- 1Department of Orthopedics, Dandong Central Hospital, China Medical University, Dandong, China
- 2Department of Oncology, Dandong Central Hospital, China Medical University, Dandong, China
Purpose: Postoperative urinary tract infections (UTIs) worsen the prognosis of elderly patients with hip fractures. This study aimed to assess the predictive ability of blood-based biomarkers, specifically the glucose-albumin ratio (GAR), in predicting postoperative UTIs.
Methods: A retrospective observational study of 1,231 patients from a Level I trauma center was conducted. We evaluated the prognostic and predictive value of 15 biomarkers, including the glucose-albumin ratio, in elderly patients with hip fractures. The primary outcome measure was the incidence of postoperative UTIs.
Results: The glucose to albumin ratio transformed into GAR was superior to any other biomarker in predicting postoperative UTIs in elderly hip fracture patients (AUC = 0.756, p < 0.001). Elevated GAR (using the best cut-off value of 0.18) was independently associated with postoperative UTIs (OR 3.20, 95% CI 2.23–4.58). Further analysis dividing GAR levels into four groups according to quartiles showed that compared to patients with GAR levels of Q1 (< 0.14), GAR levels of Q2 (0.14–0.17; OR 2.11, 95% CI 1.07–4.15), Q3 (0.17–0.21; OR 3.36, 95% CI 1.74–6.52) and Q4 (> 0.21; OR 7.55, 95% CI 3.84–14.83) patients had significantly higher odds of UTIs.
Conclusion: GAR holds potential as a novel biomarker for predicting postoperative UTIs in elderly patients with hip fractures.
Introduction
The global incidence of hip fractures is on the rise, with projections stating a dramatic increase from 1.31 million cases in 1990 to an estimated 6.26 million by 2050 (1–4). This escalating prevalence places significant pressure on healthcare systems while leading to an increase in postoperative complications and mortality rates (5–8). Efficient surgical intervention and diligent postoperative management have proved effective for hip fracture patients (4, 9). Furthermore, postoperative urinary tract infections (UTIs) in elderly patients with hip fractures present significant healthcare concerns, often not given due attention (10–13). Significantly, the routine application of indwelling urinary catheters in orthopedic wards can intensify the adverse effects associated with hip fractures, leading to increased patient discomfort and unfavorable prognosis (12, 14–16). Hence, rapid identification of hip fracture patients prone to UTI is paramount through certain indicators.
Post-traumatic hyperglycemia commonly observed in elderly patients upon admission with hip fractures is a contributing factor to the occurrence of UTIs (17, 18). While it is widely recognized that diabetic patients are at an increased risk of urinary tract infections, the association between stress-induced hyperglycemia upon admission due to trauma and the risk of urinary tract infections remains unclear (19–21). In addition to investigating blood glucose levels, attention must also be directed toward serum albumin as a significant indicator of nutritional status (22, 23). Hypoalbuminemia (serum albumin < 35 g/L), a manifestation of malnutrition commonly observed in elderly hip fracture patients, is intrinsically associated with postoperative complications and premature mortality (24, 25). Its development is influenced by several factors, particularly chronic disease, inflammatory response, and insulin resistance (22, 26). Hypoalbuminemia exacerbates insufficient nutritional intake, hindering recovery and increasing infection risk (27).
The existing literature suggests an association between post-traumatic hyperglycemia, hypoalbuminemia, and increased susceptibility to infection, but well-designed studies directly linking these factors to the risk of UTIs are limited (18, 28, 29). This gap underscores the clinical importance of the proposed study to explore the link between hyperglycemia, hypoalbuminemia, and the occurrence of UTIs in elderly hip fracture patients. With the aforementioned considerations, the objective of this paper is to examine the potential of the glucose-albumin ratio (GAR) as a novel biomarker for predicting postoperative UTIs in elderly patients with hip fractures, thereby contributing to preoperative risk assessment and postoperative nutritional intervention strategies to improve surgical outcomes and providing valuable insights to improve patient prognosis.
Materials and methods
Study design and data sources
We conducted a retrospective analysis of elderly patients with hip fractures who were admitted to our institution between March 2013 and June 2023. The study was approved by the Dandong Central Hospital Ethics Committees (No. DDZX-20230711), and informed consent was waived. Our study cohort comprises patients undergoing surgical treatment for hip fractures, all of whom underwent dual diagnostic evaluation with preoperative X-ray imaging and three-dimensional CT reconstruction. However, patients meeting any of the following criteria were excluded: (1) age below 60 years; (2) presence of old fractures, pathologic fractures, multiple fractures, periprosthetic fractures, or open fractures; (3) previous conservative treatment, revision surgery, reoperation, or long-term use of immunosuppressive agents, such as glucocorticoids; (4) preoperative diagnosis of urinary tract infections; (5) lack of urinary culture during hospitalization or laboratory tests, including urinalysis; (6) in-hospital mortality or incomplete information available for any reason.
Variables
The demographic data collected for analysis included age, gender, smoking status, and alcoholism. Past comorbidities considered were hypertension, diabetes, cardiovascular disease, stroke, chronic kidney disease, prostatic hyperplasia, urolithiasis, vesicoureteral disease, and a history of neoplasms. The baseline health information of these patients was routinely collected and recorded in the hospital’s electronic system by nursing staff upon the patients’ admission to our medical institution. Additionally, the data encompassed the type of hip fracture, classification based on the American Society of Anesthesiologists (ASA), surgery method, the utilization of indwelling catheterization, duration of indwelling catheterization, duration of the surgery, and length of bed rest. In our study, the calculation of patient bed rest duration began upon admission and ended when patients were capable of bearing weight and standing post-surgery.
Laboratory indicators
Blood samples were collected from patients with hip fractures within 24 h of admission to obtain relevant biomarker data. The biomarkers collected included: blood glucose levels (mmol/L), albumin levels (g/L), D-dimer levels (μg/L), alanine aminotransferase levels (IU/L), high-density lipoprotein levels (mmol/L), erythrocyte count (× 1012/L), leukocyte count (× 109/L), neutrophil count (× 109/L), lymphocyte count (× 109/L), platelet count (× 109/L), mean platelet volume (fL), red blood cell distribution width (%), aspartate aminotransferase levels (IU/L), cholesterol levels (mmol/L), low-density lipoprotein levels (mmol/L), triglyceride levels (mmol/L), hemoglobin levels (g/L), creatinine levels (μmol/L), uric acid levels (μmol/L), and blood urea nitrogen levels (mmol/L).
Definition of outcome
The primary outcome measured in this study was the incidence of postoperative UTIs. To exclude preadmission urinary tract infection in patients with hip fractures, urine analysis and culture results upon admission were analyzed. After undergoing orthopedic surgical treatment, routine urine cultures were performed every three days (on Tuesdays and Fridays). To ensure standardized urine collection, clinical nurses in the orthopedic unit received regular training from the hospital’s infection committee on aseptic techniques, standard disinfection methods, and prompt sample delivery to the microbiology laboratory. In accordance with the guidelines from the Centers for Disease Control and Prevention (30), a UTI was defined if patients met the following criteria: (1) fever (> 38 degrees Celsius or 100.4 degrees Fahrenheit), urgency, frequency, urethral pain, suprapubic pressure, and lumbar and dorsal horn pain or tenderness; and (2) a positive urine culture (bacteriuria > 105 CFU/mL) or positive urinalysis results (leukocyte esterase and nitrite in mid-stream urine specimens). Prior to the commencement of the study, three researchers underwent standardized training in systematic operation and interpretation of UTIs indicators (WY, WW, and WT). The determination of infection events was independently performed by three members, with any disagreements being resolved by senior researchers (QL and WD). Finally, patients diagnosed with UTIs routinely received antibiotic treatment.
