- Institute of Respiratory and Critical Care Medicine, The First Hospital of China Medical University, Shenyang, Liaoning, China
Background: Obstructive sleep apnea (OSA) and metabolic syndrome (MetS) often coexist, and the causal relationship between them is not yet clear; treatments for OSA include continuous positive airway pressure (CPAP), mandibular advancement device (MAD), surgery, and lifestyle intervention and so on. However, the effects of different treatments on metabolic syndrome in OSA patients are still under debate.
Objectives: Review the effects of different treatments on metabolic syndrome in OSA patients by meta-analysis.
Methods: we searched articles in PubMed, Embase, Cochrane Library, CNKI, CBM, and Wanfang data from database construction to Feb. 2024.RevMan5.4 and Stata software were used to conduct a meta-analysis of 22 articles.
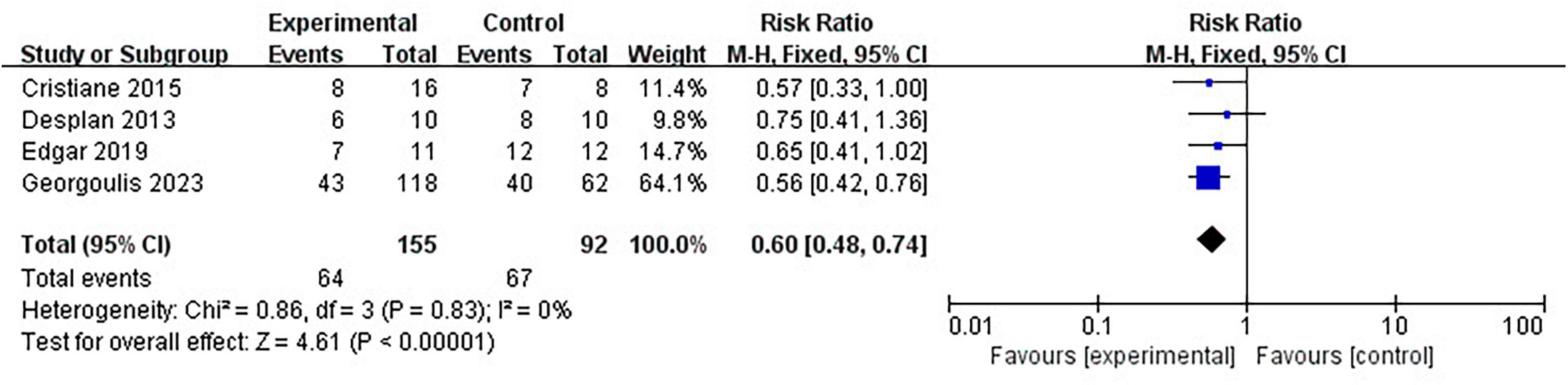
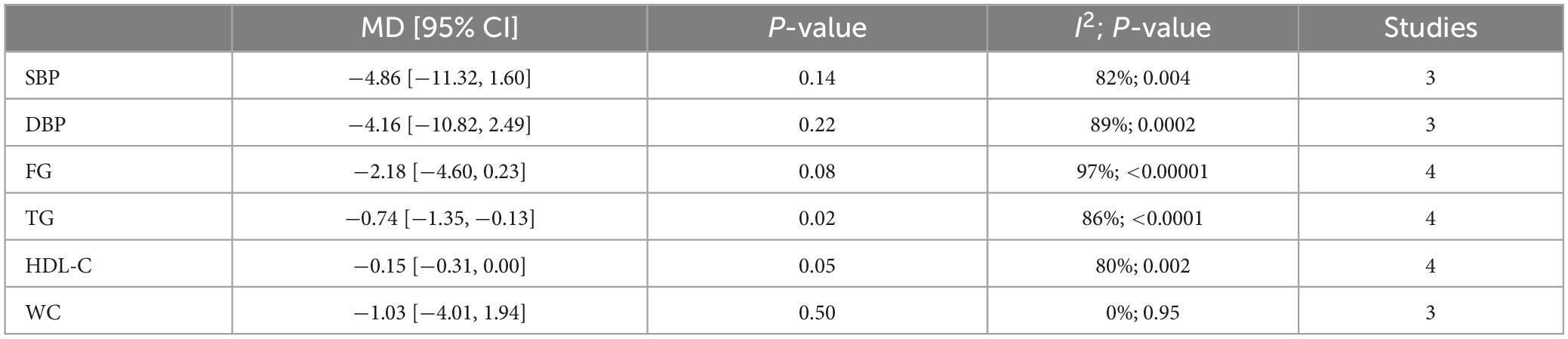
Results: A total of 22 articles were finally included. The results showed that CPAP treatment could reduce the prevalence of metabolic syndrome in OSA patients in randomized controlled trials (RCTs) (RR = 0.82 [95% CI, 0.75 to 0.90]; p < 0.01) and single-arm studies (RR = 0.73 [95% CI, 0.63 to 0.84]; p < 0.01). As for metabolic syndrome components, CPAP treatment reduces blood pressure, fasting glucose (FG), triglycerides (TG), and waist circumference (WC) but can’t affect high-density lipoprotein cholesterol (HDL-C) levels. Lifestyle intervention could significantly reduce the prevalence of metabolic syndrome in OSA patients (RR = 0.60 [95% CI, 0.48 to 0.74]; p < 0.01) and can lower blood pressure, fasting glucose, and waist circumference but can’t affect the lipid metabolism of OSA patients. Upper airway surgery can only reduce TG levels in OSA patients (MD = −0.74 [95% CI, −1.35 to −0.13]; p = 0.02) and does not affect other components of metabolic syndrome. There is currently no report on the impact of upper airway surgery on the prevalence of metabolic syndrome. No study has reported the effect of MAD on metabolic syndrome in OSA patients.
Conclusion: We confirmed that both CPAP and lifestyle intervention can reduce the prevalence of MetS in OSA patients. CPAP treatment can lower blood pressure, fasting glucose, waist circumference, and triglyceride levels in OSA patients. Lifestyle intervention can lower blood pressure, fasting glucose, and waist circumference in OSA patients. Upper airway surgery can only reduce TG levels in OSA patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022326857.
1 Introduction
Obstructive sleep apnea is a common disease characterized by repetitive airway collapse during sleep, which can lead to decreased blood oxygen saturation, causing chronic intermittent hypoxia (CIH) and sleep fragmentation. The main symptoms include snoring, sleep apnea, and daytime sleepiness (1). MetS is a common comorbidity of OSA; the main diagnostic criteria include abdomen obesity, hypertension, hyperglycemia, and dyslipidemia (2). A growing number of studies have shown that OSA is associated with an increased risk of MetS (3, 4). As the leading cause of metabolic disorders, CIH can promote sympathetic nerve activation, thus increasing blood pressure (5). CIH increases triglycerides and phospholipid synthesis, inhibits liver cholesterol uptake, and reduces triglycerides clearance, thus contributing to hyperlipidemia (6–8). As for glucose metabolism, CIH induces insulin resistance by causing β cell dysfunction and adipose tissue inflammation (9–11). Among adults aged 30 to 70, approximately 13% of men and 6% of women have moderate to severe OSA (12). Studies have shown that the prevalence of MetS in the Chinese population over 15 years old is 24.5% (13). It is estimated that 50–60% of obese people with MetS have OSA. Many previous studies have shown that both the prevalence of OSA in patients with MetS and the prevalence of MetS in OSA patients were at a high level (between 60 and 70%) (14–16), and both OSA and MetS were associated with an increased risk of cardiovascular disease.
The overlap in health outcomes in individuals with MetS and OSA makes it difficult to disentangle cause and effect and whether OSA treatments, such as CPAP, MAD, upper airway surgery, and weight loss, can improve these outcomes (17). As the first-line treatment for moderate and severe patients with OSA (18), CPAP treatment can effectively reduce the collapse of the upper airway, alleviate hypoxia, and improve clinical symptoms, including snoring and excessive daytime sleepiness (9). The randomized controlled trials conducted by Giampa et al. (19, 20) showed a significant decrease in the incidence of MetS in OSA patients after CPAP treatment compared to the control group. However, other studies have reached the opposite conclusion, suggesting that CPAP did not change the prevalence of MetS (21, 22). Among the various components of MetS, previous studies have only reached a consensus on reducing blood pressure after CPAP treatment (23). There were many controversies regarding its impact on other components of MetS, such as fasting glucose, blood lipids, and obesity. Upper respiratory surgery is a standard treatment for OSA. It is suitable for OSA patients who cannot tolerate CPAP or have apparent upper respiratory tract structural abnormalities. Traditional surgical methods include Uvulopalatopharyngoplasty (UPPP) and related soft tissue procedures, maxillomandibular advancement, tracheostomy (rarely used), and hypoglossal nerve stimulation. Upper airway surgery can reduce the apnea and hypopnea index of OSA patients, thereby improving their quality of life. Surgical intervention is an irreplaceable way to improve OSA for patients with severe OSA who are complicated with anatomical abnormalities (24). Surgery undoubtedly can improve the condition of OSA, but its improvement of metabolic disorders complicated with OSA was controversial in previous studies. The study of Warner et al. (25) proved that upper airway surgery can reduce blood pressure and fasting glucose levels in OSA patients but can’t affect the reduction of blood lipids. However, some studies have suggested that upper airway surgery can significantly reduce triglyceride levels in OSA patients (26), while previous studies have failed to report the impact of surgery on the prevalence of MetS. Lifestyle intervention for OSA patients includes a low-calorie diet and exercise training. Lifestyle intervention can improve the severity of OSA, improve the co-existing metabolic disorders in patients, and reduce the risk of obesity, hypertension, and diabetes (27–29). Lifestyle intervention is recommended for obese OSA patients of any severity. It can be used as a primary treatment and combined with other therapies (18). MAD applies to patients with mild and moderate OSA. Some studies have shown that applying MAD can reduce patients’ blood pressure but does not affect fasting glucose and blood lipids (30, 31). There is no study report on the effect of MAD on the prevalence of MetS in OSA patients. As for the impact of different MetS treatments on OSA patients, there is heterogeneity among the results of previous studies. In this study, we conducted a meta-analysis to investigate whether rational treatment of OSA improves the co-existing MetS in patients.
