- 1Department of Anesthesiology, Peking University First Hospital, Beijing, China
- 2Department of Anesthesiology, Sichuan Provincial People’s Hospital, Chengdu, China
- 3Department of Anesthesiology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 4Department of Anesthesiology, Yibin Second People’s Hospital, Yibin, China
- 5Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, China
- 6Department of Anesthesiology, Dongguan People’s Hospital, Dongguan, China
- 7Department of Anesthesiology, Tianjin Medical University General Hospital, Tianjin, China
- 8Department of Anesthesiology, Beijing Obstetrics and Gynecology Hospital, Beijing, China
- 9Haisco Pharmaceutical Group Co., Ltd., Chengdu, China
- 10Outcomes Research Consortium, Cleveland, OH, United States
Objective: Ciprofol (also known as cipepofol and HSK3486), is a compound similar to propofol in chemical structure and hypnotic effect. Herein we evaluated the efficacy and safety of ciprofol for sedation in outpatient gynecological procedures.
Methods: This phase III multicenter randomized trial with a non-inferiority design was conducted in nine tertiary hospitals. We enrolled 135 women aged 18–65 years who were scheduled for ambulatory gynecological procedures. Patients were randomly assigned to receive either ciprofol (0.4 mg/kg for induction and 0.2 mg/kg for maintenance) or propofol (2.0 mg/kg for induction and 1.0 mg/kg for maintenance) sedation in a 2:1 ratio. Patients and investigators for data collection and outcome assessment were blinded to study group assignments. The primary outcome was the success rate of sedation, defined as completion of procedure without remedial anesthetics. The non-inferiority margin was set at −8%. Secondary outcomes included time to successful induction, time to full awake, time to meet discharge criteria, and satisfaction with sedation assessed by patients and doctors. We also monitored occurrence of adverse events and injection pain.
Results: A total of 135 patients were enrolled; 134 patients (90 patients received ciprofol sedation and 44 patients propofol sedation) were included in final intention-to-treat analysis. The success rates were both 100% in the two groups (rate difference, 0.0%; 95% CI, −4.1 to 8.0%), i.e., ciprofol was non-inferior to propofol. When compared with propofol sedation, patients given ciprofol required more time to reach successful induction (median difference [MD], 2 s; 95% CI, 1 to 7; p < 0.001), and required more time to reach full awake (MD, 2.3 min; 95% CI, 1.4 to 3.1; p < 0.001) and discharge criteria (MD, 2.3 min; 95% CI, 1.5 to 3.2; p < 0.001). Fewer patients in the ciprofol group were dissatisfied with sedation (relative risk, 0.21; 95% CI, 0.06 to 0.77; p = 0.024). Patients given ciprofol sedation had lower incidences of treat-emergent adverse events (34.4% [31/90] vs. 79.5% [35/44]; p < 0.001) and injection pain (6.7% [6/90] vs. 61.4% [27/44]; p < 0.001).
Conclusion: Ciprofol for sedation in ambulatory gynecological procedures was non-inferior to propofol, with less adverse events and injection pain.
Clinical trial registration: ClinicalTrials.gov, identifier NCT04958746.
Introduction
Propofol, chemically named 2, 6-diisopropyl phenol, produces hypnotic effect mainly by enhancing gamma-aminobutyric acid type A (GABAA) receptor-mediated inhibitory synaptic currents. Although propofol is broadly used for sedation and general anesthesia, the related adverse effects including injection pain, respiratory suppression, and circulatory inhibition cannot be ignored. Compounds chemically like propofol were produced after incorporating cyclopropyl group into the 2,6-side chain to increase its lipophilicity, and introducing one or more chiral centers to break the structure symmetry. The aim was to reduce propofol-related side effects while retaining sedative efficacy. Among these compounds, ciprofol (also known as cipepofol and HSK3486) was identified as a novel anesthetic agent (1). Like propofol, ciprofol exerts hypnotic effects by acting on GABAA receptors and produces rapid-onset action and clear wake-up with similar pharmacokinetic characteristics of absorption, distribution, metabolism (2).
Ciprofol has been evaluated in clinical trials from phase I (3, 4), phase II (5–7), to phase III (8–10). In a phase I trial, ciprofol 0.4–0.9 mg/kg produced rapid onset of anesthesia and rapid recovery of consciousness, and was well tolerated by subjects (4). In a phase II trial, the efficacy and safety of ciprofol 0.4–0.5 mg/kg were comparable to propofol 2 mg/kg in adults under 65 years of age (6). In phase III trials, the success rates of ciprofol were all 100% when used for sedation in digestive endoscopy (6, 8) and fiberoptic bronchoscopy (9), for anesthesia induction in elective gynecological surgery (11), and for anesthesia maintenance in elective surgery (12, 13). Furthermore, ciprofol induction produced a more stable change of bispectral index and slighter variations of blood pressure and heart rate (10). The indications for sedation in digestive endoscopy (approval number H20200013) and general anesthesia (approval number H20210007) have been approved by the National Medical Products Administration of China.1 Up to now, ciprofol has been used for anesthesia in aged patients (14), patients with mild to moderate liver injury (15), and patients undergoing kidney transplantation (16); it has also been used for sedation in intensive care unit patients with mechanical ventilation (7).
As a novel anesthetic agent, the efficacy and safety of ciprofol requires further demonstration. Outpatient gynecological procedures usually last for 2 to 30 min under sedation. The use of ciprofol for sedation in outpatients undergoing gynecological procedure has not been evaluated. We therefore designed this non-inferiority trial to compare the efficacy and safety of ciprofol versus propofol in gynecological outpatients.
Materials and methods
This phase III multicenter randomized parallel-group trial was conducted in nine tertiary hospitals across China. The trial protocol was approved by the Biomedical Research Ethics Committee of Peking University First Hospital (2021-052, June 25, 2021) and other participating centers and was prospectively registered with ClinicalTrials.gov (NCT04958746; June 23, 2021). Written informed consent was obtained from each participating patient.
Participants
We included patients aged between 18–65 years who had a body mass index (BMI) between 18–30 kg/m2 and an American Society of Anesthesiologists (ASA) classification I to II and were scheduled for gynecological procedures under intravenous sedation. We excluded patients who had contraindications to general anesthesia or a history of previous anesthesia incidents; were allergic to propofol injection, ciprofol injection, excipients of study drugs, and opioids or their drug ingredients; had positive urine or blood human chorionic gonadotropin (HCG) test (except abortion, curettage, or other outpatient procedures for pregnancy termination); or were in lactating period. Detailed inclusion and exclusion criteria are listed in Supplementary File S1.
