
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 22 May 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1361437
This article is part of the Research Topic Delirium in Older Persons View all 11 articles
A correction has been applied to this article in:
Corrigendum: Frailty index and risk of delirium in hospitalized patients: a two-sample Mendelian randomization study
Objective: Observational studies suggest that the frailty index (FI) is closely related to delirium, but the relationship between them is still uncertain due to the influence of various confounding factors. Therefore, two-sample Mendelian randomization (MR) was used to explore the causal relationship between the FI and delirium risk.
Methods: This study obtained pooled statistics for the FI and delirium from two of the most extensive genome-wide association studies. To make the results more robust and reliable, supplementary analyses were performed using several robust analytical methods (inverse-variance weighting, MR-Egger regression, and weighted median). In addition, this study used the MR-Egger intercept test, Cochran’s Q test, funnel plots and the leave-one-out method to evaluate the pleiotropy and heterogeneity among the abovementioned genetic variation instrumental variables.
Results: Frailty might increase the relative risk of delirium, as shown by IVW (OR = 1.849, 95% CI 0.027∼2.067, P = 0.044), weighted median (OR = 1.726, 95% CI −0.178∼2.664, P = 0.083), MR-Egger regression (OR = 1.768, 95% CI −3.08∼6.171, P = 0.525) and leave-one-out sensitivity analysis (P = 0.058). Although the WME method and MR–Egger regression analysis showed no statistically significant causal relationship between the FI and the risk of delirium, the direction of the causal effect was consistent with the IVW method.
Conclusion: There is a notable correlation between a higher FI and an elevated risk of delirium. This indicates that healthcare providers should take proactive measures to prevent delirium in hospitalized patients with a higher FI.
With the arrival of an aging society, frailty has received increasing attention in clinical nursing work, and previous observational studies have shown that frailty can increase the incidence of delirium, leading to poor prognosis. Both frailty and delirium are complications that require attention in daily nursing work. Therefore, this study intends to use Mendelian randomization method to explore the causal relationship between frailty and delirium, thereby providing a theory for improving the quality of clinical nursing care.
Delirium, a profound and sudden-onset neuropsychiatric condition, is distinguished by compromised focus and awareness, an ever-changing trajectory, and general cognitive decline (1). Greatly prevalent among the elderly and critically ill, this syndrome has numerous predisposing factors and frequently manifests as a complication of sudden illness, substance overconsumption or cessation, surgical intervention, or disturbances in electrolyte or metabolic levels; it can even arise solely due to hospitalization (2). Despite the high frequency of delirium cases, its identification, diagnosis, and treatment in clinical practice are often neglected, misjudged, or inadequately handled.
Observational studies conducted in Italy aimed to examine the potential association between frailty and delirium in hospitalized older adults and in different contexts (3), and others were investigated the impact of these syndromes on outcomes including maintaining attention, functional status and mortality (4, 5). Different researchers also conducted systematic reviews and meta-analyses of the literature to address this issue, showing that frailty and delirium are common in geriatric practice, but their association with each other and their effect on short-term mortality in the general population hospitalized for acute conditions remains largely unknown (6, 7). The findings of this study suggest that frailty is indeed associated with delirium in hospitalized elderly patients. Furthermore, the presence of both conditions, either alone or in combination, leads to increased short-term mortality rates.
Notably, the connection between frailty and delirium is frequently assessed through observation-based studies. The currently debated aspect of the frailty-delirium relationship stems from the biases, confounding factors, and constraints inherent in observational studies. The utilization of Mendelian randomization (MR) provides a robust method for identifying causal links between risk factors and diseases by leveraging genetic variation as an instrumental variable (8). Given that genetic variation is determined at conception, it is generally less susceptible to external factors. Additionally, MR research mitigates concerns surrounding the causal sequence, a critical aspect of causal inference and the foundation of establishing causality (9). Hence, this study incorporates a two-sample MR analysis utilizing publicly available data from genome-wide association studies (GWAS) to investigate the causal association between the FI and the susceptibility to delirium.
To assess whether there is a causal relationship between frailty and delirium, a two-sample MR was used in this study.
