- 1Department of Intensive Care, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
- 2Department of Internal Medicine, University Hospital of Patras, Patras, Greece
Background: Limiting the fluid bolus (FB) volume may attenuate side effects, including hemodilution and increased filling pressures, but it may also reduce hemodynamic responsiveness. The minimum volume to create hemodynamic effects is considered to be 4 mL/kg. In critically ill patients, the hemodynamic effects of FB with this volume have not been adequately investigated and compared to higher quantities. We hypothesized that a standardized FB approach using 4 mL/kg has comparable hemodynamic and metabolic effects to the common practice of physician-determined FB in critically ill patients.
Methods: We conducted post hoc analysis of two trials in non-selected critically ill patients with central venous-to-arterial CO2 tension (PvaCO2) >6 mmHg and no acute bleeding. All patients received crystalloids either at a physician-determined volume and rate or at 4 mL/kg pump-administered at 1.2 L/h. Cardiac index (CI) was calculated with transthoracic echocardiogram, and arterial and venous blood gas samples were assessed before and after FB. Endpoints were changes in CI and oxygen delivery (DO2) >15%.
Results: A total of 47 patients were eligible for the study, 15 of whom received physician-determined FB and 32 of whom received standardized FB. Patients in the physician-determined FB group received 16 (12–19) mL/kg at a fluid rate of 1.5 (1.5–1.9) L/h, compared to 4.1 (3.7–4.4) mL/kg at a fluid rate of 1.2 (1.2–1.2) L/h (p < 0.01) in the standardized FB group. The difference in CI elevations between the two groups was not statistically significant (8.8% [−0.1–19.9%] vs. 8.4% [0.3–23.2%], p = 0.76). Compared to physician-determined FB, the standardized FB technique had similar probabilities of increasing CI or DO2 by >15% (odds ratios: 1.3 [95% CI: 0.37–5.18], p = 0.66 and 1.83 [95% CI: 0.49–7.85], p = 0.38).
Conclusion: A standardized FB protocol (4 mL/kg at 1.2 L/h) effectively reduced the volume of fluid administered to critically ill patients without compromising hemodynamic or metabolic effects.
Introduction
Fluid bolus (FB) is an essential technique used in the management of critically ill patients that involves the rapid administration of intravenous fluids under strict control and evaluating the patient’s hemodynamic response (1). The primary objectives of this technique are to increase cardiac output and, thus, oxygen delivery via the Frank–Starling law (2). The amount and rate of fluid infusion are essential components of this technique (1). Insufficient volume and rate may not result in a significant preload increase, leading to misleading results and suboptimal treatment of fatal hypovolemia (3, 4). On the other hand, increased volume can significantly increase cardiac pressures, possibly leading to pulmonary or peripheral edema, which can be detrimental for critically ill patients (5). Consequently, it is clinically significant to investigate the optimal fluid volume and rate for performing FB.
The optimal fluid volume and rate for a fluid challenge in critically ill patients has not yet been fully determined. In one study, an FB of approximately 1 L administered over 5–10 min significantly increased the cardiac index (CI) and filling pressures in healthy volunteers (6). According to standard practice, 500 mL of fluid is administered to critically ill patients at a rate of 1 L/h (7). Slower rates of FB have not been proven superior in terms of patients’ outcomes (8). Nonetheless, blood volume can vary according to body measurement. Consequently, a constant fluid dose for all patients may be insufficient or excessive for some patients. Prior research has shown that 4 mL/kg of crystalloid fluids is sufficient to increase mean systemic filling pressure and cause a significant elevation in CI in cardiac surgery patients (4). Nevertheless, the extent to which this finding may be applied to the wider population of critically ill individuals requiring FB to address insufficient CI for meeting their metabolic requirements has not been thoroughly examined.
A substantial increase in the difference between mixed venous and arterial CO2 partial pressure, particularly when it surpasses 6 mmHg, is a crucial marker of insufficient peripheral perfusion in clinical settings (9). This measure, obtained from Fick’s equation for CO2, is directly linked to the ratio of CO2 generation (VCO2) to cardiac output, assuming a wide but linear correlation between CO2 partial pressure and CO2 content within physiological limits (10). A high PvaCO2 value indicates that the cardiac output is not enough to fulfill the metabolic needs, as seen by the inadequate removal of CO2 related to its production (11). In such instances in the context of critically ill patients, it becomes imperative to consider the administration of a FB in order to enhance cardiac output (11, 12). Hence, it is crucial to evaluate the efficacy of FB techniques in patients with high PvaCO2 levels, since improper use of FB in these patients may result in less than ideal hemodynamic and/or metabolic results.
This study’s objective was to assess the hemodynamic and metabolic effects of the FB technique using a physician-determined volume and rate or a standardized volume of 4 mL/kg at a rate of 1.2 L/h. We hypothesized that in critically ill patients with limited peripheral perfusion, as indicated by elevated central venous-to-arterial CO2 tension (PvaCO2), a standardized fluid challenge technique adapted to body weight could yield comparable hemodynamic outcomes to existing practices, but with a reduced volume of fluid administered.