2 Methods
2.1 Search strategy
We systematically searched English articles using PubMed, Embase, and the Cochrane Library and searched the Chinese ones using CNKI, CBM, and Wanfang data. The keywords used for the search included “Metabolic Syndrome,” “obstructive sleep apnea,” “sleep-disordered breathing,” “OSA,” “OSAHS,” and corresponding Chinese words. The search time of studies was from the time of database construction to Feb.2024.2 Independent reviewers performed the study searching and screening process, and disagreements were resolved by discussion.
2.2 Inclusion and exclusion criteria
The inclusion criteria were: (1) published articles on OSA patients combined with MetS; (2) only adults (aged 18 years) were included; (3) CPAP, surgery, or lifestyle intervention was applied, and the duration of CPAP therapy was ≥2 weeks; and (4) Reported the number of patients with MetS or one of the following indicators before and after treatment: triglycerides, high-density lipoprotein cholesterol, blood pressure, fasting glucose, and waist circumference
The following studies were excluded: (1) Reviews, abstracts, case reports, letters, and non-human studies. (2) Insufficient information provided.
2.3 Quality assessment
Two independent reviewers (J. N. Liu and J. H. Xu) evaluated the included studies using the quality assessment method, and disagreements were resolved via discussion. The Cochrane Collaboration for Systematic Reviews of Interventions was used to assess randomized controlled trials, the methodological index for non-randomized studies (MINORS) was used to evaluate non-randomized clinical trials, and the Newcastle-Ottawa Scale (NOS) was used to assess the quality of single-arm studies. Agreements between reviewers were resolved by discussion to reach a consensus.
2.4 Data extraction
We extracted data from 22 studies. These data were the first author, year of publication, study design, body mass index (BMI), AHI or RDI values of the participants, age, number of subjects, the duration of intervention, the number of patients with MetS, the levels of TG, HDL-C, SBP, DBP, fasting glucose and waist circumference, data from both the experimental groups and control groups in controlled trials, before and after treatment in single-arm studies. Our final analysis examined the differences in these values between them. Two independent reviewers performed this process.
2.5 Data synthesis
We used mean differences (MDs) and standard deviations for continuous data such as TG, HDL-C, SBP, DBP, fasting glucose, and waist circumference. RR was used to estimate the impact of different treatments on the prevalence of MetS. The associated CIs were calculated with p-values < 0.05, indicating significance. We also use funnel plots and Egger’s test to assess publication bias. Heterogeneity was assessed using the Cochrane Q and chi-square tests with I-squared index tests. I-square >0% with p-values < 0.05 was considered high heterogeneity, using the random-effects model; otherwise, the fixed-effects model was used. Cochrane Collaboration’s RevMan 5.4 and Stata software were used to analyze all data.
3 Results
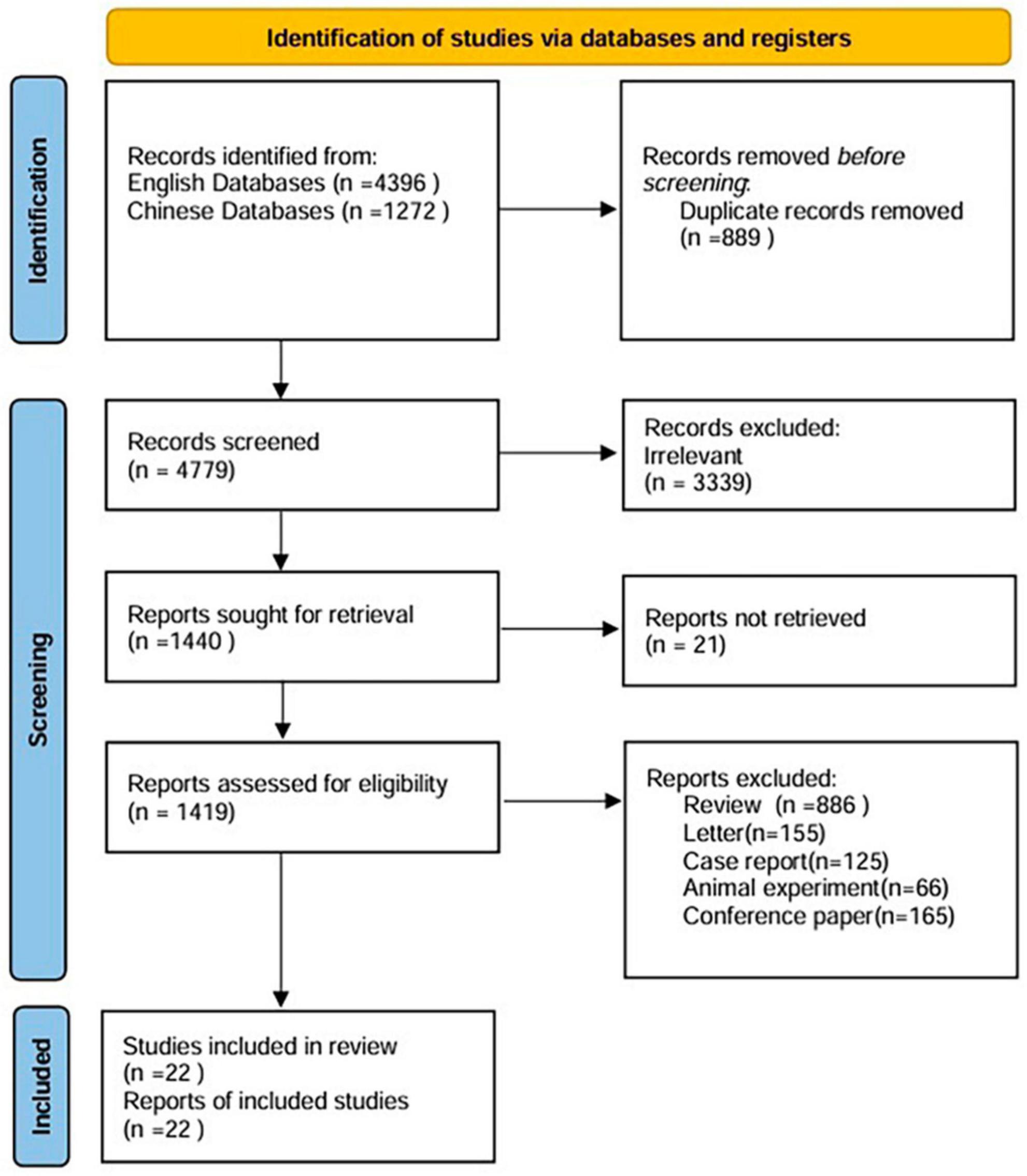
A total of 5,668 studies were identified from different sources. Finally, data were taken from 22 articles, including 9 Chinese and 13 English articles. Figure 1 shows the process of the literature search. The characteristics of the studies are summarized in Table 1. According to the funnel plots and Egger’s test outcome, the included studies had no significant publication bias.
3.1 Qualitative analysis
All included articles were published from 2007 to 2023. Of the 22 articles, 8 were RCTs, 2 were non-randomized controlled clinical trials, and the other 12 were single-arm studies. The mean age ranged between 35 and 70. The average Body Mass Index (BMI) ranged between 26.7 and 49.3 kg/m2. The mean AHI ranged between 14 and 68.3/h. The duration of CPAP treatment and lifestyle intervention was 4 weeks to 12 months. The evaluation of surgical effectiveness is 6 or 12 months later.
3.2 Quality assessment
Newcastle-Ottawa Scale was used to evaluate the quality of single-arm studies. The eight single-arm studies’ scores were six points or above, indicating high research quality. The MINORS scale was used to evaluate the study quality of non-randomized clinical trials. The scores of the two non-randomized clinical trials included were 21 points, indicating high study quality. The results of the Cochrane Bias risk assessment of RCTs are shown in Figure 2.