Statistical analysis
Continuous variables were reported as median ± interquartile range (IQR), while categorical variables were presented as absolute numbers and percentages. Statistical significance was defined as two-sided P-values < 0.05. Five blood-based biomarkers were identified and tested. Two of these biomarkers (blood glucose and D-dimer) exhibited an increase in adverse outcomes, while the remaining three biomarkers (alanine aminotransferase, albumin, and high-density lipoprotein) showed a decrease in adverse outcomes. All these factors were employed to identify the combination (glucose-albumin ratio) that yielded the highest predictive accuracy for postoperative UTIs in patients with hip fractures.
Receiver operating characteristic (ROC) curves and their corresponding area under the curve (AUC) were calculated to evaluate the predictive performance of different biomarkers for postoperative UTIs. To ascertain the optimal cutoff, we employed the Youden index, computed as the sum of sensitivity and specificity minus one. We systematically derived Youden index values by calculating corresponding sensitivity and specificity at various points along the ROC curve. The optimal cutoff was identified as the point on the ROC curve that maximizes the Youden index.
The covariates were analyzed using multivariate logistic regression to calculate the odds ratio (OR) and corresponding 95% confidence interval (CI). Variables with a p-value < 0.10 in the univariate regression were included in the multivariate logistic regression analysis. The covariates included in the logistic regression models were selected based on previous studies (31, 32) and clinical expertise. E-values were employed to evaluate the strength of observed associations considering potential unmeasured confounding (33). These e-values were computed utilizing an online e-value calculator available at https://www.evalue-calculator.com/evalue/. Moreover, patients were categorized into four groups (Q1: < 0.14, Q2: 0.14–0.17, Q3: 0.17–0.21, and Q4: > 0.21) based on the quartile distribution of the target biomarkers, aiming to enhance the assessment of the dose-effect relationship with postoperative UTIs. Lastly, propensity score matching was conducted as a sensitivity analysis (34). In our PSM 1:1 matching process, we utilized the nearest neighbor matching algorithm to match the high-level blood sugar or albumin group with the normal group, with a caliper value set at 0.1 standard deviation. We employed the standardized mean difference (SMD) to assess any imbalance between the two groups, considering SMD ≥ 0.10 as indicative of imbalance. Statistical analyses were performed using R software version 4.0.3.
Results
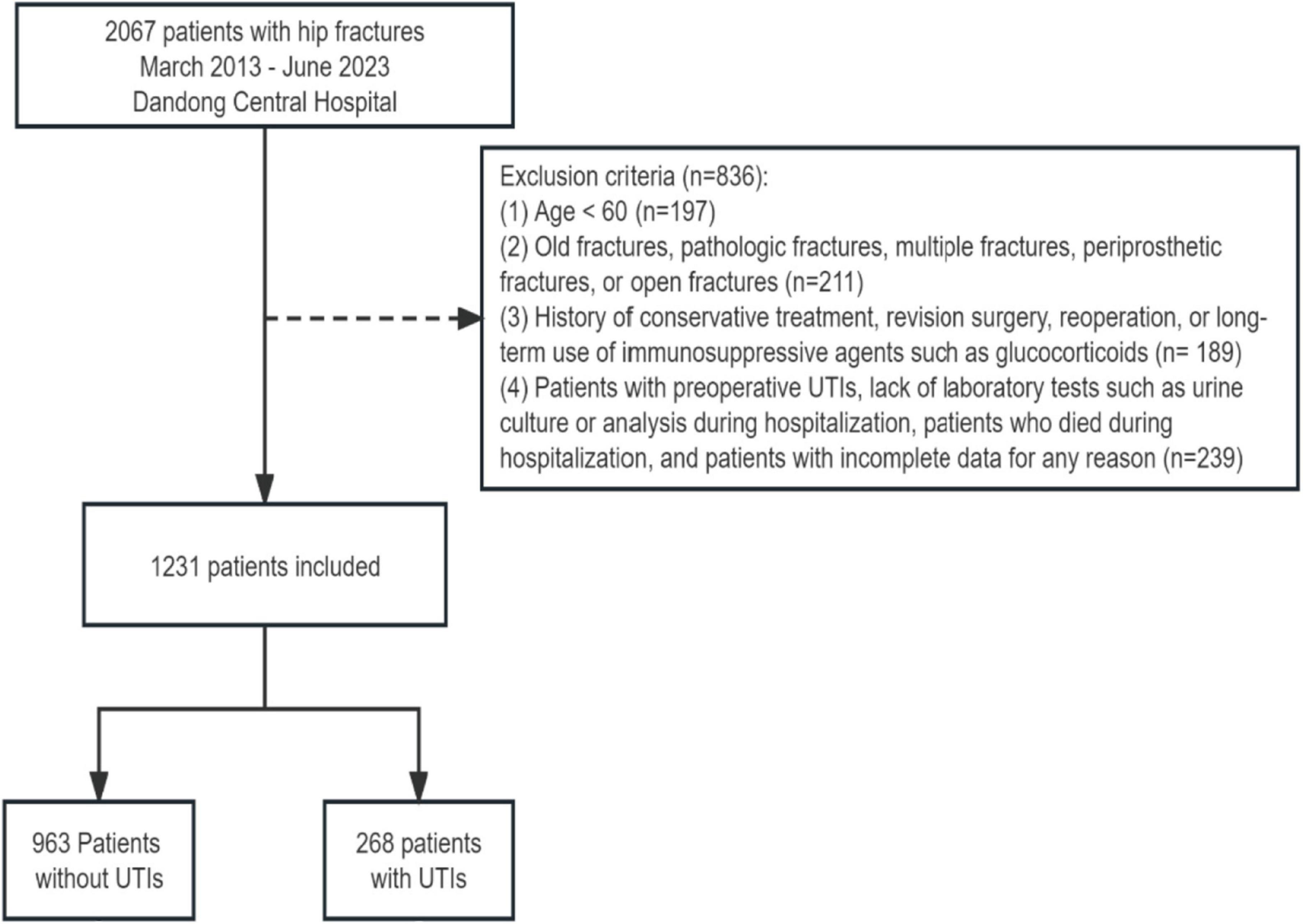
