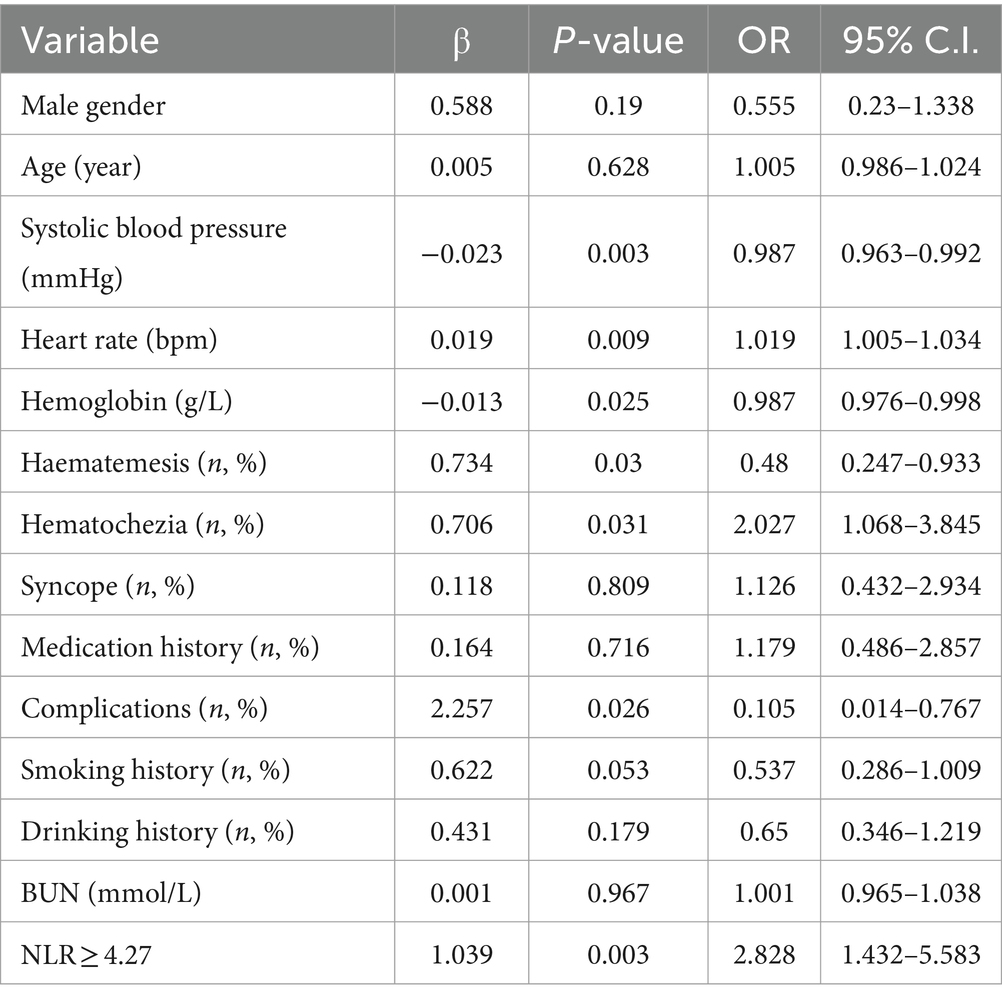
Table 3. Univariate analysis of risk factors affecting mortality from upper gastrointestinal bleeding.
3.5 Pearson correlation analysis of NLR compared with GBS and FRS
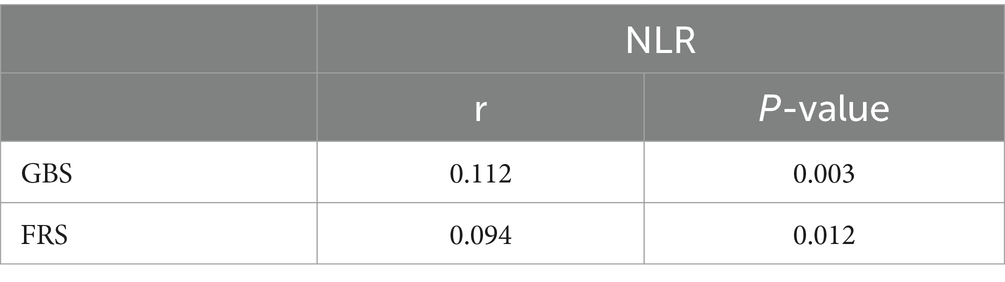
The Pearson correlation analysis was conducted to examine the relationship between NLR and both GBS and FRS. The results revealed a positive correlation between NLR and GBS (r = 0.112, p < 0.05), as well as a positive correlation between the NLR and FRS (r = 0.094, p < 0.05) (Table 5).