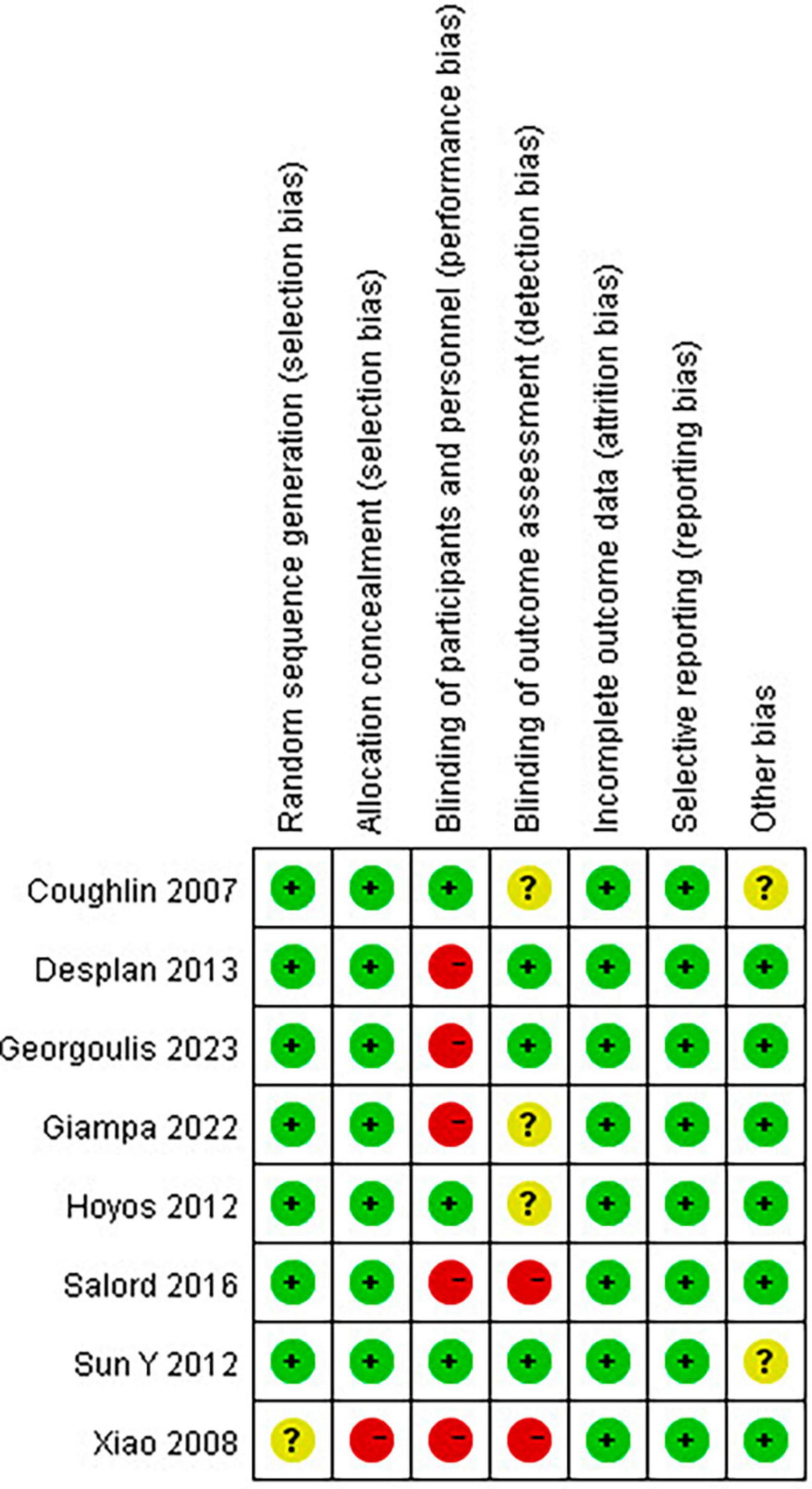
Figure 2. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.3 Pooled analysis on MetS prevalence
3.3.1 Pooled analysis of the impact of CPAP on MetS prevalence
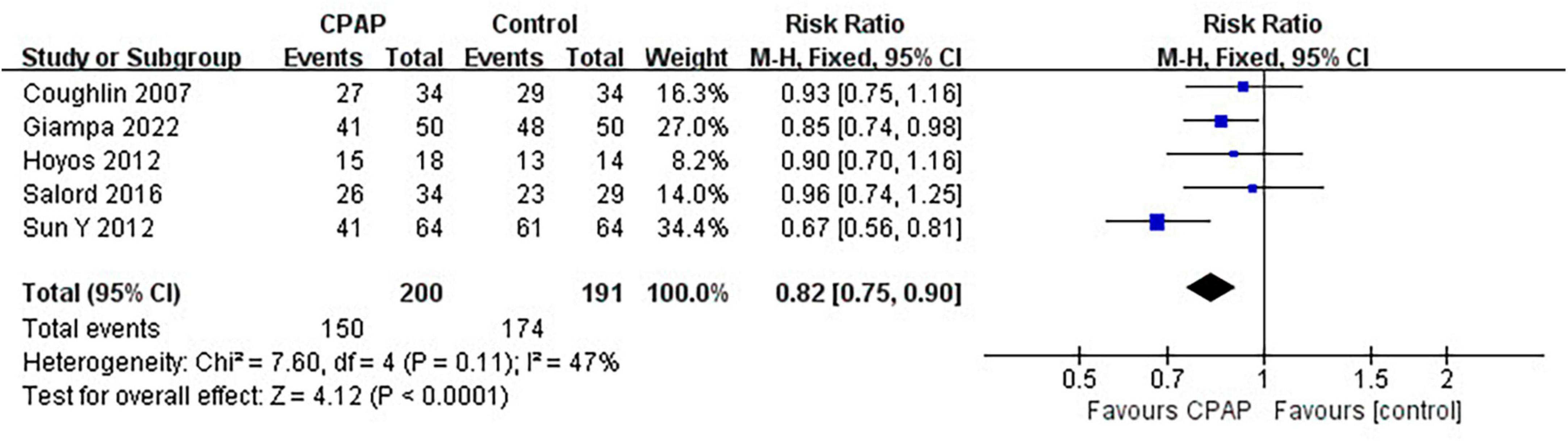
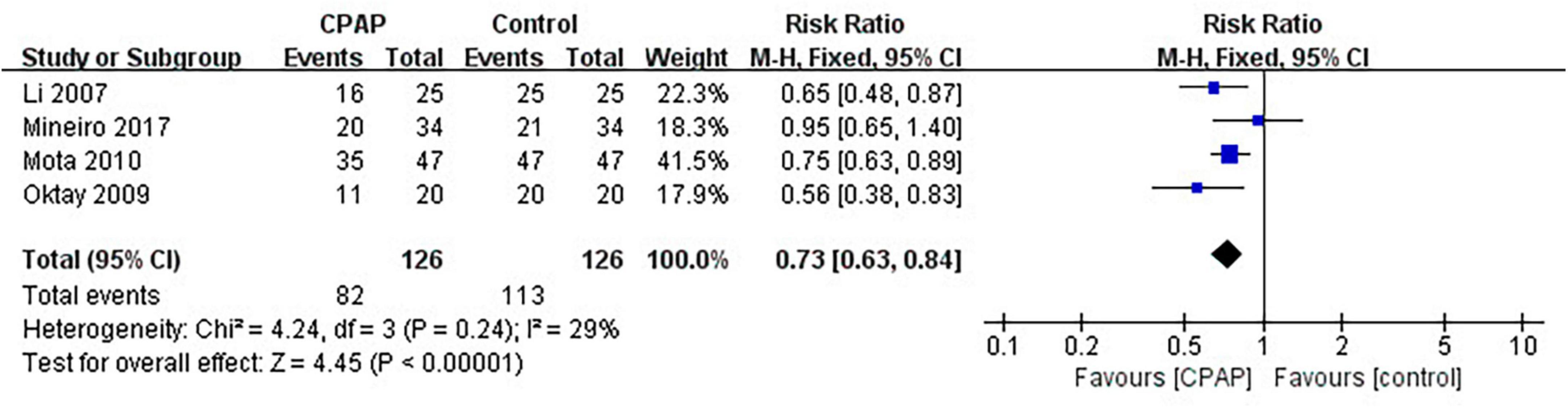
Nine studies have reported the number of patients diagnosed with MetS before and after CPAP treatment, five of which were randomized controlled trials. Pooled meta-analysis showed that compared with untreated patients, CPAP treatment could reduce the prevalence of MetS in OSA patients in both RCTs (RR = 0.82 [95% CI, 0.75 to 0.90]; p < 0.01) (Figure 3) and single-arm studies (RR = 0.73 [95% CI, 0.63 to 0.84]; p < 0.01) (Figure 4).
3.3.2 Pooled analysis of the impact of lifestyle intervention on MetS prevalence
Four clinical trials have reported the number of patients diagnosed with MetS before and after lifestyle intervention. Pooled meta-analysis showed that lifestyle intervention could significantly reduce the prevalence of MetS in OSA patients (RR = 0.60 [95% CI, 0.48 to 0.74]; p < 0.01) (Figure 5).
3.3.3 Pooled analysis of the impact of surgery and MAD on MetS prevalence
No study has reported the effect of surgery or MAD on MetS prevalence in OSA patients.