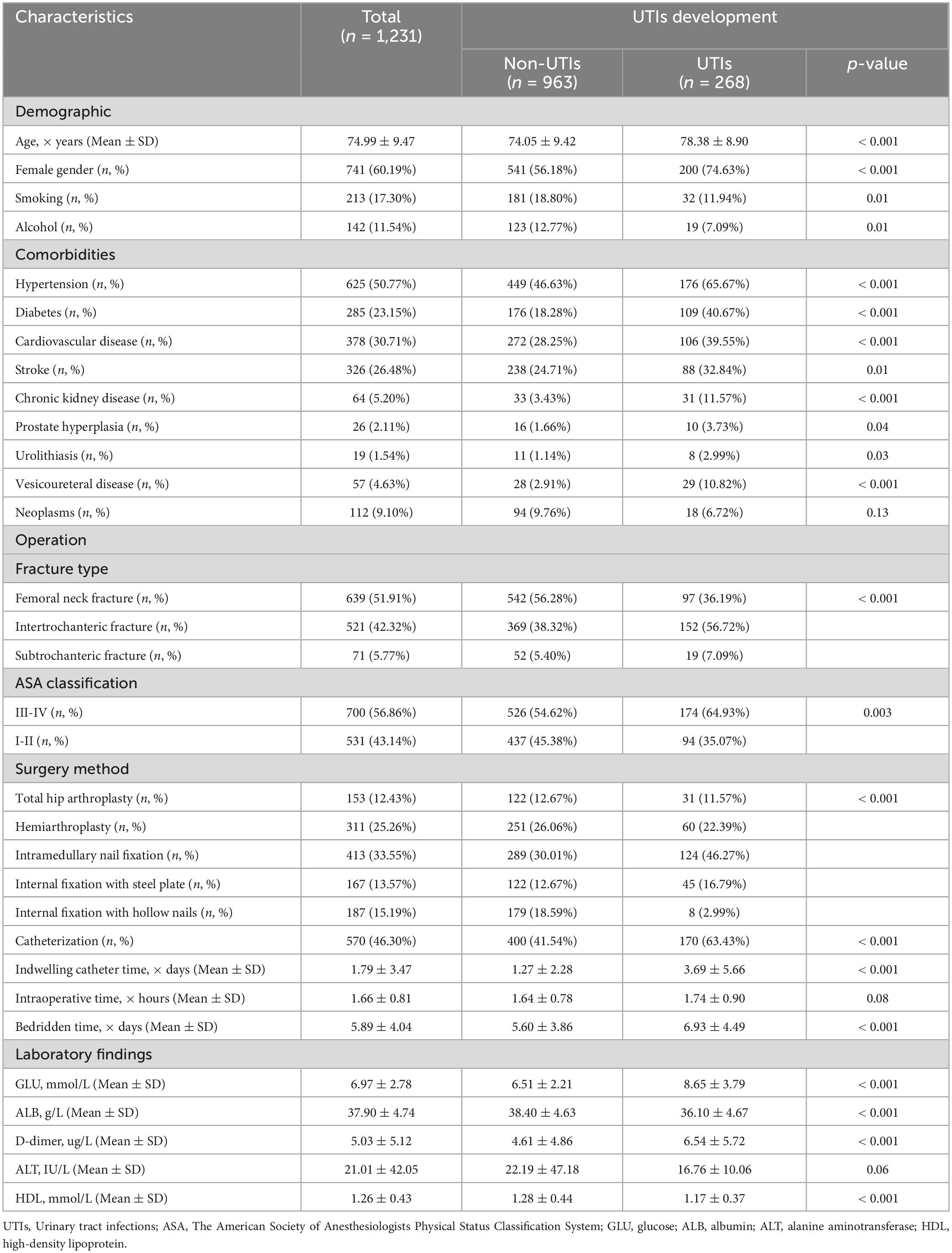
We initially identified 2,067 patients with hip fractures occurring between March 2013 and June 2023. Following a rigorous exclusion process, a total of 836 patients were excluded from the study. Specifically, patients were excluded if they were under 60 years old (n = 197), presented with old fractures, pathologic fractures, multiple fractures, periprosthetic fractures, or open fractures (n = 211), had a history of conservative treatment, revision surgery, reoperation, or long-term use of immunosuppressive agents such as glucocorticoids (n = 189), or if they had preoperative urinary tract infections (UTIs), lacked laboratory tests such as urine culture or analysis during hospitalization, died during hospitalization, or had incomplete data for any reason (n = 239). After applying these exclusion criteria, a final cohort of 1,231 elderly patients with hip fractures was included in the study. Among them, 268 patients (21.77%) developed postoperative UTIs (Figure 1). Table 1 presents the baseline characteristics of elderly patients with hip fractures, including a mean age of 74.99 years and 741 (60.19%) female patients. Patients in the UTIs group were significantly older (p < 0.001), had a higher proportion of females (p < 0.001), a higher prevalence of indwelling urinary catheters and longer catheterization duration (p < 0.001), a longer period of bedridden status (p < 0.001), a higher prevalence of comorbidities, and a higher preoperative ASA grading (p < 0.01) compared to those in the non-UTIs group. Furthermore, patients with intertrochanteric fractures exhibited a higher incidence of UTIs compared to patients with femoral neck and proximal femur fractures (p < 0.001). Likewise, patients who underwent intramedullary nail fixation showed a higher incidence of UTIs than those who underwent alternative surgical approaches (p < 0.001).
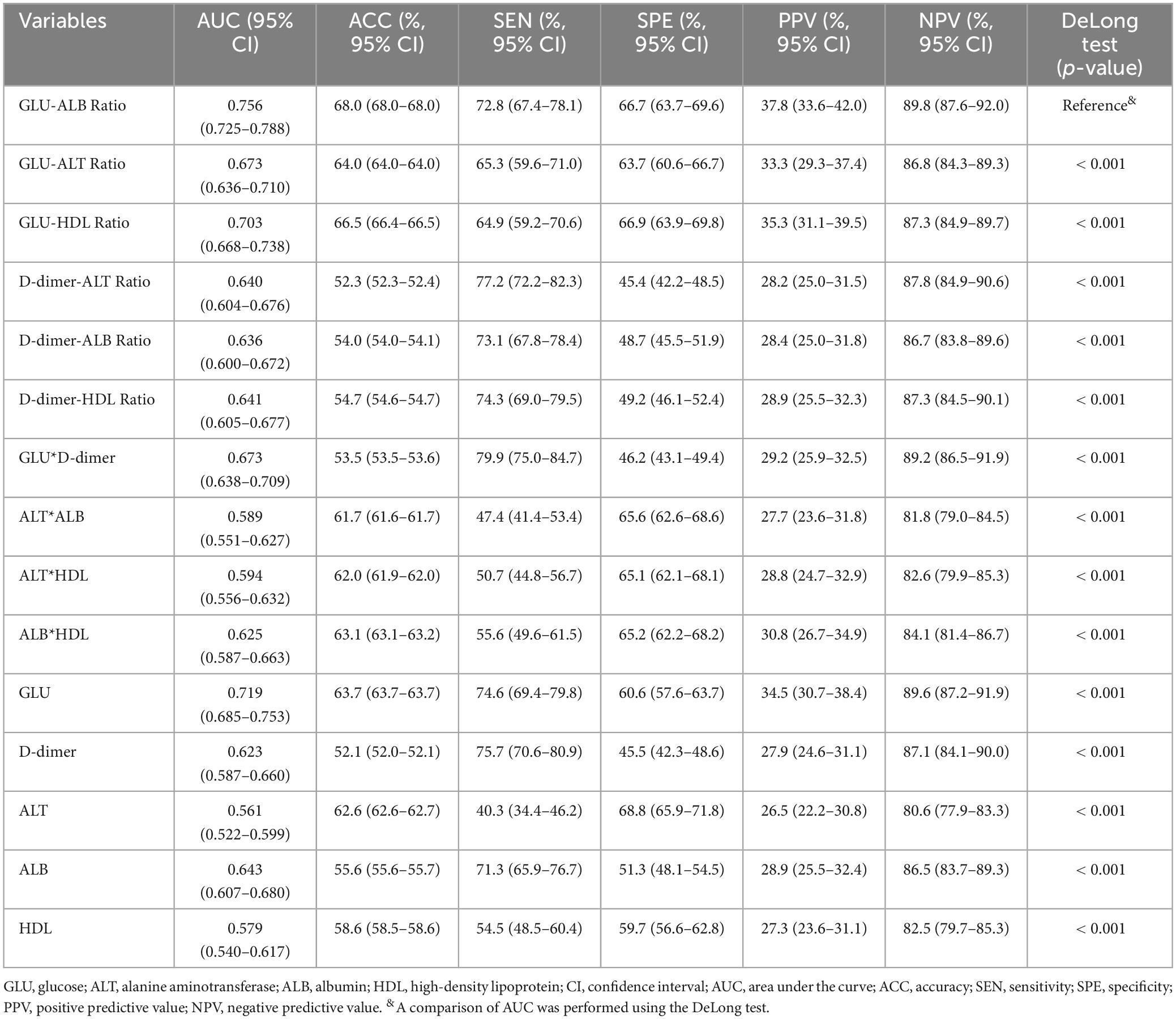
The collected biomarkers and their baseline levels are presented in Figure 2A. Subsequently, after a screening process, 5 blood biomarkers and 10 potential combinations were selected for further investigation, as depicted in Figure 2B. Out of these combinations, the prediction of postoperative UTIs in elderly hip fracture patients was most accurately achieved by the GAR (AUC = 0.756, Figure 3C), surpassing both individual biomarkers and their combinations (Figure 3). Significantly, the GAR exhibited superior accuracy compared to other biomarkers (p < 0.001, Table 2). The optimal cut-off value for the GAR was determined to be 0.18. Additionally, the Data Supplementary Material enclosed in Supplementary Figures 1, 2 summarizes the predictive capabilities of individual biomarkers for postoperative UTIs.
