- 1Gastroenterology and Liver Diseases Institute, Shamir Medical Center, Zerifin, Israel
- 2Pre Clinical Department, Shamir Medical Center, Zerifin, Israel
Introduction: There is limited data on the histopathological effects of hemostatic agents (HAs) used during endoscopic submucosal dissection (ESD). We used an in vivo porcine model to compare the tissue effects of tranexamic acid (TXA) and adrenaline (epinephrine) compared to controls.
Methods: Standard ESD, using a 2 mm flash-knife, was performed in three pigs. Four resections were performed in the stomach and rectum of each pig. Injectate contained 4% succinylated gelatin solution and indigo carmine, plus either TXA, adrenaline, or neither. Pigs were euthanized after 7 days and evaluated by two blinded pathologists.
Results: Twenty-four defects were analyzed. Within each animal no significant histopathological changes were noted between the defects, but differences were noted between the animals. In the stomachs of the TXA and adrenaline pigs, pathology revealed a clear ulcer in the mucosa/submucosa with abundant granulation tissue, while the muscular layer was unaffected. In the control pig’s stomach, the lesions were deeper, transmurally distributed, with inflammation of the muscular and adventitia layers accompanied by severe inflammation and necrosis. Fewer differences were noted in the rectum.
Conclusion: For ESD, HAs such as TXA and adrenaline may have protective effects on the depth and extent of injury to the underlying tissue.
Introduction
Endoscopic submucosal dissection (ESD) has become a common minimally invasive therapy for early colonic and gastric neoplasia. One of the more serious adverse events is immediate or delayed bleeding which can occur in up to 11.9% of cases (1, 2). To prevent bleeding, several techniques are used including vessel coagulation and the use of adrenaline (epinephrine) as part of injection gel (1). While adrenaline is the most commonly used hemostatic agent, it may lead to increased postprocedural pain and prolong patient observation after the procedure (3).
Tranexamic acid (TXA) is a synthetic derivative of lysine that exerts antifibrinolytic effects by inhibition of lysine binding sites on plasminogen molecules and therefore stabilizes the fibrin meshwork produced by secondary hemostasis (4). During the past few years, TXA has been “rediscovered” and is now used in several fields of medicine to reduce bleeding and transfusion requirements (5–7).
The effectiveness of systemic TXA in gastrointestinal bleeding is controversial. A large, randomized controlled trial of intravenous TXA found that it is unlikely to confer additional benefits beyond the current standard of care in all patients with severe upper or lower GI bleeding (8).
Topical use of TXA may be more beneficial than systemic use as it may provide a higher drug concentration on the wound surface without causing systemic side effects (9). Moreover, according to a few animal studies, it may have a positive effect on wound healing and may reduce the development of fibrosis (10, 11). In gastroenterology (GI) practice, there is only limited data for the topical use of TXA, and there is no data on how TXA affects histopathological changes in GI tract.
Recently, there has been increased attention to the possible histological effects of injectates used during ESD. For example, a commonly used submucosal lifting agent (Orise) was found to be associated with a foreign body-like granulomatous reaction (12).
Therefore, in this study we primarily aimed to assess the safety and histopathological changes related to the submucosal injection of TXA in ESD specimens. This was performed in an in vivo porcine model and compared with submucosal injections containing adrenaline and a control group. The secondary aim of the study was to assess differences in intraprocedural and postprocedural bleeding between the injectate substances and their influence on surgical field visualization.
Materials and methods
This study was designed as a prospective, blinded, controlled pilot study in live animals. All animal experiments were performed at the animal endoscopy center of Shamir Medical Center after the experimental protocol was approved by the Institutional Animal Care and Use Committee of Shamir Medical Center (Ethics number 54/2021). The study was conducted between January and February 2022.
Animal pre-procedure preparation
Three female domestic pigs with a mean weight of 48 kg were used. The animals were fasted from solid food for 24 h before ESD but were allowed full access to water. For bowel preparation, each animal received 3 L of polyethylene glycol via orogastric tube 24 h prior to the procedure.
Endoscopy and ESD
General anesthesia was provided by a veterinarian and performed by using ketamine and isoflurane, nitrous oxide, and O2 after endotracheal intubation. Continuous pulse oximetry and electrocardiograph findings were monitored throughout the procedure.