3.4 Pooled analysis of the changes in MetS components
3.4.1 Pooled analysis of the impact of CPAP on SBP
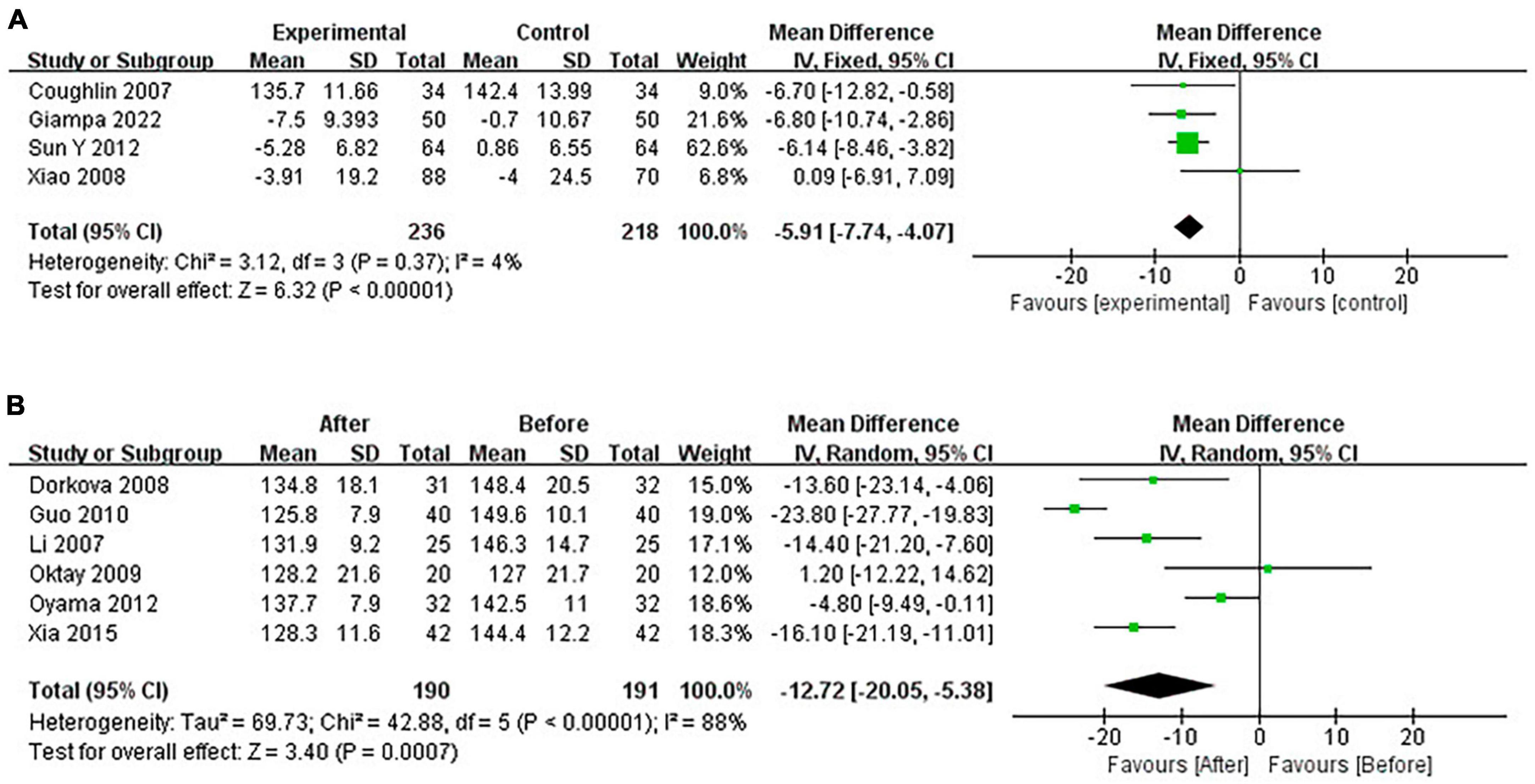
Continuous positive airway pressure reduced SBP in OSA patients. A total of 4 RCTs (MD = −5.91 [95% CI, −7.74 to −4.07]; p < 0.01) and 6 single-arm studies (MD = −12.72 [95% CI, −20.05 to −5.38]; p < 0.01) were included. The difference was statistically significant (Figure 6).
3.4.2 Pooled analysis of the impact of CPAP on DBP
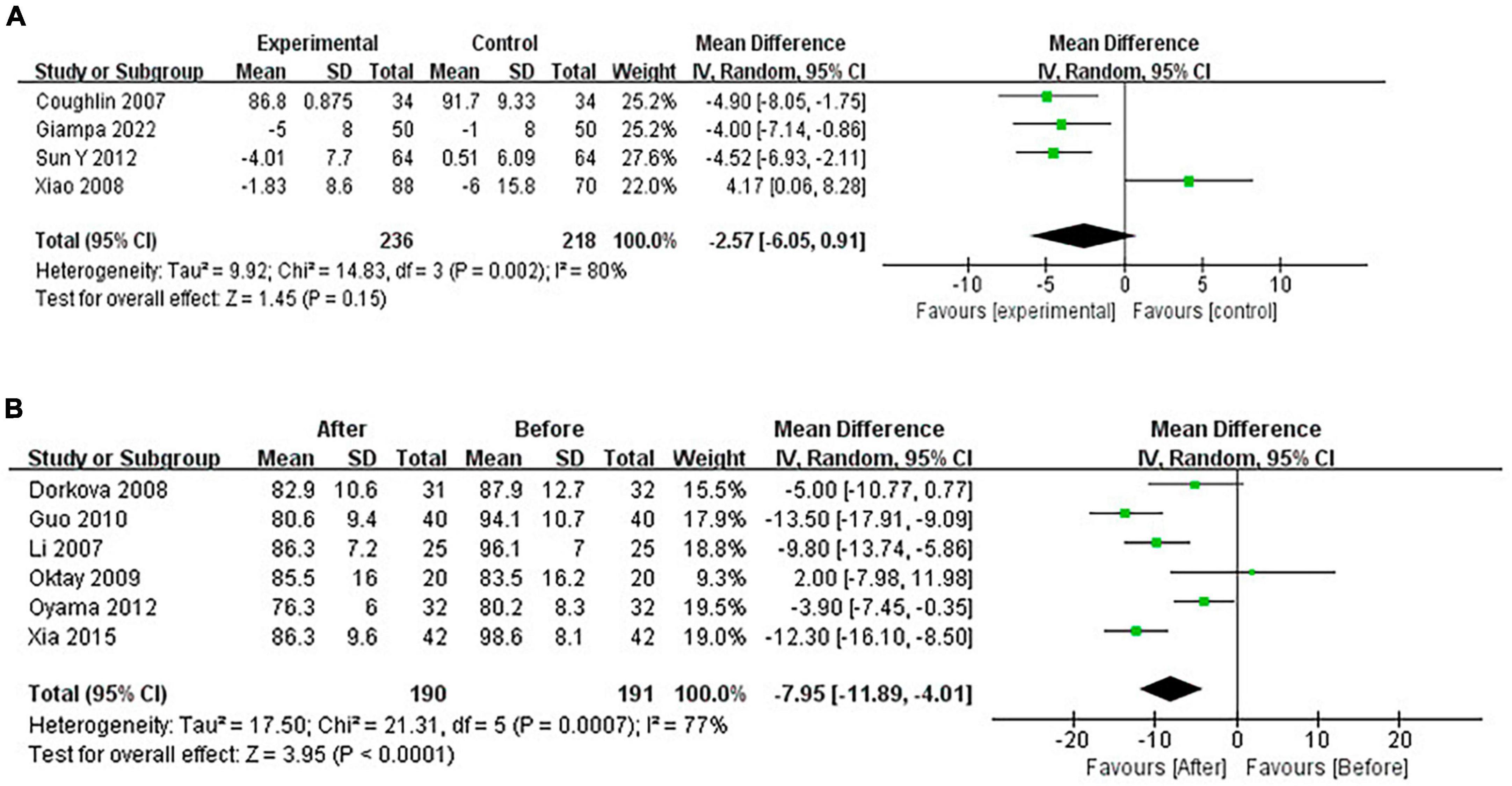
Continuous positive airway pressure reduced DBP in OSA patients. A total of 4 RCTs (MD = −2.57 [95% CI, −6.05 to 0.91]; p = 0.15) and 6 single-arm studies (MD = −7.95 [95% CI, −11.89 to −4.01]; p < 0.01) were included. The difference in single-arm studies was statistically significant (Figure 7).
3.4.3 Pooled analysis of the impact of CPAP on FG
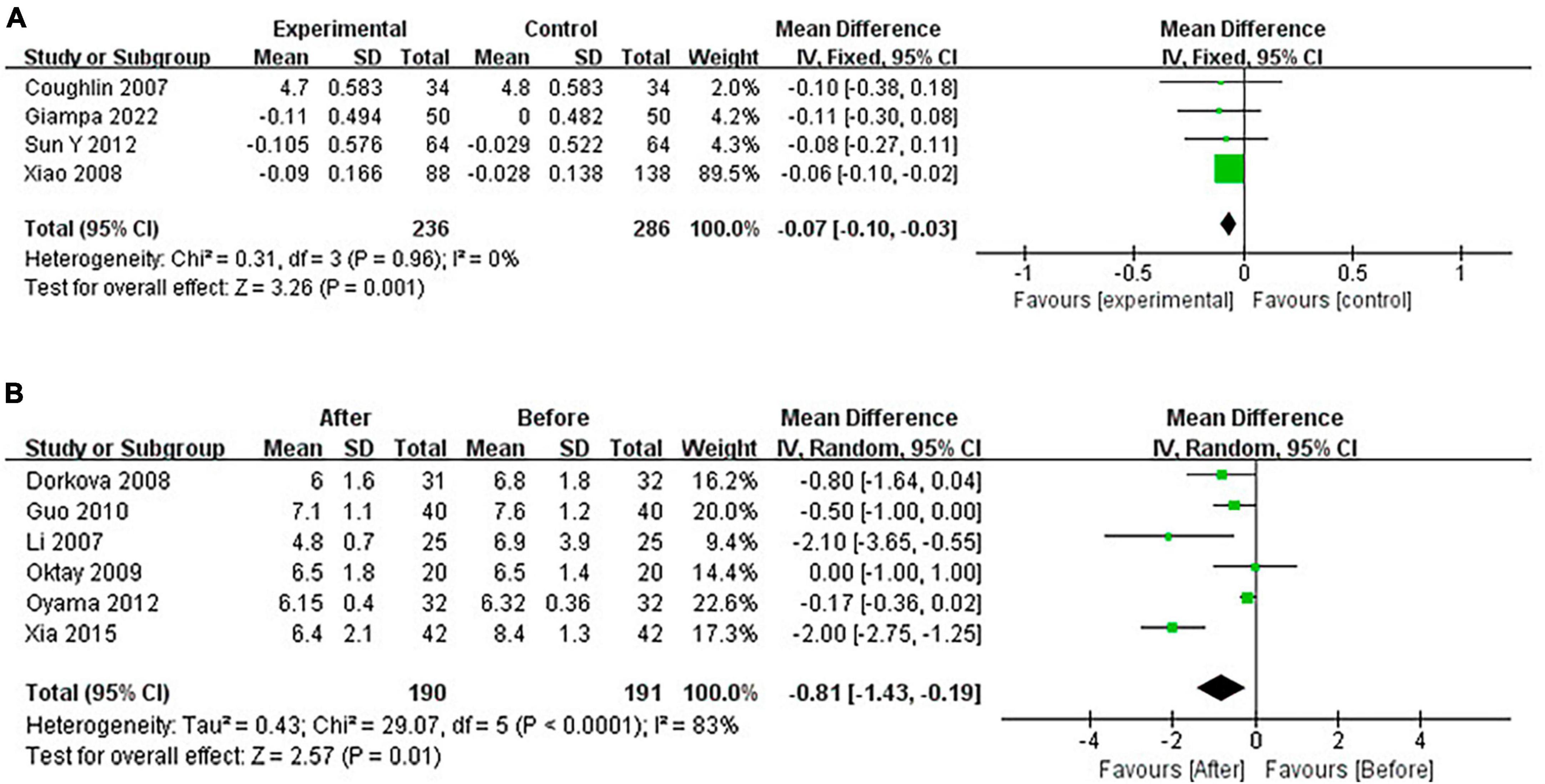
Continuous positive airway pressure reduced FG in OSA patients. A total of 4 RCTs (MD = −0.07 [95% CI, −0.10 to −0.03]; p = 0.001) and 6 single-arm studies (MD = −0.81 [95% CI, −1.43 to −0.19]; p = 0.01) were included. The difference was statistically significant (Figure 8).