For the purpose of this analysis, single-nucleotide polymorphisms (SNPs) associated with the FI were obtained from the European Bioinformatics Institute (EBI) as instrumental variables. The working variable (ID: ebi-a-GCST90020053) originates from a Genome-wide association study (GWAS) on frailty (10), which included 164,610 samples of individuals aged 60 to 70 years. The sample population consisted of 84,819 females (51.3%), the detail information is in the Supplementary Table 1. The relevant SNP data on delirium was obtained from the Finnish database. This dataset mainly comes from the European population, contains a total of 16,380,452 SNPs and the inclusion criteria were acute or subacute: (1) brain syndrome; (2) confusional state (nonalcoholic); (3) infective psychosis; (4) organic reaction; (5) psycho-organic syndrome. Delirium cases were excluded if they were described as delirium tremens, alcohol-induced, or unspecified. Delirium was diagnosed based on the patient’s ICD-10 code at discharge, and the database did not specify which delirium assessment scale was used to assess the patient during the hospitalization. And the detail basal information of delirium datasets is in the Supplementary Table 2 and Supplementary Figures 1, 2.
First, this study used Plink software to screen out SNPs with P < 5 × 10–8, a genetic distance of 10,000 kb and linkage disequilibrium (LD) r2 < 0.001 from the FI database (11). Second, the catalog and PhenoScanner databases were also used to further verify whether the above included SNPs were related to other confounding factors (12). Finally, the F statistic was used to evaluate whether the included SNPs were affected by weak instrumental variables (13). If the F statistic of SNPs is less than 10, it indicates that there is a possibility of weak instrumental variable bias in the SNPs, and then it will be eliminated to avoid affecting the results.
This study mainly used the inverse-variance weighted (IVW) method, wherein the existence of the intercept item is not considered in the regression and the reciprocal of the outcome variance is used as the weight for fitting (14). Among the different IVW approaches, the IVW fixed-effects model was mainly used in the absence of any underlying heterogeneity of horizontal pleiotropic effects. If heterogeneity existed, a random-effects model was used. Second, this study used methods such as MR-Egger regression and the weighted median estimator (WME) to further supplement the above conclusions. The biggest difference between the MR-Egger method and the IVW method is that the existence of the intercept item is considered in the MR–Egger regression, and it also uses the reciprocal of the outcome variance as the weight for fitting (15). The weighted median method was defined as the median of the weighted empirical density function of ratio estimates, from which causality was assessed if at least 50% of the information in the analysis came from valid tools.
This study used the “leave-one-out” sensitivity analysis by removing individual SNPs one-at-a-time to assess whether that variation drove the association between exposure and outcome variables. Second, to clarify whether there was horizontal pleiotropy in the MR analysis, this study also carried out MR-Egger intercept detection. If the intercept item in the MR–Egger intercept analysis had obvious statistical significance, then it indicated that the study had obvious significance. Finally, this study also used Cochran’s Q statistic to detect heterogeneity. Significant heterogeneity in the results of the analysis was demonstrated if the Cochran’s Q test result was statistically significant. P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using R program (version 4.2.0), including packages such as TwoSampleMR.
In this study, 14 genome-wide significant SNPs closely related to frailty were selected as instrumental variables (see Table 1). The included SNPs explained approximately 7.41% of the phenotypic variation, and the F values of the included SNPs were all greater than 10, which proved that the study was not easily affected by weak instrumental variables. The MR-Egger regression intercept and leave-one-out sensitivity analysis showed no horizontal pleiotropy for any instrumental variable (see Table 2).
Frailty might increase the relative risk of delirium, as demonstrated by IVW (OR = 1.849, 95% CI 0.027∼2.067, P = 0.044), the weighted median (OR = 1.726, 95% CI −0.178∼2.664, P = 0.083) and MR-Egger regression (OR = 1.768, 95% CI −3.08∼6.171, P = 0.525). Although the WME method and MR–Egger regression analysis showed no statistically significant causal relationship between the FI and the risk of delirium, the direction of the causal effect was consistent with the IVW method (see Figures 1, 2).
