- 1Department of Ophthalmology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 2Key Laboratory of Ocular Fundus Diseases, Chinese Academy of Medical Sciences, Beijing, China
Background: Central retinal vein occlusion (CRVO) is a rare adverse effect related to the use of tyrosine kinase inhibitors (TKIs) in patients with metastatic malignancies, which has only been reported in several case reports.
Case presentation: We reported the case series of three CRVO patients on regular regimens of TKIs as part of targeted therapies for metastatic malignancies, all of whom were otherwise healthy with no or well-controlled systemic conditions. All these patients received injections of intravitreal dexamethasone implant (IDI) and achieved a fluid-free macula at the end of the visit. In addition, we reviewed the existing literature on this subject and present here an updated analysis of the related TKIs, ocular presentation, treatment, and prognosis.
Conclusion: All patients diagnosed with CRVO on TKIs received dexamethasone implant treatment and obtained a fluid-free macula. We would like to raise awareness among our colleague oncologists about the possibility of CRVO related to TKI use and the necessity for patients to be screened regularly by a retinal specialist.
Introduction
Retinal vein occlusions (RVOs) are a group of vision-threatening disorders with the second-highest rate of retinal vascular blindness after diabetic retinopathy (1) and a worldwide prevalence of approximately 0.1–0.5% (1–3). Those whose obstruction occurs before the major bifurcation of the central retinal vein fall into the clinical entity of central retinal vein occlusions (CRVOs), characterized by engorgement and tortuosity of the retinal veins in all quadrants with retinal hemorrhages, cotton wool spots (CWSs), swelling of the optic disc, and macular edema (ME) (4). Instead of venous compression at the arteriovenous crossing site often observed in branch RVOs (5), thrombosis of the central retinal vein posterior to the lamina cribrosa is typically the cause of CRVO (6).
Advancing age, hyperlipidemia, hypertension (HTN), and primary open-angle glaucoma are well-established systemic and ocular risk factors for CRVO, while diabetes mellitus (DM) is less so (6, 7). Other conditions are less commonly associated with CRVO, including sleep apnea, homocystinuria, stroke, and thrombophilia, which should be given special attention, especially for younger patients (8, 9).
CRVO, though uncommon, is recognized as a severe adverse event related to the use of targeted anti-cancer drugs, especially for mitogen-activated protein kinase kinase (MEK) and human gene that encodes a protein (BRAF) inhibitors, usually for the treatment of metastatic melanoma (10, 11). It is even more rarely reported in the use of tyrosine kinase inhibitors (TKIs) and fibroblast growth factor receptor (FGFR) inhibitors (12), providing sporadic evidence for the retinal toxicity of these drugs. Herein, we report the case series of three CRVO patients on regular regimens of TKIs as part of targeted therapies for metastatic malignancies, all of whom were otherwise healthy with no or well-controlled systemic conditions.
Case series presentation
Case 1
A 65-year-old man presented with suddenly decreased vision in his right eye for 20 days. He was on a regular regimen of sorafenib 400 mg twice a day for metastatic renal cell carcinoma (RCC) for more than 2 years after left radical nephrectomy. No ocular or systemic diseases or systemic medications were reported. On physical examination, the visual acuity (VA) of the right eye was counting fingers. The intraocular pressure (IOP) of the right eye was recorded at 14 mmHg. A mild cataract was noticed. Funduscopic examination, fundus photographs, and optical coherence tomography (OCT) B-scan (Figure 1) showed severe ME with tortuous retinal veins, scattered retinal hemorrhagic, and CWSs in the posterior pole with no obvious non-perfusion area or neovascularization. The diagnosis of CRVO of the right eye was made. Homocysteine, protein C and S, activated protein C, and antithrombase levels were screened for systemic risk factors of CRVO and thrombophilia, which were within the normal range. Long-term use of sorafenib was considered the probable major cause of CRVO after ruling out other risk factors. Injection of sustained-release intravitreal dexamethasone implant (IDI, Ozurdex, Allergan) was prescribed. One month after the injection, ME of the right eye completely resolved but recurred at the next visit 2 months later. In total, he received three more injections, at a 3-month interval, due to repeated absorption and recurrence of ME, which became less severe than the previous recurrence. After four injections, the macula remained fluid-free until the last visit 9 months later, with his VA improving to 20/133.

Figure 1. Retinal and optical coherence tomography (OCT) images of patient 1. (A,B) Before and after four injections of intravitreal dexamethasone implant (IDI). (C) Before the first injection. (D) One month after the first injection, the macular edema (ME) completely resolved. (E) Three months after the first injection, the ME recurred, and the second injection was prescribed. (F) Two months after the second injection, the ME completely resolved. (G) Three months after the second injection, ME recurred, and the third injection was prescribed. (H) Two months after the third injection, the ME completely resolved. (I) Three months after the third injection, macular injection recurred, and the fourth injection was prescribed. (J) OCT image of the last visit. The macula remained fluid-free for nine months after the fourth injection, until the last visit.