3.6 Predictive performance of different scoring systems for mortality in patients with UGIB
The ROC curve analysis was performed using the occurrence of in-hospital mortality in UGIB patients as the status variable (non-survival = 1, survival = 0), and NLR, GBS, FRS, GBS + NLR as the test variables. The results revealed that there was a statistically significant difference in AUC between FRS + NLR and FRS (Z = 2.033, p = 0.042). Additionally, the AUC for GBS + NLR was found to be higher than that for GBS with a statistically significant difference (Z = 20.072, p = 0.0383) (Table 6; Figure 3).
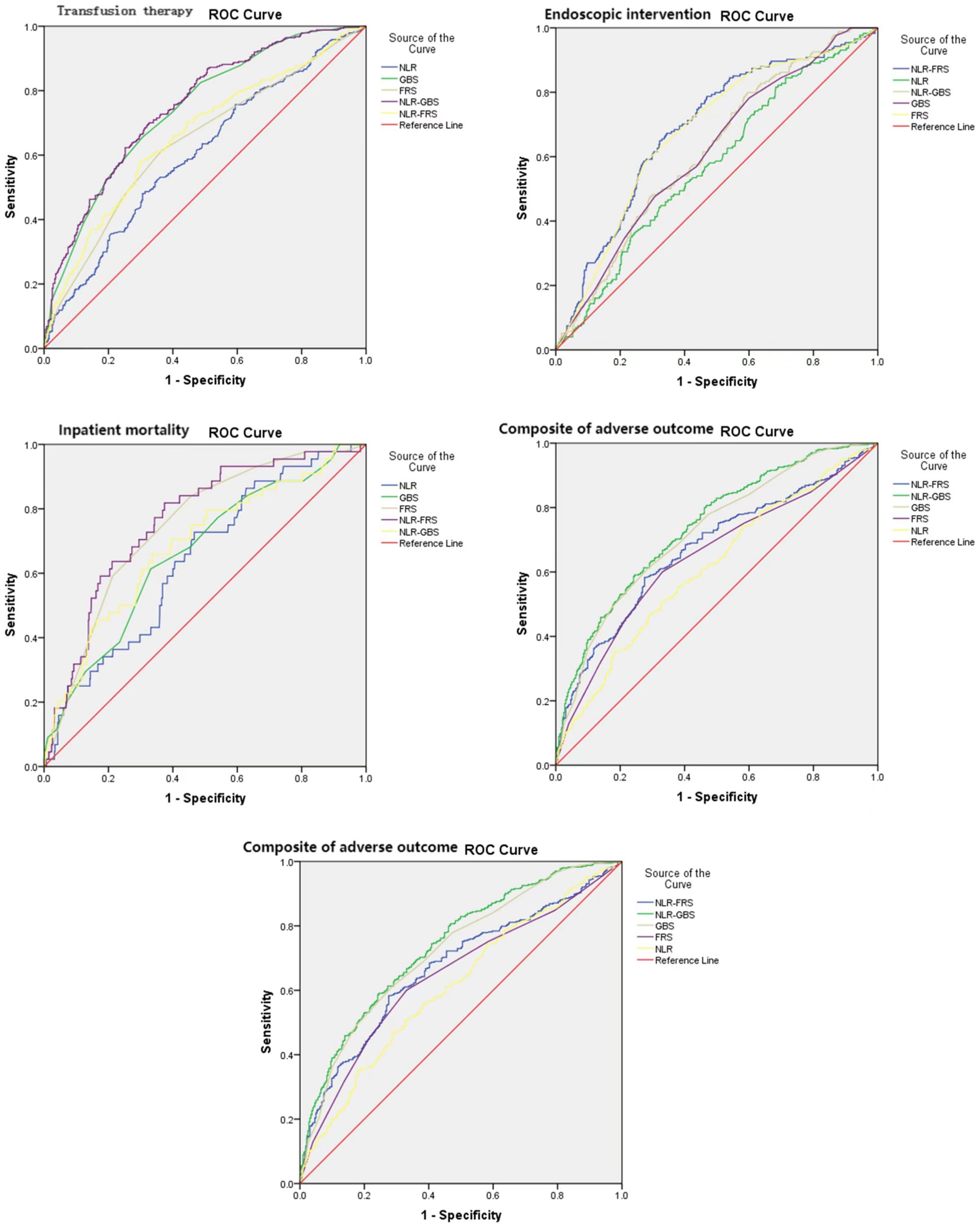
Figure 3. Reciever-operating characteristic curves (AUROCs) for the NLR, GBS, FRS, NLR-GBS, NLR, in predicting in-hospital mortality, transfusion therapy, endoscopic therapy, and composite of adverse outcome.