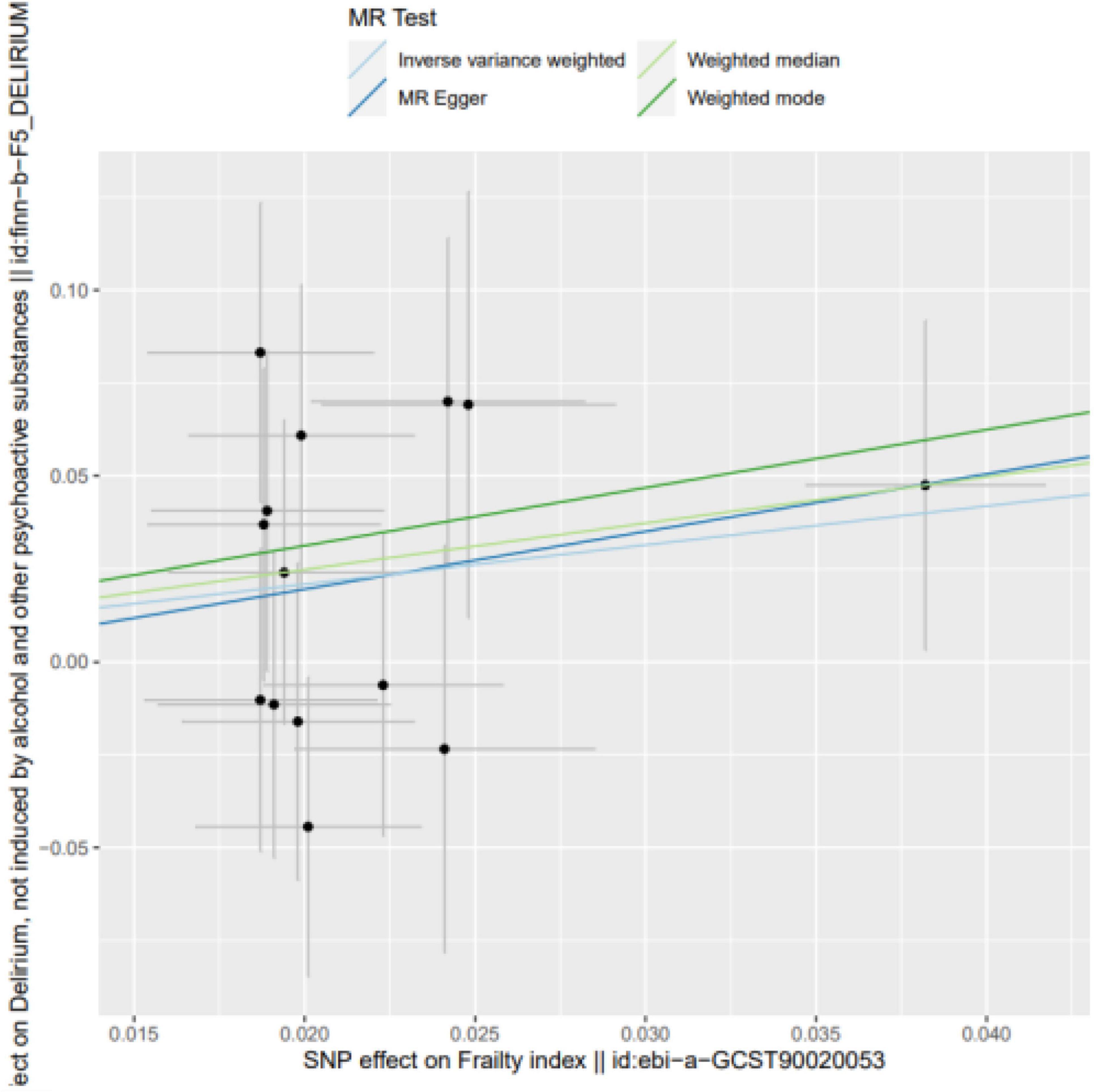
Figure 1. SNP effects on the outcome against SNP effects on the exposure. Frailty might increase the relative risk of delirium, as demonstrated by IVW (OR = 1.849, 95% CI 0.027∼2.067, P = 0.044), the weighted median (OR = 1.726, 95% CI –0.178∼2.664, P = 0.083) and MR-Egger regression (OR = 1.768, 95% CI –3.08∼6.171, P = 0.525).