Each animal was assigned a different treatment substance (either adrenaline, TXA, or the control group with no hemostatic agent).
For the first animal (Pig1), the standard 9 mL submucosal injectate comprising succinylated gelatin (Gelofusine; B. Braun, Crissier, Switzerland), 1:100000 adrenaline (epinephrine) and indigo carmine, with 1 mL of saline 0.9% was used.
For the second pig (Pig2), 9 mL submucosal injectate comprising succinylated gelatin and indigo carmine with 1 mL of TXA (100 mg/mL) was used.
For the third pig (Pig3), 10 mL of submucosal injectate comprising succinylated gelatin and indigo carmine.
ESD procedures were performed by two attending advanced endoscopists. The endoscopists were blinded to the randomization of the injection substrates until after all study procedures were performed. The syringes for submucosal injection were prepared outside of the endoscopy room. The same volume (20 mL) of injectate was used for each ESD resection.
All procedures were performed by using a commercially available endoscope (Pentax EC-380LKp). Before resection areas median size 3 cm in diameter each were marked at 4 sites in the body of the stomach and 4 in the rectum, mimicking lesions and were easily accessible by the endoscope. Standard ESD using a 2 mm flush-knife (Fuji Flush Knife BTS, Fujifilm, Tokyo, Japan) was used to resect each lesion. Circumferential incisions with standard dissection were applied for gastric lesions, while tunneling techniques were applied for colorectal lesions. At the end of the procedure, all visible vessels were coagulated. No antacids or proton pump inhibitors were given to the pigs.
Post-ESD evaluation
For the week following the ESD, daily assessments of general health parameters including monitoring for signs of overt bleeding were performed on the animals. Complete blood counts were taken before the procedure and on day 3 after procedure. Diagnostic gastroscopy and colonoscopy were performed after 1 week of follow up (day 7); afterwards, the animals were euthanized by a veterinarian. The 1 week length of time was chosen as it would ensure that ulcers were still visible at each ESD site, while also falling within the time that post-procedure complications such as bleeding often occur.
Samples from the gastric and rectal ESD sites (two samples for each ESD site) from each of the 3 pigs were harvested and fixed in 4% formaldehyde. The tissues were trimmed, placed in embedding cassettes, and processed routinely for paraffin embedding. A total of 48 cassettes were prepared per animal. Paraffin sections (4 microns thick) were cut, placed on glass slides, and stained with Hematoxylin & Eosin (H&E).
Outcome parameters and statistics
During the ESD procedures, an assessment of intraprocedural bleeding was performed. Bleeding less than 1 min was defined as minor, lasting more than 1 min with partial interference in visualization or in which coagulation treatment was needed was defined as moderate, and pulsatile bleeding or complete interference with visualization was defined as severe.
The slides were subjected to histopathological evaluation by two expert veterinary pathologists who were blinded to the study groups. Additionally, each pathologist graded the histopathology slides while blinded from the scores given by the other pathologist. The histological slides were examined, described, and scored using a semi-quantitative grading scale. This established 5-point scale graded the severity of the histopathological changes in terms of inflammation, necrosis, and granulation (13).
Grade 1—Minimal inflammation/necrosis/granulation tissue. Findings (<10 inflammatory cells per ×20 magnification, up to 25% necrosis/granulation tissue).
Grade 2—Mild inflammation/necrosis/granulation tissue. Findings (>10 <25 inflammatory cells per ×20 magnification, 25–50% necrosis/granulation tissue).
Grade 3—Moderate inflammation/necrosis/granulation tissue. Findings (>25 <50 inflammatory cells per ×20 magnification, 50–75% necrosis/granulation tissue).
Grade 4—Severe inflammation/necrosis/granulation tissue. Findings (>50 inflammatory cells per ×20 magnification, 75–100% necrosis/granulation tissue).
Finally, a global score (from 0 to 4) was given to the stomach and rectum of each animal as a summary of the severity of the histopathological findings.
In the event of a difference in any of the scores between the two blinded pathologists, the pathologists would discuss the findings until a consensus score was agreed upon.
Given the small number of pigs used, the study was not powered for formal statistical comparisons between the groups.