3.4.4 Pooled analysis of the impact of CPAP on WC
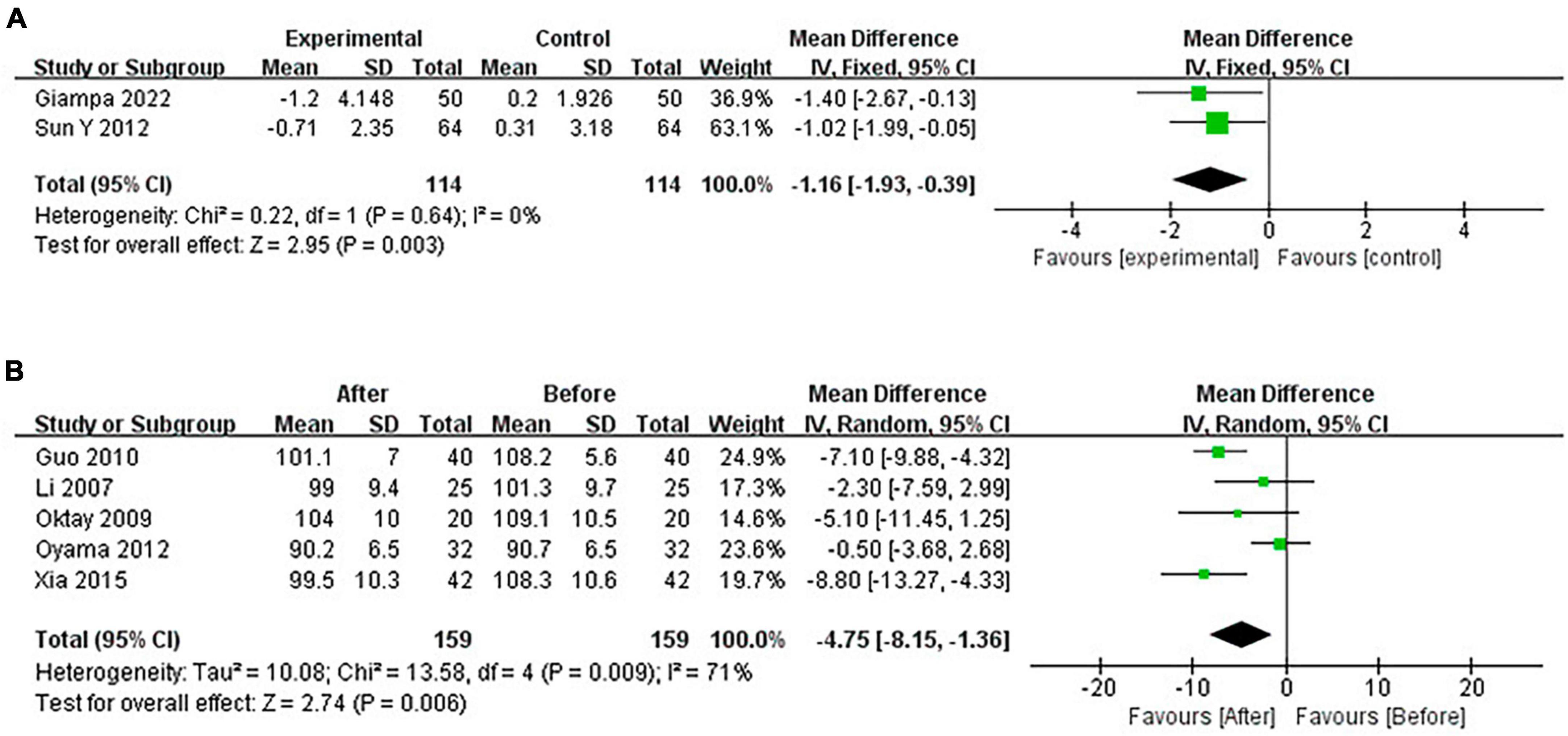
Continuous positive airway pressure reduced WC in OSA patients. A total of 2 RCTs (MD = −1.16 [95% CI, −1.93 to −0.39], p = 0.003; p = 0.001) and 5 single-arm studies (MD = −4.75 [95% CI, −8.15 to −1.36], p = 0.006) were included. The difference was statistically significant (Figure 9).
3.4.5 Pooled analysis of the impact of CPAP on TG
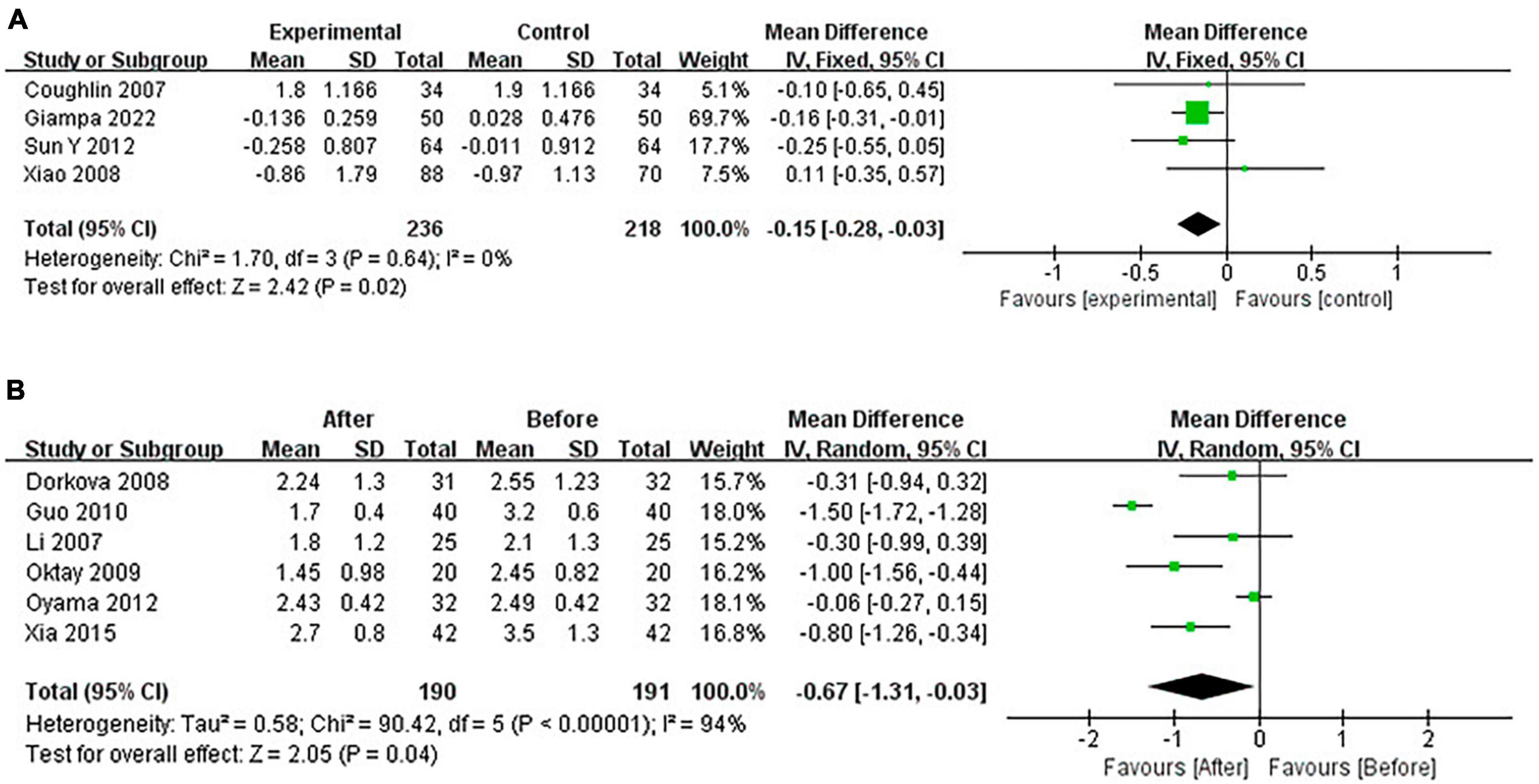
Continuous positive airway pressure reduced TG in OSA patients. A total of 4 RCTs (MD = −0.15 [95% CI, −0.28 to −0.03], p = 0.02) and 6 single-arm studies (MD = −0.67 [95% CI, −1.31 to −0.03], p = 0.04) were included. The difference was statistically significant (Figure 10).