Randomization, masking, and study drug administration
Random allocations were generated in a 2:1 ratio with a block size of nine by an independent statistician using the SAS 9.4 software (SAS Institute, United States). During the study period, random numbers were obtained from the Rave Data Management/Randomization and Trial Supply Management (RTSM; MediData Institute, United States) by non-blinded pharmacists who prepared the study drugs but were otherwise not involved in the trial. The study drugs, either ciprofol (0.25% ciprofol injection, 20 mL/ampoule; Haisco, Xingcheng, Liaoning, China) or propofol (1% propofol injection, 20 mL/ampoule; AstraZeneca, Wuxi, Jiangsu, China), were provided as emulsion for injection with identical appearance to responsible anesthesiologists. In this way the enrolled patients were randomly assigned to receive either ciprofol or propofol anesthesia in a 2:1 ratio. For each participant, a non-blinded anesthesiologist was designated for study drug administration. All patients, other health-care team members, and investigators who were responsible for data collection and outcomes assessment were blinded to study group assignments.
Intraoperative monitoring included electrocardiogram, noninvasive blood pressure, respiratory rate, and pulse oxygen saturation (SpO2). Anesthesia was induced after mask oxygen inhalation at 5 L/min for 3 min. Fentanyl 50 μg was injected over 10 ± 5 s. Ciprofol 0.4 mg/kg or propofol 2.0 mg/kg was then injected over 30 ± 5 s. The initiation of study drug administration was marked as 0 min and followed by evaluation with Modified Observer’s Assessment of Alert/Sedation (MOAA/S; Supplementary Table S1) every 30 ± 10 s. If the required depth of sedation (MOAA/S score of ≤1) was not reached in 2 min, additional ciprofol 0.2 mg/kg or propofol 1.0 mg/kg was injected over 10 ± 5 s until the required depth was achieved. If adequate sedation was not achieved with the above study drug doses, propofol was added as a remedy. Gynecological procedures began after successful induction (MOAA/S ≤1). During the procedure, 5 L/min oxygen inhalation was given via anesthetic mask and patients were manually ventilated, when necessary, without the use of endotracheal tube or laryngeal mask.
During the procedures (started with speculum placement and ended with speculum removal), additional ciprofol 0.2 mg/kg or propofol 1.0 mg/kg was injected when there were clinical signs indicating light sedation (body movement or eye opening). In case that more than 5 additional doses of study drugs were required within any consecutive 15 min, propofol would be administered as a remedy. For each case, an independent anesthesiologist who was not involved in the trial monitored vital signs and assured safety. At the end of procedures, patients were monitored in the operating room until regain consciousness, and then in the post-anesthesia care unit (PACU) for at least 30 min until fully awake and Aldrete score ≥9. Patients were then discharged to home.
Data collection and outcome assessment
Baseline data included demographic and morphometric characteristics, previous comorbidities, history of smoking and alcohol drinking, and results of relevant laboratory tests and other examinations. Intraoperative data included doses of study drugs and remedial medications, vital signs, verbal rating scale (VRS) (0-no pain; 1-mild pain; 2-moderate pain; 3-severe pain) for injection pain, and type and duration of procedure. Intraoperative vital signs, including mean arterial pressure (MAP), heart rate (HR), and pulse oxygen saturation (SpO2), were monitored every 2 min before induction, every minute during study drug administration until awaking, and every 2 min after awaking. Averages of the first three recorded values under resting state before induction were adopted as baseline vital sign values.
Our primary outcome was the success rate of sedation, defined as completion of procedure without remedial anesthetics. Secondary outcomes included (1) time to successful induction (defined as interval from the first study drug administration to the first MOAA/S score ≤1); (2) time to full awake (defined as interval from the last study drug administration to completely awaking, as indicated by the first MOAA/S = 5 in three consecutive MOAA/S = 5 assessments); (3) time to meet discharge criteria (defined as interval from the last study drug administration to the first Aldrete score ≥9 in three consecutive Aldrete score ≥9 assessments); (4) satisfaction with sedation as assessed by patients (Supplementary Table S2), anesthesiologists (Supplementary Table S3) and surgeons (Supplementary Table S4).
Safety outcomes including treatment-emergent adverse events (TEAEs) and treatment-related adverse events (TRAEs) were followed up and recorded from the screening period until 2–4 days after surgery. The severity of adverse events was classified into five grades, i.e., grade 1 (mild), grade 2 (moderate), grade 3 (severe), grade 4 (life-threatening) and grade 5 (causing death), according to the Common Terminology Criteria for Adverse Events (CTCAE version 5.0). For each individual patient, the most serious adverse event was recorded.
Predefined adverse events potentially related to study drug administration included respiratory depression (respiratory rate <8 breaths/min), desaturation (SpO2 <95% with oxygen inhalation), apnea (absence of breathing action for more than 10 s), tachypnea (respiratory rate >20 breaths/min), hypotension (systolic pressure <90 mmHg, diastolic pressure <50 mmHg, or MAP decrease of ≥20% from baseline), bradycardia (heart rate <50 beats/min or a decrease of >30% from baseline), tachycardia (heart rate >100 beats/min or an increase of >30% from baseline), and new-onset arrhythmia requiring therapy. We also recorded nausea, vomiting, dizziness, injection pain, postoperative pain, skin rash, and other adverse events that emerged after study drug administration. Injection pain was evaluated during administration of the first dose of study drugs with a 4-point scale (0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain). Postoperative pain was evaluated after full awake with a 10-point verbal rating scale (0 = no pain and 10 = the worst pain).
Statistical analysis
Sample size estimation
The success rate of propofol sedation was assumed to be 99% and the non-inferiority margin was set at −8%. A sample size of 135 patients was required to test the difference between two groups with 80% power at a one-sided significance level of 0.025, considering a dropout rate of 10%. We assigned patients to the ciprofol group (N = 90) or the propofol group (N = 45) in a ratio of 2:1. Sample size calculation was done with the SAS 9.4 software.
Data analysis
Outcome analyses were primarily performed in the intention-to treat population, that is all patients were analyzed in the group to which they were randomized. For the primary outcome, analysis was also performed in the per-protocol population, excluding patients who dropped out of the trial. Safety analyses were based on the safety population, including patients who had received at least one study drug administration.
Baseline balance was assessed using absolute standardized difference (ASD), that is the absolute differences in means, mean ranks, or proportions divided by the pooled standard deviation. Variables with an ASD >0.360 (calculated by ; n1 and n2 were the number of patients in each randomized group) were considered imbalanced (17).
For the primary outcome, the difference of successful sedation rates between groups was presented as rate difference and 95% confidence interval (CI), and compared with the non-inferiority margin.