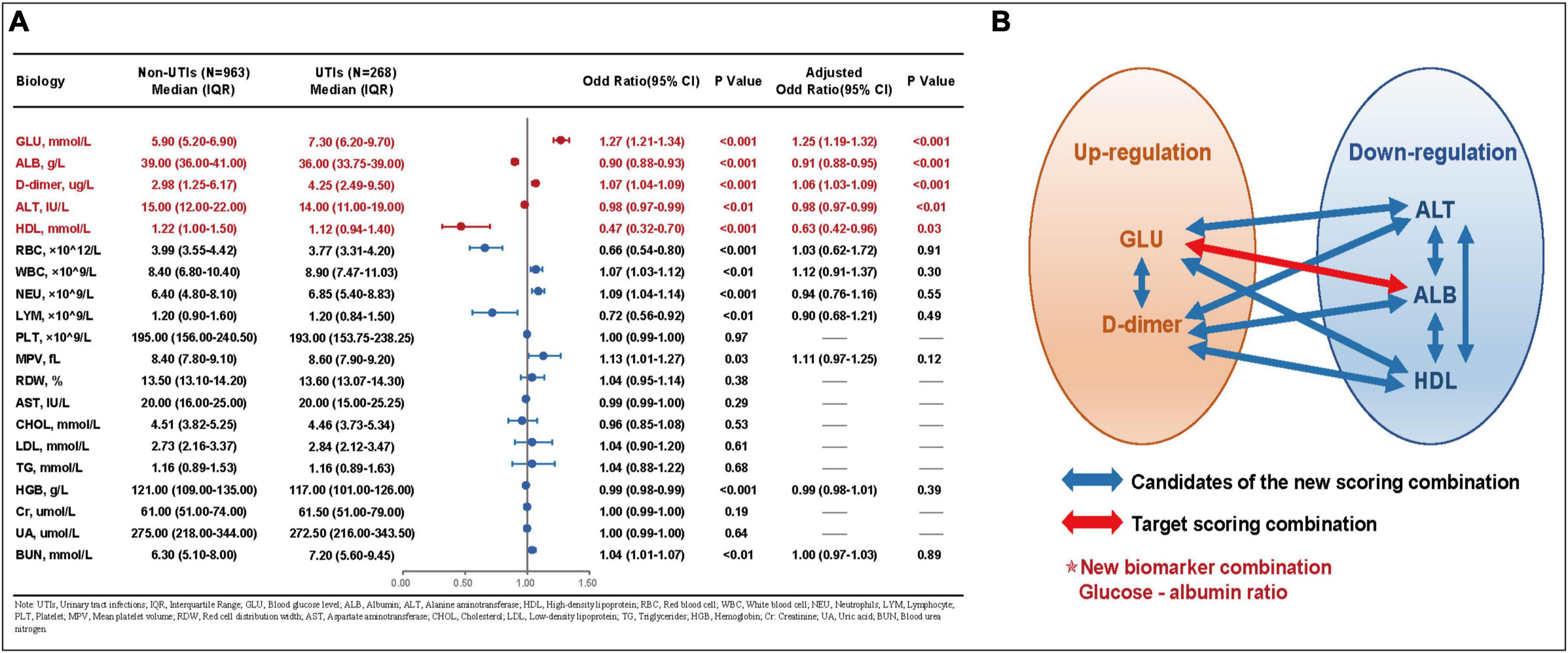
Figure 2. (A) Baseline data and univariate and multivariate analysis for each laboratory factors. (B) Schematic chart for the combination of laboratory factors in this study. UTIs, urinary tract infections; IQR, interquartile range; GLU, blood glucose lever; ALB, albumin; ALT, alanine aminotransferase; HDL, high-density lipoprotein; RBC, red blood cell; WBC, white blood cell; NEU, neutrophils; LYM, lymphocyte; PLT, platelet; MPV, mean platelet value; RDW, red cell distribution width; AST, aspartate aminotransferase; CHOL, cholesterol; LDL, low-density lipoprotein; TG, triglycerides; HGB, hemoglobin; Cr, creatinine; UA, uric acid; BUN, blood urea nitrogen.
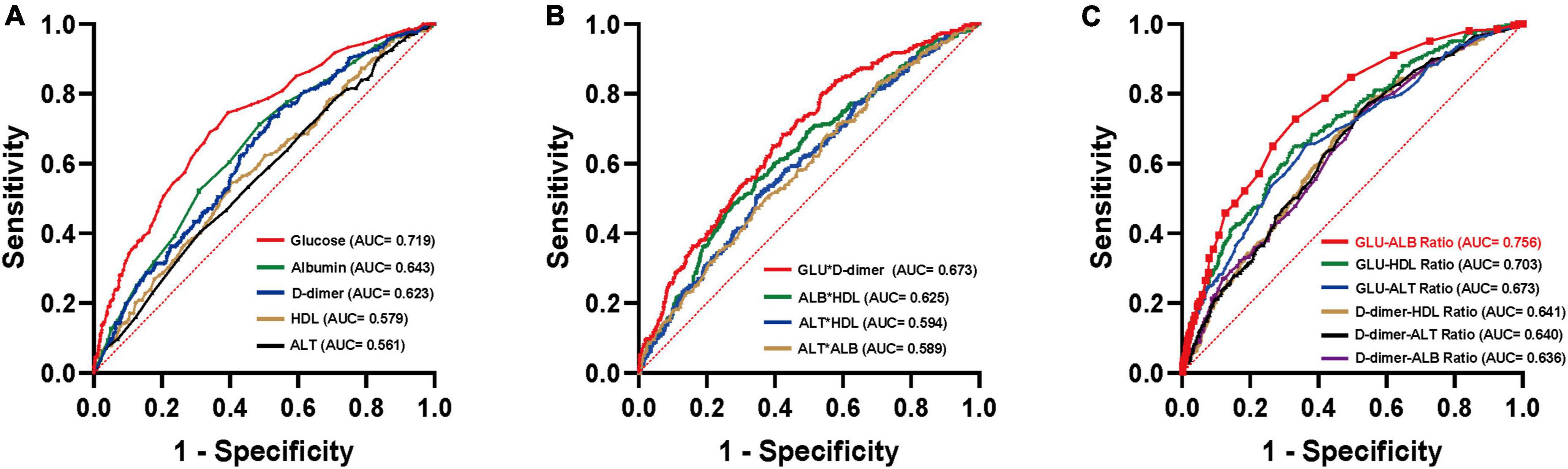
Figure 3. ROC curves analysis to evaluate the predictive value of each combination for postoperative UTIs in patients with hip fractures: GLU-ALB Ratio (C) showed the highest accuracy for the prediction of postoperative UTIs compared with established scores including other laboratory factors (A,B) in hip fracture patients. ROC, receiver operating characteristic; AUC, area under the curve.