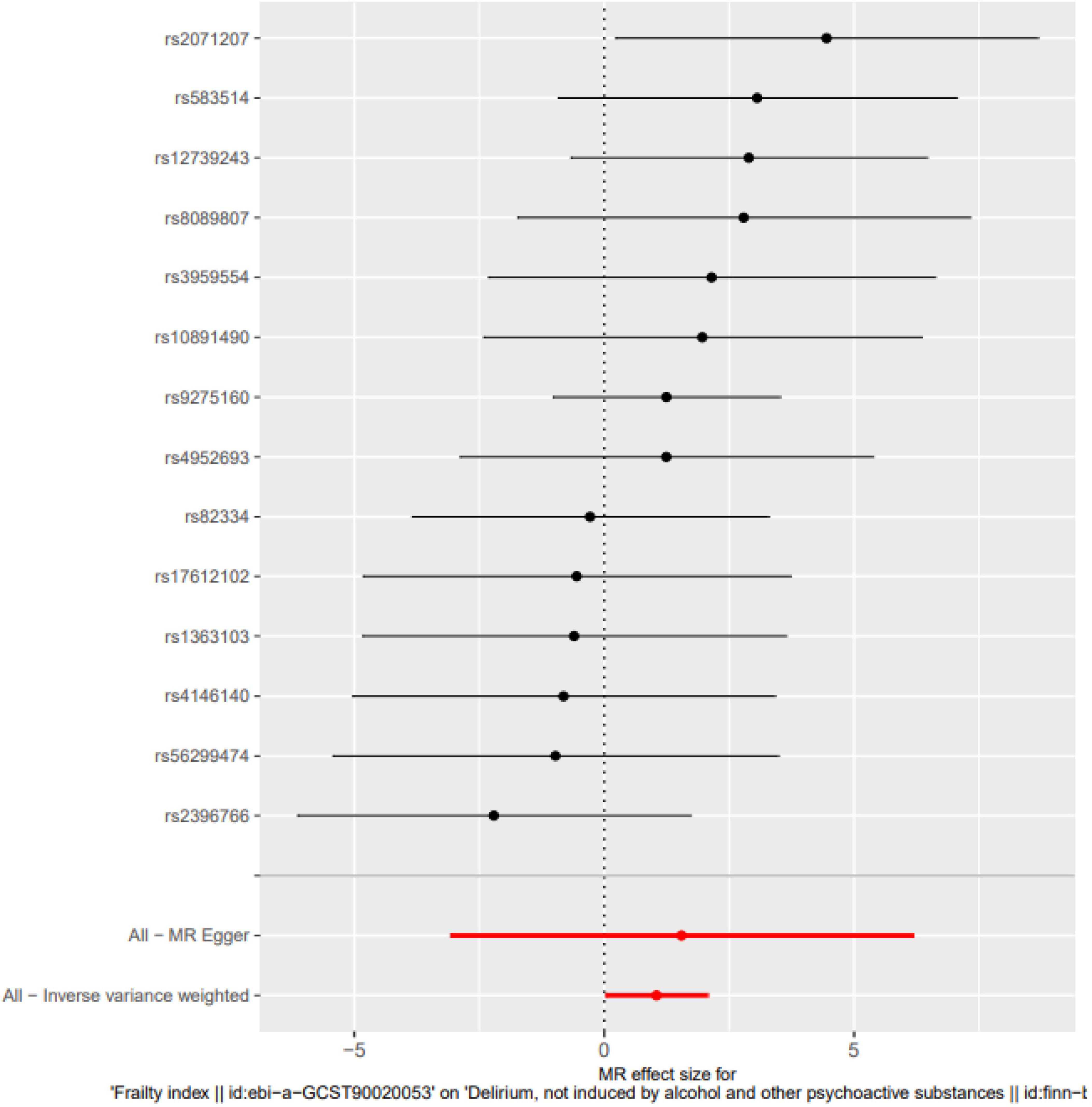
Figure 2. Single SNP analysis. The Single SNP analysis showed IVW (OR = 1.849, 95% CI 0.027∼2.067, P = 0.044), and MR-Egger regression (OR = 1.768, 95% CI –3.08∼6.171, P = 0.525).
MR-Egger intercept analysis showed that there was no horizontal pleiotropic effect in this study (P = 0.832). Second, Cochran’s Q test showed that there was no certain heterogeneity in the study results (P = 0.583), and the “leave-one-out” sensitivity analysis and funnel plot also showed that the included SNPs had no significant impact on the results (see Figures 3, 4).