3.4.6 Pooled analysis of the impact of CPAP on HDL-C
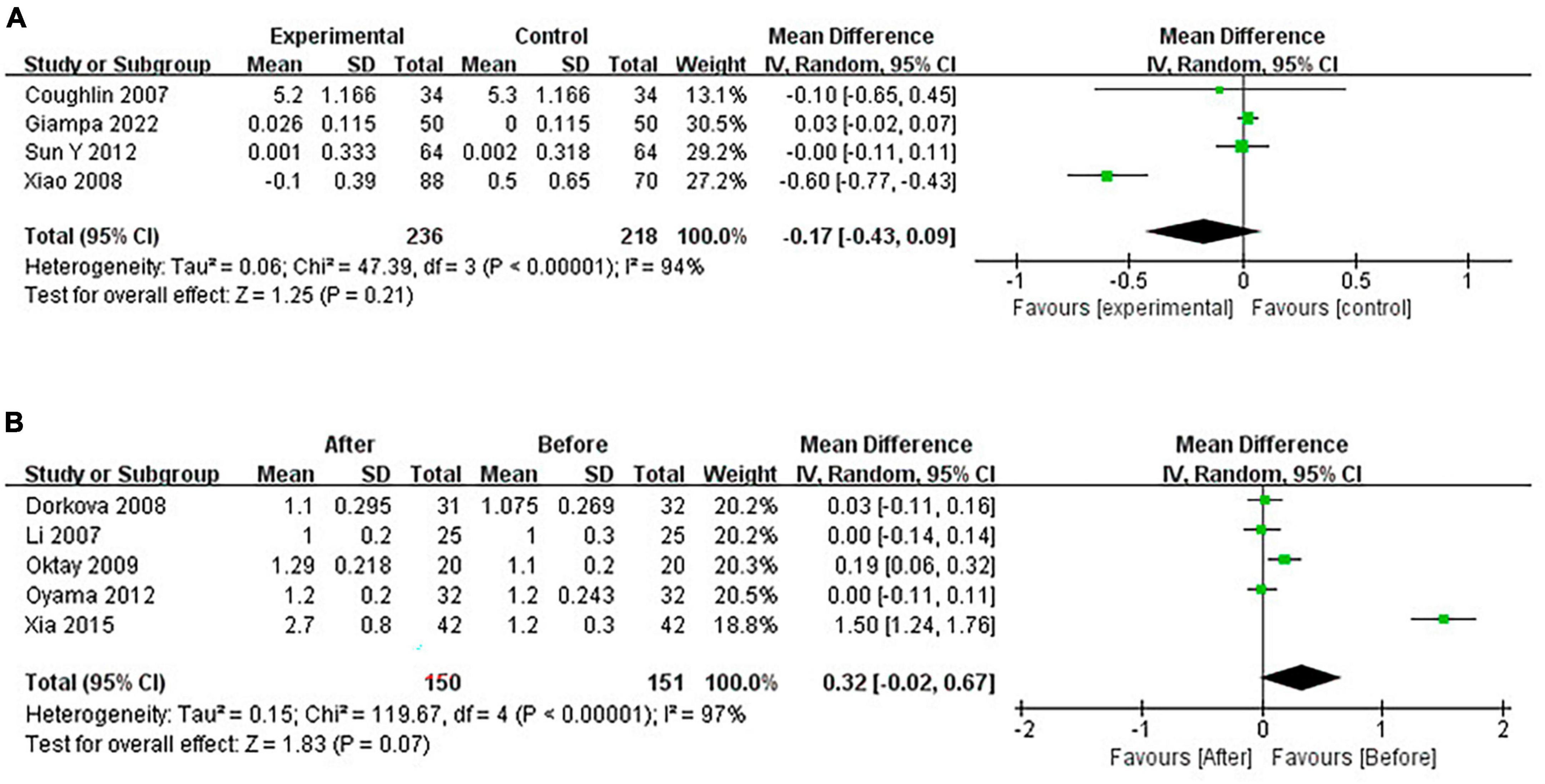
Continuous positive airway pressure can’t affect HDL-C levels in OSA patients. A total of 4 RCTs (MD = −0.17 [95% CI, −0.43 to 0.09], p = 0.21) and 5 single-arm studies (MD = 0.32 [95% CI, −0.02 to 0.67], p = 0.07) were included. The difference was not statistically significant (Figure 11).
3.4.7 Pooled analysis of the impact of lifestyle intervention on MetS components
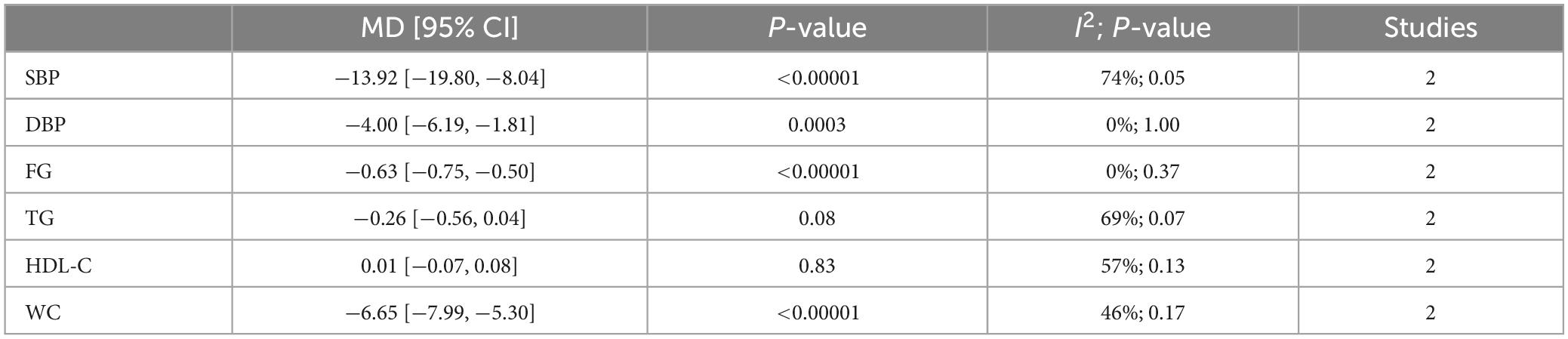
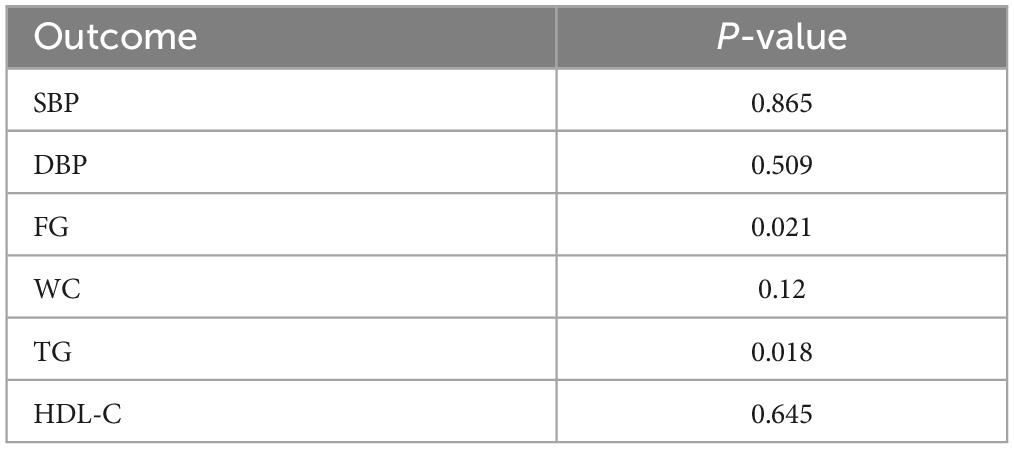
Lifestyle intervention could lower blood pressure, fasting glucose, and waist circumference but can’t affect the lipid metabolism of OSA patients (Table 2).
3.4.8 Pooled analysis of the impact of surgery on MetS components
Upper airway surgery can only reduce TG levels in OSA patients and does not affect other components of MetS (Table 3).
3.4.9 Heterogeneity and publication bias
Due to the heterogeneity of some included studies, this study conducted sensitivity analysis by successively excluding some studies. It was found that in the study on the effect of CPAP on DBP in OSA patients, after excluding the study of Xiao et al. (32), there is a significant change in the results (MD = −4.48 [95% CI, −6.11 to −2.85]; p < 0.00001) (Figure 12).
Funnel plots were used to evaluate publication bias; if the funnel plots showed apparent asymmetry, we used Egger’s test for the quantitative evaluation. The results of Egger’s test showed that the P-value of the test result of CPAP’s effect on fasting blood glucose and triglyceride was <0.05, indicating the existence of publication bias. However, after trim and filling analysis, it was found that the publication bias had little impact on the study results. Publication bias was not present in the remaining outcomes. The funnel plots and Egger’s test outcome are shown in Figure 13 and Table 4.