Results
Procedures
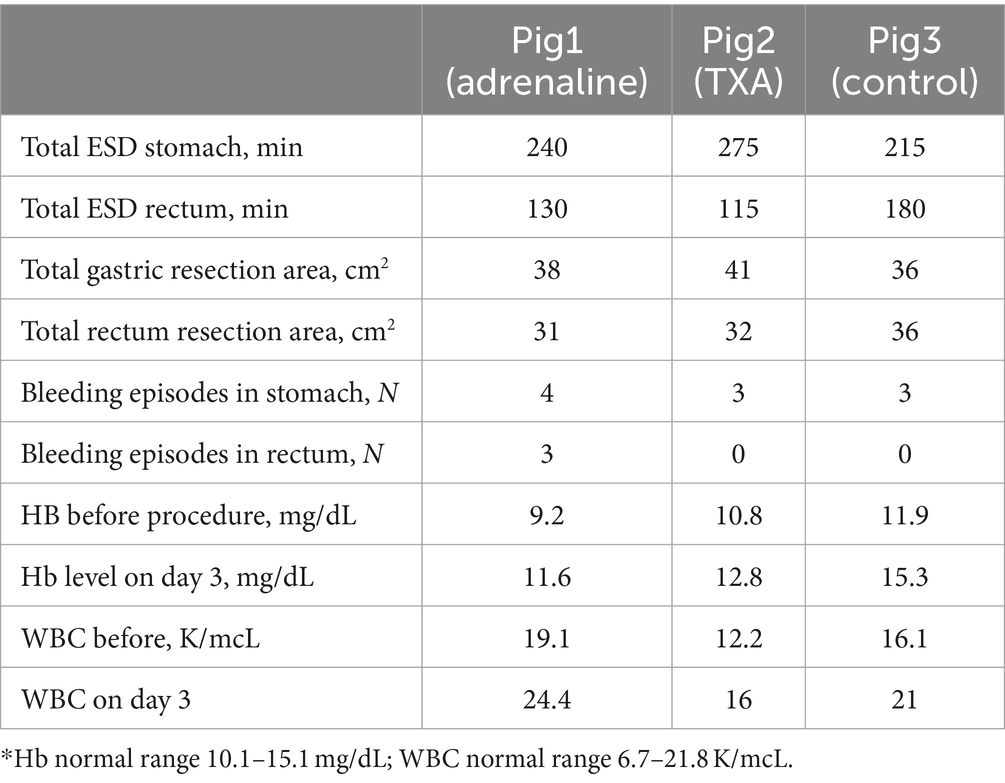
Eight ESDs were performed on each animal, 4 in the proximal stomach body and 4 in the rectum. Details of the procedures can be found in Table 1.
Repeat endoscopies on day 7
Endoscopic examinations were performed after 1 week of follow-up. There was no significant difference in the gross appearance of the wound defects between the groups. All defects had the appearance of clean-based ulcers.
Histology
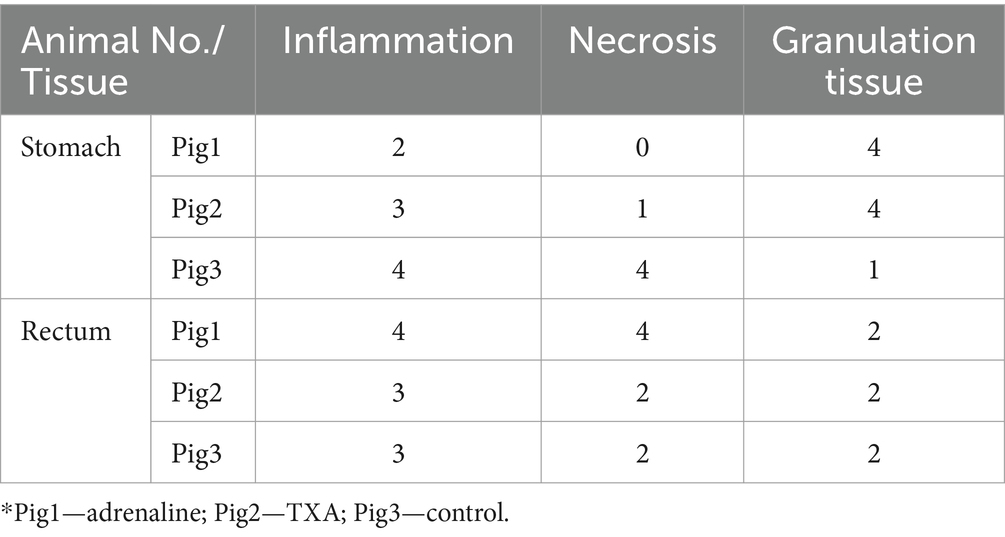
Within each animal, there were no significant histopathological changes noted between the defects. However, differences were noted in histopathological changes between the animals. In the stomach of first 2 animals (adrenaline and TXA), pathological examination revealed a clear ulcer in the mucosa and submucosa with an abundant amount of granulation tissue and mild inflammation, while the muscular layer was unaffected and intact (Figures 1a,b). There was a marked edema in the junction between the lesion and the intact mucosa and submucosa. In the third animal’s stomach (control group), the lesions were deeper, transmurally distributed, with inflammation of the muscular and adventitia layers accompanied by severe inflammation and necrosis (Figure 1c). Thus, in the stomach, Pig3 had higher inflammation and necrosis scores, and lower granulation score, than the other animals (Table 2).