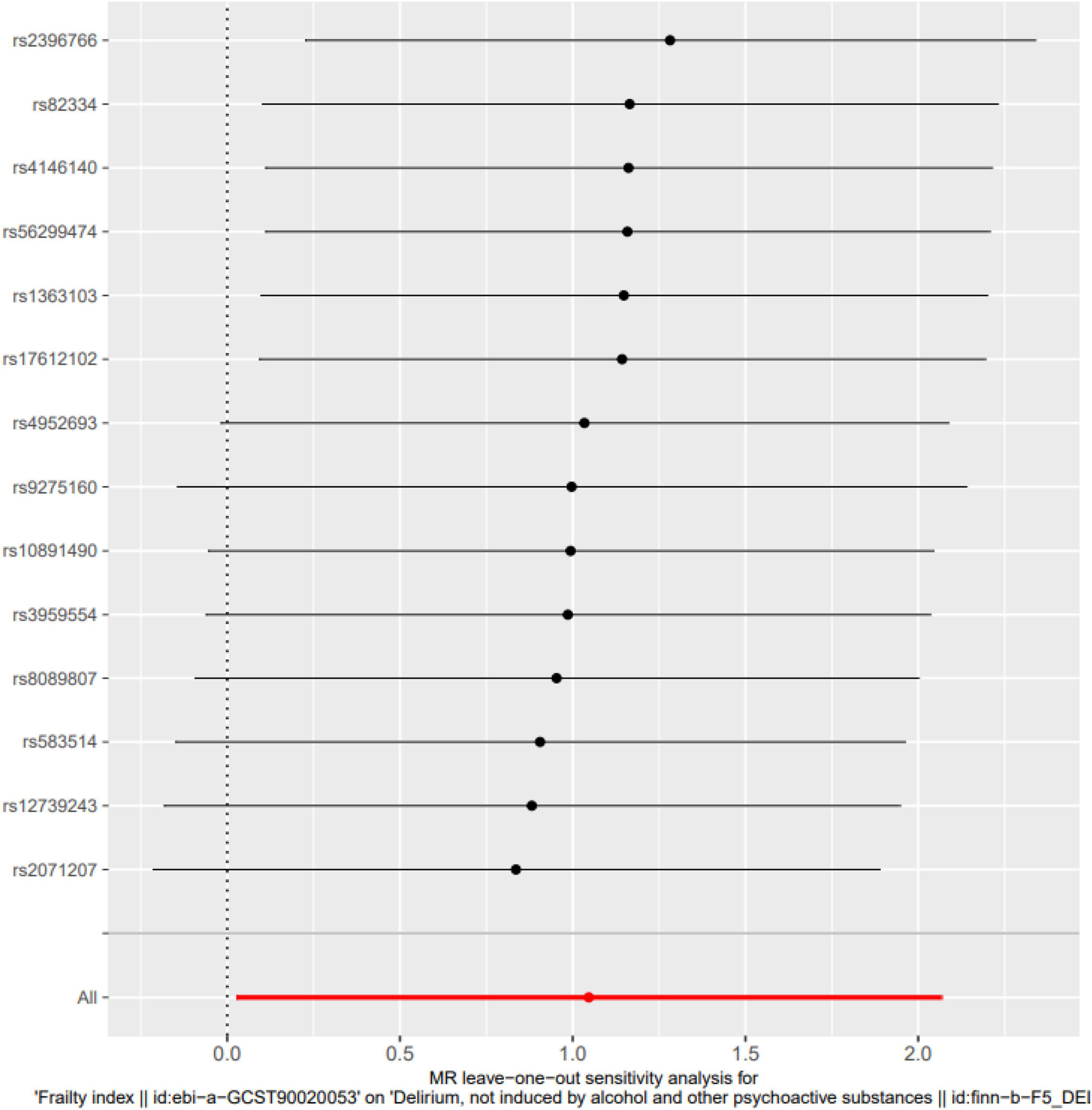
Figure 3. Leave-one-out sensitivity analysis. The “leave-one-out” sensitivity analysis showed that the included SNPs had no significant impact on the results (P = 0.058).