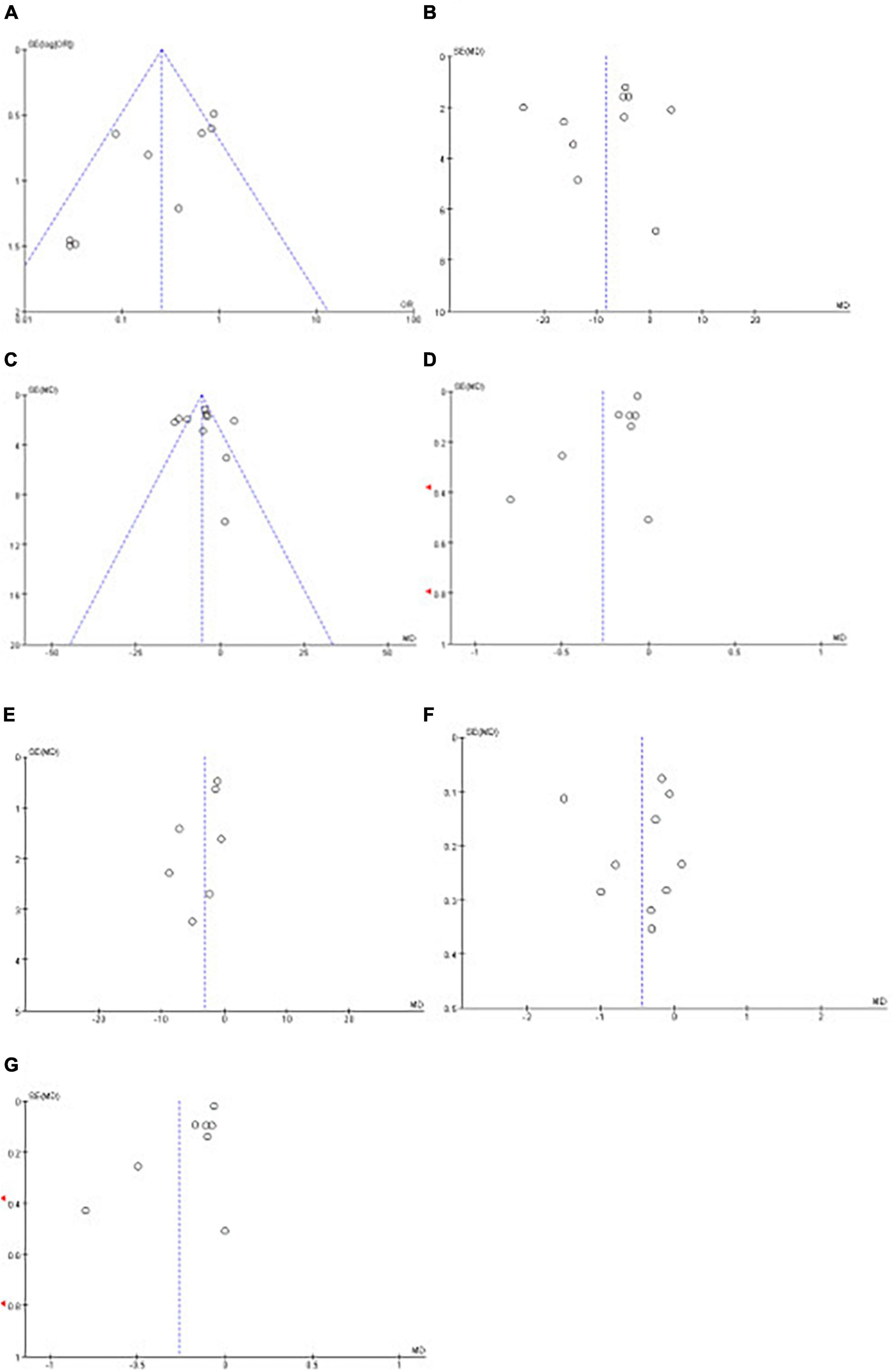
Figure 13. Funnel plots of the impact of CPAP. Funnel plots of the impact CPAP [(A): MetS, (B): SBP, (C): DBP, (D): FG, (E): WC, (F): TG, (G): HDL-C].
4 Discussion
Previous studies have shown that OSA can promote the occurrence of MetS (3, 4, 33). CIH and sleep fragmentation mainly characterize OSA. CIH promotes the activation of the sympathetic nerves, thereby increasing blood pressure; the increased level of circulating inflammatory products and reactive oxygen results in hyperglycemia and dyslipidemia (7, 9). OSA is more common in obese patients (34). Patients with OSA are 6–9 times more likely to have MetS than the general population (35–37). The existing research on the impact of different treatments for OSA on coexisting MetS in patients has inconsistent conclusions. This study found through meta-analysis that CPAP and lifestyle intervention could reduce blood pressure, fasting glucose, and waist circumference in OSA patients, thereby reducing the prevalence of co-existing MetS. However, upper airway surgery can only reduce the triglycerides level of patients.
This meta-analysis confirmed that CPAP can reduce the prevalence of MetS, but the improvement of MetS with CPAP alone is limited (RCT: RR = 0.82; Single-arm studies: RR = 0.73). The formation of MetS is related to many factors, such as genetics, diet habits, exercise, and co-existing diseases, and OSA is only one of the many risk factors (38). In contrast, lifestyle interventions had a more dominant effect on MetS in OSA patients (RR = 0.60). Lifestyle intervention is an essential treatment for MetS (39), and the Mediterranean diet has been shown to reduce the risk of MetS and improve every component of it (40, 41). In various studies exploring lifestyle intervention, the prevalence of MetS was notably reduced in subjects receiving CPAP therapy simultaneously, suggesting that CPAP combined lifestyle intervention is essential in improving disease prognosis in OSA patients with MetS. There is an interaction between components of MetS, and weight loss can lead to changes in other components, such as blood lipid and fasting glucose (2). Therefore, lifestyle intervention is considered an essential and effective treatment for OSA (18). Our study showed that lifestyle intervention, including low-calorie diet and exercise, could reduce patients’ blood pressure, fasting glucose, and waist circumference, and the impact on waist circumference was particularly significant. Lifestyle intervention can’t affect lipid metabolism, which differs from previous studies (41, 42). This may be related to the short intervention time. The intervention time of the studies included in our report was only 4 months. Reducing blood lipid levels is a long-term process, so a short intervention may not seem to have a significant outcome. So far, no study has reported whether MAD and surgery can impact the prevalence of MetS in OSA patients.
Continuous positive airway pressure is the first-line treatment for patients with moderate to severe OSA. CPAP can effectively reduce CIH caused by airway collapse (37), but the therapeutic effect of CPAP depends on compliance. CPAP can reduce sympathetic nerve activation and vascular damage caused by intermittent hypoxia, thereby reducing blood pressure (42). Studies have proved that CPAP can significantly improve the condition of patients with refractory hypertension and can play an essential clinical role in the management of hypertension in these patients (23). Similarly, our study confirmed that CPAP can reduce blood pressure in OSA patients with MetS. There is much debate about the effects of CPAP on blood lipids in patients. Our study demonstrated a decrease in triglycerides after CPAP treatment. The formation of dyslipidemia in OSA patients with MetS is a long-term process influenced by many factors, of which OSA is only one. Triglycerides are more sensitive to reducing oxidative stress caused by OSA treatment (43), while HDL-C is mainly influenced by genetic factors and not easily changed by various intervention methods (44). When it comes to fasting glucose, we agree with previous studies. CPAP can reduce fasting glucose in OSA patients, regardless of whether they have MetS (45).
Surgery is the preferred treatment for patients who are intolerant to CPAP or have anatomic abnormalities in the upper airway. Intrapharyngeal surgery (soft tissue surgery) is widely used in treating OSA. Uvulopalatopharyngoplasty is the most effective method to solve oropharyngeal obstruction in OSA patients (18). Previous studies have confirmed the effectiveness of surgery in OSA patients (46). However, there is controversy in previous studies regarding the impact of surgery on MetS in OSA patients, and no RCTs have been published in relevant fields in the past. The studies included are all single-arm clinical trials. The meta-analysis showed that only TG was reduced after upper airway surgery. A liquid diet for some time after surgery can also reduce energy intake to some extent, thus lowering TG levels. Most of the included subjects were obese patients; after the surgery, the BMI of the patients all decreased to varying degrees, while TG levels were closely related to BMI decrease (47). All the above reasons could cause TG changes.
Our study had some limitations. First, only a few RCTs could be included, and the total number of samples was relatively small. Therefore, there were single-arm studies in our included studies. However, even so, existing studies have sufficiently confirmed the role of different treatments in reducing the incidence of MetS in OSA patients. This study analyzed RCTs and single-arm studies, respectively, and the conclusions were consistent. Secondly, in some of the studies, the duration of treatment was relatively short, which may not be sufficient to impact MetS, leading to significantly increased heterogeneity between studies. Therefore, future research requires RCTs with larger patient samples and longer follow-up times. Finally, although some studies have reported the effects of MAD on blood pressure, blood glucose, and blood lipids in OSA patients (30), no study was conducted among patients with both OSA and MetS. Therefore, this study failed to conduct a comprehensive discussion on this aspect.
In conclusion, we confirmed that both CPAP treatment and lifestyle intervention could reduce the prevalence of MetS in OSA patients. CPAP can lower blood pressure, fasting glucose, waist circumference, and triglyceride levels. Lifestyle intervention can lower blood pressure, fasting glucose, and waist circumference in OSA patients. Upper airway surgery can only reduce TG levels in OSA patients. Research suggests that for patients with OSA combined with MetS, appropriate treatment should be selected promptly based on the condition to improve the patient’s prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL: Data curation, Formal Analysis, Methodology, Resources, Software, Writing – original draft. JX: Investigation, Resources, Supervision, Validation, Writing – original draft. SG: Data curation, Investigation, Methodology, Visualization, Writing – original draft. WW: Conceptualization, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82270107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1354489/full#supplementary-material
References
1. Jordan A, McSharry D, Malhotra A. Adult obstructive sleep apnoea. Lancet. (2014) 383:736–47. doi: 10.1016/S0140-6736(13)60734-5
2. Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, et al. Diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
3. Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. (2007) 131:1387–92.
