- Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Background: Pulmonary actinomycosis (PA) is a rare type of Actinomyces infection that can be challenging to diagnose since it often mimics lung cancer.
Methods: Published case reports and case series of PA in patients with suspicion of lung cancer were considered, and data were extracted by a structured search through PubMed/Medline.
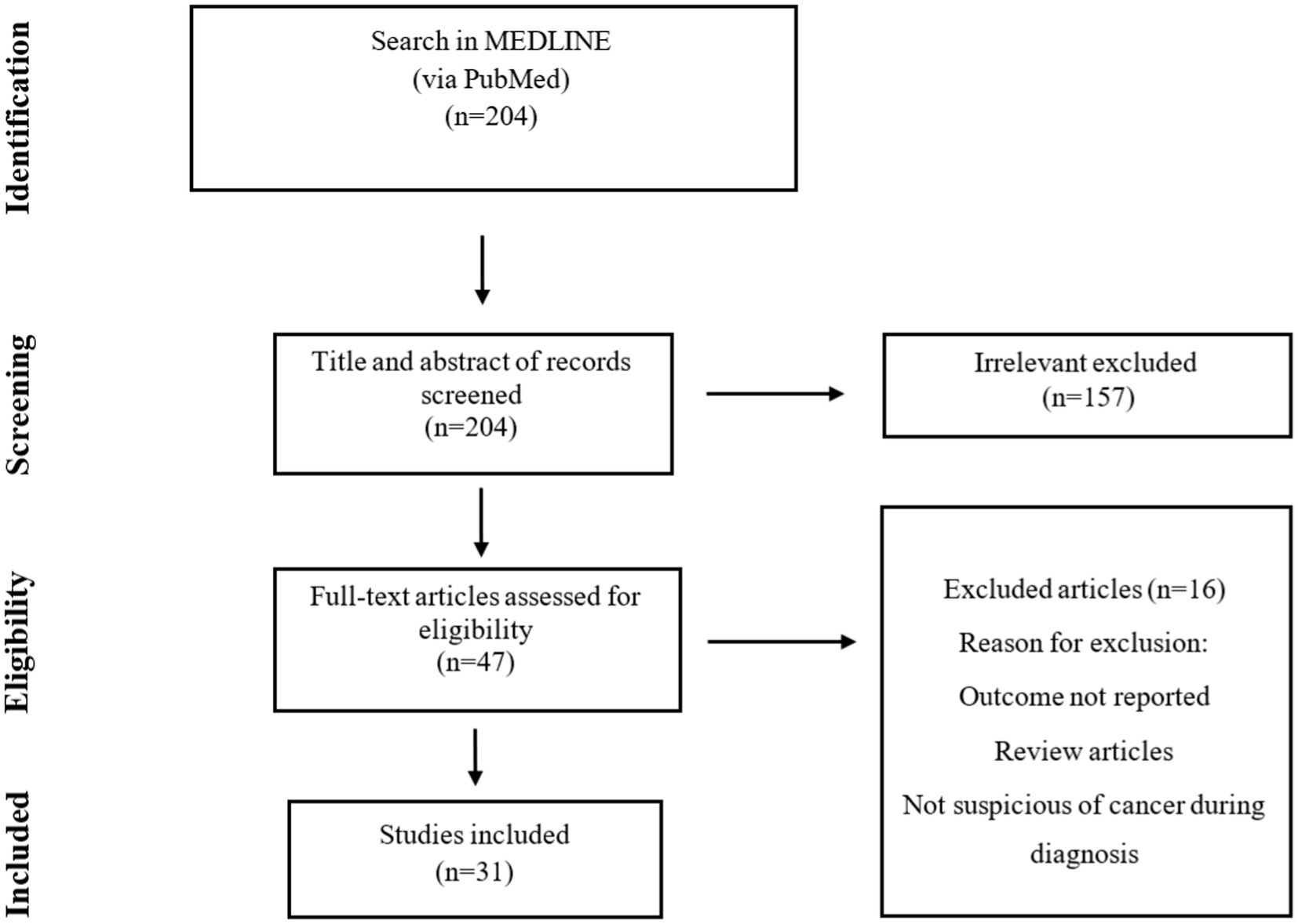
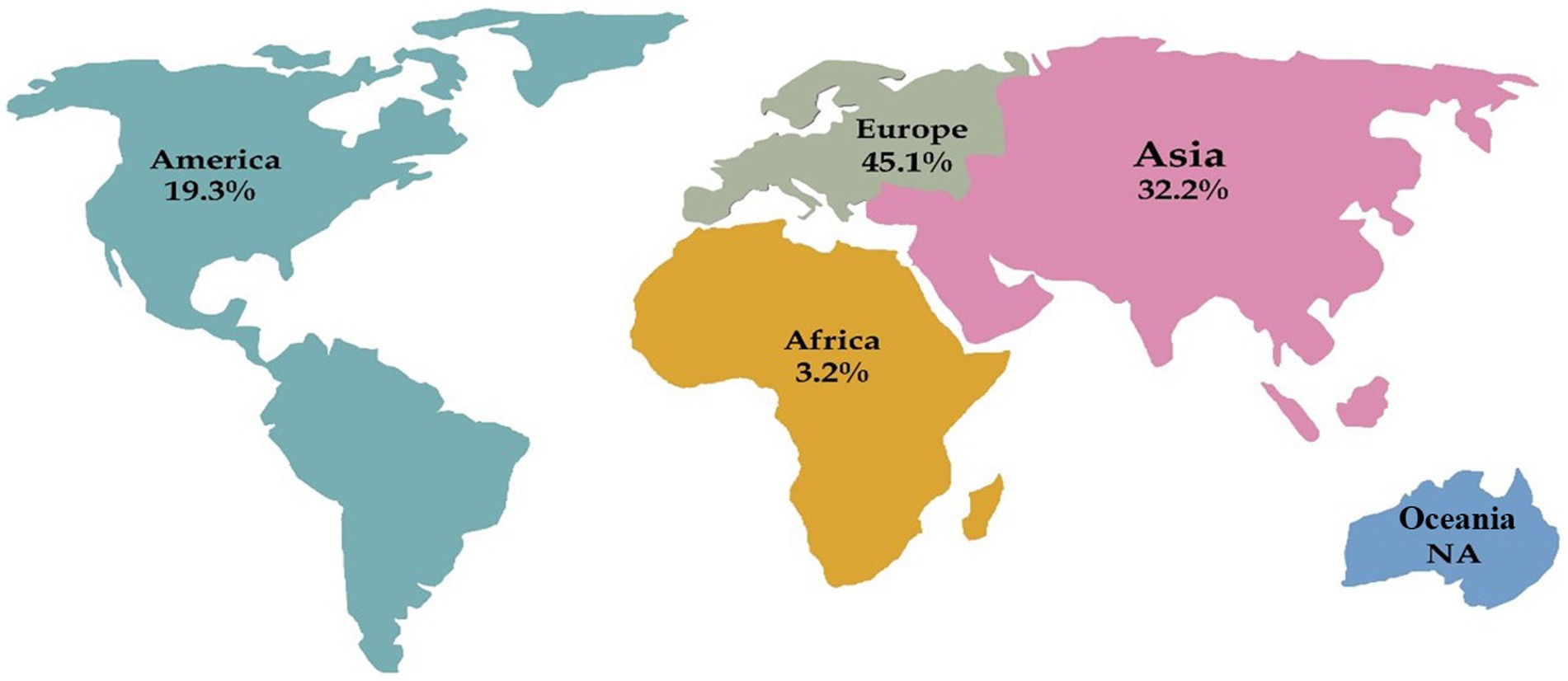
Results: After analyzing Medline, 31 studies were reviewed, from which 48 cases were extracted. Europe had the highest prevalence of reported cases with 45.1%, followed by Asia (32.2%), America (19.3%), and Africa (3.2%). The average age of patients was 58.9 years, and 75% of all patients were above 50 years old. Male patients (70%) were predominantly affected by PA. The overall mortality rate was 6.25%. In only eight cases, the causative agent was reported, and Actinomyces odontolyticus was the most common isolated pathogen with three cases. Based on histopathological examination, 75% of the cases were diagnosed, and the lobectomy was performed in 10 cases, the most common surgical intervention. In 50% of the cases, the selective antibiotics were intravenous and oral penicillin, followed by amoxicillin (29.1%), amoxicillin-clavulanic acid, ampicillin, levofloxacin, and doxycycline.