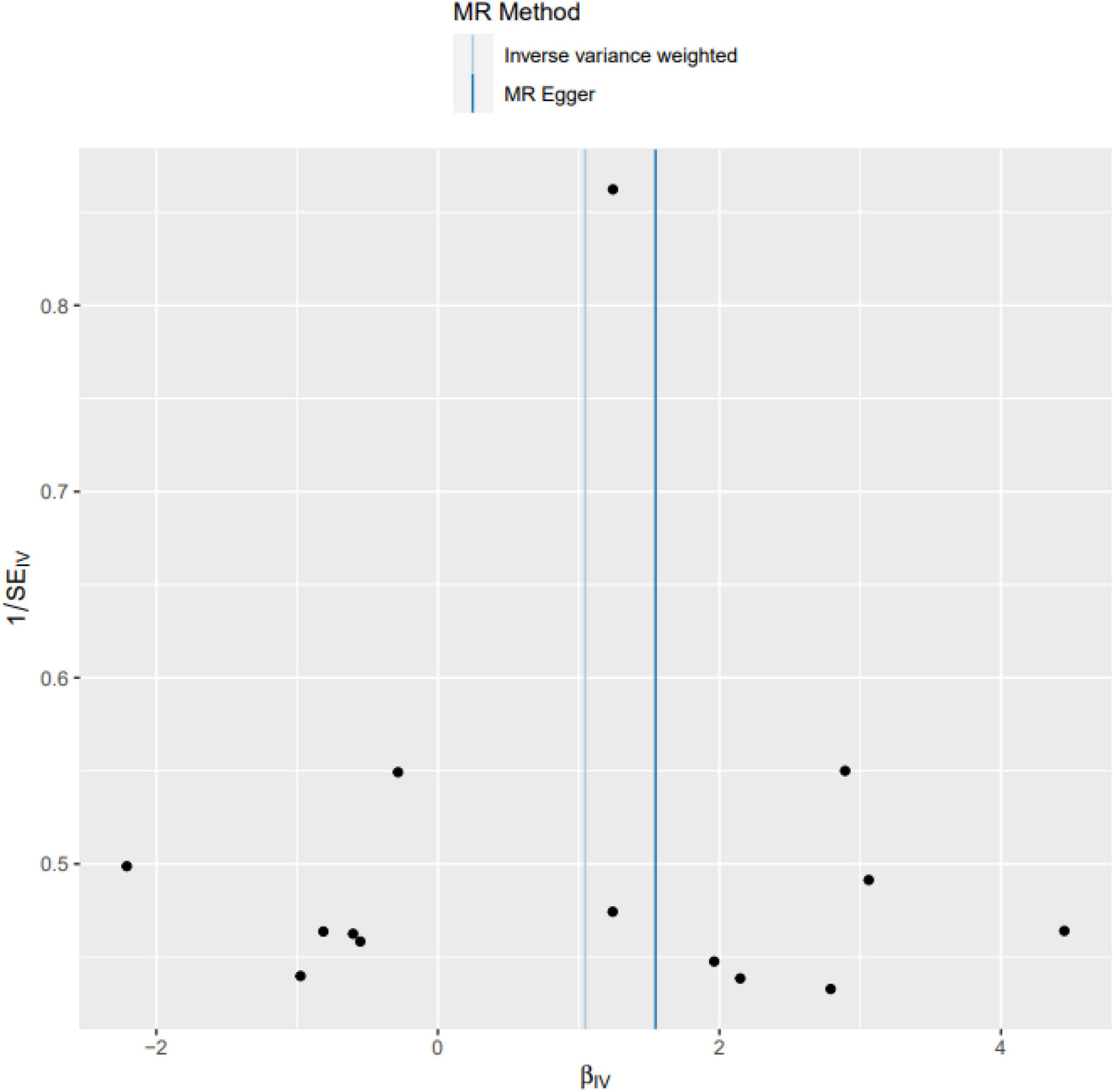
Figure 4. Funnel plot. The funnel plot also showed that the included SNPs had no significant impact on the results.