For secondary outcomes, discrete variables were analyzed with the Mann–Whitney U test; median difference (95% CI) was calculated with the Hodges–Lehmann estimator. Categorical variables were analyzed with chi-square or Fisher exact tests and the differences between groups were presented as relative risks and 95% CIs. Missing data were not replaced.
For all hypotheses, two-tailed p-values <0.05 were considered statistically significant. Statistical analyses were performed with the SPSS 25.0 (IBM SPSS Inc., United States) software package.
Results
Patients
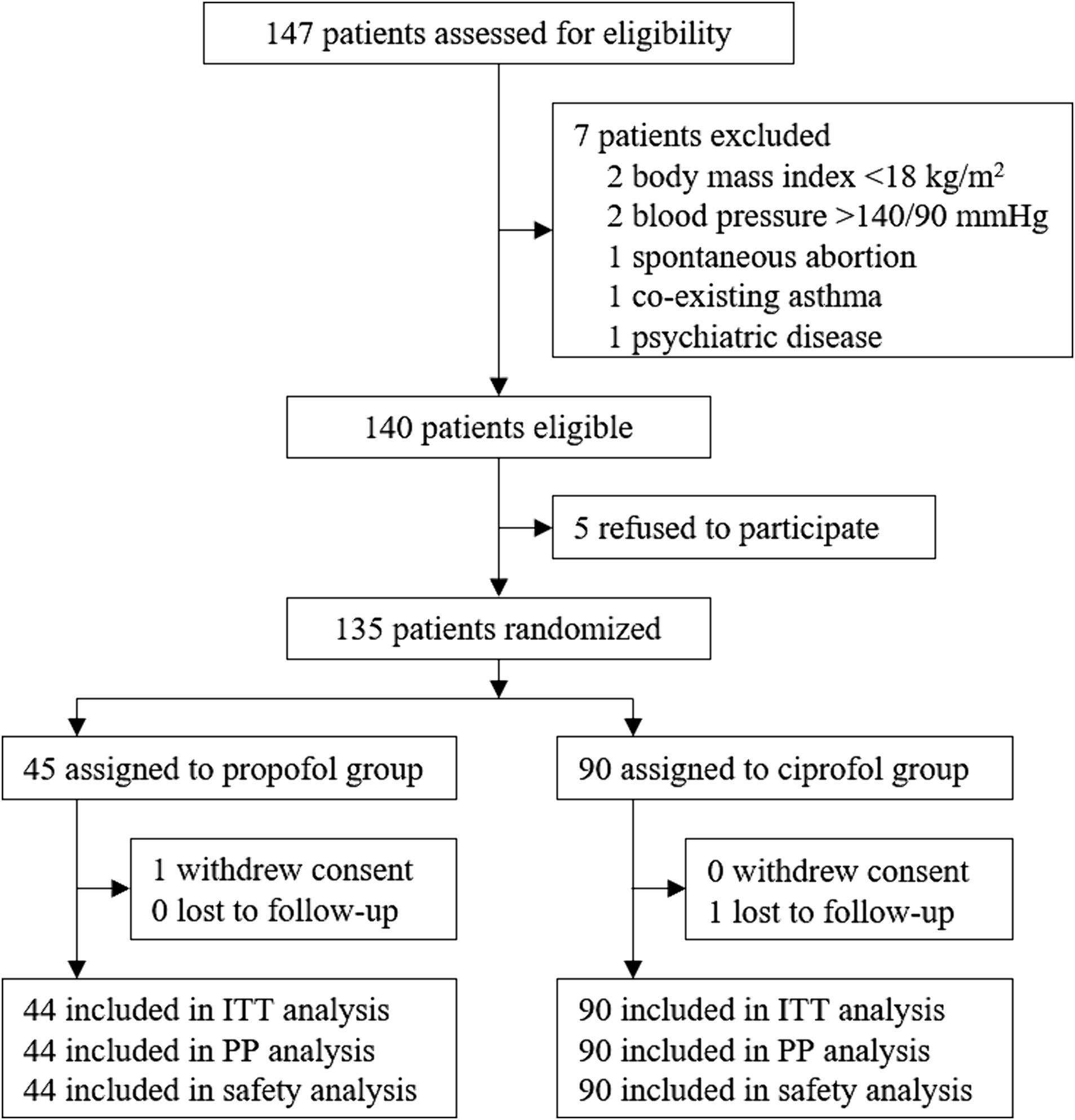
From July 26, 2021 to September 30, 2021, a total of 147 patients were screened for eligibility. Among them, 140 patients met the inclusion/exclusion criteria; 135 patients were enrolled and randomly assigned to the propofol (N = 45) or the ciprofol group (N = 90). One patient in the propofol group withdrew consent before receiving study drug. Thus, 134 patients received at least one study drug dose and were included in the intention-to-treat, per-protocol, and safety analyses (Figure 1).