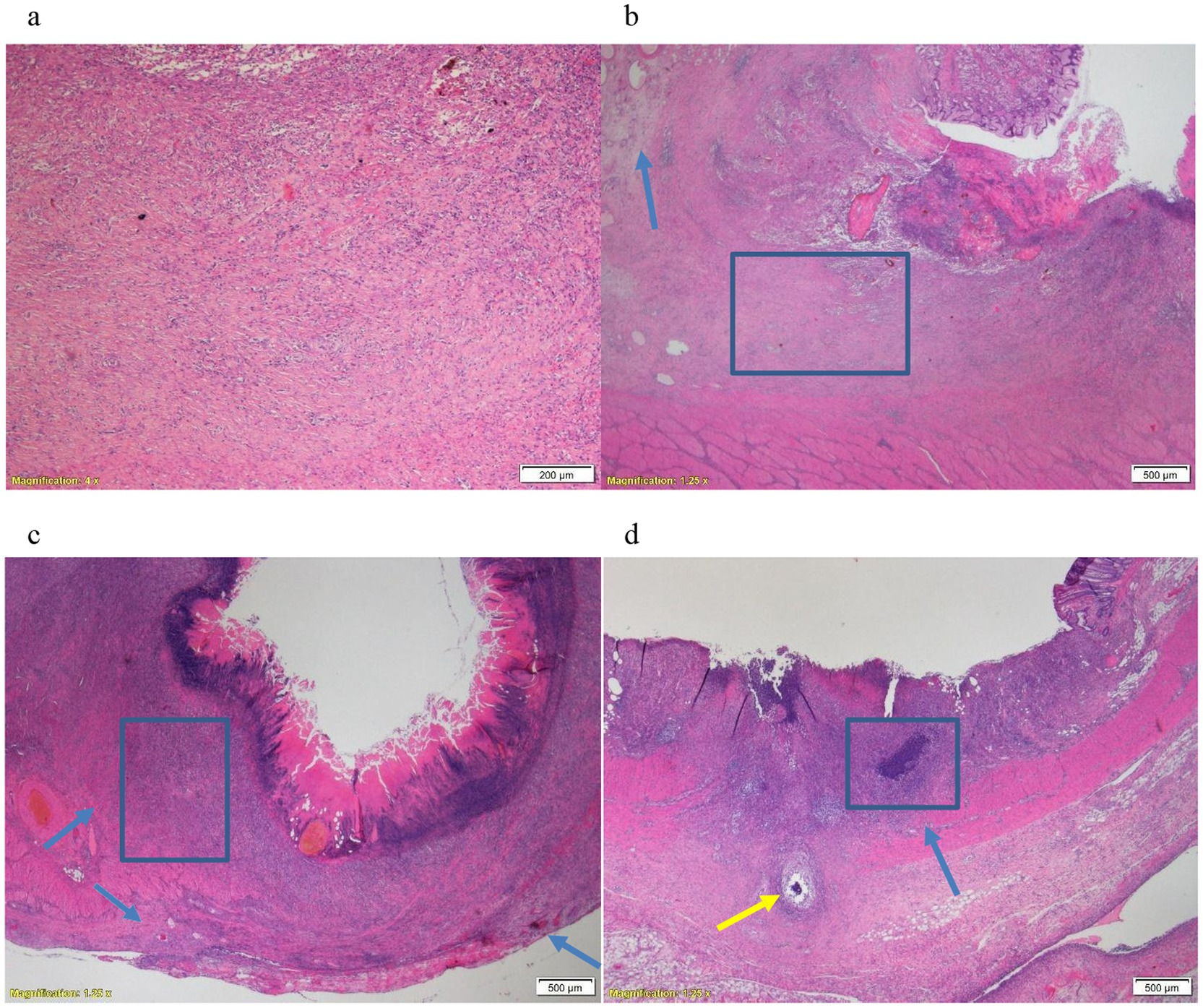
Figure 1. (a) Animal Pig2, stomach. Junction of the lesion, abundant amount of granulation tissue and a moderate inflammatory reaction with no apparent necrosis. X4, H&E. (b) Animal Pig2, stomach. Junction of the lesion, edema under the intact mucosa (arrow) and an intact muscular layer. X1.25, H&E. (c) Animal Pig3, stomach. Very deep lesion, transmural involving the muscular layer and the adventitia with severe inflammation and necrosis (arrows). X1.25, H&E. (d) Animal Pig1, colorectum. Deep lesion with severe inflammation and necrosis (blue arrow) and perforation of the muscular layer (yellow arrow). X1.25, H&E.