This scientific investigation employed a Mendelian randomization analysis using a two-sample approach to evaluate the causality between the FI and the likelihood of experiencing delirium. The study uncovered a potential exacerbating impact of frailty on the risk of delirium in hospitalized patients, providing a basis for establishing a causal relationship between frailty and delirium. Delirium, characterized by impaired attention and confusion, represents a frequently encountered complication among hospitalized individuals, particularly among the elderly The occurrence of delirium can ascend to as high as 60%. A pharmacoepidemiological study was to describe the pattern of use of antidepressant medication the elderly population. Meantime, pharmacological treatment of delirium is receiving increasing attention (16). Concurrently, frailty is highly prevalent among elderly patients admitted to hospitals. Nonetheless, the presence of a causal association between these two phenomena remains ambiguous. The significance of the link between frailty and delirium warrants discussion. Despite the seemingly intuitive nature of this relationship, the current body of literature providing evidence for it is lacking, with most studies focusing on surgical contexts (17–19). And the relevant relationship between frailty and cardiovascular diseases and sarcopenia were also got the same results (20, 21). To shed light on this matter, Zhang et al. (6) executed a meta-analysis in 2021 exploring the interplay between frailty and delirium. The analysis divulged that the incidence of delirium in frail hospitalized patients was approximately three times higher compared to their non-frail counterparts (6). It is essential to consider, however, that the majority of studies included in the meta-analysis were of an observational nature, engendering a high level of heterogeneity among the included studies, thus limiting the ability to definitively establish a causal connection between frailty and delirium. A single-center study examined the impact of frailty and delirium on patient survival, revealing that frail individuals with delirium faced a higher long-term mortality risk compared to those who were fit (22). This finding aligns with the current study’s results and with previous research highlighting the significant influence of frailty on mortality (23, 24).
Our study possesses various strengths in contrast to observational studies. Firstly, we employed a comprehensive large-sample genome-wide association study, enabling a thorough analysis of delirium events. Secondly, we utilized several alternative methods that produced consistent results. However, it is crucial to exercise caution when interpreting these findings as certain limitations exist. Initially, the MR study employed GWAS data sourced exclusively from the European population, necessitating further studies to determine the generalizability of our research to other populations. Additionally, our study employed three statistical methods, with only the IVW method yielding statistically significant results. This may possibly be attributed to the fact that our study only included European population, leading to reduced statistical power in the causality assessment. Hence, it is imperative to conduct additional causality assessments encompassing diverse racial backgrounds in order to attain more reliable conclusions.
In conclusion, this study is the first to comprehensively explore the causal relationship between the FI and the risk of delirium in hospitalized patients through two-sample MR analysis. The results showed that there was a notable correlation between a higher FI and an elevated risk of delirium. This indicates that healthcare providers should take proactive measures to prevent delirium in hospitalized patients with a higher FI.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ebi.ac.uk/metagenomics/,ebi-a-GCST90020053.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YC: Writing – original draft, Writing – review & editing. FF: Funding acquisition, Writing – review & editing. QL: Methodology, Resources, Writing – review & editing. HG: Software, Writing – review & editing. LZ: Software, Writing – review & editing. JL: Conceptualization, Supervision, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Gansu Province (No. 23JRRA0965) and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-BJ-A10).
We would like to thank AJE for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1361437/full#supplementary-material
Supplementary Table 1 | The detail information of frailty datasets.
Supplementary Table 2 | The detail basal information of delirium datasets.
Supplementary Figure 1 | Survival analyses between endpoints of delirium datasets.
Supplementary Figure 2 | Cumulative incidence of delirium.
1. Patnode CD, Henderson JT, Melnikow J, Coppola EL, Durbin S, Thomas RUS. Preventive Services task force evidence syntheses, formerly systematic evidence reviews. Interventions for Tobacco cessation in adults, including pregnant women: An evidence update for the US preventive services task force. Rockville, MD: Agency for Healthcare Research and Quality (US) (2021).