4 Discussion
Upper gastrointestinal bleeding is a common disease in the emergency department, with various etiologies and presentations. It is defined as blood loss originating proximal to the ligament of Treitz, in the esophagus, stomach, or duodenum (18). UGIB exhibits a substantial morbidity and mortality rate; therefore, early assessment and prediction of UGIB condition hold paramount clinical significance for enhancing patient prognosis. Currently, various scoring systems are employed to evaluate the condition and prognosis of UGIB patients (19). However, these systems possess inherent subjectivity, necessitating the urgent development of safer, more efficient, and objective biochemical markers that can aid in early prognostic prediction for UGIB patients. Such markers would greatly assist in guiding timely treatment interventions for UGIB.
The cost-effectiveness and widespread use of NLR as a composite inflammatory marker, reflecting the interplay between neutrophil and lymphocyte immune responses, make it a valuable tool for disease diagnosis. Elevated levels of NLR may indicate an increase in various pro-inflammatory cytokines concentration (20). More research evidence confirms the value of NLR in the assessment of disease and prognosis of cirrhosis (21), hepatocellular carcinoma (13), gastric cancer (14), allergic purpura combined with gastrointestinal hemorrhage, and ischemic stroke (22), etc., and Makay et al. found that the NLR values of patients with UGIB were significantly higher than those of patients without gastrointestinal hemorrhage (15).
The expression of NLR was significantly elevated in the non-survivor group with UGIB (p < 0.001). Moreover, it was observed that an admission peripheral blood NLR ≥4.27 independently contributed to the risk of in-hospital mortality in individuals with UGIB (p = 0.028). The NLR value of patients with UGIB in this study was divided into quartiles, and the clinical characteristics and prognosis were compared among patients in G1-4 groups. The results demonstrated a progressive increase in mortality rates from the G1 to G4 group (G1-4 groups, 2.2% vs. 4.4% vs. 8.8% vs. 8.8%, p = 0.015). Moreover, there was a gradual rise in the proportion of blood transfusion treatment from the G1 to G4 group (G1-4 groups, 34.8% vs. 44.5% vs. 48.4% vs. 58%, p < 0.001), along with an increasing trend in the proportion of endoscopic therapy (G1-4 groups, 14.9% vs. 25.4% vs. 24.6% vs. 30.9%, p = 0.004). These findings are consistent with those reported by Ramazan et al. (9). UGIB are affected by various stress factors and excessive bleeding, changes in levels of inflammatory factors in blood vessels, aggravating local damage at the bleeding site. Inflammatory reactions run through the entire course of the disease, and sustained inflammatory reactions are important factors that make it difficult for patients to stop bleeding, and even lead to death. The persistent inflammatory response is a crucial factor contributing to difficulties in achieving hemostasis and even mortality (23, 24). Koseoglu (25) and Kong (26) et al. reported that leukocyte values were higher in the group that died of UGIB than in the group that survived. Scholar Dertli found that high NLR level on admission was an independent risk factor for length of hospitalization and death in patients with non-variceal upper gastrointestinal bleeding (9), and high leukocyte values could be a predictor of high morbidity and mortality in patients with UGIB and could reflect the severity of bleeding and blood loss in patients (27, 28).