Baseline and perioperative data
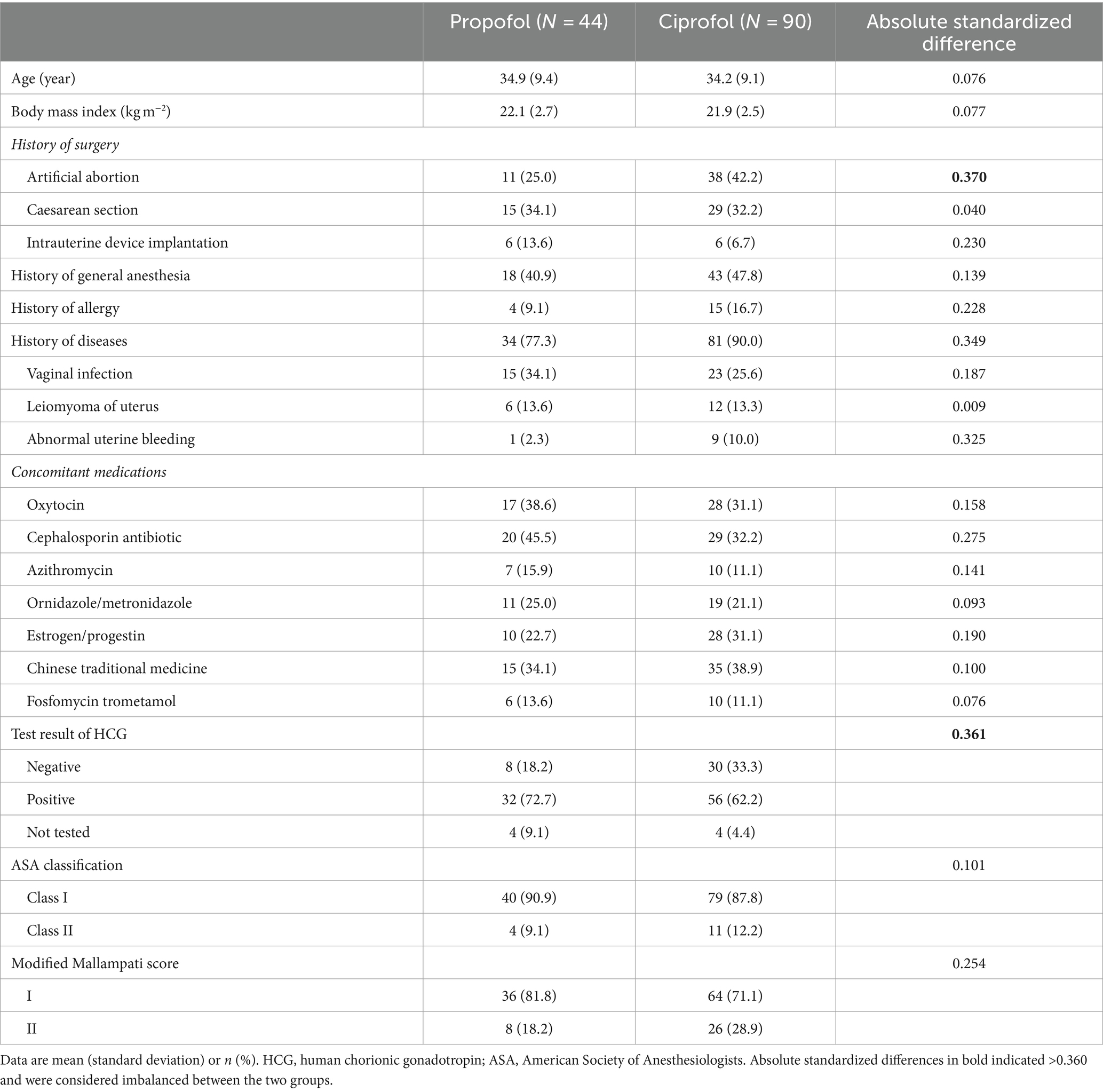
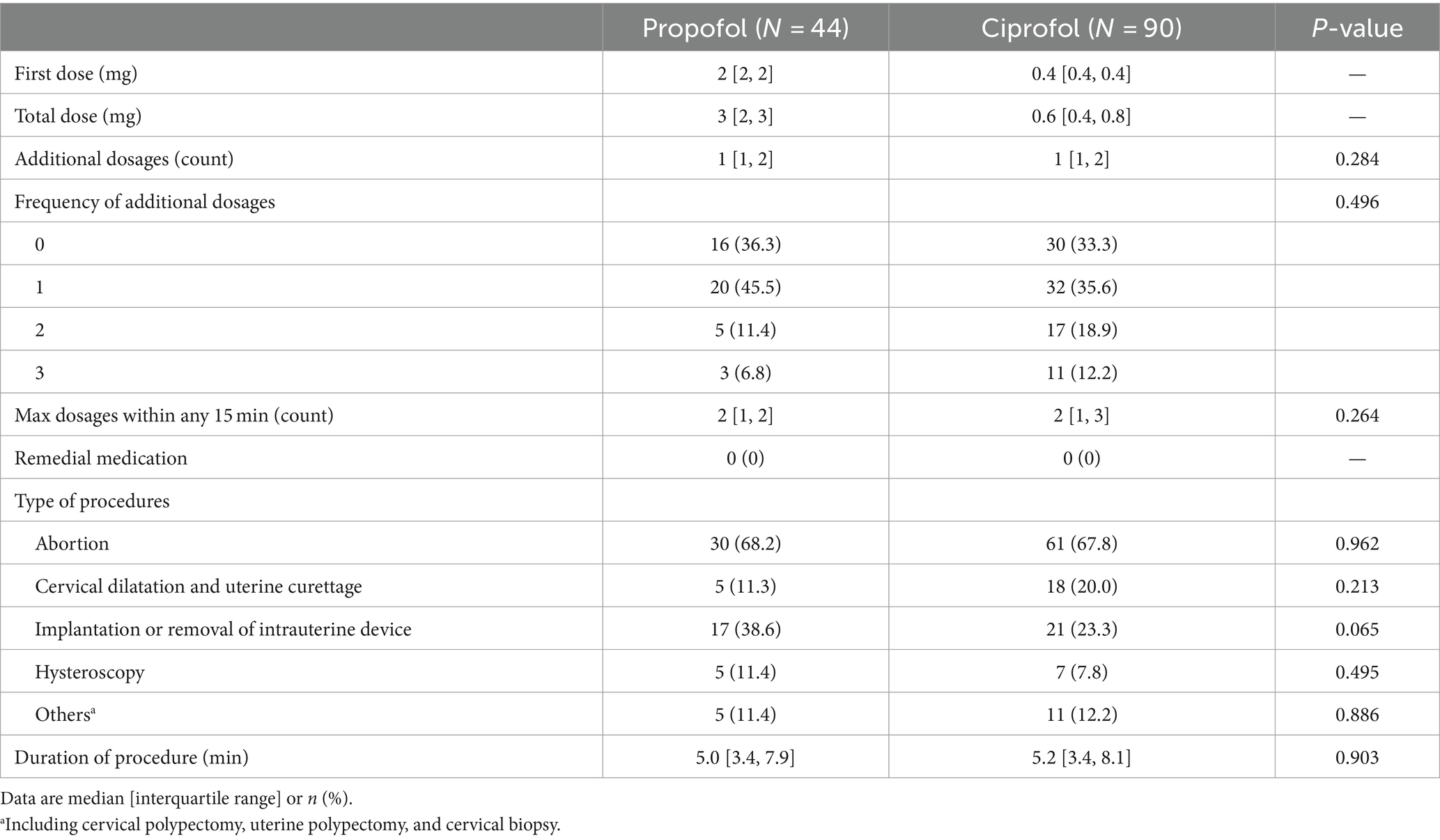
Baseline characteristics were generally balanced between the two groups, except that the proportions with histories of artificial abortion and with negative HCG-test results were higher in the ciprofol group (Table 1). Intraoperative data including the number of additional study drug dosages and the number of maximal study drug dosages within any 15 min were comparable between the two groups (Table 2).