The differences between the lesions in the rectum were less observable and prominent between the animals compared to the stomach lesions. Overall, there was more severe inflammation and necrosis in Pig1 (adrenaline) (Figure 1d).
There was good agreement between the two blinded pathologists in terms of these findings. Of the 18 scores given in Table 2, only two scores differed between the pathologists, and these only differed by 1 grade.
The summary semi-quantitative histological score is shown in Figure 2. There were no differences between the two pathologists in terms of these scores.
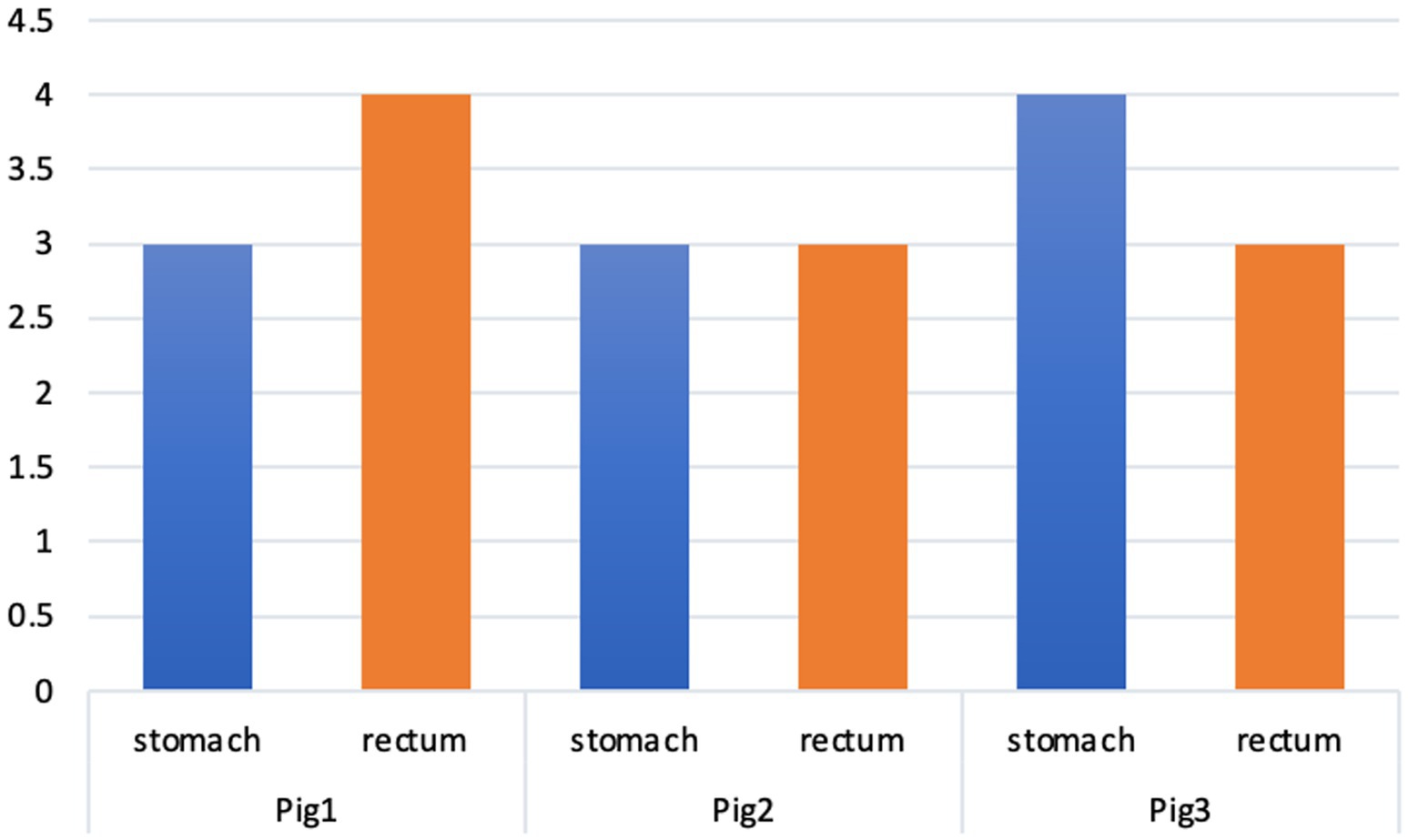
Figure 2. Summary of the histopathological scores in the different animals. *Pig1—adrenalin; Pig2—TXA; Pig3—control.
The intact tissue surrounding the lesions appeared normal in all samples. Some edema was noted on the edges of the lesion in some samples. There was no evidence of small vessel thrombosis in any specimens.
Bleeding
There were no episodes of severe intraprocedural bleeding, but several moderate bleeding episodes occurred (Table 1). There was a similar number between the groups in the stomach, but in the rectum they only occurred in the adrenaline group. All intraprocedural bleeding either stopped spontaneously or with the use of coagulation from the ESD knife.
The coagulation hemostatic grasper was not required. There was no evidence of overt GI bleeding from any of the animals during the 7 days of follow-up, nor was there any difference in general health parameters. Hemoglobin and WBC increased in all animals on day 3 of follow-up.
Discussion
This is the first study performed in an in vivo porcine model to evaluate the histopathologic changes and safety of TXA injected during mucosal resection. In this blinded, randomized, and controlled study, adding a hemostatic agent to the submucosal injectate was associated with less inflammation and necrosis and significant more granulation tissue formation. The positive effects were more prominent in the animals that received TXA and appeared universally in all resection locations. TXA was not associated with significant injury or capillary thrombosis in the tissue adjacent to the resection specimens.