The correlation between GAR levels and postoperative UTIs in elderly hip fracture patients was assessed, and the results are displayed in Table 3. In a univariate regression analysis, a high GAR level was found to be associated with postoperative UTIs (OR 5.34, 95% CI 3.96–7.22). Even after adjusting for confounding factors (age, gender, smoking, alcohol, hypertension, diabetes mellitus, cardiovascular disease, stroke, chronic kidney disease, prostatic hyperplasia, urolithiasis, vesicoureteral disease, type of hip fracture, ASA classification, surgical modality, indwelling catheterization, indwelling catheterization time, surgery method, and bed rest time), this correlation remained significant (OR 3.20, 95% CI 2.23–4.58). When analyzed as a continuous variable, each 1-SD increase in GAR resulted in a corrected OR of 1.81 (95% CI 1.51–2.17; Table 3) for postoperative UTIs. Furthermore, when GAR levels were examined in quartiles, patients in Q2 (0.14–0.17; OR 2.11, 95% CI 1.07–4.15), Q3 (0.17–0.21; OR 3.36, 95% CI 1.74–6.52), and Q4 (> 0.21; OR 7.55, 95% CI 3.84–14.83) demonstrated significantly higher odds of developing UTIs, compared to patients in Q1 (< 0.14). Notably, a dose-response relationship between GAR levels and postoperative UTIs was observed (p for trend < 0.001; Table 3 and Figure 4).
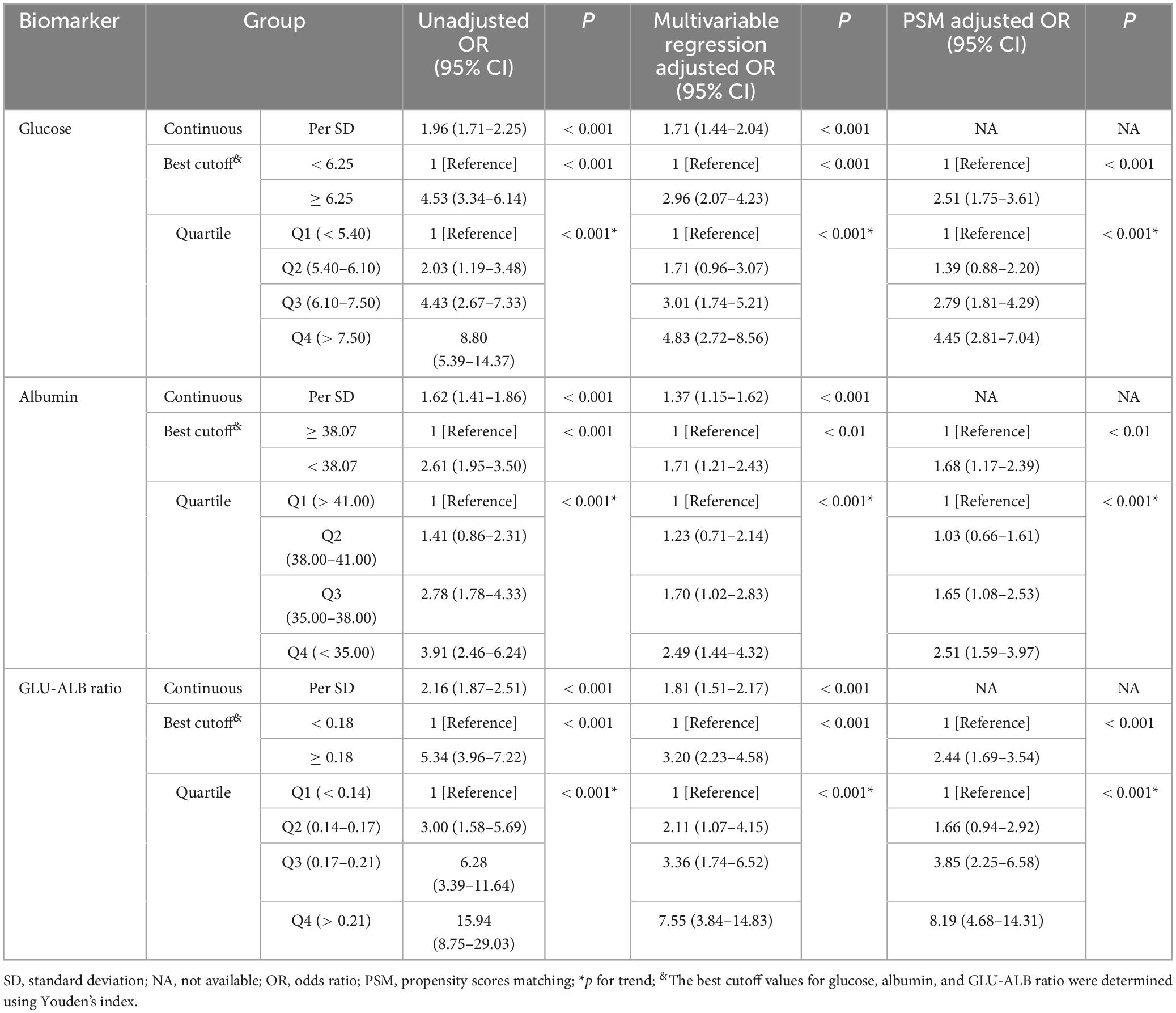
Table 3. Unadjusted and adjusted associations between postoperative UTIs and GLU-ALB ratio based on different cut-off values.
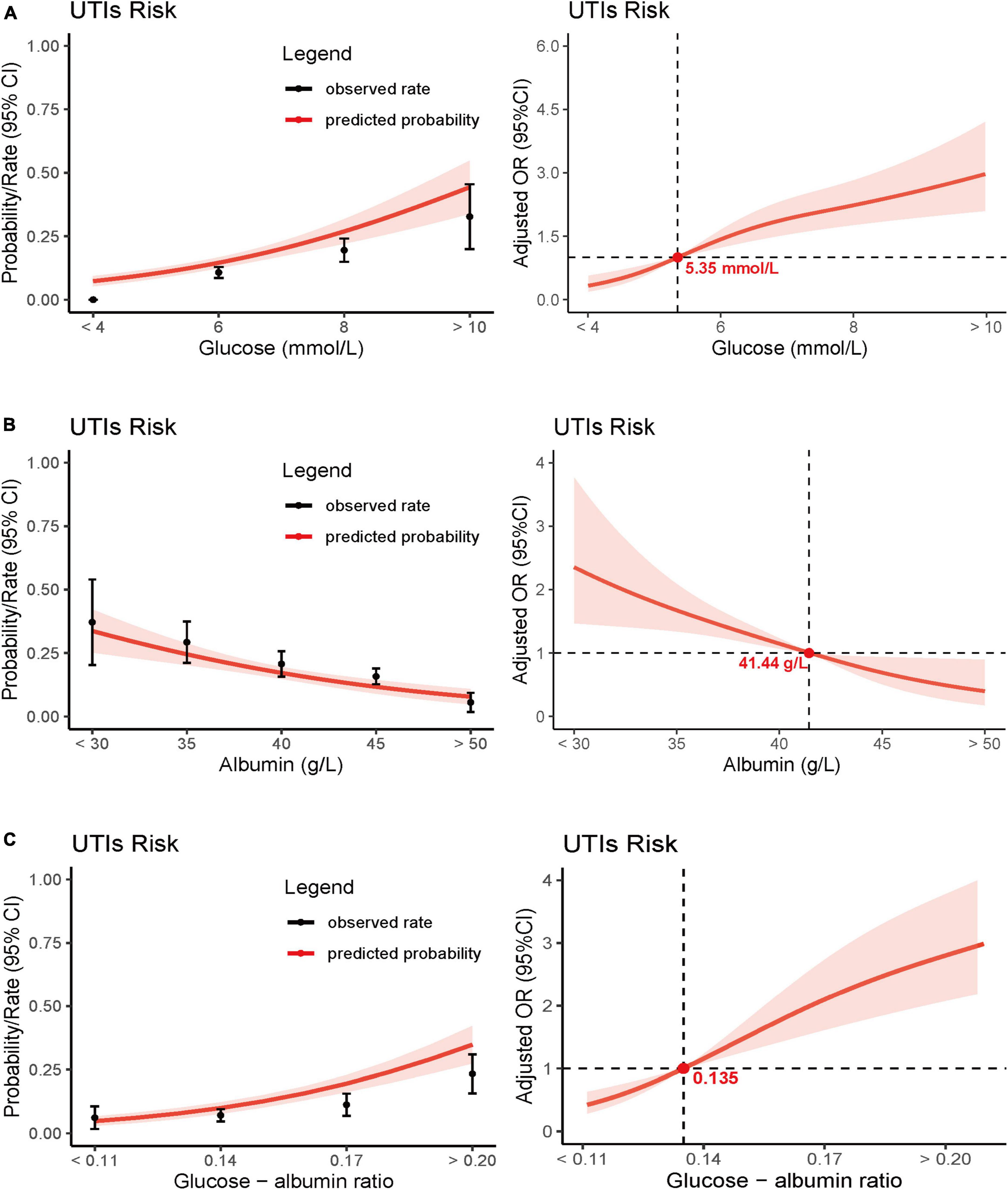
Figure 4. Predicted probabilities and the observed rate of postoperative UTIs: glucose (A), albumin (B), and glucose - albumin ratio (C). Adjusted odds ratio (OR) for postoperative UTIs according to levels of glucose (A), albumin (B), and glucose - albumin ratio (C) on a continuous scale.