The most prevalent cause of UGIB is peptic ulcer, followed by bleeding from esophageal and gastric varices. This finding aligns with previous reports (29, 30).The level of NLR in patients presenting with UGIB demonstrated a significant correlation with the etiology of the hemorrhage (p = 0.026). Furthermore, our study revealed a progressive increase in the proportion of patients experiencing esophagogastric variceal bleeding (EGVB) across G1-4 groups. EGVB can induce the production of various inflammatory mediators, thereby promoting the development of acute inflammation (31), which in turn may lead to an acute systemic inflammatory response and increase the risk of mortality (32). Lin et al. demonstrated that the NLR independently predicted 30-day mortality in patients with acute decompensated cirrhosis (33), while Rice Jonathan et al. revealed a significant association between elevated NLR and increased all-cause mortality at 1 year in cirrhotic patients (34).
The expression level of NLR was higher in UGIB patients with a history of nonsteroidal anti-inflammatory drugs, and the proportion of patients with such history gradually increased from G1 to G4 (9.4% vs.13.7% vs. 18.7% vs. 20.4%, p = 0.016). Patients with UGIB mostly take NSAIDs (35), NSAIDs inhibit cyclooxygenase activity and decrease prostaglandin synthesis in the body, leading to a reduction in prostaglandin levels that can compromise the mucosal barrier and cause ischemia. The ischemic effect of NSAIDs induces inflammation, leukocyte migration, and vascular damage (9, 23).
The present study revealed a positive correlation between NLR and both GBS and FRS in patients with UGIB. In terms of predicting in-hospital mortality, the NLR-FRS model demonstrated superior performance compared to FRS (0.763 vs. 0.747, p = 0.042), while the NLR-GBS model outperformed GBS (0.687 vs. 0.662, p = 0.0383). Notably, the NLR-FRS model exhibited the highest AUC for predicting in-hospital mortality specifically in patients with UGIB, thereby providing a comprehensive reflection of changes in their clinical status.
There are some limitations in this study, such as this is a retrospective study, some patients were excluded due to the lack of necessary data, there may be a selection bias, and this is a single-center study, the results may not apply to other medical centers, the above conclusions need to be verified by additional studies.
5 Conclusion
In conclusion, the level of NLR is closely related to the prognosis of UGIB patients, which can play an early warning role for the risk of death in UGIB patients, and the combination with GBS and FRS can significantly improve the prediction efficacy, which can help to guide the clinic and nursing care in the early stage of risk stratification and the development of therapeutic care plan for the patients. With the advantages of simplicity, speed, and low cost, NLR deserves to be popularized.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XC: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. XL: Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. GZ: Conceptualization, Formal analysis, Supervision, Writing – review & editing. WX: Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded with Wenzhou Association of Science and Technology Service Innovation Project (kjfw23).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kamboj, AK, Hoversten, P, and Leggett, CL. Upper gastrointestinal bleeding: etiologies and management. Mayo Clin Proc. (2019) 94:697–703. doi: 10.1016/j.mayocp.2019.01.022
2. Kichler, A, and Jang, S. Endoscopic hemostasis for non-variceal upper gastrointestinal bleeding: new Frontiers. Clin Endosc. (2019) 52:401–6. doi: 10.5946/ce.2018.103
3. Haddad, FG, El Imad, T, Nassani, N, Kwok, R, Al Moussawi, H, Polavarapu, A, et al. In-hospital acute upper gastrointestinal bleeding: what is the scope of the problem? World J Gastrointest Endosc. (2019) 11:561–72. doi: 10.4253/wjge.v11.i12.561
4. Antunes, C, and Copelin, IE. Upper gastrointestinal bleeding. Treasure Island, FL: StatPearls (2022).
5. Shung, DL, Au, B, Taylor, RA, Tay, JK, Laursen, SB, Stanley, AJ, et al. Validation of a machine learning model that outperforms clinical risk scoring Systems for Upper Gastrointestinal Bleeding. Gastroenterology. (2020) 158:160–7. doi: 10.1053/j.gastro.2019.09.009
6. Wilkins, T, Wheeler, B, and Carpenter, M. Upper gastrointestinal bleeding in adults: evaluation and management. Am Fam Physician. (2020) 101:294–300.