Our findings are consistent with some other studies in animals in which using TXA as a hemostatic agent was associated with an improvement in the healing process. Degirmenci et al. (14) found that topical application of TXA on experimentally induced osteochondral defects in rabbits improved the healing time and was associated with more organized regeneration of subchondral and cartilage tissues. Çevik et al. (15) showed that the topical use of TXA in rats with diaphyseal femoral fractures after fixation exhibited significantly better bone healing than the control group or systemic use of TXA. Additionally, Yuan et al. (16) conducted a study to determine whether TXA could repair the skin barrier by means of tight intercellular junctions using damaged skin models and revealed that TXA can accelerate skin barrier recovery.
It has been proposed that these positive effects on healing may be related to the fibrin-independent effect of TXA on immune function via the plasminogen pathway which is known as an inflammatory regulator that can accelerate wound healing (17–19). For example, it has been shown that administration of TXA in heterozygous Plg+/− mice can restore plasminogen’s effect and improve healing of radiation-induced dermatitis (20). Another recent study evaluated the effects of TXA in patients following cardiac surgery. This study revealed that TXA-mediated plasmin blockade modulates the immune system, reduces injury-induced immunosuppression, and enhanced the expression of immune-activating markers (21).
The beneficial effects of TXA on wound healing may also be related to its beneficial effects on preventing infection. Two studies have shown that TXA reduced the rate of post-operative infections and the need for prolonged antibiotics in patients after orthopedic surgery and cardiac surgery (21, 22).
Epinephrine injection has also been shown to produce positive histopathologic changes during wound healing in animal models and humans. Shirazi et al. (23) performed an experimental hypospadias surgery with topical epinephrine administration as a hemostatic agent. They revealed higher volumes of collagen bundles, and a larger population of fibroblasts were in the histological sections when epinephrine was used which suggested better postoperative structural outcomes. Gacto et al. (24) also showed that subcutaneous administration of adrenaline-lidocaine reduced intraoperative bleeding and accelerated re-epithelialization.