Case 2
A 66-year-old man presented with decreased VA and metamorphopsia in his left eye for 2 weeks. He had been on sorafenib 400 mg twice a day for metastatic RCC for 4 years, before which he had left radical nephrectomy 3 years ago. No systemic comorbidities or other systemic medications were reported. Upon initial examination, the VA of his left eye was measured at 20/200, and the IOP of the left eye was recorded at 16 mmHg. On physical examination, optic disc edema, flame-like disc hemorrhages, “dot and blot” hemorrhages, and ME were noticed (Figure 2), and the diagnosis of CRVO of the left eye was made. Hyperhomocysteinemia and thrombophilia were screened and excluded. After one injection of IDI, his VA improved to 20/40, and photocoagulation of the non-perfusion area in the temporal retina was demonstrated by OCT angiography, after which he lost follow-up due to the coronavirus disease 2019 (COVID-19) pandemic.
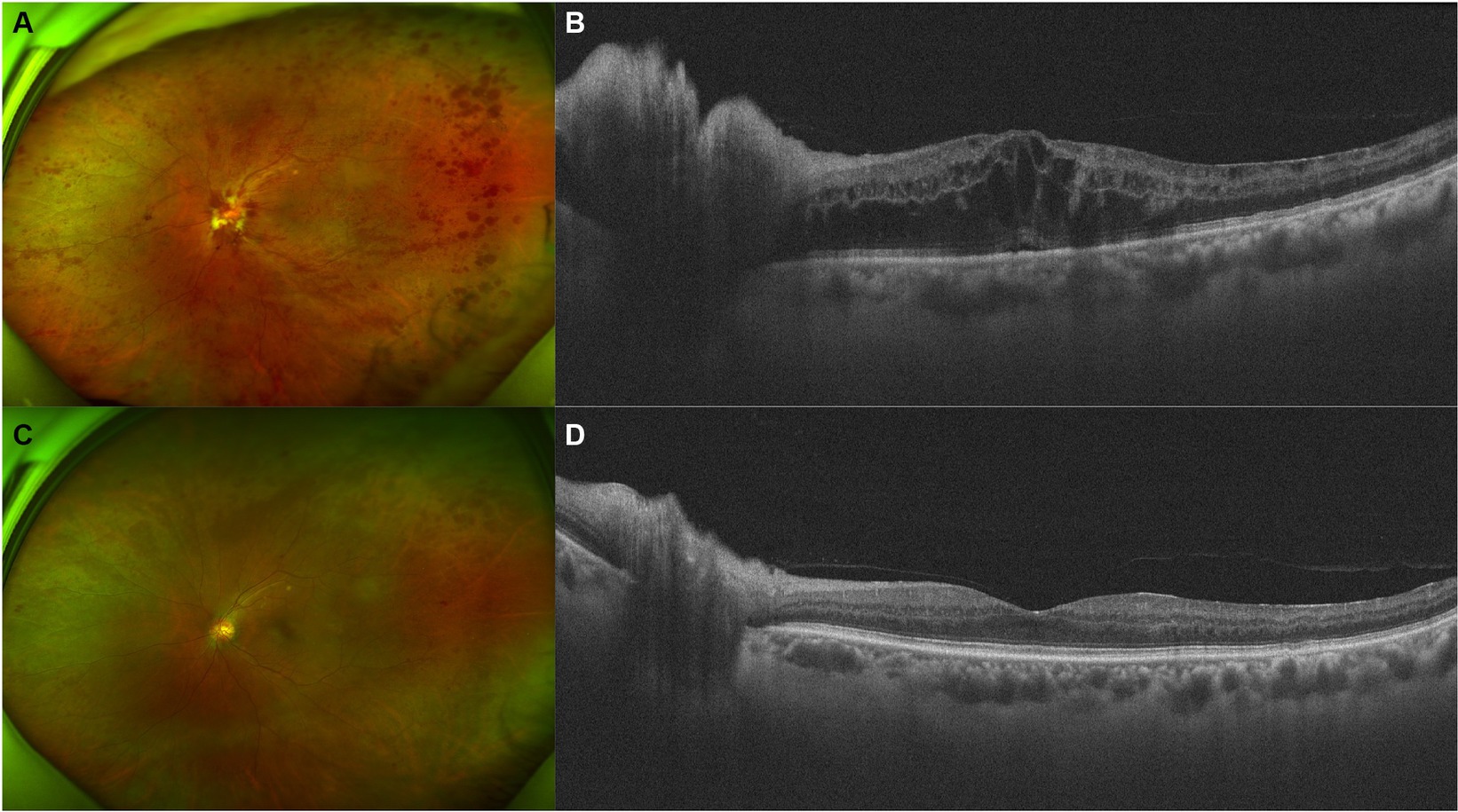
Figure 2. Retinal and optical coherence tomography (OCT) images of patient 2. (A,B) Retinal and OCT images on presentation. Flame-like disc hemorrhages as well as “dot and blot” hemorrhages over the whole retina were obvious. OCT demonstrated severe macular and disc edema. (C,D) Five months after injection of intravitreal dexamethasone implant (IDI). Most of the hemorrhages and the macular edema resolved.