Conclusion: The non-specific symptoms resemble lung cancer, leading to confusion between PA and cancer in imaging scans. Radiological techniques are helpful but have limitations that can lead to unnecessary surgeries when confusing PA with lung cancer. Therefore, it is important to raise awareness about the signs and symptoms of PA and lung cancer to prevent undesirable complications and ensure appropriate treatment measures are taken.
Introduction
Actinomyces species are Gram-positive bacteria with anaerobic and facultative microaerophilic metabolism that typically colonize the oropharynx, urogenital tract, or gastrointestinal system (1, 2). Actinomycosis is generally considered an endogenous infection. Although the bacteria are initially colonized on the surface of the mucosa, they can reach the deeper tissues through any disruption of the mucosal barrier caused by procedures such as trauma, surgical intervention, or foreign bodies (2–4). Actinomycosis is a rare and granulomatous disease that progresses slowly and creates sinus tract fistulae in a chronic form with a slow progression that creates sinus tract fistulae in a chronic form. It has been known for more than 150 years, and the most common causative agent is Actinomyces israelii (5, 6). In recent years, the frequency of all forms of actinomycosis has decreased, possibly as a result of the enhancement of oral hygiene and antibiotic therapy upon infection suspicion (1). However, there is no solid proof to support the effectiveness of such actions in reducing the incidence of colonization and mild periodontal infection with Actinomyces species (4, 6).
The common forms of actinomycosis are cervicofacial, abdominal, pelvic, and pulmonary. Moreover, on rare occasions, the spread of local infection through hematogenous dissemination may lead to the development of actinomycotic lesions in the lungs. Despite anatomic barriers, Actinomyces can spread and eventually invade the pleura, resulting in empyema formation.
With the improvement of oral hygiene and the availability of effective antibiotics, the severity of PA manifestation has become less severe. Furthermore, if diagnosis and treatment are not performed correctly, it can spread into the chest wall and create a pleuro-cutaneous fistula and destruction of vertebrae and ribs (1, 2, 7, 8). The diagnosis of pulmonary actinomycosis (PA) is quite challenging, and the delay in diagnosis can last for 6 months. PA usually results in the formation of nodules, consolidation, or mass that can often be mistaken for lung cancer. Therefore, PA could be misdiagnosed as lung cancer, lung abscess, or tuberculosis (9, 10). Due to non-specific laboratory and clinical features, it is usually challenging to differentiate PA from lung malignancy. Moreover, the most common initial diagnosis of PA among physicians is lung cancer (10–14). The common signs and symptoms of PA are fever, chest pain, hemoptysis, shortness of breath, and a productive cough (15, 16). Furthermore, the severity of PA manifestations has become less severe with the enhancement of oral hygiene and the availability of effective antibiotics.
Dealing with PA can be challenging due to its difficult diagnosis. However, if more people are aware of this infection, it could lead to an easier diagnosis and prevent undesired complications such as unnecessary surgeries and treatment with the wrong medication. To study this, we conducted a scoping review that explored the clinical, epidemiological, diagnostic, and therapeutic features of PA cases that were initially suspected of lung cancer.
Methods
Search strategy
In the current study, a Medline search (via PubMed) was performed on 4 December 2022. The keywords were chosen from the National Library of Medicine’s Medical Subject Heading (MeSH) terms, titles, and abstracts through Boolean operators (and/or) including “Pulmonary Neoplasms” or “Lung Neoplasm” or “Lung Cancer” or “Pulmonary Cancer” or “Cancer of Lung,” and (Actinomyc*). The present study was conducted according to the PRISMA extension for scoping reviews.
Inclusion and exclusion criteria
All case reports and case series studies were included where the cancer was initially suspected in the diagnosis process, and irrelevant articles (review articles, conference abstracts, and studies with unclear results and insufficient data) were excluded.
Study selection and data extraction
The titles, abstracts, and full texts of all included studies were reviewed independently by two authors (AKH and SHM). The search was limited to English-published studies, and any disagreements among authors were resolved through discussion and consensus. The data extracted from each study included the first author’s name, publication year, country, sex, age, Actinomyces species, treatment, surgery or puncture drainage for biopsy, diagnosis method, radiologic finding, patient outcome, and additional findings.
Results
Epidemiology
Our search of the Medline database yielded a total of 204 hits, we reached 31 studies, of which 48 cases were included in the final analysis (Figure 1). These cases were reported from Poland, Malaysia, India, Korea, Germany, Italy, and the Netherlands (one study), China, Spain, and Turkey (two studies), Japan and Greece (four studies), and the USA (six studies). Furthermore, there were four case series from Japan, Italy, Germany, and Tunisia (one study). Accordingly, Europe had the highest share of reported studies with 45.1% (14 studies), followed by Asia with 32.2% (10 studies), America with 19.3% (6 studies), and Africa with 3.2% (one study). No cases were identified from Oceania (Figure 2).
Outcome and etiology
Overall, PA showed low mortality, and only three patients died. A 59-year-old female developed lung adenocarcinoma following an initial PA diagnosis. The patient died eventually after chemotherapy failure. In another 50-year-old male patient, recovery happened, although, after 1.5 years, the patient died from a massive gastrointestinal hemorrhage. The third patient was an 83-year-old male who died despite antibiotic treatment with penicillin.
Our results showed that only 25 and 70% of the patients were female and male, respectively, and in two cases, sex was not reported. The mean age of patients was 58.9, within the range of 36–86, and 75% of all patients were above 50 years old (Tables 1, 2).