2. Faulkner KM, Uchmanowicz I, Lisiak M, Cichoń E, Cyrkot T, Szczepanowski R. Cognition and frailty in patients with heart failure: A systematic review of the association between frailty and cognitive impairment. Front Psychiatry. (2021) 12:713386. doi: 10.3389/fpsyt.2021.713386
3. Mazzola P, Tassistro E, Di Santo S, Rossi E, Andreano A, Valsecchi MG, et al. The relationship between frailty and delirium: Insights from the 2017 delirium day study. Age Ageing. (2021) 50:1593–9. doi: 10.1093/ageing/afab042
4. Bellelli PG, Biotto M, Morandi A, Meagher D, Cesari M, Mazzola P, et al. The relationship among frailty, delirium and attentional tests to detect delirium: A cohort study. Eur J Intern Med. (2019) 70:33–8. doi: 10.1016/j.ejim.2019.09.008
5. Gandossi CM, Zambon A, Oliveri G, Codognola M, Szabo H, Cazzulani I, et al. Frailty, post-operative delirium and functional status at discharge in patients with hip fracture. Int J Geriatr Psychiatry. (2021) 36:1524–30.
6. Zhang XM, Jiao J, Xie XH, Wu XJ. The association between frailty and delirium among hospitalized patients: An updated meta-analysis. J Am Med Dir Assoc. (2021) 22:527–34.
7. Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, et al. Frailty and delirium in older adults: A systematic review and meta-analysis of the literature. J Am Geriatr Soc. (2018) 66:2022–30.
8. Cai J, He L, Wang H, Rong X, Chen M, Shen Q, et al. Genetic liability for prescription opioid use and risk of cardiovascular diseases: A multivariable Mendelian randomization study. Addiction. (2022) 117:1382–91. doi: 10.1111/add.15767
9. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8.
10. Atkins JL, Jylhävä J, Pedersen NL, Magnusson PK, Lu Y, Wang Y, et al. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell. (2021) 20:e13459. doi: 10.1111/acel.13459
11. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
12. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
13. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64.
14. Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int J Epidemiol. (2015) 44:496–511.
15. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89.
16. Giovannini S, Onder G, van der Roest HG, Topinkova E, Gindin J, Cipriani MC, et al. Use of antidepressant medications among older adults in European long-term care facilities: A cross-sectional analysis from the SHELTER study. BMC Geriatr. (2020) 20:310. doi: 10.1186/s12877-020-01730-5
17. Yamanashi T, Iwata M, Crutchley KJ, Sullivan EJ, Malicoat JR, Anderson ZM, et al. New cutoff scores for delirium screening tools to predict patient mortality. J Am Geriatr Soc. (2021) 69:140–7.
18. Basinski JR, Alfano CM, Katon WJ, Syrjala KL, Fann JR. Impact of delirium on distress, health-related quality of life, and cognition 6 months and 1 year after hematopoietic cell transplant. Biol Blood Marrow Transplant. (2010) 16:824–31. doi: 10.1016/j.bbmt.2010.01.003
19. Mahanna-Gabrielli E, Zhang K, Sieber FE, Lin HM, Liu X, Sewell M, et al. Frailty is associated with postoperative delirium but not with postoperative cognitive decline in older noncardiac surgery patients. Anesth Analg. (2020) 130:1516–23.
20. Giovannini S, Brau F, Forino R, Berti A, D’Ignazio F, Loreti C, et al. Sarcopenia: Diagnosis and management, state of the art and contribution of ultrasound. J Clin Med. (2021) 10:5552. doi: 10.3390/jcm10235552
21. Liperoti R, Vetrano DL, Palmer K, Targowski T, Cipriani MC, Lo Monaco MR, et al. Association between frailty and ischemic heart disease: A systematic review and meta-analysis. BMC Geriatr. (2021) 21:357. doi: 10.1186/s12877-021-02304-9
22. Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. (2012) 41:412–6.
23. Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. (2016) 45:353–60.
Keywords: frailty index, delirium, Mendelian randomization study, intensive care, nursing care
Citation: Chen Y, Feng F, Li Q, Guo H, Zhang L and Liu J (2024) Frailty index and risk of delirium in hospitalized patients: a two-sample Mendelian randomization study. Front. Med. 11:1361437. doi: 10.3389/fmed.2024.1361437
Received: 26 December 2023; Accepted: 08 May 2024;
Published: 22 May 2024.
Edited by:
Paolo Mazzola, University of Milano-Bicocca, ItalyReviewed by:
Alessio Greco, ASST Lecco, ItalyCopyright © 2024 Chen, Feng, Li, Guo, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, bWVkZWNpbmxpdUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.