Case 3
A 68-year-old man presented with decreased vision in his left eye for 1 week. He had been on a regular dose of apatinib 250 mg once a day and camrelizumab 3 mg/kg every 2 weeks for metastatic thyroid cancer for 1 year before discontinuing the camrelizumab therapy 1 week ago due to abnormal liver function. He was diagnosed with HTN for over 10 years, for which he took nifedipine 30 mg once a day and valsartan 80 mg three times a day. His blood pressure (BP) was 128/79 mmHg at presentation, with a self-reported BP range over the previous 2-year period of 90–130/65–85 mmHg. He was diagnosed with DM for 1 year. He took metformin hydrochloride 0.5 g three times a day with a self-reported fasting blood glucose (BG) range of 4–5.5 mmol/L and a postprandial BG range of 6.5–7.5 mmol/L. In addition, he used levothyroxine sodium 175 μg once a day as postoperative treatment. On presentation, the VA of the left eye was 20/2000, compared to 20/60 during his last consultation for a cataract 2 months ago. The IOP of the left eye was 13 mmHg. On fundus examination, the disc was edematous, with scattered CWSs and “dot and blot” hemorrhages of the posterior pole and a grayish edematous retina in the upper nasal quadrant (Figure 3). The diagnosis of CRVO and retinal artery occlusion of the left eye was made. Hyperhomocysteinemia and thrombophilia were also screened and excluded. He was prescribed one intravitreal injection of ranibizumab, after which the ME did not resolve. Therefore, another injection of IDI was prescribed. One month after the injection, the ME improved significantly, and subsequent PRP was finished. OCT showed that the retinal condition was stable, despite little improvement of his VA. The patient lost follow-up afterward due to the COVID-19 pandemic.
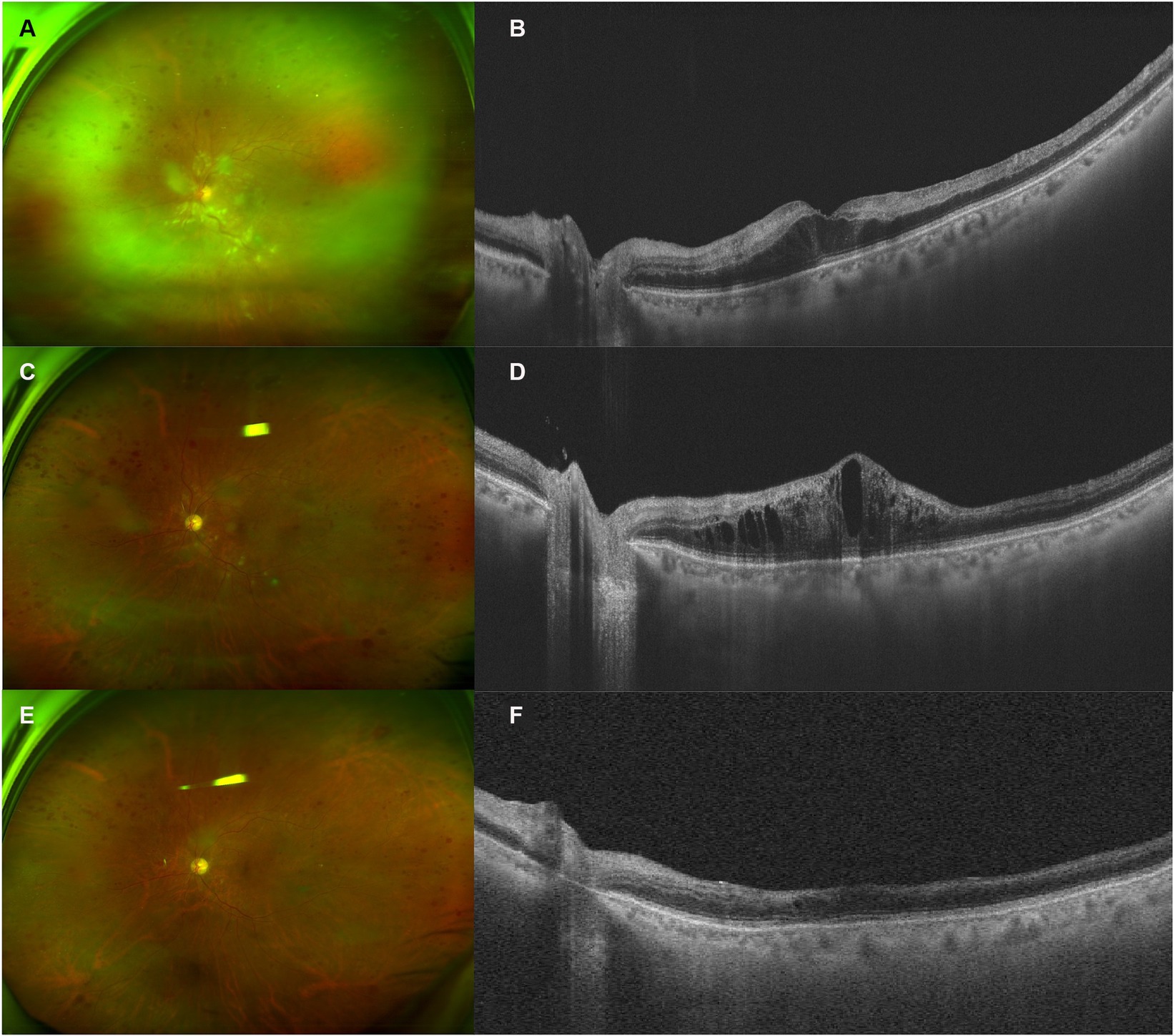
Figure 3. Retinal and optical coherence tomography (OCT) images of patient 3. (A,B) Retinal and OCT images on presentation. Notice the edematous disc, scattered cotton wool spots (CWSs) in the posterior pole, and “dot and blot” retinal hemorrhages. The white retinal vessels in the periphery indicated extensive retinal ischemia, corresponding to the hyper-reflective band spanning the inner nuclear layer (INL). (C,D) Two months after the intravitreal injection of ranibizumab, the CWS and hemorrhage were partially absorbed. However, OCT demonstrated a recurrence of macular edema (ME). (E,F) One month after the injection of intravitreal dexamethasone implant (IDI), most CWSs and ME resolved, with some remnant of retinal hemorrhages. INL atrophy could be observed.