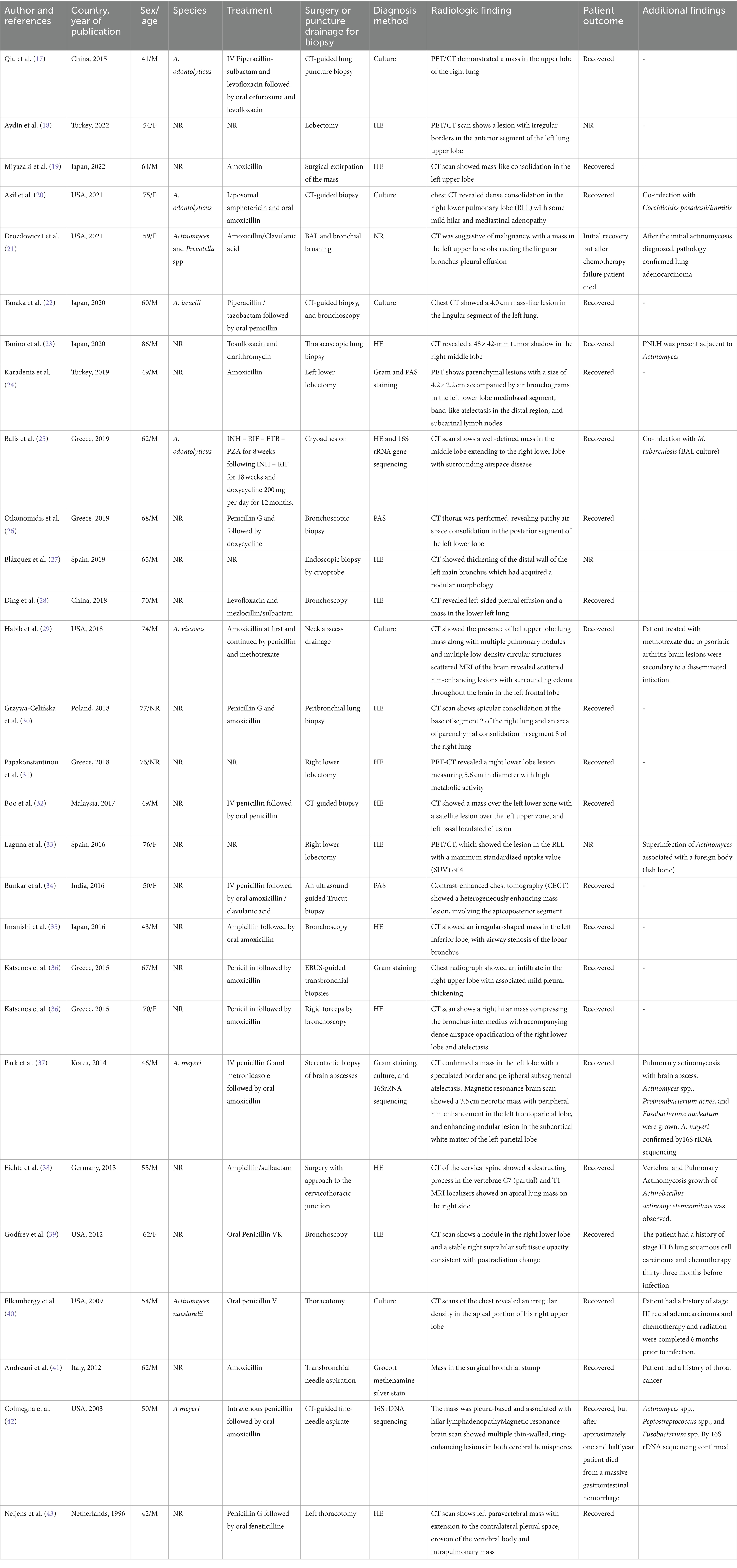
Table 1. Epidemiological, clinical, diagnosis, and therapeutic features of patients with pulmonary actinomycosis from individual case reports.
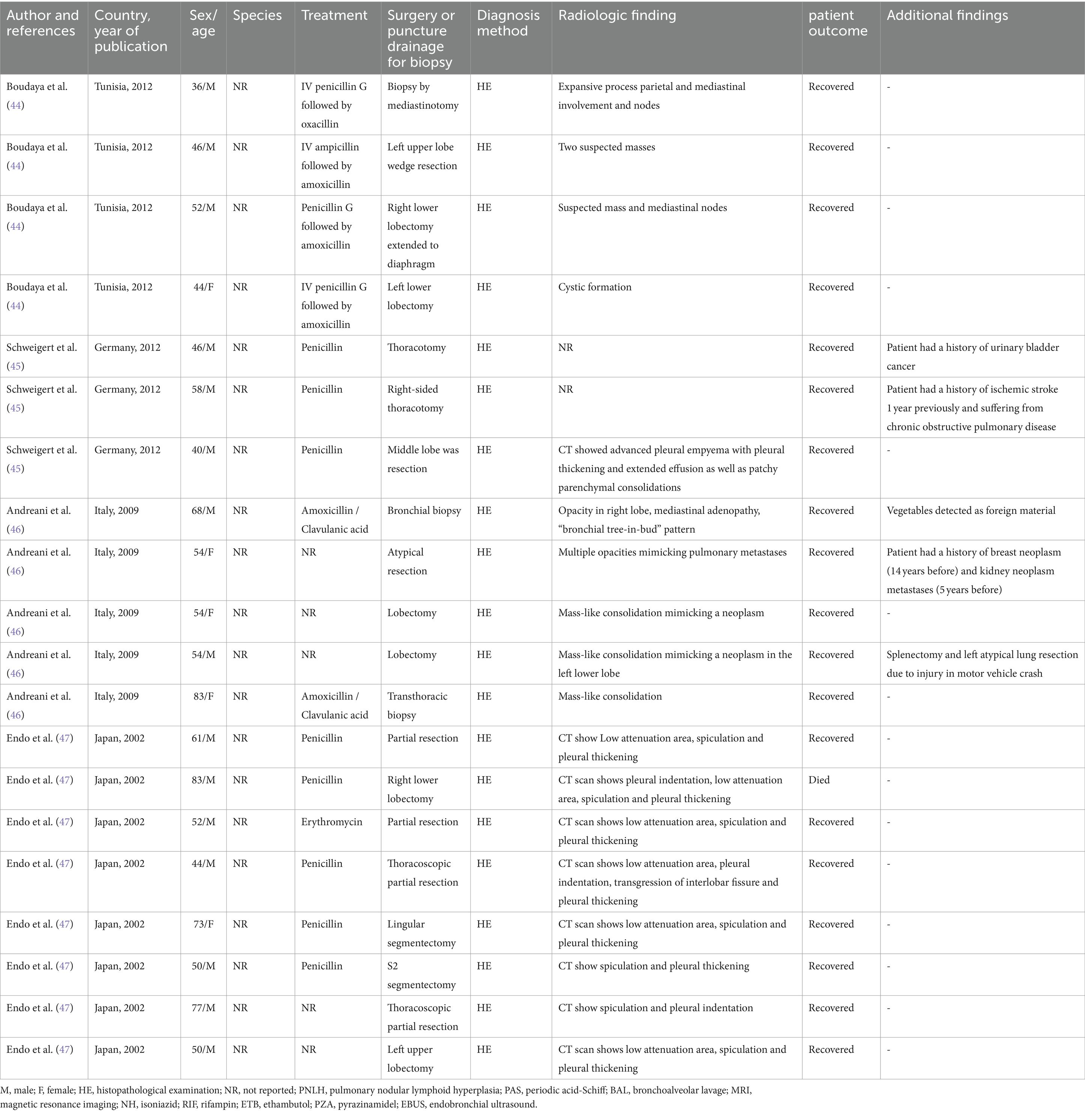
Table 2. Epidemiological, clinical, diagnosis, and therapeutic features of patients with pulmonary actinomycosis from individual case series.