Efficacy outcomes
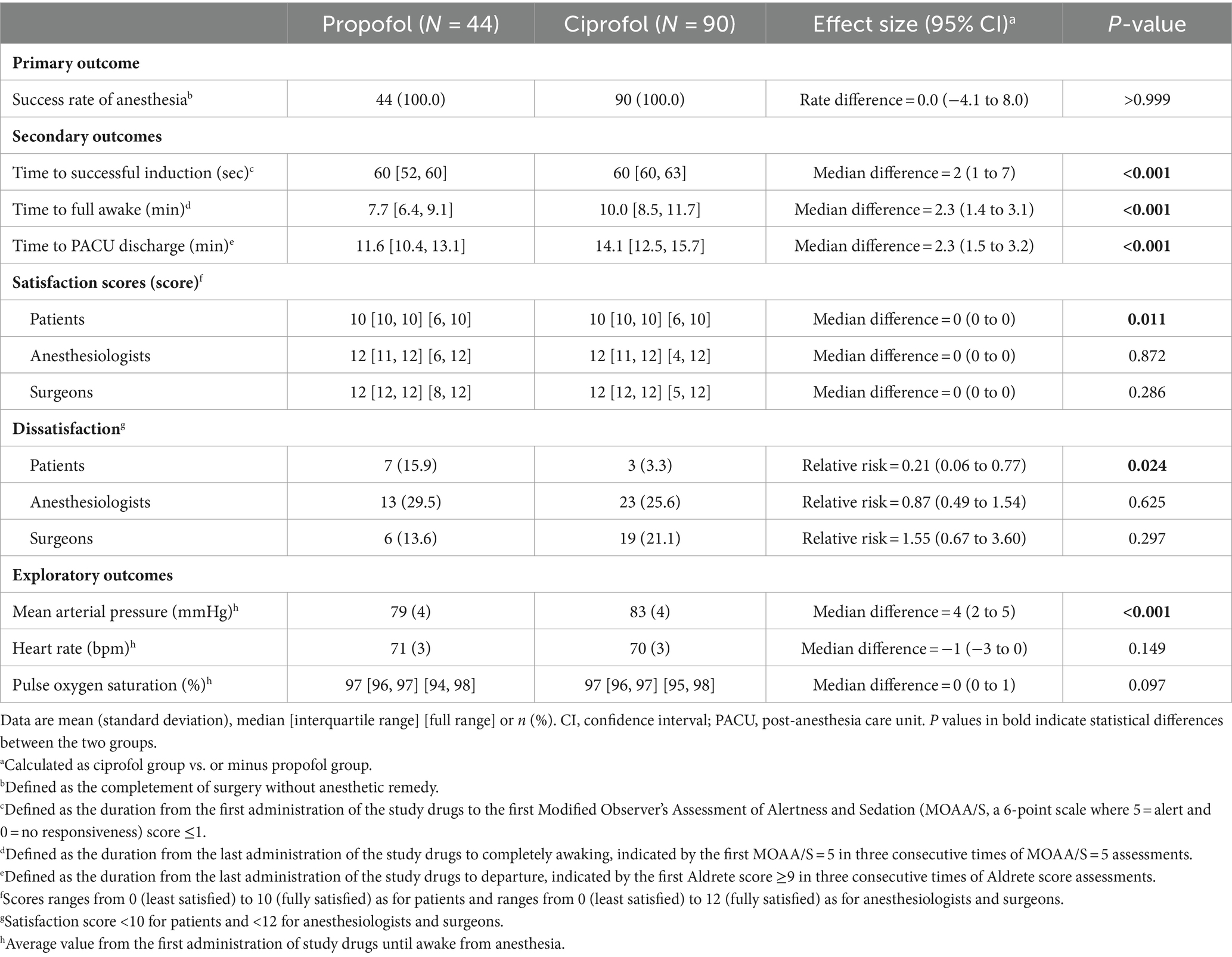
The success rate of sedation was 100% in both groups (rate difference, 0.0%; 95% CI, −4.1 to 8.0%; p > 0.999) (Table 3). With the lower limit of 95% CI greater than the preset non-inferiority margin −8%, the assumption that ciprofol was not inferior to propofol in anesthetic efficacy was manifested.
Among secondary outcomes, time to successful induction was longer in patients receiving ciprofol (median 60 s (interquartile range 60 to 63) with ciprofol vs. 60 s [52 to 60] with propofol; median difference, 2 s; 95% CI, 1 to 7; p < 0.001). After procedure, patients in the ciprofol group required more time to reach full awake (10 min [8.5 to 11.7] with ciprofol vs. 7.7 min [6.4 to 9.1] with propofol; median difference, 2.3 min; 95% CI, 1.4 to 3.1; p < 0.001) and criteria for PACU discharge (14.1 min [12.5 to 15.7] with ciprofol vs. 11.6 min [10.4 to 13.1] with propofol; median difference, 2.3 min; 95% CI, 1.5 to 3.2; p < 0.001). Patients given ciprofol showed lower dissatisfaction rate (3.3% [3 of 90] with ciprofol vs. 15.9% [7 of 44] with propofol; relative risk, 0.21; 95% CI, 0.06 to 0.77; p = 0.024). There were no significant differences in satisfaction scores and dissatisfaction rates of both anesthesiologists and surgeons between the two groups (Table 3).
Among exploratory outcomes, patients receiving ciprofol had higher MAP during the procedure (83 ± 4 mmHg with ciprofol vs. 79 ± 4 mmHg with propofol; mean difference, 4 mmHg; 95% CI, 2 to 5; p < 0.001). There were no significant differences in HR and SpO2 during the procedure between the two groups (Table 3 and Supplementary Figures S1–S3).