Discussion
TKIs function by inhibiting corresponding kinases from catalyzing downstream phosphorylation (13). Since being approved in 2001 by the Food and Drug Administration (FDA) of the United States, TKIs have greatly improved the life expectancy of numerous cancer patients. They are highly potent in stabilizing tumor progression with fewer side effects compared to cytotoxic chemotherapeutic agents (14). Currently, TKIs are designed to target multiple pathways for cumulative antitumor efficacy. Although most TKIs are designed to be highly selective, other targets can be covered unexpectedly (14), which partially explains the ocular side effects of targeted therapy (15).
Cases reporting CRVO and other retinal vascular abnormalities related to the use of TKIs are summarized in Table 1 (16–21). Since 2012, several cases have been reported sporadically, four of which were CRVO. Of these cases, two were related to the use of sorafenib (16, 17), three were related to axitinib (19–21) and one was related to regorafenib (18). Interestingly, the TKIs used in previously reported cases (Table 1) and in our patients all belong to vascular endothelial growth factor receptor (VEGFR)-associated multitargeted TKIs (22), which target multiple receptors of endothelial cells, including VEGFR, platelet-derived growth factor receptor, FGFR, c-Kit, and c-Met. These TKIs block the kinase activity and the downstream transduction pathways involving angiogenesis, proliferation, migration, and cell survival (23), significantly improving the overall survival of some types of solid malignancies, including RCC and hepatocellular carcinoma. Some of these pathways, including the VEGF pathways, are shared by normal retinal tissues, contributing to retinal toxicity. Agents targeting VEGF pathways have been reported to increase the risk of ocular and systemic thromboembolic events (TEs), partially contributed by the dysregulation of endothelial cells and a decrease in prostaglandin-I 2 (PG-I 2) and nitric oxide (NO) (24). On the other hand, with endothelial cell death, exposure to phospholipid and extracellular matrix increases the tendency for thrombosis (25).
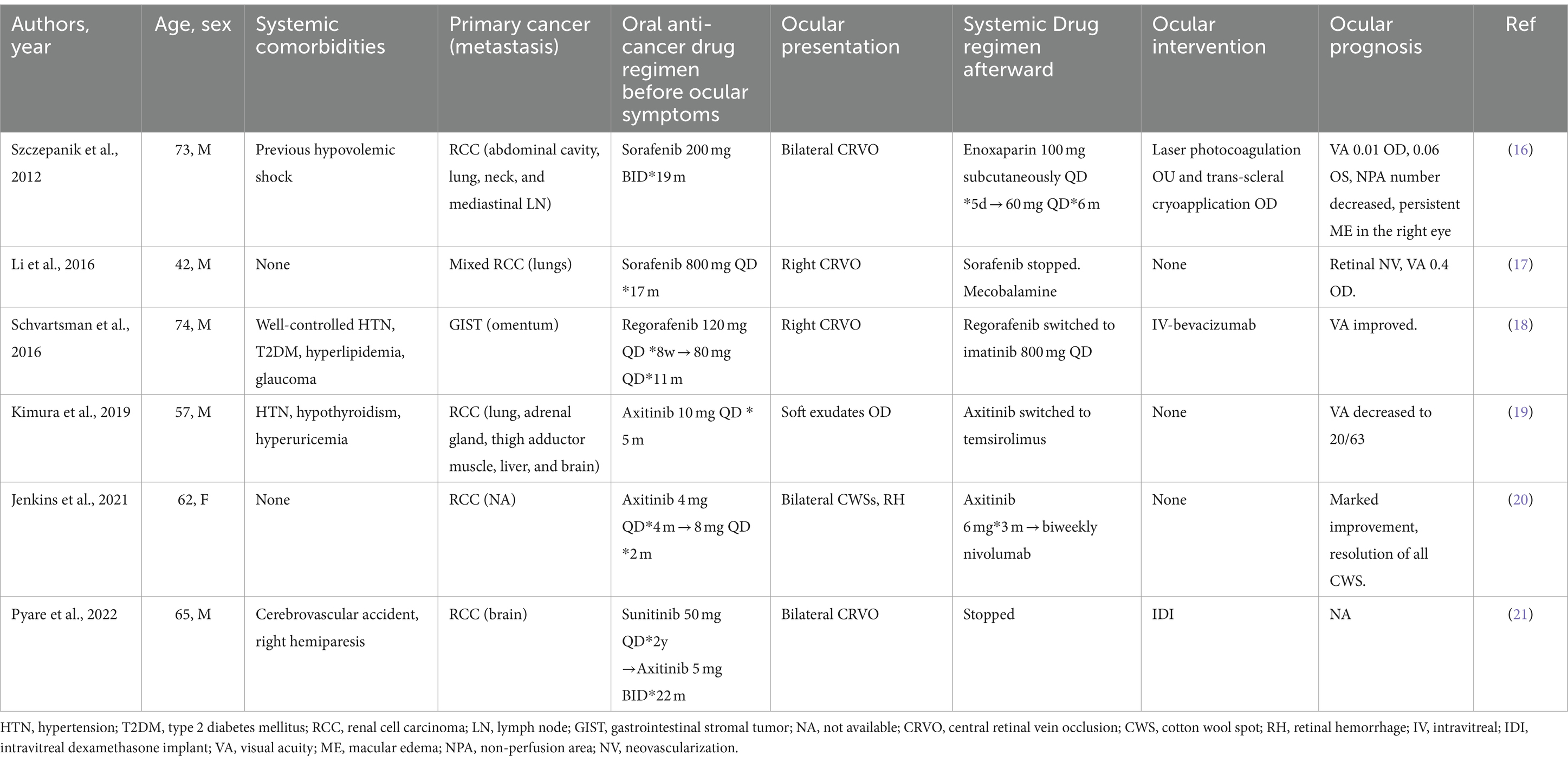
Table 1. Previous cases of retinal vein occlusion and retinal vascular abnormalities related to the use of tyrosine kinase inhibitors.
It may seem paradoxical to relate RVO to the systemic use of anti-VEGF agents, as intravitreal injection of anti-VEGF agents is the first-line therapy for RVO. In fact, local vascular toxicity has been observed with intravitreal injections. In patients with diabetic retinopathy, anti-VEGF agents showed enlargement of the foveal avascular zone, with no efficacy in improving retinal perfusion in some studies (26, 27). In patients with RVO, aggravation of retinal perfusion and development of the new non-perfusion area