Several patients in the study had various medical conditions in addition to PA. In one patient, pulmonary nodular lymphoid hyperplasia (PNLH) was diagnosed adjacent to the Actinomyces lesion, and lung adenocarcinoma was detected in one patient after PA diagnosis. Moreover, one of the patients had a history of treatment for lung squamous cell carcinoma, 33 months before the PA diagnosis. Another case had a history of treatment for rectal adenocarcinoma 6 months before the infection. Moreover, there were two patients with a history of throat and urinary bladder cancer, as well as one patient with a history of breast neoplasm (14 years before the infection) and kidney neoplasm metastases (5 years before the infection). Another patient had a history of treatment with methotrexate as well as a brain lesion following the disseminated infection. One patient had PA with a brain abscess. One patient tested positive for tuberculosis by culture of bronchoalveolar lavage fluid. Furthermore, another patient was involved in vertebral and PA with the growth of Actinobacillus actinomycetemcomitans. Additionally, in one patient, co-infection with Coccidioides posadasii/immitis was reported.
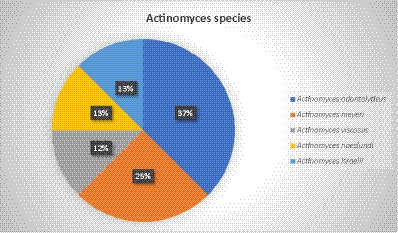
In addition to Actinomyces, Prevotella spp., Propionibacterium acnes, Fusobacterium nucleatum, Peptostreptococcus spp., and Fusobacterium spp. were also found in some patients. One patient had an ischemic stroke 1 year before the infection and suffered from chronic obstructive pulmonary disease. Another patient who was involved in a motor vehicle crash underwent splenectomy and atypical lung resection. The Actinomyces infection seemed to be related to foreign bodies, such as fish bones and vegetables, which were found in two patients. Most of the patients (79%) with PA were immunocompetent. Accordingly, in 83% of the cases, Actinomyces at the species level were not detected, and species identification was reported in only 8 cases. The most reported species was Actinomyces odontolyticus, with three cases, followed by Actinomyces meyeri, with two cases. Actinomyces viscosus, Actinomyces naeslundii, and Actinomyces israelii were found in only one case each. Culture was the most common detection method, while 16SrRNA sequencing was performed in two cases and 16SrDNA sequencing was used in one case (Figure 3).
Diagnosis method
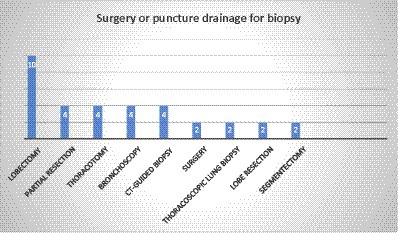
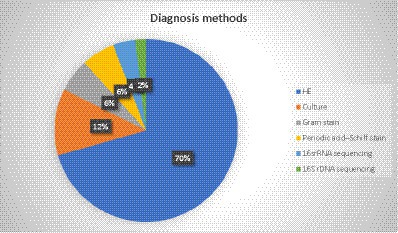
In most of the cases (75%, 36 out of 48), the diagnosis was based on the histopathologic examination (HE) of different types of specimens. The most common specimen was a lobectomy (10 cases), while partial resection, thoracotomy, bronchoscopy, and CT-guided biopsy were reported in 4 cases each. Moreover, surgery, thoracoscopic lung biopsy, lobe resection, and segmentectomy were observed in two cases (Figure 4). In two patients with brain involvement, a stereotactic biopsy of brain abscesses and neck abscess drainage was performed.
The bacterial culture was diagnostic only in 12.5% of the patients (six cases), as Actinomyces species are difficult to grow. Gram staining and periodic acid–Schiff stain were also reported in three cases, 16SrRNA sequencing in two cases, and 16SrDNA sequencing was reported in one case. Additionally, the diagnosis method was not reported in one case. Figure 5 represents the different methods used to diagnose PA.
Treatment
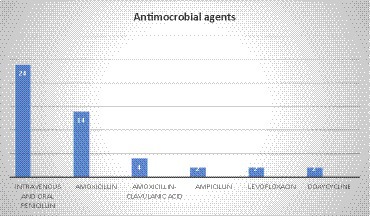
In a total of 48 cases, the antimicrobial treatment was not mentioned in 9 cases. In the majority of cases (50%, 24 cases), treatment was administered via both intravenous and oral penicillin. Amoxicillin was the second most common agent with 29.1% (14 cases, 29.1%), followed by amoxicillin–clavulanic acid (4 cases), and ampicillin, levofloxacin, and doxycycline, each with 2 cases. Additionally, feneticilline, erythromycin, oxacillin, ampicillin/sulbactam, piperacillin–sulbactam, mezlocillin /sulbactam, metronidazole, tosufloxacin, clarithromycin, cefuroxime, and piperacillin /tazobactam were each reported in one case (Figure 6).
In another case, a patient had a co-infection with Coccidioides posadasii/immitis. The treatment plan consisted of liposomal amphotericin and oral amoxicillin, which proved to be successful in leading to the patient’s recovery. Another patient had a co-infection with Mycobacterium tuberculosis, and treatment was started with rifampin, ethambutol, pyrazinamide, and isoniazid for 8 weeks. It was followed by rifampin and isoniazid for 18 weeks and doxycycline for 12 months.
Discussion
PA is a rare actinomycosis disease with a slow-progressing form of pulmonary infection, with a prevalence of 15% in all actinomycosis cases. This infection is often associated with the aspiration of oropharyngeal or gastrointestinal secretions (31, 48). It can involve both sexes and any age, but our findings showed that most of the infected patients were men and that 75% of patients were over 50 years of age. This is in correlation with other studies declaring that PA was more common in male patients and that the peak incidence of infection reached in the fourth to fifth decades of age (12, 24, 48). The high incidence of PA in male patients could be partly related to poorer oral hygiene and the occurrence of more facial trauma (9, 48).