Safety outcomes
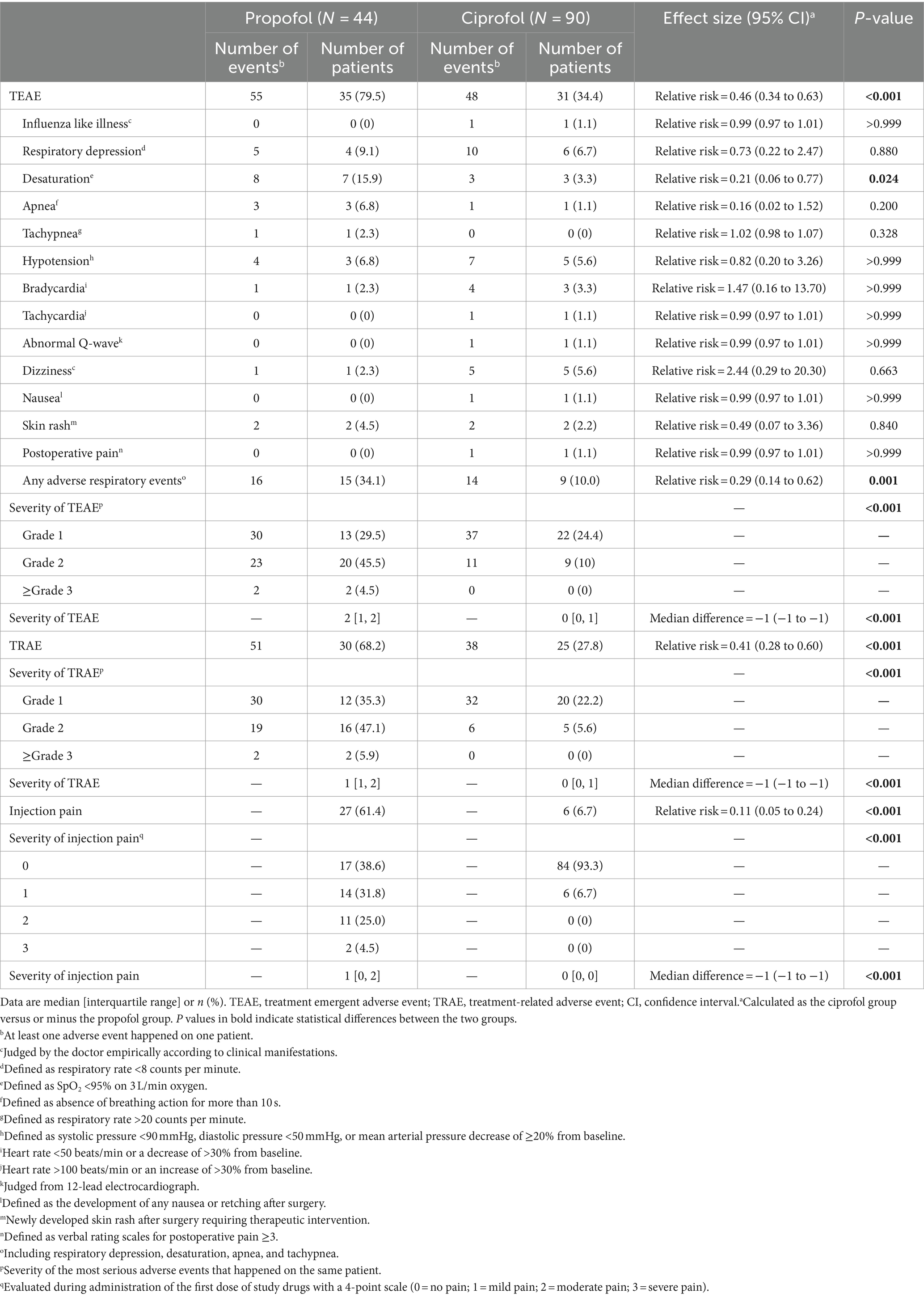
The incidence of TEAE (34.4% [31 of 90] with ciprofol vs. 79.5% [35 of 44] with propofol; relative risk, 0.46; 95% CI, 0.34 to 0.63; p < 0.001) and the severity of TEAE (median 0 [interquartile range 0, 1] with ciprofol vs. 2 [1, 2] with propofol; median difference, −1; 95% CI, −1 to −1; p < 0.001) were both lower in the ciprofol group than in the propofol group. Specifically, patients given ciprofol developed less desaturation (relative risk, 0.21; 95% CI, 0.06 to 0.77; p = 0.024) and any respiratory adverse events (relative risk, 0.29; 95% CI, 0.14 to 0.62; p < 0.001). The incidence of TRAE (27.8% [25 of 90] with ciprofol vs. 68.2% [30 of 44] with propofol; relative risk, 0.41; 95% CI, 0.28 to 0.60; p < 0.001) and the severity of TRAE (0 [0, 1] with ciprofol vs. 1 [1, 2] with propofol; median difference, −1; 95% CI, −1 to −1; p < 0.001) were also both lower in the ciprofol group. Patients in ciprofol group experienced less injection pain (6.7% [6 of 90] with ciprofol vs. 61.4% [27 of 44] with propofol; relative risk, 0.11; 95% CI, 0.05 to 0.24; p < 0.001) and lower severity of injection pain (0 [0, 0] with ciprofol vs. 1 [0, 2] with propofol; median difference, −1; 95% CI, −1 to −1; p < 0.001; Table 4).
Discussion
Results of this phase III randomized trial showed that, for adult patients undergoing ambulatory gynecological procedures, the efficacy of ciprofol used for induction and maintenance of sedation was not inferior to propofol. Ciprofol sedation required more time to reach adequate depth during induction and to reach full awake and PACU discharge criteria during recovery. However, these prolongations were clinically acceptable. On the other hand, ciprofol sedation was associated with less dissatisfaction of patients, less decrease in MAP, less injection pain, and less adverse events especially respiratory events.
Patients undergoing gynecological/obstetrical procedures usually have a high level of anxiety (18, 19). Our finding that the efficacy of ciprofol sedation was non-inferior to propofol was in line with previous studies. In a trial of 109 patients undergoing intubated general anesthesia, three different doses of ciprofol (0.3, 0.4, and 0.5 mg/kg) were used for anesthesia induction and achieved 100% success rate which was comparable to propofol (2.0 and 2.5 mg/kg) (5). The non-inferiority of ciprofol 0.4 mg/kg to propofol 2.0 mg/kg in anesthesia induction was also confirmed in a multi-center trial including 176 patients for elective surgery (10).
In the present study, the median time to successful induction was 2 s longer with ciprofol. Similar results were reported by others. For example, when compared with propofol 2.0 mg/kg in patients undergoing colonoscopy, the mean time to colonoscope insertion was longer with ciprofol 0.4 mg/kg (1.9 min vs. 1.5 min, p < 0.01) but was similar with a higher dose ciprofol 0.5 mg/kg (1.5 min) (6). Using midazolam (0.03 mg/kg) and sufentanil (0.3 μg/kg) ahead of ciprofol 0.4 mg/kg or propofol 2 mg/kg resulted in shortened and comparable induction time between the two groups (34.8 ± 15.5 s vs. 35.4 ± 9.5 s, p = 0.83) (11). So, with ciprofol, time required to achieve successful induction could be reduced by increasing drug dose or adding other sedatives and analgesics.
Of our patients, median time intervals to full awake and PACU discharge were both 2.3 min longer in the ciprofol group. A previous trial including 267 patients who underwent fiberoptic bronchoscopy also found that time required to full alertness (median 8.50 min vs. 6.00 min, p = 0.012) and discharge (median 13.00 min vs. 9.87 min, p = 0.002) were longer in patients given ciprofol 0.4 mg/kg than in those given propofol 2.0 mg/kg (9). The recovery time of ciprofol anesthesia is dose-dependent, that is a higher dose requires a longer time of recovery (4). The situation is especially true in patients with renal failure such as those undergoing kidney transplantation (16). However, when anesthesia maintenance was guided by BIS monitoring, recovery time was comparable between ciprofol and propofol anesthesia (13).