and CWSs after intravitreal injections were reported (28, 29). In addition, side effects of the posterior segment, such as RVO (18), ME, and central serous retinopathy, have been reported with systemic anti-VEGF agents (30), which are consistent with the results of animal experiments showing decreased local blood flow secondary to retinal vascular endothelial cell dysfunction with systemic anti-VEGF treatment (31). Bevacizumab, a commonly used anti-VEGF agent in systemic chemotherapy, is also the first generation of anti-VEGF agents for intravitreal injections. It increases the risk of venous thromboembolism development in cancer patients (32), and RVO is reported as an adverse effect when combined with chemotherapy for metastatic colorectal cancer (33). Although the underlying mechanism has not been fully elucidated, these observations justify the causal relationship between anti-VEGF agents and RVO.
Theoretically, hypercoagulation and thrombosis, which are commonly observed in patients with malignancies (34), seem to increase the risk of CRVO. However, up to now, the direct causal relationship between malignancies and CRVO was not well-established (35), and the results of previous studies were not consistent. The study by Kim et al. (36) identified an increased risk of subsequent cancer in patients with RVO. In other studies, thrombosis of retinal veins was not identified as a significant clinical marker for occult cancer (37), and RVO did not show an increased frequency of thrombophilia (38). The study by Toft-Petersen et al. (39) established the association between cancer and RVO, but the authors stated that the relationship reflects common risk factors instead of causal relationships. In addition, primary malignancy may trigger the elevation of anticardiolipin and antiphospholipid, resulting in RVO (40).
In fact, TEs related to the use of TKIs in other systems are not scarce. Cardiotoxicity is a known adverse effect of TKIs targeting VEGFR (41). Pantaleo et al. (42) reported coronary artery stenosis in a 58-year-old man on regular sorafenib for metastatic RCC (mRCC) for 2 years, whose cardiac function was normal and with no common cardiovascular risk factors. Axitinib was reported in relation to the cerebrovascular accident (CVA) (21, 43) and pulmonary embolism (43). The exact mechanism of TKI-related endothelial dysfunction has not been elucidated, yet due to the vital role of VEGF signaling pathways in the maintenance of retinal endothelial cell homeostasis and function, it is a fair guess that blockade of VEGF results in abnormal vascular integrity, which may increase the tendency for embolisms. Additionally, animal studies of sunitinib-related cardiac side effects suggest the impairment of mitochondrial function and the compromise of cellular energy homeostasis might be involved in TKI-related toxicity (41).