7. Orpen-Palmer, J, and Stanley, AJ. A review of risk scores within upper gastrointestinal bleeding. J Clin Med. (2023) 12:3678. doi: 10.3390/jcm12113678
8. Hess, JJ, Wallenstein, J, Ackerman, JD, Akhter, M, Ander, D, Keadey, M, et al. Scribe impacts on provider experience, operations, and teaching in an academic emergency medicine practice. West J Emerg Med. (2015) 16:602–10. doi: 10.5811/westjem.2015.6.25432
9. Dertli, R, Toka, B, Asıl, M, Kayar, Y, Karakarcayıldız, A, Göktepe, MH, et al. Can neutrophil-lymphocyte ratio predict mortality in acute non-variceal upper gastrointestinal bleeding? Ulus Travma Acil Cerrahi Derg. (2022) 28:626–33. doi: 10.14744/tjtes.2021.42900
10. Lee, KR, Park, SO, Kim, SY, Hong, DY, Kim, JW, Baek, KJ, et al. Red cell distribution width as a novel marker for predicting high-risk from upper gastro-intestinal bleeding patients. PLoS One. (2017) 12:e0187158. doi: 10.1371/journal.pone.0187158
11. Ham, SY, Yoon, HJ, Nam, SB, Yun, BH, Eum, D, and Shin, CS. Prognostic value of neutrophil/lymphocyte ratio and mean platelet volume/platelet ratio for 1-year mortality in critically ill patients. Sci Rep. (2020) 10:21513. doi: 10.1038/s41598-020-78476-y
12. Liu, Y, Zheng, J, Zhang, D, and Jing, L. Neutrophil-lymphocyte ratio and plasma lactate predict 28-day mortality in patients with sepsis. J Clin Lab Anal. (2019) 33:e22942. doi: 10.1002/jcla.22942
13. Xiao, W-K, Chen, D, Li, S-Q, Fu, SJ, Peng, BG, and Liang, LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. (2014) 14:117. doi: 10.1186/1471-2407-14-117
14. Zhang, X, Zhang, W, and Feng, L-J. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One. (2014) 9:e111906. doi: 10.1371/journal.pone.0111906
15. Makay, B, Gücenmez, ÖA, Duman, M, and Ünsal, E. The relationship of neutrophil-to-lymphocyte ratio with gastrointestinal bleeding in Henoch-Schonlein purpura. Rheumatol Int. (2014) 34:1323–7. doi: 10.1007/s00296-014-2986-2
16. Rockall, TA, Logan, RF, Devlin, HB, and Northfield, TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. (1996) 38:316–21. doi: 10.1136/gut.38.3.316
17. Blatchford, O, Murray, WR, and Blatchford, M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. (2000) 356:1318–21. doi: 10.1016/S0140-6736(00)02816-6
18. Laine, L, Barkun, AN, Saltzman, JR, Martel, M, and Leontiadis, GI. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. (2021) 116:899–917. doi: 10.14309/ajg.0000000000001245
19. Lee, HA, Jung, HK, Kim, TO, Byeon, JR, Jeong, ES, Cho, HJ, et al. Clinical outcomes of acute upper gastrointestinal bleeding according to the risk indicated by Glasgow-Blatchford risk score-computed tomography score in the emergency room. Korean J Intern Med. (2022) 37:1176–85. doi: 10.3904/kjim.2022.099
20. Templeton, AJ, McNamara, M, Šeruga, B, Vera-Badillo, FE, Aneja, P, Ocaña, A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
21. Lin, B, Geng, L, Zheng, Z, Jia, J, Shen, T, Zhang, J, et al. The predictive value of blood neutrophil-lymphocyte ratio in patients with end-stage liver cirrhosis following ABO-incompatible liver transplantation. J Res Med Sci. (2016) 21:69. doi: 10.4103/1735-1995.189653
22. Sharma, D, Spring, KJ, and Bhaskar, SMM. Neutrophil-lymphocyte ratio in acute ischemic stroke: immunopathology, management, and prognosis. Acta Neurol Scand. (2021) 144:486–99. doi: 10.1111/ane.13493