Besides its positive effects on histopathology, our study’s secondary outcomes showed other areas for which TXA injection during ESD appears promising. In our study, the use of TXA was not associated with any significant difference in procedure times, technical difficulties, or surgical field visualization in TXA. Additionally, there was no difference in post-procedural bleeding between groups. These results suggest that TXA is a promising agent and safe for future studies in humans, despite this study being limited by the small number of animals and ESD resections performed.
This study has some limitations. Unfortunately, due to the small number of animals used, formal statistical comparisons between the groups could not be performed, and extrapolating these findings from a small number of animals should be done with caution. Additionally, as with all pathological evaluations, there is an element of subjectivity to reporting the histopathological findings. However, the utilization of an established scoring system used by two blinded pathologists helps mitigate this concern.
In conclusion, we have shown that the topical use of TXA, as well as epinephrine, as an injectate during endoscopic procedures is safe. TXA may also have positive histological effects on post-procedural wound healing.
Future studies assessing its effects during ESD in humans are certainly warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of Shamir Medical Center. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AB: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. SM: Writing – original draft, Investigation, Data curation. DC: Writing – review & editing, Writing – original draft, Supervision. VR: Writing – review & editing. YS-T: Writing – review & editing, Methodology, Investigation, Data curation. HS: Writing – review & editing, Supervision. SV: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Special thanks to Niva & Moti Volovelsky for supporting the research study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park, CH, and Lee, SK. Preventing and controlling bleeding in gastric endoscopic submucosal dissection. Clin Endosc. (2013) 46:456–62. doi: 10.5946/ce.2013.46.5.456
2. Akintoye, E, Kumar, N, Aihara, H, Nas, H, and Thompson, CC. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open. (2016) 4:E1030–44. doi: 10.1055/s-0042-114774
3. Rex, DK, Lahr, RE, Peterson, MM, and Vemulapalli, KC. Impact of including epinephrine in the submucosal injectate for colorectal EMR on postprocedural pain: a randomized controlled trial. Gastrointest Endosc. (2022) 95:535–539.e1. doi: 10.1016/j.gie.2021.11.043
4. Cai, J, Ribkoff, J, Olson, S, Raghunathan, V, Al-Samkari, H, DeLoughery, TG, et al. The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol. (2020) 104:79–87. doi: 10.1111/ejh.13348
5. Shakur, H, Roberts, I, Bautista, R, Caballero, J, Coats, T, Dewan, Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. (2010) 376:23–32. doi: 10.1016/S0140-6736(10)60835-5
6. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. (2017) 389:2105–16. doi: 10.1016/S0140-6736(17)30638-4
7. Wu, Y, Zeng, Y, Bao, X, Xiong, H, Fan, X, and Shen, B. Application of tranexamic acid and diluted epinephrine in primary total hip arthroplasty. Blood Coagul Fibrinolysis. (2018) 29:451–7. doi: 10.1097/MBC.0000000000000743
8. HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. (2020) 395:1927–36. doi: 10.1016/S0140-6736(20)30848-5
9. Ausen, K, Pleym, H, Liu, J, Hegstad, S, Nordgård, HB, Pavlovic, I, et al. Serum concentrations and pharmacokinetics of tranexamic acid after two means of topical administration in massive weight loss skin-reducing surgery. Plast Reconstr Surg. (2019) 143:1169e–78e. doi: 10.1097/PRS.0000000000005620
10. Björlin, G, and Nilsson, IM. The effect of antifibrinolytic agents on wound healing. Int J Oral Maxillofac Surg. (1988) 17:275–6. doi: 10.1016/S0901-5027(88)80056-0