The findings of a chest computed tomography scan (CT scan) of actinomycosis are non-specific and resemble necrotic lung malignancy. This condition is characterized by chronic segmental airspace consolidation with low-attenuation areas that have peripheral enhancement (7, 10, 49). Additionally, cavitations, shadowing, and pleural effusion with cavitary lesions are also typical features of PA that can be misdiagnosed as tuberculosis (28). Similarly, our results revealed that in most of the cases, CT scans were useful for diagnosis, but not always conclusive. The scan showed air space consolidation, mass in the middle lobes that could be misinterpreted as malignancy, pleural empyema, and opacity in the lobes, which could mimic pulmonary metastases. Moreover, positron emission tomography-computed tomography (PET-CT) is a helpful imaging technique to differentiate benign lesions from malignant ones, but there is limited information on PET-CT findings about PA (10, 50).
This technique was used in five cases and showed lesions and masses in lung lobes. Nevertheless, there is minimal information about PET-CT findings on PA. This diagnostic method has also encountered some issues. According to Choi et al., PET-CT is not an ideal tool for the differentiation of PA from lung cancer because of its high fluorodeoxyglucose (FDG) uptake. Consequently, physicians may mistake the high FDG uptake in favor of lung malignancy over PA. Therefore, clinicians must carefully evaluate the need for lung resection surgery when PA is suspected (17, 41, 51).
Some pulmonary infections, such as tuberculosis, aspergilloma, and histoplasmosis, can create false positive results due to their high metabolic uptake. Furthermore, FDG uptake has been observed in actinomycosis, leading to a mimicry of pulmonary malignancies (10, 24, 45, 52).
Although the diagnosis of PA could be delayed, our results showed that the mortality rate was only 6.25% and the overall outcome was acceptable. Similarly, a recent study in China showed that 75.9% of patients fully recovered, while another study in Korea reported a 98% recovery rate among 94 patients with PA (12). Therefore, PA seemed to have a good prognosis with a low rate of mortality because of antibiotic treatment and surgical intervention.
Actinomycosis might coexist with lung cancer, making the diagnosis even harder (9). Among the patients we studied, six had a history of cancer, and two had lung cancer. Although actinomycosis is unusual in immunosuppressed patients, immune system abnormalities may be a facilitating factor for the development of infection. However, the exact relationship between the two conditions is not yet fully understood (53). Interestingly, we found three cases in our research where PA involvement was diagnosed after cancer treatment (54–56). In one of the cases, the patient has been treated with bevacizumab for advanced non-small-cell lung cancer. After 36 months of bevacizumab maintenance, the patient was diagnosed with actinomycosis in the right lung. Bevacizumab was discontinued, and the patient was treated with amoxicillin–clavulanic acid. Unfortunately, the patient passed away after 3 months (54). The diagnostic method was culture in only 12% of the cases. Currently, positive culture in PA is rare due to the challenges of culturing anaerobic bacteria. Previous antibiotic treatments and bacterial overgrowth can also complicate matters. In addition, the evidence suggests that using normal saline, which is usually used for bronchoalveolar lavage, can prevent Actinomyces growth (9, 45, 57, 58). On the other hand, isolating Actinomyces may be crucial to distinguishing nocardiosis or botryomycosis from actinomycosis, which is usually difficult to differentiate morphologically. As a result, the direct culture of biopsy material in both aerobic and anaerobic blood culture media can improve culture sensitivity (59). The accurate diagnosis of PA depends on HE, as radiologic imaging and culture may not be conclusive. Without histological or microbiological confirmation, misdiagnosis can be fairly common (48, 60). However, sulfur granules in biopsy can be essential and suggestive, but not specific. On the other hand, when a small amount of tissue is biopsied, sulfur granules can be missed (60, 61). Nevertheless, granulomas and multinucleated giant cells can be observed in some cases. These morphological shapes are not specific, and other pathogens such as Nocardia spp. and some fungal and parasitic infections can cause similar observations. Furthermore, Grocott methenamine silver staining can identify the branching microorganism that is specific for the existence of actinomycosis infection (6, 62, 63).
Furthermore, surgical intervention may be necessary for diagnosis and treatment if lung cancer cannot be ruled out (31, 47). Endo et al. declared that a conclusive differential diagnosis between PA and necrotic lung cancer might be possible only when the surgical restriction specimen is sent for HE (47).
Furthermore, it has been shown that surgery can be avoided in most cases of thoracic actinomycosis, and long-term intravenous penicillin therapy leads to a good prognosis. However, early surgical intervention may lead to equally good or better outcomes by shortening the antibiotic therapy period (13, 64).
Our results showed that in 75% of the cases diagnosed with HE, and similarly, in 94 cases in Korea, all PA patients were diagnosed with HE (12). Altogether, HE is an essential method for the correct diagnosis of PA (17). Moreover, the new approach of using molecular methods in diagnosis can be helpful in the detection of PA, as in one case, HE and 16SrRNA sequencing were used together for diagnosis. This was a complicated case, and the patient had a co-infection with PA and tuberculosis. The molecular method also led to the identification of a bacterial species, which was A. odontolyticus.
Moreover, 16SrRNA sequencing helped the diagnosis of A. meyeri in a complicated case of PA involvement with a brain abscess. In another case, the diagnosis made by 16SrDNA demonstrated that A. meyeri was a causative agent of PA. Furthermore, 16SrRNA is a component of the 30S ribosomal subunit in prokaryotic cells, and it is transcribed as a single-stranded ribosomal RNA molecule. On the other hand, the 16SrDNA is the gene that encodes the 16SrRNA, and it consists of double-stranded chromosomal DNA. 16SrRNA sequencing is used to detect and identify bacterial pathogens in clinical specimens from patients with a suspicion of infection. 16SrDNA is applied to identify microorganisms and determine microbial communities.
Recently, molecular techniques, including 16SrRNA sequencing, have been used to reach fast and precise results in reference or research laboratories, and such methods are now recommended in challenging conditions such as PA infection.
Furthermore, our results showed that beta-lactam antibiotics were used in the majority of cases (91%) as a selective drug, with intravenous and oral penicillin being used in half of the cases, followed by amoxicillin, amoxicillin–clavulanic acid, and ampicillin. This result is predictable, as antibiotic resistance is not considered a problem in actinomycosis.
Usually, Actinomyces spp. are susceptible to beta-lactams, and in particular, penicillin G and amoxicillin are considered the desirable drugs for actinomycosis treatment. Since Actinomyces spp. do not produce beta-lactamases, combining amoxicillin with beta-lactam inhibitors such as clavulanic acid is not usually necessary unless there are co-pathogens such as Enterobacteriaceae presumed in the infection (3, 65, 66).
Furthermore, in a retrospective analysis from China, 46% of the cases were treated with penicillin G (60). A recent study was conducted in Turkey on 37 PA patients, and it was reported that most cases (73%) were treated with penicillin G and ampicillin-sulbactam, 13% with cefuroxime and ceftriaxone, and 5.4% with clarithromycin, levofloxacin, and moxifloxacin (1). Contrastingly, ampicillin/sulbactam was used only in one case. Cefuroxime, erythromycin, and clarithromycin were used in one case. Additionally, macrolides are considered useful alternatives (65).