4. Hirotsu C, Haba-Rubio J, Togeiro S, Marques-Vidal P, Drager L, Vollenweider P, et al. Obstructive sleep apnoea as a risk factor for incident metabolic syndrome: A joined Episono and HypnoLaus prospective cohorts study. Eur Respir J. (2018) 52:1801150. doi: 10.1183/13993003.01150-2018
5. Chen L, Einbinder E, Zhang Q, Hasday J, Balke C, Scharf S. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. (2005) 172:915–20. doi: 10.1164/rccm.200504-560OC
6. Li J, Thorne L, Punjabi N, Sun C, Schwartz A, Smith P, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. (2005) 97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9
7. Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser A, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. (2008) 103:1173–80. doi: 10.1161/CIRCRESAHA.108.178533
8. Drager L, Li J, Shin M, Reinke C, Aggarwal N, Jun J, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. (2012) 33:783–90. doi: 10.1093/eurheartj/ehr097
9. Shin M, Han W, Joo H, Bevans-Fonti S, Shiota M, Stefanovski D, et al. Effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia in the frequently sampled intravenous glucose tolerance test. J Appl Physiol. (1985) 122:767–74. doi: 10.1152/japplphysiol.00975.2016
10. Polak J, Shimoda L, Drager L, Undem C, McHugh H, Polotsky V, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: Partial improvement with cessation of the exposure. Sleep. (2013) 36:1483–90. doi: 10.5665/sleep.3040
11. Iiyori N, Alonso L, Li J, Sanders M, Garcia-Ocana A, O’Doherty R, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. (2007) 175:851–7. doi: 10.1164/rccm.200610-1527OC
12. Peppard P, Young T, Barnet J, Palta M, Hagen E, Hla K. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. (2013) 177:1006–14. doi: 10.1093/aje/kws342
13. Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu J, et al. Prevalence of metabolic syndrome in Mainland China: A meta-analysis of published studies. BMC Public Health. (2016) 16:296. doi: 10.1186/s12889-016-2870-y
14. Parish J, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. (2007) 3:467–72.
15. Drager L, Bortolotto L, Maki-Nunes C, Trombetta I, Alves M, Fraga R, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. (2010) 208:490–5.
16. Drager L, Lopes H, Maki-Nunes C, Trombetta I, Toschi-Dias E, Alves M, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. (2010) 5:e12065. doi: 10.1371/journal.pone.0012065
17. Gaines J, Vgontzas A, Fernandez-Mendoza J, Bixler E. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. (2018) 42:211–9. doi: 10.1016/j.smrv.2018.08.009
18. Gottlieb D, Punjabi N. Diagnosis and management of obstructive sleep apnea: A review. JAMA. (2020) 323:1389. doi: 10.1001/jama.2020.3514
20. Giampa S, Freitas L, Furlan S, Macedo T, Lebkuchen A, Cardozo K, et al. Effects of CPAP on metabolic syndrome in patients with obstructive sleep apnea: The TREATOSA-MS randomized controlled trial. Am J Respir Crit Care Med. (2020) 201:1370–81. doi: 10.1016/j.chest.2021.12.669
21. Coughlin S, Mawdsley L, Mugarza J, Wilding J, Calverley P. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. (2007) 29:720–7. doi: 10.1183/09031936.00043306
22. Salord N, Fortuna A, Monasterio C, Gasa M, Pérez A, Bonsignore M, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep. (2016) 39:35–41. doi: 10.5665/sleep.5312
23. Labarca G, Schmidt A, Dreyse J, Jorquera J, Enos D, Torres G, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Med Rev. (2021) 58:101446. doi: 10.1016/j.smrv.2021.101446
24. American Sleep Disorders Association and Sleep Research Society. Practice parameters for the treatment of obstructive sleep apnea in adults: The efficacy of surgical modifications of the upper airway. Sleep. (1996) 19:152–5. doi: 10.1093/sleep/33.10.1408
27. Georgoulis M, Yiannakouris N, Kechribari I, Lamprou K, Perraki E, Vagiakis E, et al. Sustained improvements in the cardiometabolic profile of patients with obstructive sleep apnea after a weight-loss Mediterranean diet/lifestyle intervention: 12-month follow-up (6 months post-intervention) of the ‘MIMOSA’ randomized clinical trial. Nutr Metab Cardiovasc Dis. (2023) 33:1019–28. doi: 10.1016/j.numecd.2023.02.010
28. Laterza M, de Matos L, Trombetta I, Braga A, Roveda F, Alves M, et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. (2007) 49:1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548
29. Trombetta I, Batalha L, Rondon M, Laterza M, Kuniyoshi F, Gowdak M, et al. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. (2003) 285:H974–82. doi: 10.1152/ajpheart.01090.2002
30. Belanche Monterde A, Zubizarreta-Macho Á, Lobo Galindo A, Albaladejo Martínez A, Montiel-Company J. Mandibular advancement devices decrease systolic pressure during the day and night in patients with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. (2024). doi: 10.1007/s11325-023-02984-0 [Epub ahead of print].
31. Uniken Venema J, Knol-de Vries G, van Goor H, Westra J, Hoekema A, Wijkstra P. Cardiovascular and metabolic effects of a mandibular advancement device and continuous positive airway pressure in moderate obstructive sleep apnea: A randomized controlled trial. J Clin Sleep Med. (2022) 18:1547–55. doi: 10.5664/jcsm.9908
33. Bonsignore M, Esquinas C, Barceló A, Sanchez-de-la-Torre M, Paternó A, Duran-Cantolla J, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J. (2012) 39:1136–43. doi: 10.1183/09031936.00151110
34. Wang S, Keenan B, Wiemken A, Zang Y, Staley B, Sarwer D, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am J Respir Crit Care Med. (2020) 201:718–27. doi: 10.1164/rccm.201903-0692OC
35. Gruber A, Horwood F, Sithole J, Ali N, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. (2006) 5:22. doi: 10.1186/1475-2840-5-22
36. Coughlin S, Mawdsley L, Mugarza J, Calverley P, Wilding J. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. (2004) 25:735–41. doi: 10.1016/j.ehj.2004.02.021
37. Kayikcioğlu M, Oto A. Control and management of cardiovascular disease in Turkey. Circulation. (2020) 141:7–9. doi: 10.1016/j.ajpc.2021.100184
38. Huang P. A comprehensive definition for metabolic syndrome. Dis Model Mech. (2009) 2:231–7. doi: 10.1242/dmm.001180
39. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. IJMS. (2022) 23:786. doi: 10.3390/ijms23020786
40. Asoudeh F, Fallah M, Aminianfar A, Djafarian K, Shirzad N, Clark C, et al. The effect of Mediterranean diet on inflammatory biomarkers and components of metabolic syndrome in adolescent girls. J Endocrinol Invest. (2023) 46:1995–2004. doi: 10.1007/s40618-023-02027-1
41. Esposito K, Kastorini C, Panagiotakos D, Giugliano D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev Endocr Metab Disord. (2013) 14:255–63. doi: 10.1007/s11154-013-9253-9
42. Labarca G, Gower J, Lamperti L, Dreyse J, Jorquera J. Chronic intermittent hypoxia in obstructive sleep apnea: A narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. (2020) 24:751–60. doi: 10.1007/s11325-019-01967-4
43. Robinson G. Circulating cardiovascular risk factors in obstructive sleep apnoea: Data from randomised controlled trials. Thorax. (2004) 59:777–82. doi: 10.1136/thx.2003.018739
44. Assmann G. Genes and dyslipoproteinaemias. Eur Heart J. (1990) 11:4–8. doi: 10.1093/eurheartj/11.suppl_h.4
45. Shang W, Zhang Y, Wang G, Han D. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: A meta-analysis. Diabetes Obes Metab. (2021) 23:540–8. doi: 10.1111/dom.14247
46. Smith D, Cohen A, Ishman S. Surgical management of OSA in adults. Chest. (2015) 147:1681–90. doi: 10.1007/s13311-012-0141-x
47. McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: A matched controlled study. Am J Respir Crit Care Med. (2007) 175:190–5.
48. Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. (2012) 67:1081–9.
49. Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques J. A. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath. (2011) 15:665–72.
50. Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg. (2009) 64:329–34.
51. Mineiro MA, Silva PMD, Alves M, Papoila AL, Marques Gomes MJ, Cardoso J. The role of sleepiness on arterial stiffness improvement after CPAP therapy in males with obstructive sleep apnea: a prospective cohort study. BMC Pulm Med. (2017) 17:182.
52. Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. (2008) 134:686–92. doi: 10.1378/chest.08-0556
53. Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. (2012) 35:231–6.
56. Desplan M, Mercier J, Sabaté M, Ninot G, Prefaut C, Dauvilliers Y. A comprehensive rehabilitation program improves disease severity in patients with obstructive sleep apnea syndrome: a pilot randomized controlled study. Sleep Med. (2014) 15:906–12.
57. Maki-Nunes C, Toschi-Dias E, Cepeda FX, Rondon MU, Alves MJ, Fraga RF, et al. Diet and exercise improve chemoreflex sensitivity in patients with metabolic syndrome and obstructive sleep apnea. Obesity (Silver Spring), (2015) 23:1582–90. doi: 10.1002/oby.21126
58. Toschi-Dias E, Trombetta IC, Silva VJD, Maki-Nunes C, Cepeda FX, Alves M, et al. Diet associated with exercise improves baroreflex control of sympathetic nerve activity in metabolic syndrome and sleep apnea patients. Sleep Breath. (2019) 23:143–51. doi: 10.1007/s11325-018-1675-x
Keywords: obstructive sleep apnea, metabolic syndrome, continuous positive airway pressure, exercise, diet, surgery, meta-analysis
Citation: Liu J, Xu J, Guan S and Wang W (2024) Effects of different treatments on metabolic syndrome in patients with obstructive sleep apnea: a meta-analysis. Front. Med. 11:1354489. doi: 10.3389/fmed.2024.1354489
Received: 12 December 2023; Accepted: 22 February 2024;
Published: 07 March 2024.
Edited by:
Huiguo Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Ou Qiong, Guangdong Provincial People’s Hospital, ChinaKe Hu, Renmin Hospital of Wuhan University, China
Copyright © 2024 Liu, Xu, Guan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d3dieWNtdUAxMjYuY29t
 Jianing Liu
Jianing Liu Jiahuan Xu
Jiahuan Xu Shibo Guan
Shibo Guan Wei Wang
Wei Wang