Patients receiving propofol for procedure sedation are at risk of adverse events due to limited circumstance and staff (20, 21). When ciprofol 0.4 mg/kg was used in gastroscopy and colonoscopy, the reported incidences of adverse events ranged from 31.3 to 48.4% (6, 8). In our results, both the incidence and severity of either treatment-emergent or treatment-related adverse events were lower in the ciprofol group than those in the propofol group. Importantly, patients receiving ciprofol sedation were less likely to develop desaturation and had higher MAP during the procedures. This indicated that ciprofol dose adopted in our trial was relatively safe considering its dose-dependent respiratory and circulated depression effects (12, 13, 22).
According to previous studies, 37 to 82.3% of patients experienced injection pain during propofol administration (23–25). Intravenous lidocaine could alleviate propofol-related injection pain (24), but was associated with metallic taste which negatively affected patients’ satisfaction (26). Injection pain of propofol was determined by many factors, including the site of injection, size of vein, speed of injection, propofol concentration in the aqueous phase, and the buffering effect of blood (27). As an oil-in-water emulsion, ciprofol has a high hydrophobicity and a low blood plasma concentration, which might explain the reduction of injection pain (1, 2). In accord with previous studies (3, 22, 28), we also found a much lower incidence of injection pain during the induction phase. Low rate of patient dissatisfaction with ciprofol might also be attributed to less injection pain during induction since 52 to 90.9% of patients could recall injection pain after awakening (29–31).
According to our results and previous studies, ciprofol may be a suitable alternative of propofol when used for procedure sedation in patients who are at risk of circulatory instability or respiratory suppression, and those who are sensitive to injection pain. There are some limitations in the present trial. One is that only fentanyl was used in combination with the study drugs. The mutual interaction of ciprofol with other drugs cannot be inferred from this trial. In consideration of patients’ safety, anesthesiologists who were responsible for study drug administration were not blind to treatment assignments. So, the determination to provide supplemental dose might be influenced by personal experience, causing bias to the results. We only enrolled patients with few comorbidities. This limited the generalizability of our results, for example, to aged or critically ill patients.
Conclusion
Ciprofol was not inferior to propofol when used for sedation in adult patients undergoing ambulatory gynecological procedures. Ciprofol sedation required longer time during induction and recovery, although clinically acceptable, but produced less injection pain and adverse events.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Committee of Peking University First Hospital and other participating centers. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JX: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. MY: Investigation, Writing – original draft. YZ: Investigation, Writing – original draft. X-HZ: Investigation, Writing – original draft. J-HR: Investigation, Writing – original draft. ZX: Investigation, Writing – original draft. H-HX: Investigation, Writing – original draft. Y-HY: Investigation, Writing – original draft. M-JX: Investigation, Writing – original draft. WC: Resources, Writing – original draft. D-XW: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study received funding from Haisco Pharmaceutical Group Co., Ltd. The open access fee was also paid by Haisco Pharmaceutical Group Co., Ltd.
Conflict of interest
The authors declare that this study received funding from Haisco Pharmaceutical Group Co., Ltd. The funder had the following involvement in the study: data collection, analysis and draft writing.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1360508/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Variations of MAP during sedation and recovery period.
SUPPLEMENTARY FIGURE S2 | Variations of HR during sedation and recovery period.
SUPPLEMENTARY FIGURE S3 | Variations of SpO2 during sedation and recovery period.
Footnotes
References
1. Qin, L, Ren, L, Wan, S, Liu, G, Luo, X, Liu, Z, et al. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. (2017) 60:3606–17. doi: 10.1021/acs.jmedchem.7b00254
2. Bian, Y, Zhang, H, Ma, S, Jiao, Y, Yan, P, Liu, X, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. (2021) 87:93–105. doi: 10.1111/bcp.14363
3. Hu, C, Ou, X, Teng, Y, Shu, S, Wang, Y, Zhu, X, et al. Sedation effects produced by a ciprofol initial infusion or bolus dose followed by continuous maintenance infusion in healthy subjects: a phase 1 trial. Adv Ther. (2021) 38:5484–500. doi: 10.1007/s12325-021-01914-4
4. Teng, Y, Ou, MC, Wang, X, Zhang, WS, Liu, X, Liang, Y, et al. Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open-label, single-arm dose-escalation phase 1 study. Am J Transl Res. (2021) 13:13791–802.
5. Zhu, Q, Luo, Z, Wang, X, Wang, D, Li, J, Wei, X, et al. Efficacy and safety of ciprofol versus propofol for the induction of anesthesia in adult patients: a multicenter phase 2a clinical trial. Int J Clin Pharm. (2023) 45:473–82. doi: 10.1007/s11096-022-01529-x
6. Teng, Y, Ou, M, Wang, X, Zhang, W, Liu, X, Liang, Y, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. (2021) 164:105904. doi: 10.1016/j.ejps.2021.105904
7. Liu, Y, Yu, X, Zhu, D, Zeng, J, Lin, Q, Zang, B, et al. Safety and efficacy of ciprofol vs. propofol for sedation in intensive care unit patients with mechanical ventilation: a multi-center, open label, randomized, phase 2 trial. Chin Med J. (2022) 135:1043–51. doi: 10.1097/CM9.0000000000001912
8. Li, J, Wang, X, Liu, J, Wang, X, Li, X, Wang, Y, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. (2022) 131:138–48. doi: 10.1111/bcpt.13761