11. Sarı, A, Dinçel, YM, Karabağ, S, and Çetin, MÜ. Histopathological and immunohistochemical investigation of the local and systemic effects of tranexamic acid on the healing of the Achilles tendon in rats. Jt Dis Relat Surg. (2021) 32:152–61. doi: 10.5606/ehc.2021.76301
12. Colak, Y, Hasan, B, Tandon, K, Khan, S, Wadhwa, V, Bejarano, PA, et al. Potential clinical complications of Orise™ gel use, a new submucosal lifting agent: experience from a tertiary care center and review of the literature. Ann Gastroenterol. (2022) 35:407–13. doi: 10.20524/aog.2022.0726
13. Schafer, KA, Eighmy, J, Fikes, JD, Halpern, WG, Hukkanen, RR, Long, GG, et al. Use of severity grades to characterize histopathologic changes. Toxicol Pathol. (2018) 46:256–65. doi: 10.1177/0192623318761348
14. Degirmenci, E, Ozturan, KE, Sahin, AA, Yilmaz, F, and Kaya, YE. Effects of tranexamic acid on the recovery of osteochondral defects treated by microfracture and acellular matrix scaffold: an experimental study. J Orthop Surg Res. (2019) 14:105. doi: 10.1186/s13018-019-1144-7
15. Çevik, HB, Eceviz, E, Çilingir Kaya, ÖT, Ercan, F, and Çeçen, GS. The effect of topical and systemic tranexamic acid on fracture healing in rats. Acta Orthop Traumatol Turc. (2020) 54:207–12. doi: 10.5152/j.aott.2020.02.44
16. Yuan, C, Wang, XM, Yang, LJ, and Wu, PL. Tranexamic acid accelerates skin barrier recovery and upregulates occludin in damaged skin. Int J Dermatol. (2014) 53:959–65. doi: 10.1111/ijd.12099
17. Shen, Y, Guo, Y, Mikus, P, Sulniute, R, Wilczynska, M, Ny, T, et al. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood. (2012) 119:5879–87. doi: 10.1182/blood-2012-01-407825
18. Shen, Y, Guo, Y, Wilczynska, M, Li, J, Hellström, S, and Ny, T. Plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. J Transl Med. (2014) 12:5. doi: 10.1186/1479-5876-12-5
19. Sulniute, R, Shen, Y, Guo, YZ, Fallah, M, Ahlskog, N, Ny, L, et al. Plasminogen is a critical regulator of cutaneous wound healing. Thromb Haemost. (2016) 115:1001–9. doi: 10.1160/TH15-08-0653
20. Fallah, M, Shen, Y, Brodén, J, Bäckman, A, Lundskog, B, Johansson, M, et al. Plasminogen activation is required for the development of radiation-induced dermatitis. Cell Death Dis. (2018) 9:1051. doi: 10.1038/s41419-018-1106-8
21. Draxler, DF, Yep, K, Hanafi, G, Winton, A, Daglas, M, Ho, H, et al. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. (2019) 3:1598–609. doi: 10.1182/bloodadvances.2019000092
22. Gupta, A, Singh, M, Raina, P, Singh, S, Ahmad, S, Imran, S, et al. Post-surgical wound care in orthopedics: role of tranexamic acid. J Evol Med Dent Sci. (2015) 4:5716–20. doi: 10.14260/jemds/2015/835
23. Shirazi, M, Rajabalian, MB, Noorafshan, A, Karbalay-Doust, S, Jahanabadi, Z, and Ahmed, F. The comparison of the effect of phenylephrine and epinephrine on penile spongy tissue in rats after experimental hypospadias surgery: a quantitative stereological study. BMC Urol. (2019) 19:55. doi: 10.1186/s12894-019-0483-3
Keywords: endoscopic submucosal dissection, endoscopy, tranexamic acid, bleeding, complications
Citation: Bermont A, Matalon S, Cohen DL, Richter V, Siman-Tov Y, Shirin H and Vosko S (2024) The effects of tranexamic acid on the histopathology of defect healing in an in vivo porcine model after gastric and colonic endoscopic submucosal dissection. Front. Med. 11:1352967. doi: 10.3389/fmed.2024.1352967
Edited by:
Hung Leng Kaan, National University Hospital, SingaporeReviewed by:
Eiji Kawamoto, Mie University, JapanSai Sailesh Kumar Goothy, NRI Institute of Medical Sciences, India
Copyright © 2024 Bermont, Matalon, Cohen, Richter, Siman-Tov, Shirin and Vosko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton Bermont, YmVybW9udEBkb2N0b3IuY29t
 Anton Bermont
Anton Bermont Shay Matalon1
Shay Matalon1 Daniel L. Cohen
Daniel L. Cohen Sergei Vosko
Sergei Vosko