Furthermore, the presence of A. actinomycetemcomitans, Prevotella spp., P. acnes, F. nucleatum, Peptostreptococcus spp., and Fusobacterium spp. alongside Actinomyces spp. was reported in some cases. It seems that treatment with beta-lactam agents can effectively lead to the successful treatment of PA. However, it is important to note that metronidazole has no in vitro activity against Actinomyces (65). Therefore, combination therapy with penicillin G was observed in one case of PA, where P. acnes and F. nucleatum were grown simultaneously. Hoca et al. reported the combination of metronidazole with other antibiotics in four cases of PA with co-infection (1).
Treatment with piperacillin–tazobactam was observed in only one case and could be related to the fact that although piperacillin–tazobactam, meropenem, and imipenem are considered active against Actinomyces spp., their use should be limited to prevent the acquisition of resistant flora, as they have broad-spectrum effects (65). Interestingly, feneticilline, which is not approved, was used in one case from the Netherlands after initial treatment with penicillin G. Finally, antibiotic therapy is administered for a prolonged duration because of the chance of recurrence in PA. Patients with no surgical intervention and a shorter period of 3 months of antibiotic therapy are at a higher risk of recurrence (13, 58, 67). Furthermore, no study currently suggests the period for follow-up of recurrent infection, although some studies suggested 3 months, 6 months, and 1-year follow-ups (9, 60, 67, 68). In summary, the treatment duration should be implemented in each case based on the main factors, such as severity and possible changes in the follow-up imaging.
Limitations
In the current study, we only used available studies on PubMed/Medline, and only English studies were included. Therefore, the relevant publications decreased. Additionally, discussing the bias, risks, and individual limitations in the studies was not possible, as they were not reported.
Conclusion
PA is a rare form of infection that is challenging to diagnose due to its non-specific symptoms, failure to detect pathogens, and resemblance to lung cancer. Although it can show similar imagining results as malignancy, which should be differentiated by the presence of nodules, in the sinus tract on the chest wall. Radiological techniques can be helpful in diagnosing PA but have their limitations. The limited available information about PA means that it can be easily confused with other diseases, leading to unnecessary surgeries.
Therefore, clinicians should be aware of the overlapping of signs and symptoms between PA and lung cancer. As antibiotic therapy may be adequate to treat this lung infection, biopsy specimen and histopathological examination should be considered before any surgical operation (i.e., lobectomy).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AK: Writing – original draft, Writing – review & editing. NA: Writing – review & editing. SM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hoca, N, Berktaş, M, Söyler, Y, Celep, C, and Tanrıkulu, F. Clinical features and treatment outcomes of pulmonary actinomycosis. Eur Rev Med Pharmacol Sci. (2022) 26:8064–72. doi: 10.26355/eurrev_202211_30160
2. Könönen, E, and Wade, WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. (2015) 28:419–42. doi: 10.1128/CMR.00100-14
3. Wong, VK, Turmezei, T, and Weston, V. Actinomycosis. BMJ. (2011) 343:d6099. doi: 10.1136/bmj.d6099
4. Smego, RA Jr, and Foglia, G. Actinomycosis. Clin Infect Dis. (1998) 26:1255–61. doi: 10.1086/516337
5. Joshi, V, Koulaouzidis, A, McGoldrick, S, Tighe, M, and Tan, C. Actinomycotic liver abscess: a rare complication of colonic diverticular disease. Ann Hepatol. (2010) 9:96–8. doi: 10.1016/S1665-2681(19)31688-6
6. Chegini, Z, Didehdar, M, Tabaeian, SP, Khoshbayan, A, and Shariati, A. A systematic review of case reports of hepatic actinomycosis. Orphanet J Rare Dis. (2021) 16:1–13. doi: 10.1186/s13023-021-01821-5
7. Kim, TS, Han, J, Koh, W-J, Choi, JC, Chung, MJ, Lee, JH, et al. Thoracic actinomycosis: CT features with histopathologic correlation. Am J Roentgenol. (2006) 186:225–31. doi: 10.2214/AJR.04.1749
8. Yung, BC, Cheng, JC, Chan, TT, Loke, TK, Lo, J, and Lau, PY. Aggressive thoracic actinomycosis complicated by vertebral osteomyelitis and epidural abscess leading to spinal cord compression. Spine. (2000) 25:745–8. doi: 10.1097/00007632-200003150-00017
9. Mabeza, GF, and Macfarlane, J. Pulmonary actinomycosis. Eur Respir J. (2003) 21:545–51. doi: 10.1183/09031936.03.00089103
10. Choi, H, Lee, H, Jeong, SH, Um, S-W, Kown, OJ, and Kim, H. Pulmonary actinomycosis mimicking lung cancer on positron emission tomography. Ann Thoracic Med. (2017) 12:121–4. doi: 10.4103/1817-1737.203752
11. Choi, J, Koh, W-J, Kim, TS, Lee, KS, Han, J, Kim, H, et al. Optimal duration of IV and oral antibiotics in the treatment of thoracic actinomycosis. Chest. (2005) 128:2211–7. doi: 10.1016/S0012-3692(15)52624-X
12. Kim, SR, Jung, LY, Oh, I-J, Kim, Y-C, Shin, K-C, Lee, MK, et al. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis. (2013) 13:1–8. doi: 10.1186/1471-2334-13-216
13. Song, J-U, Park, HY, Jeon, K, Um, S-W, Kwon, OJ, and Koh, W-J. Treatment of thoracic actinomycosis: a retrospective analysis of 40 patients. Ann Thoracic Med. (2010) 5:80–5. doi: 10.4103/1817-1737.62470
14. Park, JY, Lee, T, Lee, H, Lim, H-J, Lee, J, Park, JS, et al. Multivariate analysis of prognostic factors in patients with pulmonary actinomycosis. BMC Infect Dis. (2014) 14:1–7. doi: 10.1186/1471-2334-14-10
15. Machi, T. Chest pain with induration of the chest wall. Postgrad Med J. (1998) 74:283–5. doi: 10.1136/pgmj.74.871.283
16. Petroianni, A, Conti, V, and Terzano, C. A thoracic mass infiltrating the chest wall. Eur Rev Med Pharmacol Sci. (2011) 15:345–8.