It should be extremely cautious to establish the cause of CRVO with targeted therapy. An important reason is that severe adverse events such as CRVO alter the treatment protocols of oncologists, which may influence the prognosis of systemic malignancy. Most patients in previous literature and in our case series were otherwise healthy, with normal lab results and well-controlled or no systemic comorbidities. Particularly, Li et al. (17) reported a 42-year-old man with no comorbidities. Jenkins et al. (20) reported bilateral hemorrhagic and ischemic retinopathy in a 62-year-old woman who was on a regular regimen of axitinib, another kind of TKI, for metastatic RCC. She was otherwise healthy, with no systemic or ocular comorbidities. Her retinal condition significantly improved with the cessation of axitinib without ocular treatment. Other reported cases mostly had well-controlled comorbidities such as HTN and hyperlipidemia. For these patients, RVO could hardly be explained other than the use of systemic TKIs, despite the presence of primary malignancies.
In addition to apatinib, camrelizumab, a programmed cell death receptor 1 (PD-1) inhibitor, was used in combination with our third patient. A synergistic antitumor effect of immunotherapy and anti-angiogenesis is well-recognized and often used as neoadjuvant therapy (44). Up to date, only one case of CRVO was reported with anti-PD-1 therapy (45), which is rarer than TKI. Although the possibility of camrelizumab-induced CRVO cannot be excluded, it is likely that apatinib contributes more to the disease onset in this patient.
All our patients developed CRVO during the use of systemic anti-VEGF agents, namely TKIs. The initial treatment of the third patient was local anti-VEGF agents, but the ME did not resolve, compromising faith in intravitreal anti-VEGF agents. On the other side, the poor responses to anti-VEGF agents indicate that TKIs might be the trigger of the retinal toxic reactions, resulting in CRVO. In comparison, IDI demonstrated good efficacy as the initial treatment or after the regimen switch, implicating the role of inflammation over VEGF in the pathogenesis of TKI-induced CRVO. In the setting of retinal endothelial injuries that might be related to the blockade of VEGF signaling pathways by systemic TKIs, IDI may be preferred in future patients with similar presentations.
Our study had the inherent limitations of a case series with a small sample size. The exact mechanism of the retinal toxicity of TKIs cannot be elucidated at this point, and the contribution of malignancy to CRVO is hard to evaluate. In addition, we lacked a control group to compare the efficacy of anti-VEGF agents and IDIs. However, to the best of our knowledge, this may be the first review on the topic of CRVO related to the use of systemic TKIs. Since the condition is rare, joint efforts should be made to reveal the incidence of CRVO and retinal toxicities in patients on systemic TKIs for metastatic malignancies.
Conclusion
In this case series report, all patients diagnosed with CRVO on TKIs received dexamethasone implant treatment and obtained a fluid-free macula until the last visits, indicating intravitreal injection of IDIs may be preferred over anti-VEGF agents. Oncologists should be aware of the possibility of CRVO in patients on TKIs for metastatic malignancies and inform them of the potential retinal toxicity. Regular screening by a retinal specialist is necessary.
Author contributions
ML: Data curation, Formal analysis, Methodology, Writing – original draft. LS: Data curation, Writing – original draft. RD: Writing – review & editing. YC: Writing – review & editing. CW: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-061) for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ip, M, and Hendrick, A. Retinal vein occlusion review. Asia Pac J Ophthalmol (Phila). (2018) 7:40–5. doi: 10.22608/apo.2017442
2. Klein, R, Klein, BE, Moss, SE, and Meuer, SM. The epidemiology of retinal vein occlusion: the beaver dam eye study. Trans Am Ophthalmol Soc. (2000) 98:133–41; discussion 141-3.