Moreover, we conducted separate analyses for the correlation between blood glucose levels and albumin levels with postoperative UTIs. Results indicated that both of these biomarkers were independently associated with postoperative UTIs, and a dose-response relationship was evident (p for trend < 0.001; Table 3 and Figure 4). For comprehensive details, the multiple regression analyses for the three biomarkers are presented in Supplementary Tables 1, 3, 5 of the Data Supplementary. The E-value for the association between GAR and postoperative UTIs was calculated to be 2.98, with a lower limit of 2.35.
As part of a sensitivity analysis, propensity score matching was conducted. Following the matching process, all covariates exhibited balanced differences (SMD < 0.1, see Supplementary Tables 2, 4, 6). Importantly, the propensity-matched analysis confirmed the significant correlation between GAR and postoperative UTIs (OR 2.44, 95% CI 1.69–3.54; Table 3), thus enhancing the robustness of the findings.
Discussion
This retrospective cohort study aimed to assess the prognostic value of different biomarker combinations in predicting postoperative UTIs among elderly patients with hip fractures. Our findings indicate that a high GAR with a threshold of 0.18 is significantly associated with an increased risk of postoperative UTIs. Among the tested biomarker combinations, GAR demonstrated the highest predictive accuracy for postoperative UTIs.
Urinary tract infections (UTIs) consistently present as significant complications in diabetic patients, a trend supported by numerous studies (19–21). Diabetes, a chronic illness, affects various organ systems within the human body and often leads to complications, including infections (35, 36). Several mechanisms contribute to the development of UTIs within this demographic (20, 37, 38). A hyperglycemic environment intrinsic to diabetic patients hinders their immune response. This inhibition underscores the increased invasiveness and proliferation of pathogens whilst also curtailing the secretion of critical cytokines, such as interleukins (20, 37). Hyperglycemia compromises the functions of leukocytes, granulocytes, and macrophages, thereby expediting bacterial growth in glucose-rich environments (38). An in vitro study illustrated faster bacterial growth in urine containing a high-glucose level than in glucose-free human urine (39). Additionally, a recent pivotal study by Murtha et al. (40) suggested that lowered insulin receptor expression coupled with restricted insulin signaling in diabetic patients could significantly impact the immune system’s innate function in the urothelium and renal epithelium, thus heightening the proneness to UTIs.
Prior research has extensively examined the correlation between diabetic patients and UTI, although the influence of admission hyperglycemia levels on acute trauma patients’ susceptibility to UTI remains under-discussed (18, 41). This study endeavored to explain the connection between post-traumatic admission hyperglycemia and UTIs in patients with hip fractures. The findings pointed to a direct relation between admission hyperglycemia and increased UTI probability, demonstrating a dose-response relationship. Here, rising glucose levels were invariably tied to an amplified risk of infection. Several recent studies suggested post-traumatic injuries provoke specific autonomic and endocrine reactions, raising catecholamine and glycogen levels that potentially harm immune function (42, 43). Moreover, Karunakar and Staples (41) indicated stress-induced hyperglycemia as a significant risk factor for infectious complications in orthopedic trauma patients, including those without a diabetes history.
Recent years have witnessed an increasing utilization of serum albumin levels in hip fracture patients, demonstrating their association with perioperative complications and long-term postoperative mortality (24, 25). Our research indicated a connection between hypoalbuminemia at the time of admission and a rise in the likelihood of urinary tract infections, exhibiting a dose-response relationship where lower albumin levels corresponded with a heightened risk of infection. Commonly, hypoalbuminemia is viewed as a manifestation of malnutrition (22, 23, 26). Emerging evidence suggests that the significant decline in serum albumin levels in hip fracture patients might be attributed to inflammation, rather than pre-existing malnutrition (26, 44). Albumin, exclusively synthesized by the liver, represents the most abundant plasma protein. However, the comprehensive understanding of its metabolic functions remains incomplete (45). Apart from its established role in maintaining fluid-electrolyte balance, albumin may possess immunomodulatory functions (46). Circulating albumin interacts with various inflammatory mediators, promoting neutrophil degranulation and enhancing phagocytosis (47). Consequently, diminished serum albumin levels can impair immune system efficiency, resulting in an increased susceptibility to infectious complications (48). Furthermore, hypoalbuminemia serves as a straightforward indicator of malnutrition, which is a prominent cause of immune response impairment and a robust predictor of hospital-acquired infections (49).
While the novel combination of glucose and albumin ratio (GAR) identified in this study introduces new insight, previous literature has extensively discussed the relationship between glucose, albumin, and UTIs (14, 50–52). A meta-analysis by Huang et al. (50), reviewing 183,588 stroke patients, revealed that high glucose levels were linked to a higher likelihood of developing UTIs (OR 2.53, 95% CI 1.45–4.42). Similarly, Shanks et al. (51) demonstrated that intraoperative glucose levels exceeding 8.3 mmol/L were linked to an increased risk of postoperative infections, based on a study involving 3,150 patients undergoing non-cardiac surgery. In a study of 286 elderly patients with UTIs, Kitano et al. (14) observed that hypoalbuminemia significantly correlated with UTI development. Furthermore, Tal et al. (52) discovered a strong association between low serum albumin levels and mortality in elderly patients with UTIs (p < 0.002). Nonetheless, the relationship between GAR and postoperative UTIs is intricate, potentially confounded by various factors. Thus, further investigations are necessary to explore this potential association thoroughly and determine the optimal utilization of GAR as a predictor of postoperative UTIs following hip fracture.
Glucose and albumin, widely used biomarkers in clinical settings, provide a cost-effective and readily available approach to identifying high-risk patients. Importantly, both biomarkers can be modified, enabling physicians to take proactive interventions. Currently, researchers are exploring the management of postoperative UTIs in hip patients and recognizing the significance of controlling UTIs through insulin or hypoglycemic agents in combination with antimicrobial therapy (53, 54). Simultaneously, early nutritional optimization may enhance patient prognosis by providing supplemental calories and protein, modulating immune function, and maintaining normal cellular metabolism (55). Preoperative nutritional intervention has been shown to significantly improve the prognosis of infection-related complications following fracture surgery (56). Additionally, preoperative oral nutritional supplementation (ONS) has demonstrated its preventive effects on exacerbating complications in elderly patients undergoing hip fracture surgery (57). Consequently, when appropriately selected, prophylactic use of antibiotics, hypoglycemic agents, and early nutritional optimization can effectively reduce UTI incidence and overall hospital stay (Supplementary Figures 3–5).