9. Luo, Z, Tu, H, Zhang, X, Wang, X, Ouyang, W, Wei, X, et al. Efficacy and safety of HSK3486 for anesthesia/sedation in patients undergoing fiberoptic bronchoscopy: a multicenter, double-blind, propofol-controlled, randomized, phase 3 study. CNS Drugs. (2022) 36:301–13. doi: 10.1007/s40263-021-00890-1
10. Wang, X, Wang, X, Liu, J, Zuo, YX, Zhu, QM, Wei, XC, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. (2022) 26:1607–17. doi: 10.26355/eurrev_202203_28228
11. Chen, BZ, Yin, XY, Jiang, LH, Liu, JH, Shi, YY, and Yuan, BY. The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study. BMC Anesthesiol. (2022) 22:245. doi: 10.1186/s12871-022-01782-7
12. Zeng, Y, Wang, DX, Lin, ZM, Liu, J, Wei, XC, Deng, J, et al. Efficacy and safety of HSK3486 for the induction and maintenance of general anesthesia in elective surgical patients: a multicenter, randomized, open-label, propofol-controlled phase 2 clinical trial. Eur Rev Med Pharmacol Sci. (2022) 26:1114–24. doi: 10.26355/eurrev_202202_28101
13. Liang, P, Dai, M, Wang, X, Wang, D, Yang, M, Lin, X, et al. Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anaesthesia: a multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial. Eur J Anaesthesiol. (2023) 40:399–406. doi: 10.1097/EJA.0000000000001799
14. Li, X, Yang, D, Li, Q, Wang, H, Wang, M, Yan, P, et al. Safety, pharmacokinetics, and pharmacodynamics of a single bolus of the γ-aminobutyric acid (GABA) receptor potentiator HSK3486 in healthy Chinese elderly and non-elderly. Front Pharmacol. (2021) 12:735700. doi: 10.3389/fphar.2021.735700
15. Hu, Y, Li, X, Liu, J, Chen, H, Zheng, W, Zhang, H, et al. Safety, pharmacokinetics and pharmacodynamics of a novel γ-aminobutyric acid (GABA) receptor potentiator, HSK3486, in Chinese patients with hepatic impairment. Ann Med. (2022) 54:2757–68. doi: 10.1080/07853890.2022.2129433
16. Qin, K, Qin, WY, Ming, SP, Ma, XF, and Du, XK. Effect of ciprofol on induction and maintenance of general anesthesia in patients undergoing kidney transplantation. Eur Rev Med Pharmacol Sci. (2022) 26:5063–71. doi: 10.26355/eurrev_202207_29292
17. Austin, PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
18. Arena, A, Orsini, B, Degli Esposti, E, Manzara, F, Ambrosio, M, Raimondo, D, et al. The unbearable burden of endometriosis: results from a large cohort about anxiety reduction during the first outpatient evaluation. J Psychosom Res. (2021) 147:110512. doi: 10.1016/j.jpsychores.2021.110512
19. Tenório, PJ, Katz, L, and Amorim, MMR. Symptoms of anxiety and depression in women with gestational trophoblastic disease compared to women who had a miscarriage: a cross-sectional study. J Psychosom Obstet Gynaecol. (2023) 44:2210747. doi: 10.1080/0167482X.2023.2210747
20. Burton, FM, Lowe, DJ, Millar, JE, Corfield, AR, Watson, MJ, Shaw, M, et al. Effect of target-controlled propofol infusion to reduce the incidence of adverse events for procedural sedation in the emergency department: a systematic review. Eur J Emerg Med. (2020) 27:253–9. doi: 10.1097/MEJ.0000000000000655
21. Wu, F, Zhan, L, Xu, W, and Bian, J. Effect of intravenous lidocaine on outcomes in patients receiving propofol for gastrointestinal endoscopic procedures: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. (2023) 80:39–52. doi: 10.1007/s00228-023-03589-y
22. Chen, L, Xie, Y, Du, X, Qin, W, Huang, L, Dai, J, et al. The effect of different doses of ciprofol in patients with painless gastrointestinal endoscopy. Drug Des Devel Ther. (2023) 17:1733–40. doi: 10.2147/DDDT.S414166
23. Yao, Y, Guan, J, Liu, L, Fu, B, Chen, L, and Zheng, X. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomised, double-blind trial. Eur J Anaesthesiol. (2022) 39:911–7. doi: 10.1097/EJA.0000000000001715
24. Xing, J, Liang, L, Zhou, S, Luo, C, Cai, J, and Hei, Z. Intravenous lidocaine alleviates the pain of propofol injection by local anesthetic and central analgesic effects. Pain Med. (2018) 19:598–607. doi: 10.1093/pm/pnx070
25. Larsen, B, Beerhalter, U, Biedler, A, Brandt, A, Doege, F, Brün, K, et al. Less pain on injection by a new formulation of propofol? A comparison with propofol LCT. Anaesthesist. (2001) 50:842–5. doi: 10.1007/s00101-001-0234-0
26. Moon, YE, Lee, MY, and Kim, DH. Preventive effect of a vapocoolant spray on propofol-induced pain: a prospective, double-blind, randomized study. J Anesth. (2017) 31:703–8. doi: 10.1007/s00540-017-2386-3
27. Tan, CH, and Onsiong, MK. Pain on injection of propofol. Anaesthesia. (1998) 53:468–76. doi: 10.1046/j.1365-2044.1998.00405.x
28. Lan, H, Shan, W, Wu, Y, Xu, Q, Dong, X, Mei, P, et al. Efficacy and safety of ciprofol for sedation/anesthesia in patients undergoing hysteroscopy: a randomized, parallel-group, controlled trial. Drug Des Devel Ther. (2023) 17:1707–17. doi: 10.2147/DDDT.S414243
29. Nathanson, MH, Gajraj, NM, and Russell, JA. Prevention of pain on injection of propofol: a comparison of lidocaine with alfentanil. Anesth Analg. (1996) 82:469–71. doi: 10.1097/00000539-199603000-00006
30. Tian, S, Zhang, D, Zhou, W, Tan, C, Shan, Q, Ma, R, et al. Median effective dose of lidocaine for the prevention of pain caused by the injection of propofol formulated with medium- and long-chain triglycerides based on lean body weight. Pain Med. (2021) 22:1246–52. doi: 10.1093/pm/pnaa316
Keywords: ciprofol, propofol, sedation, outpatient, gynecological procedure
Citation: Xu J, Yang M, Zeng Y, Zou X-H, Ren J-H, Xia Z, Xie H-H, Yu Y-H, Xu M-J, Chen W and Wang D-X (2024) Efficacy and safety of ciprofol for sedation in outpatient gynecological procedures: a phase III multicenter randomized trial. Front. Med. 11:1360508. doi: 10.3389/fmed.2024.1360508
Edited by:
Somchai Amornyotin, Mahidol University, ThailandReviewed by:
Abhijit Nair, Ministry of Health, OmanJesus Rico-Feijoo, Hospital Universitario Río Hortega, Spain
Luis Laranjeira, Eli Lilly, Portugal
Copyright © 2024 Xu, Yang, Zeng, Zou, Ren, Xia, Xie, Yu, Xu, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Xin Wang, d2FuZ2Rvbmd4aW5AaG90bWFpbC5jb20=; ZHh3YW5nNjVAYmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Jing Xu
Jing Xu Mengchang Yang
Mengchang Yang Yuan Zeng1
Yuan Zeng1 Zhongyuan Xia
Zhongyuan Xia Dong-Xin Wang
Dong-Xin Wang