17. Qiu, L, Lan, L, Feng, Y, Huang, Z, and Chen, Y. Pulmonary actinomycosis imitating lung cancer on 18F-FDG PET/CT: a case report and literature review. Korean J Radiol. (2015) 16:1262–5. doi: 10.3348/kjr.2015.16.6.1262
18. Aydin, Y, Arslan, R, and Filik, M. Pulmonary actinomycosis mimicks lung cancer. Rev Soc Bras Med Trop. (2022) 55:e0195. doi: 10.1590/0037-8682-0195-2022
19. Miyazaki, S, Fujito, T, Kondo, Y, Kuno, Y, Mori, S, Yamashita, R, et al. Pulmonary actinomycosis mimicking lung cancer on 18F-fluorodeoxyglucose positron emission tomography: a case report. J Med Case Rep. (2022) 16:1–5. doi: 10.1186/s13256-022-03481-w
20. Asif, AA, Roy, M, and Ahmad, S. More than valley fever: pulmonary Actinomycosis and Coccidioidomycosis co-infection in a patient. European J Case Rep Intern Med. (2021) 8:2664. doi: 10.12890/2021_002664
21. Drozdowicz, K, Marquez, HA, Burks, EJ, and Suzuki, K. Lung adenocarcinoma and pulmonary actinomycosis: a cautionary tale. Tumori J. (2021) 107:NP77–80. doi: 10.1177/03008916211010225
22. Tanaka, S, Araki, H, Yoshizako, T, Kitagaki, H, and Isobe, T. Pulmonary actinomycosis mimicking pulmonary cancer on fluorine-18 fluorodeoxyglucose PET-CT. Cureus. (2020) 12:12306. doi: 10.7759/cureus.12306
23. Tanino, A, Tsubata, Y, Hamaguchi, S, Sutani, A, Nagase, M, and Isobe, T. Antibiotic-induced reduction of abnormal lung shadow in pulmonary nodular lymphoid hyperplasia. Respirol Case Rep. (2020) 8:e00522. doi: 10.1002/rcr2.522
24. Karadeniz, G, Polat, G, Ucsular, F, and Yalnız, E. A difficult disease to diagnosis: pulmonary actinomycosis. Clin Respir J. (2020) 14:416–8. doi: 10.1111/crj.13136
25. Balis, E, Kakavas, S, Kompogiorgas, S, Kotsifas, K, and Boulbasakos, G. Presentation of pulmonary tuberculosis and Actinomyces co-infection as a lung mass: a literature review and unique case report. Monaldi Arch Chest Dis. (2019) 89:1180. doi: 10.4081/monaldi.2019.1180
26. Oikonomidis, P, Fousekis, F, Kotsaftis, P, Pilios, I, Dimas, D, and Giannoulis, G. A case of pulmonary actinomycosis presented with endobronchial involvement. Respir Med Case Rep. (2019) 28:100930. doi: 10.1016/j.rmcr.2019.100930
27. Posadas Blázquez, TJ, Ferrando Gabarda, JR, and Briones, GA. Bronchopulmonary Actinomycosis mimicking lung Cancer. Arch Bronconeumol (Engl Ed). (2020) 56:522. doi: 10.1016/j.arbres.2019.07.007
28. Ding, X, Sun, G, Fei, G, Zhou, X, Zhou, L, and Wang, R. Pulmonary actinomycosis diagnosed by transbronchoscopic lung biopsy: a case report and literature review. Exp Ther Med. (2018) 16:2554–8. doi: 10.3892/etm.2018.6483
29. Habib, S, Siddiqui, AH, Azam, M, Siddiqui, F, and Chalhoub, M. Actinomyces viscosus causing disseminated disease in a patient on methotrexate. Respir Med Case Rep. (2018) 25:158–60. doi: 10.1016/j.rmcr.2018.08.009
30. Grzywa-Celińska, A, Emeryk-Maksymiuk, J, Szmigin-Millanowska, K, Czekajska-Cherhab, E, and Milanowski, J. Pulmonary actinomycosis-the great imitator. Ann Agric Environ Med. (2018) 25:211–2. doi: 10.26444/aaem/75652
31. Papakonstantinou, NA, Vlachou, G, Vourlakou, C, and Zisis, C. Pulmonary actinomycosis masquerading as lung cancer: keep it in mind. ANZ J Surg. (2018) 89:966–8. doi: 10.1111/ans.14397
32. Boo, YL, How, KN, Pereira, DS, Chin, PW, Foong, KK, and Lim, SY. Pulmonary actinomycosis masquerading as lung cancer: a case report. Med J Malaysia. (2017) 72:246–7.
33. Laguna, S, Lopez, I, Zabaleta, J, and Aguinagalde, B. Actinomycosis associated with foreign body simulating lung Cancer. Archivos de Bronconeumolog a (English Edition). (2017) 53:284–5. doi: 10.1016/j.arbr.2017.03.008
34. Bunkar, ML, Gupta, PR, Takhar, R, Rajpoot, GS, and Arya, S. Pulmonary actinomycosis masquerading as lung cancer: case letter. Lung India. (2016) 33:460–2. doi: 10.4103/0970-2113.184944
35. Imanishi, S, Shinohara, T, Naruse, K, and Ogushi, F. Overlapping lung parenchymal and bronchial lesion and hilar lymphadenopathy in pulmonary actinomycosis mimicking lung cancer. Case Reports. (2016) 2016:bcr2016216308. doi: 10.1136/bcr-2016-216308
36. Katsenos, S, Galinos, I, Styliara, P, Galanopoulou, N, and Psathakis, K. Primary bronchopulmonary actinomycosis masquerading as lung cancer: apropos of two cases and literature review. Case Rep Infect Dis. (2015) 2015:1–5. doi: 10.1155/2015/609637
37. Park, HJ, Park, K-H, Kim, S-H, Sung, H, Choi, S-H, Kim, YS, et al. A case of disseminated infection due to Actinomyces meyeri involving lung and brain. Infect Chemother. (2014) 46:269–73. doi: 10.3947/ic.2014.46.4.269
38. Fichte, S, Brodhun, M, Göttinger, S, Rosahl, S, Klisch, J, and Gerlach, R. Vertebral and pulmonary actinomycosis mimicking metastatic lung cancer. Journal of neurological surgery part a: central European. Neurosurgery. (2013) 74:e188–92. doi: 10.1055/s-0033-1342930
39. Godfrey, AMK, Diaz-Mendoza, J, Ray, C, and Simoff, MJ. Endobronchial Actinomycosis after airway stenting. J Bronchol Intervent Pulmonol. (2012) 19:315–8. doi: 10.1097/LBR.0b013e31826a3aed
40. Elkambergy, H, Irani, F, Okoli, K, and Jamal, R. Pulmonary actinomycosis: the great masquerader. BMJ Case Rep. (2009) 2009:bcr0720080374. doi: 10.1136/bcr.07.2008.0374
41. Andreani, A, Rossi, G, Giovannini, M, and Cappiello, GF. Unexpected positron emission tomography-positive Actinomyces-related mass of the bronchial stump. Can Respir J. (2012) 19:77–9. doi: 10.1155/2012/502041
42. Colmegna, I, Rodriguez-Barradas, M, Young, EJ, Rauch, R, and Clarridge, J. Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci. (2003) 326:152–5. doi: 10.1097/00000441-200309000-00010
43. Neijens, V, van Heerde, P, van der Heijden, A, and Baas, P. Actinomycosis, a sheep in wolves' clothes. Lung Cancer. (1996) 15:131–5. doi: 10.1016/0169-5002(96)00578-8
44. Boudaya, MS, Smadhi, H, Marghli, A, Mouna, M, Charmiti, F, Ismail, O, et al. Surgery in thoracic actinomycosis. Asian Cardiovasc Thorac Ann. (2012) 20:314–9. doi: 10.1177/0218492312439310
45. Schweigert, M, Meyer, C, Stadlhuber, R, Dubecz, A, Kraus, D, and Stein, H. Surgery for inflammatory tumor of the lung caused by pulmonary actinomycosis. Thorac Cardiovasc Surg. (2012) 60:156–60. doi: 10.1055/s-0030-1271180
46. Andreani, A, Cavazza, A, Marchioni, A, Richeldi, L, Paci, M, and Rossi, G. Bronchopulmonary actinomycosis associated with hiatal hernia. Mayo Clinic Proc. (2009) 84:123–8. doi: 10.4065/84.2.123
47. Endo, S, Murayama, F, Yamaguchi, T, Yamamoto, S-i, Otani, S-i, Saito, N, et al. Surgical considerations for pulmonary actinomycosis. Ann Thorac Surg. (2002) 74:185–90. doi: 10.1016/S0003-4975(02)03616-0
48. Sun, X-F, Wang, P, Liu, H-R, and Shi, J-H. A retrospective study of pulmonary actinomycosis in a single institution in China. Chin Med J. (2015) 128:1607–10. doi: 10.4103/0366-6999.158316
49. Cheon, J-E, Im, J-G, Kim, MY, Lee, JS, Choi, GM, and Yeon, KM. Thoracic actinomycosis: CT findings. Radiology. (1998) 209:229–33. doi: 10.1148/radiology.209.1.9769836
50. Patz, EF Jr, and Goodman, PC. Positron emission tomography imaging of the thorax. Radiol Clin North Am. (1994) 32:811–23. doi: 10.1016/S0033-8389(22)00410-9
51. Tokuyasu, H, Harada, T, Watanabe, E, Touge, H, Kawasaki, Y, Isowa, N, et al. A case of endobronchial actionomycosis evaluated by FDG-PET. Nihon Kokyuki Gakkai Zasshi. (2008) 46:650–4.