23. Bjarnason, I, Scarpignato, C, Holmgren, E, Olszewski, M, Rainsford, KD, and Lanas, A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. (2018) 154:500–14. doi: 10.1053/j.gastro.2017.10.049
24. Lanas, A, Dumonceau, JM, Hunt, RH, Fujishiro, M, Scheiman, JM, Gralnek, IM, et al. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. (2018) 4:18020. doi: 10.1038/nrdp.2018.20
25. Koseoglu, Z, Ozkan, OV, Semerci, E, Aslan, A, Yetim, I, Ucar, E, et al. The relationship between mortality and inflammation in patients with gastrointestinal bleeding. J Int Med Res. (2009) 37:1508–14. doi: 10.1177/147323000903700528
26. Kong, T, In, S, Park, YS, Lee, HS, Lee, JW, You, JS, et al. Usefulness of the Delta neutrophil index to predict 30-day mortality in patients with upper gastrointestinal bleeding. Shock. (2017) 48:427–35. doi: 10.1097/SHK.0000000000000878
27. Chalasani, N, Patel, K, Clark, WS, and Wilcox, CM. The prevalence and significance of leukocytosis in upper gastrointestinal bleeding. Am J Med Sci. (1998) 315:233–6.
28. Turkmen, K, Guney, I, Yerlikaya, FH, and Tonbul, HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. (2012) 34:155–9. doi: 10.3109/0886022X.2011.641514
29. Lu, M, Sun, G, Zhang, XM, Xv, YQ, Chen, SY, Song, Y, et al. Peptic ulcer is the Most common cause of non-variceal upper-gastrointestinal bleeding (NVUGIB) in China. Med Sci Monit. (2018) 24:7119–29. doi: 10.12659/MSM.909560
30. Kaviani, MJ, Pirastehfar, M, Azari, A, and Saberifiroozi, M. Etiology and outcome of patients with upper gastrointestinal bleeding: a study from south of Iran. Saudi J Gastroenterol. (2010) 16:253–9. doi: 10.4103/1319-3767.70608
31. Wang, XY. Clinical value of MELD-Na score combined with NLR in predicting Rebleeding after esophageal and gastric varices rupture and hemorrhage in cirrhosis (in Chinese). J Rare Uncommon Dis. (2024) 31:70–2. doi: 10.3969/j.issn.1009-3257.2024.5.029
32. Chikamori, F, Ito, S, and Sharma, N. Percutaneous transhepatic obliteration for life-threatening bleeding after endoscopic variceal ligation in a patient with severe esophagogastric varices. Radiol Case Rep. (2023) 18:624–30. doi: 10.1016/j.radcr.2022.10.105
33. Lin, L, Yang, F, Wang, Y, Su, S, Su, Z, Jiang, X, et al. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int Immunopharmacol. (2018) 56:58–64. doi: 10.1016/j.intimp.2018.01.007
34. Rice, J, Dodge, JL, Bambha, KM, Bajaj, JS, Reddy, KR, Gralla, J, et al. Neutrophil-to-lymphocyte ratio associates independently with mortality in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. (2018) 16:1786–1791.e1. doi: 10.1016/j.cgh.2018.04.045
Keywords: upper gastrointestinal bleeding, neutrophil-lymphocyte ratio, outcome, Glasgow-Blatchford score, full Rockall score
Citation: Chen X, Li X, Zhao G and Xu W (2024) Neutrophil-lymphocyte ratio predict outcome of upper gastrointestinal bleeding in emergency. Front. Med. 11:1366715. doi: 10.3389/fmed.2024.1366715
Edited by:
Marcus Hollenbach, University Hospital Leipzig, GermanyReviewed by:
Pavel Skok, University of Maribor, SloveniaAli Taha, University Hospital Crosshouse, United Kingdom
Copyright © 2024 Chen, Li, Zhao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinqun Li, bHhxMjAwOTg4ODJAMTYzLmNvbQ==
 Xinyi Chen
Xinyi Chen Xinqun Li1*
Xinqun Li1* Wen Xu
Wen Xu