3. Mitchell, P, Smith, W, and Chang, A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains eye study. Arch Ophthalmol. (1996) 114:1243–7. doi: 10.1001/archopht.1996.01100140443012
4. McAllister, IL. Central retinal vein occlusion: a review. Clin Experiment Ophthalmol. (2012) 40:48–58. doi: 10.1111/j.1442-9071.2011.02713.x
5. Christoffersen, NL, and Larsen, M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. (1999) 106:2054–62. doi: 10.1016/s0161-6420(99)90483-9
6. Nicholson, L, Talks, SJ, Amoaku, W, Talks, K, and Sivaprasad, S. Retinal vein occlusion (RVO) guideline: executive summary. Eye. (2022) 36:909–12. doi: 10.1038/s41433-022-02007-4
7. O'Mahoney, PR, Wong, DT, and Ray, JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. (2008) 126:692–9. doi: 10.1001/archopht.126.5.692
8. Chen, TY, Uppuluri, A, Zarbin, MA, and Bhagat, N. Risk factors for central retinal vein occlusion in young adults. Eur J Ophthalmol. (2021) 31:2546–55. doi: 10.1177/1120672120960333
9. Bakhoum, CY, Madala, S, Long, CK, Adabifirouzjaei, F, Freeman, WR, Goldbaum, MH, et al. Retinal vein occlusion is associated with stroke independent of underlying cardiovascular disease. Eye (Lond). (2022) 37:764–7. doi: 10.1038/s41433-022-02038-x
10. Méndez-Martínez, S, Calvo, P, Ruiz-Moreno, O, Pardiñas Barón, N, Leciñena Bueno, J, Gil Ruiz, MR, et al. Ocular adverse events associated with MEK inhibitors. Retina. (2019) 39:1435–50. doi: 10.1097/iae.0000000000002451
11. Mettler, C, Monnet, D, Kramkimel, N, Tréluyer, JM, Mouthon, L, Brézin, A, et al. Ocular safety profile of BRAF and MEK inhibitors: data from the World Health Organization pharmacovigilance database. Ophthalmology. (2021) 128:1748–55. doi: 10.1016/j.ophtha.2021.05.008
12. Foulsham, W, Edghill, BZ, Julia Canestraro, OD, Makker, V, Konner, J, Abramson, DH, et al. Central retinal vein occlusion in the setting of fibroblast growth factor receptor inhibition. Am J Ophthalmol Case Rep. (2022) 27:101657. doi: 10.1016/j.ajoc.2022.101657
13. Zhang, J, Yang, PL, and Gray, NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. (2009) 9:28–39. doi: 10.1038/nrc2559
14. Broekman, F, Giovannetti, E, and Peters, GJ. Tyrosine kinase inhibitors: multi-targeted or single-targeted? World J Clin Oncol. (2011) 2:80–93. doi: 10.5306/wjco.v2.i2.80
15. Fortes, BH, Tailor, PD, and Dalvin, LA. Ocular toxicity of targeted anticancer agents. Drugs. (2021) 81:771–823. doi: 10.1007/s40265-021-01507-z
16. Szczepanik, S, and Kęcik, D. Bilateral central retinal vein occlusion in a patient with disseminated metastatic renal cell carcinoma treated with sorafenib. Retin Cases Brief Rep Spring. (2012) 6:148–50. doi: 10.1097/ICB.0b013e3182160965
17. Li, ZY, Fan, XX, Wang, YJ, Yao, K, Liu, ZW, Pan, WT, et al. Metastatic renal cell carcinoma: the first report of unilateral fundus hemorrhage induced by sorafenib. Oncotarget. (2016) 7:35181–7. doi: 10.18632/oncotarget.9285
18. Schvartsman, G, Wagner, MJ, Zobniw, CM, Trinh, VA, Patel, S, and Somaiah, N. An unusual case of central retinal vein occlusion and review of the toxicity profile of Regorafenib in GIST patients. Curr Oncol Rep. (2016) 18:49. doi: 10.1007/s11912-016-0536-7
19. Kimura, M, Kusuhara, S, Tagami, M, and Nakamura, M. Impaired retinal circulation during Axitinib treatment for metastatic renal cell carcinoma. Case Rep Ophthalmol. (2019) 10:5–10. doi: 10.1159/000496197
20. Jenkins, TL, Aderman, CM, and Ho, AC. Reversible retinal toxicity in a patient taking AXITINIB. Retin Cases Brief Rep. (2021) 15:239–42. doi: 10.1097/icb.0000000000000771
21. Pyare, R, Ganvir, A, Goyal, S, Rao, C, and Dutta, MP. Bilateral central retinal vein occlusion associated with Axitinib therapy: a case report. Ocul Immunol Inflamm. (2022) 31:635–7. doi: 10.1080/09273948.2022.2042318
22. Huang, L, Jiang, S, and Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J Hematol Oncol. (2020) 13:143. doi: 10.1186/s13045-020-00977-0
23. Qin, S, Li, A, Yi, M, Yu, S, Zhang, M, and Wu, K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. (2019) 12:27. doi: 10.1186/s13045-019-0718-5
24. Strumberg, D. Sorafenib for the treatment of renal cancer. Expert Opin Pharmacother. (2012) 13:407–19. doi: 10.1517/14656566.2012.654776
25. Zarbin, MA. Anti-VEGF agents and the risk of Arteriothrombotic events. Asia Pac J Ophthalmol (Phila). (2018) 7:63–7. doi: 10.22608/apo.2017495
26. Chatziralli, I, Touhami, S, Cicinelli, MV, Agapitou, C, Dimitriou, E, Theodossiadis, G, et al. Disentangling the association between retinal non-perfusion and anti-VEGF agents in diabetic retinopathy. Eye (Lond). (2022) 36:692–703. doi: 10.1038/s41433-021-01750-4