52. Dewan, NA, Gupta, NC, Redepenning, LS, Phalen, JJ, and Frick, MP. Diagnostic efficacy of PET-FDG imaging in solitary pulmonary nodules: potential role in evaluation and management. Chest. (1993) 104:997–1002. doi: 10.1378/chest.104.4.997
53. Uehara, Y, Takahashi, T, Yagoshi, M, Shimoguchi, K, Yanai, M, Kumasaka, K, et al. Liver abscess of Actinomyces israelii in a hemodialysis patient: case report and review of the literature. Intern Med. (2010) 49:2017–20. doi: 10.2169/internalmedicine.49.3700
54. Erol, C, Sendur, MAN, and Yalçin, B. An unusual infection with long-term bevacizumab treatment for advanced nonsmall-cell lung cancer: Actinomycosis. J Cancer Res Ther. (2022) 18:1809–10. doi: 10.4103/jcrt.jcrt_2083_21
55. Afsin, E, and Bacaksu, E. Endobronchial Actinomycosis: a case report. Niger J Clin Pract. (2022) 25:1758–61. doi: 10.4103/njcp.njcp_1357_21
56. Suzuki, T, Kitami, A, Kamio, Y, Hori, G, Mitsuya, T, and Higashi, Y. Sleeve lobectomy of the middle lobe for hilar lung cancer with accompanying cardiomyopathy and actinomycosis. Thorac Cardiovasc Surg. (2000) 48:157–9. doi: 10.1055/s-2000-9631
57. Lu, M-S, Liu, H-P, Yeh, C-H, Wu, Y-C, Liu, Y-H, Hsieh, M-J, et al. The role of surgery in hemoptysis caused by thoracic actinomycosis; a forgotten disease. Eur J Cardiothorac Surg. (2003) 24:694–8. doi: 10.1016/S1010-7940(03)00515-3
58. Hsieh, M-J, Liu, H-P, Chang, J-P, and Chang, C-H. Thoracic actinomycosis. Chest. (1993) 104:366–70. doi: 10.1378/chest.104.2.366
59. Runyon, BA, Canawati, HN, and Akriviadis, EA. Optimization of ascitic fluid culture technique. Gastroenterology. (1988) 95:1351–5. doi: 10.1016/0016-5085(88)90372-1
60. Zhang, M, Zhang, X, and Chen, Y. Primary pulmonary actinomycosis: a retrospective analysis of 145 cases in mainland China. Int J Tuberc Lung Dis. (2017) 21:825–31. doi: 10.5588/ijtld.16.0773
61. Ha, YJ, An, JH, Shim, JH, Yu, ES, Kim, JJ, Ha, TY, et al. A case of primary hepatic actinomycosis: an enigmatic inflammatory lesion of the liver. Clin Mol Hepatol. (2015) 21:80–4. doi: 10.3350/cmh.2015.21.1.80
62. Wang, H-K, Sheng, W-H, Hung, C-C, Chen, Y-C, Liew, P-L, Hsiao, C-H, et al. Hepatosplenic actinomycosis in an immunocompetent patient. J Formos Med Assoc. (2012) 111:228–31. doi: 10.1016/j.jfma.2012.03.001
63. Kim, HS, Park, NH, Park, KA, and Kang, SB. A case of pelvic actinomycosis with hepatic actinomycotic pseudotumor. Gynecol Obstet Investig. (2007) 64:95–9. doi: 10.1159/000100058
64. Atay, S, Banki, F, and Floyd, C. Empyema necessitans caused by actinomycosis: a case report. Int J Surg Case Rep. (2016) 23:182–5. doi: 10.1016/j.ijscr.2016.04.005
65. Valour, F, Sénéchal, A, Dupieux, C, Karsenty, J, Lustig, S, Breton, P, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. (2014) 7:183–97. doi: 10.2147/IDR.S39601
66. Smith, A, Hall, V, Thakker, B, and Gemmell, C. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J Antimicrob Chemother. (2005) 56:407–9. doi: 10.1093/jac/dki206
67. Boot, M, Archer, J, and Ali, I. The diagnosis and management of pulmonary actinomycosis. J Infect Public Health. (2023) 16:490–500. doi: 10.1016/j.jiph.2023.02.004
Keywords: actinomycosis, pulmonary actinomycosis, lung cancer, Actinomyces species, diagnosis
Citation: Khoshbayan A, Amirmozafari N and Mirkalantari S (2024) An overview of case reports and case series of pulmonary actinomycosis mimicking lung cancer: a scoping review. Front. Med. 11:1356390. doi: 10.3389/fmed.2024.1356390
Edited by:
Diego Ripamonti, Papa Giovanni XXIII Hospital, ItalyReviewed by:
Yutaka Yoshii, The Jikei University School of Medicine, JapanYubao Guan, Fifth Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2024 Khoshbayan, Amirmozafari and Mirkalantari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiva Mirkalantari, TWlya2FsYW50YXJpLnNoQGl1bXMuYWMuaXI=; c2hfbWlya2FsYW50YXJpQHlhaG9vLmNvbQ==
 Amin Khoshbayan
Amin Khoshbayan Nour Amirmozafari
Nour Amirmozafari Shiva Mirkalantari
Shiva Mirkalantari