Several limitations should be noted in this study. Firstly, we only examined basic hematological biomarkers commonly used in clinical practice. It is possible that more complex combinations may serve as more reliable indicators of poor prognosis than our newly developed GAR. Secondly, this study is based on a retrospective analysis conducted in a single-center setting. While the robustness of the results is supported by a large sample size and sophisticated statistical methods, the applicability and generalizability of GAR to other institutions need to be validated through large sample sizes from diverse institutions. Thirdly, blood glucose and albumin levels can fluctuate during hospitalization. Though we only considered baseline blood glucose and albumin levels recorded at admission to mitigate confounding effects, we did not assess these relationships over time. Lastly, it is essential to acknowledge that our study spans a data collection period of ten years, a factor that could potentially introduce unquantifiable influences on our research outcomes. In maintaining scientific integrity, it is imperative to transparently disclose this confounding variable.
Conclusion
This extensive study has identified GAR as a direct, synergistic, and readily available biomarker that predicts the risk of postoperative urinary tract infections (UTIs) in elderly patients with hip fractures. The analysis shows that GAR, obtained upon admission, enables the stratification of patients who are at a high risk of developing postoperative UTIs. Subsequent clinical trials should be carried out to evaluate the utility of this biomarker in managing hip.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Ethics Committee of Dandong Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was approved by the Ethics Committees of Dandong Central Hospital (No. DDZX-20230711) and conducted in accordance with the ethical principles of the Helsinki Declaration of 1964 and its later revisions. The Ethics Committee sought and obtained a waiver of consent for this cohort study.
Author contributions
WW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. WT: Formal analysis, Investigation, Methodology, Software, Writing – original draft. WY: Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. QL: Conceptualization, Data curation, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. WD: Conceptualization, Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1366012/full#supplementary-material
References
1. Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med. (2017) 377:2053–62. doi: 10.1056/NEJMcp1611090
2. Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury. (2018) 49:1458–60. doi: 10.1016/j.injury.2018.04.015
3. LeBlanc KE, Muncie HL Jr., LeBlanc LL. Hip fracture: Diagnosis, treatment, and secondary prevention. Am Fam Phys. (2014) 89:945–51.
4. Maffulli N, Aicale R. Proximal femoral fractures in the elderly: A few things to know, and some to forget. Medicina. (2022) 58:1341. doi: 10.3390/medicina58101314
5. Doherty WJ, Stubbs TA, Chaplin A, Reed MR, Sayer AA, Witham MD, et al. Prediction of postoperative outcomes following hip fracture surgery: Independent validation and recalibration of the nottingham hip fracture score. J Am Med Dir Assoc. (2021) 22:663–669.e662. doi: 10.1016/j.jamda.2020.07.013
6. Sheehan KJ, Guerrero EM, Tainter D, Dial B, Milton-Cole R, Blair JA, et al. Prognostic factors of in-hospital complications after hip fracture surgery: A scoping review. Osteoporos Int. (2019) 30:1339–51. doi: 10.1007/s00198-019-04976-x
7. Pincus D, Ravi B, Wasserstein D, Huang A, Paterson JM, Nathens AB, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. (2017) 318:1994–2003. doi: 10.1001/jama.2017.17606
8. Chen YP, Kuo YJ, Hung SW, Wen TW, Chien PC, Chiang MH, et al. Loss of skeletal muscle mass can be predicted by sarcopenia and reflects poor functional recovery at one year after surgery for geriatric hip fractures. Injury. (2021) 52:3446–52. doi: 10.1016/j.injury.2021.08.007
9. Quaranta M, Miranda L, Oliva F, Migliorini F, Pezzuti G, Maffulli N. Haemoglobin and transfusions in elderly patients with hip fractures: The effect of a dedicated orthogeriatrician. J Orthop Surg Res. (2021) 16:387. doi: 10.1186/s13018-021-02524-0
10. Bliemel C, Buecking B, Hack J, Aigner R, Eschbach DA, Ruchholtz S, et al. Urinary tract infection in patients with hip fracture: An underestimated event? Geriatr Gerontol Int. (2017) 17:2369–75. doi: 10.1111/ggi.13077
11. Rohold CK, Lauritzen JB, Jørgensen HL. Causes of death among 93.637 hip fracture patients– data based on the Danish national registry of causes of death. Eur J Trauma Emerg Surg. (2022) 48:1861–70. doi: 10.1007/s00068-021-01791-0
12. Saadat GH, Alsoof D, Ahmad B, Butler BA, Messer TA, Bokhari F. Incidence, risk factors and clinical implications of postoperative urinary tract infection in geriatric hip fractures. Injury. (2022) 53:2158–62. doi: 10.1016/j.injury.2022.03.012
13. Knauf T, Hack J, Barthel J, Eschbach D, Schoeneberg C, Ruchholtz S, et al. Medical and economic consequences of perioperative complications in older hip fracture patients. Arch Osteoporos. (2020) 15:174. doi: 10.1007/s11657-020-00843-z
14. Kitano H, Shigemoto N, Koba Y, Hara T, Seiya K, Omori K, et al. Indwelling catheterization, renal stones, and hydronephrosis are risk factors for symptomatic Staphylococcus aureus-related urinary tract infection. World J Urol. (2021) 39:511–6. doi: 10.1007/s00345-020-03223-x
15. Seyhan, Ak E, Özbaş A. The effect of education of nurses on preventing catheter-associated urinary tract infections in patients who undergo hip fracture surgery. J Clin Nurs. (2018) 27:e1078–88. doi: 10.1111/jocn.14160
16. Lin YC, Hsu YC, Wu WT, Lee RP, Wang JH, Chen HW, et al. The incidence of severe urinary tract infection increases after hip fracture in the elderly: A nationwide cohort study. Sci Rep. (2021) 11:3374. doi: 10.1038/s41598-021-83091-6
17. Richards JE, Kauffmann RM, Obremskey WT, May AK. Stress-induced hyperglycemia as a risk factor for surgical-site infection in nondiabetic orthopedic trauma patients admitted to the intensive care unit. J Orthop Trauma. (2013) 27:16–21. doi: 10.1097/BOT.0b013e31825d60e5
18. Yao W, Tang W, Wang W, Lv Q, Ding W. The relationship between admission hyperglycaemia and urinary tract infections in geriatric patients with hip fractures. Int Orthop. (2023) 47:2591–600. doi: 10.1007/s00264-023-05882-y
19. Nichols GA, Brodovicz KG, Kimes TM, Déruaz-Luyet A, Bartels DB. Prevalence and incidence of urinary tract and genital infections among patients with and without type 2 diabetes. J Diabetes Compl. (2017) 31:1587–91. doi: 10.1016/j.jdiacomp.2017.07.018
20. Kamei J, Yamamoto S. Complicated urinary tract infections with diabetes mellitus. J Infect Chemother. (2021) 27:1131–6. doi: 10.1016/j.jiac.2021.05.012
21. Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium-glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: A population-based cohort study. Ann Intern Med. (2019) 171:248–56. doi: 10.7326/m18-3136
22. Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: Review and meta analysis. Maturitas. (2015) 81:17–27. doi: 10.1016/j.maturitas.2015.02.009