27. Bonnin, S, Dupas, B, Lavia, C, Erginay, A, Dhundass, M, Couturier, A, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. (2019) 39:426–34. doi: 10.1097/iae.0000000000002422
28. Zhu, ZY, Meng, YA, Yan, B, and Luo, J. Effect of anti-VEGF treatment on nonperfusion areas in ischemic retinopathy. Int J Ophthalmol. (2021) 14:1647–52. doi: 10.18240/ijo.2021.11.01
29. Kida, T, Tsujikawa, A, Muraoka, Y, Harino, S, Osaka, R, Murakami, T, et al. Cotton wool spots after anti-vascular endothelial growth factor therapy for macular edema associated with central retinal vein occlusion. Ophthalmologica. (2016) 235:106–13. doi: 10.1159/000443622
30. Huillard, O, Bakalian, S, Levy, C, Desjardins, L, Lumbroso-le Rouic, L, Pop, S, et al. Ocular adverse events of molecularly targeted agents approved in solid tumours: a systematic review. Eur J Cancer. (2014) 50:638–48. doi: 10.1016/j.ejca.2013.10.016
31. Iizuka, N, Nakahara, T, Ushikubo, H, Mori, A, Sakamoto, K, and Ishii, K. Retinal region-dependent susceptibility of capillaries to high-concentration oxygen exposure and vascular endothelial growth factor receptor inhibition in neonatal mice. J Pharmacol Sci. (2015) 129:107–18. doi: 10.1016/j.jphs.2015.08.010
32. Nalluri, SR, Chu, D, Keresztes, R, Zhu, X, and Wu, S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. (2008) 300:2277–85. doi: 10.1001/jama.2008.656
33. Gilbar, P, and Sorour, N. Retinal vein thrombosis in a patient with metastatic colon cancer receiving XELOX chemotherapy combined with bevacizumab pre-hepatic resection. J Oncol Pharm Pract. (2012) 18:152–4. doi: 10.1177/1078155211401455
34. Varki, A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. (2007) 110:1723–9. doi: 10.1182/blood-2006-10-053736
35. Flaxel, CJ, Adelman, RA, Bailey, ST, Fawzi, A, Lim, JI, Vemulakonda, GA, et al. Retinal vein occlusions preferred practice pattern®. Ophthalmology. (2020) 127:P288. doi: 10.1016/j.ophtha.2019.09.029
36. Kim, MS, Cho, JH, Byun, SJ, Oh, CM, Park, KH, and Park, SJ. Increased risk of cancer in patients with retinal vein occlusion: a 12-year nationwide cohort study. Br J Ophthalmol. (2021) 105:1705–10. doi: 10.1136/bjophthalmol-2020-316947
37. Hansen, AT, Veres, K, Prandoni, P, Adelborg, K, and Sørensen, HT. Retinal vein thrombosis and risk of occult cancer: a nationwide cohort study. Cancer Med. (2018) 7:5789–95. doi: 10.1002/cam4.1803
38. Bombeli, T, Basic, A, and Fehr, J. Prevalence of hereditary thrombophilia in patients with thrombosis in different venous systems. Am J Hematol. (2002) 70:126–32. doi: 10.1002/ajh.10103
39. Toft-Petersen, AP, Muttuvelu, DV, Heegaard, S, and Torp-Pedersen, C. Correlation between retinal vein occlusion and cancer – a nationwide Danish cohort study. Acta Ophthalmol. (2018) 96:800–3. doi: 10.1111/aos.13860
40. Adrean, SD, and Schwab, IR. Central retinal vein occlusion and renal cell carcinoma. Am J Ophthalmol. (2003) 136:1185–6. doi: 10.1016/s0002-9394(03)00716-5
41. Franczyk, B, Rysz, J, Ławiński, J, Ciałkowska-Rysz, A, and Gluba-Brzózka, A. Cardiotoxicity of selected vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with renal cell carcinoma. Biomedicines. (2023) 11:181. doi: 10.3390/biomedicines11010181
42. Pantaleo, MA, Mandrioli, A, Saponara, M, Nannini, M, Erente, G, Lolli, C, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer. (2012) 12:231. doi: 10.1186/1471-2407-12-231
43. Buikhuisen, WA, Scharpfenecker, M, Griffioen, AW, Korse, CM, van Tinteren, H, and Baas, P. A randomized phase II study adding Axitinib to Pemetrexed-cisplatin in patients with malignant pleural mesothelioma: a single-center trial combining clinical and translational outcomes. J Thorac Oncol. (2016) 11:758–68. doi: 10.1016/j.jtho.2016.01.014
44. Xia, Y, Tang, W, Qian, X, Li, X, Cheng, F, Wang, K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. (2022) 10:e004656. doi: 10.1136/jitc-2022-004656
Keywords: retinal vein occlusion, macular edema, tyrosine kinase inhibitors, metastasis, intravitreal dexamethasone implant
Citation: Luo M, Sun L, Dai R, Chen Y and Wu C (2024) Central retinal vein occlusion in patients with metastatic solid tumors on tyrosine kinase inhibitors: a report of case series and literature review. Front. Med. 11:1362108. doi: 10.3389/fmed.2024.1362108
Edited by:
Georgios D. Panos, Nottingham University Hospitals NHS Trust, United KingdomReviewed by:
Weihua Yang, Jinan University, ChinaKunbei Lai, Sun Yat-sen University, China
Pingting Zhong, Guangdong Provincial People’s Hospital, China
Copyright © 2024 Luo, Sun, Dai, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan Wu, d3VjaGFuQHB1bWNoLmNu
 Mingyue Luo
Mingyue Luo Lu Sun1,2
Lu Sun1,2 Rongping Dai
Rongping Dai Youxin Chen
Youxin Chen Chan Wu
Chan Wu