23. Helminen H, Luukkaala T, Saarnio J, Nuotio M. Comparison of the mini-nutritional assessment short and long form and serum albumin as prognostic indicators of hip fracture outcomes. Injury. (2017) 48:903–8. doi: 10.1016/j.injury.2017.02.007
24. Malafarina V, Reginster JY, Cabrerizo S, Bruyère O, Kanis JA, Martinez JA, et al. Nutritional status and nutritional treatment are related to outcomes and mortality in older adults with hip fracture. Nutrients. (2018) 10:555. doi: 10.3390/nu10050555
25. O’Daly BJ, Walsh JC, Quinlan JF, Falk GA, Stapleton R, Quinlan WR, et al. Serum albumin and total lymphocyte count as predictors of outcome in hip fractures. Clin Nutr. (2010) 29:89–93. doi: 10.1016/j.clnu.2009.07.007
26. Shi H, Na Q, Zhang X, Jiang X. Correlations between the levels of acute infection markers and serum albumin in elderly patients with hip fracture. Aging Clin Exp Res. (2017) 29:435–41. doi: 10.1007/s40520-016-0585-7
27. Sigurdardottir M, Sigurdsson MI, Olafsson Y, Sverrisdottir SH, Gunnarsdottir I, Sigurdsson EL, et al. Prevalence of modifiable risk factors in primary elective arthroplasty and their association with infections. Acta Orthop. (2023) 94:38–44. doi: 10.2340/17453674.2023.8480
28. Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR. Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total hip and knee arthroplasty. J Arthroplast. (2015) 30:439–43. doi: 10.1016/j.arth.2014.10.009
29. Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. (2021) 22:4496. doi: 10.3390/ijms22094496
30. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
31. Nguyen AQ, Foy MP, Sood A, Gonzalez MH. Preoperative risk factors for postoperative urinary tract infection after primary total hip and knee arthroplasties. J Arthroplast. (2021) 36:734–8. doi: 10.1016/j.arth.2020.08.002
32. Hälleberg Nyman M, Johansson JE, Persson K, Gustafsson M. A prospective study of nosocomial urinary tract infection in hip fracture patients. J Clin Nurs. (2011) 20:2531–9. doi: 10.1111/j.1365-2702.2011.03769.x
33. Blum MR, Tan YJ, Ioannidis JPA. Use of E-values for addressing confounding in observational studies-an empirical assessment of the literature. Int J Epidemiol. (2020) 49:1482–94. doi: 10.1093/ije/dyz261
34. Liang J, Hu Z, Zhan C, Wang Q. Using propensity score matching to balance the baseline characteristics. J Thorac Oncol. (2021) 16:e45–6. doi: 10.1016/j.jtho.2020.11.030
35. Thornton GF. Infections and diabetes. Med Clin North Am. (1971) 55:931–8. doi: 10.1016/s0025-7125(16)32487-7
36. Njomnang Soh P, Vidal F, Huyghe E, Gourdy P, Halimi JM, Bouhanick B. Urinary and genital infections in patients with diabetes: How to diagnose and how to treat. Diabetes Metab. (2016) 42:16–24. doi: 10.1016/j.diabet.2015.07.002
37. Popejoy MW, Long J, Huntington JA. Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam. BMC Infect Dis. (2017) 17:316. doi: 10.1186/s12879-017-2414-9
38. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Med. (1997) 14:29–34. doi: 10.1002/(sici)1096-9136(199701)14:13.0.Co;2-v
39. Geerlings SE, Brouwer EC, Gaastra W, Verhoef J, Hoepelman AIM. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: Studies with urine from diabetic and non-diabetic individuals. J Med Microbiol. (1999) 48:535–9. doi: 10.1099/00222615-48-6-535
40. Murtha MJ, Eichler T, Bender K, Metheny J, Li B, Schwaderer AL, et al. Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J Clin Invest. (2018) 128:5634–46. doi: 10.1172/jci98595
41. Karunakar MA, Staples KS. Does stress-induced hyperglycemia increase the risk of perioperative infectious complications in orthopaedic trauma patients? J Orthop Trauma. (2010) 24:752–6. doi: 10.1097/BOT.0b013e3181d7aba5
42. McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. (2001) 17:107–24. doi: 10.1016/s0749-0704(05)70154-8
43. Losser MR, Damoisel C, Payen D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Critic Care. (2010) 14:231. doi: 10.1186/cc9100
44. Li S, Zhang J, Zheng H, Wang X, Liu Z, Sun T. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: A meta-analysis and systematic review. J Arthroplast. (2019) 34:1287–96. doi: 10.1016/j.arth.2019.02.003
45. Margarson MP, Soni N. Serum albumin: Touchstone or totem? Anaesthesia. (1998) 53:789–803. doi: 10.1046/j.1365-2044.1998.00438.x
46. Cantin AM, Paquette B, Richter M, Larivée P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am J Respir Crit Care Med. (2000) 162:1539–46. doi: 10.1164/ajrccm.162.4.9910106
47. Gorudko IV, Grigorieva DV, Shamova EV, Kostevich VA, Sokolov AV, Mikhalchik EV, et al. Hypohalous acid-modified human serum albumin induces neutrophil NADPH oxidase activation, degranulation, and shape change. Free Radic Biol Med. (2014) 68:326–34. doi: 10.1016/j.freeradbiomed.2013.12.023
48. Paillaud E, Herbaud S, Caillet P, Lejonc JL, Campillo B, Bories PN. Relations between undernutrition and nosocomial infections in elderly patients. Age Ageing. (2005) 34:619–25. doi: 10.1093/ageing/afi197
49. Schaible UE, Kaufmann SH. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. (2007) 4:e115. doi: 10.1371/journal.pmed.0040115
50. Huang YW, Yin XS, Li ZP. Association of the stress hyperglycemia ratio and clinical outcomes in patients with stroke: A systematic review and meta-analysis. Front Neurol. (2022) 13:999536. doi: 10.3389/fneur.2022.999536
51. Shanks AM, Woodrum DT, Kumar SS, Campbell DA Jr., Kheterpal S. Intraoperative hyperglycemia is independently associated with infectious complications after non-cardiac surgery. BMC Anesthesiol. (2018) 18:90. doi: 10.1186/s12871-018-0546-0
52. Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I, et al. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect. (2005) 50:296–305. doi: 10.1016/j.jinf.2004.04.004
53. Zeng G, Zhu W, Lam W, Bayramgil A. Treatment of urinary tract infections in the old and fragile. World J Urol. (2020) 38:2709–20. doi: 10.1007/s00345-020-03159-2
54. Gillespie WJ, Walenkamp GH. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochr Database Syst Rev. (2010) 2010:Cd000244. doi: 10.1002/14651858.CD000244.pub2
55. Aquilani R, Zuccarelli GC, Condino AM, Catani M, Rutili C, Del Vecchio C, et al. Despite inflammation, supplemented essential amino acids may improve circulating levels of albumin and haemoglobin in patients after hip fractures. Nutrients. (2017) 9:637. doi: 10.3390/nu9060637
56. Yokoyama K, Ukai T, Watanabe M. Effect of nutritional status before femoral neck fracture surgery on postoperative outcomes: A retrospective study. BMC Musculoskelet Disord. (2021) 22:1027. doi: 10.1186/s12891-021-04913-2
Keywords: hip fracture, elderly, postoperative urinary tract infection, prognosis, glucose-albumin ratio
Citation: Wang W, Tang W, Yao W, Lv Q and Ding W (2024) Glucose-albumin ratio (GAR) as a novel biomarker of postoperative urinary tract infection in elderly hip fracture patients. Front. Med. 11:1366012. doi: 10.3389/fmed.2024.1366012
Received: 05 January 2024; Accepted: 10 May 2024;
Published: 15 July 2024.
Edited by:
Zhenyu Zhou, Cornell University, United StatesReviewed by:
Mingxing Lei, Chinese PLA General Hospital, ChinaZhiyong Hou, Third Hospital of Hebei Medical University, China
Copyright © 2024 Wang, Tang, Yao, Lv and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbo Ding, ZHdiOTgwMDMwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Wei Wang
Wei Wang Wanyun Tang1
Wanyun Tang1 Wei Yao
Wei Yao Wenbo Ding
Wenbo Ding