Methods
Design and setting
In this post hoc analysis, we examined the data of patients enrolled in two prospective studies conducted at the 33-bed Intensive Care Unit (ICU) of Brugmann University Hospital in Brussels, Belgium, focusing on critically ill patients with PvaCO2 levels greater than 6 mmHg who received FB. In the first cohort of patients, treated between January and June 2015, a physician-determined FB technique was applied, i.e., the rate and volume of fluid administration were at the attending physician’s discretion. The second cohort of patients, treated from September 2021 to March 2023, was managed under a standardized FB protocol (standardized technique; 1.2 L/h, 4 mL/kg per actual body weight). Both cohorts received FB with Plasma-Lyte A (Baxter Healthcare, Deerfield, IL) and were assessed for fluid responsiveness using transthoracic echocardiography. The methods and the results of the first trial have been previously described (13). The second cohort of patients represents a part of an ongoing prospective observational trial (ISRCTN58464956). Briefly, similar to the first study, this second study evaluated patients receiving Plasma-Lyte A (Baxter Healthcare, Deerfield, IL), assessing fluid responsiveness through transthoracic echocardiography and venous and arterial blood gas analysis. The objective of this ongoing trial is to explore the relationship between changes in PvaCO2 and capillary refilling time during FB. The inclusion of this unpublished study is justified by its methodological consistency with the first study, while employing a different approach to FB, thus offering a unique opportunity to compare outcomes. Ethical approval for the study protocols (CE2014/122 and CE2021/29) was granted by the local ethics committee, and written informed consent was obtained from each patient or their next of kin.
Inclusion and exclusion criteria
Patients with PvaCO2 > 6 mmHg in whom the attending physician decided for an FB of crystalloids at any time of their stay in the ICU were considered eligible for this study. Each patient was assessed once.
The exclusion criteria were: (1) patients younger than 18 years old; (2) patients not equipped with jugular or subclavian venous catheter and arterial catheter; (3) measurement of cardiac output with cardiac ultrasound not possible due to lack of acoustic window; (4) patients receiving extracorporeal membrane oxygenation support; (5) partial pressure of CO2 (PCO2) exceeding 75 mmHg in venous or arterial blood gas analysis, as beyond this limit, the association between PCO2 and CO2 content may not be linear; (6) atrial fibrillation, to prevent any bias in the calculation of cardiac output through the evaluation of stroke volume using transthoracic echocardiography; (7) other simultaneous interventions (i.e., introduction or increase in inotrope dosage, previous FB, ventilator mode changes, or the introduction of mechanical ventilation) within 30 min before fluid administration; and (8) acute bleeding related to hemodynamic instability or need for transfusion of red blood cells.
Data and sample collections
Demographics, the type of fluids used for FB, the reason for FB, concomitant treatments (mechanical ventilation, inotropic agents), and laboratory data were collected for each patient. The Acute Physiology and Chronic Health Evaluation (APACHE) II score upon admission was used to assess disease severity. Sepsis was defined according to the Sepsis-3 definition (14). Before and after FB, we performed arterial and central venous blood gas analyses (which were sampled simultaneously), including hemoglobin, arterial and venous oxygen pressure (PaO2 and PvO2, respectively), and oxygen saturation (SaO2 and ScvO2). Central venous pressure (CVP) was also evaluated.
The arterial and venous oxygen content (CaO2, CvO2), oxygen delivery (DO2), oxygen consumption (VO2), and oxygen extraction ratio were computed using validated formulas (15). Also, PvaCO2 and its difference ratio with arterial–venous oxygen content (PvaCO2/CavO2) were calculated as indicators of the adequacy of peripheral perfusion and of tissue hypoxia (16), respectively.
Cardiac output calculation
An assessment of all patients was conducted using Doppler transthoracic echocardiography (GE Healthcare Vivid S5) both prior to and following the administration of FB. We determined the blood velocity time integral (VTI) of the left ventricular outflow tract (LVOT) prior to administering FB. In order to compute stroke volume and CI, the diameter of the LVOT was assessed in the parasternal long-axis view at the insertion sites of the aortic cusp., situated below the aortic valve. Immediately following FB, we repeated the measurements. Both measurements were archived for offline analysis. The mean velocity time integral was computed by averaging the values obtained from three consecutive velocity trajectories. We utilized the identical value of LVOT diameter to compute stroke volume and CI both prior to and following FB. Considering that the inter-examination least significant change in velocity time integral measurements is 11% (ranging from 5 to 18%) (17), we defined fluid responders as patients who exhibit an increase in cardiac index CI greater than 15% (18).
Study endpoints
The study’s primary endpoint was an increase of >15% in CI and DO2. The total given volume and changes in PvaCO2, ScvO2, lactate, and oxygen consumption were secondary endpoints.
Analysis plan
There was no formal power analysis conducted for this exploratory study. Instead, all available patients in the trials served as the sample size for the analysis.
R was used to conduct statistical analyses via the R-studio interface (www.r-project.org, R version 3.3.1). For each variable analyzed, descriptive statistics were computed. Absolute changes (Δ = After FB value – Before FB value) and relative changes (d = [(After FB value – Before FB value) / Before FB value] × 100) of different variables were evaluated. Continuous variables were expressed as medians with interquartile ranges, and discrete variables as counts (percentages). Categorical variables were compared using Fisher’s exact test, and a Wilcoxon signed-rank test was used to compare continuous variables. A binomial logistic regression analysis was performed to determine the impact of FB on the probability of fluid responsiveness. We calculated odds ratios with 95% confidence intervals (95% CI). A p value <0.05 was employed to define statistical significance.
Results
Study population
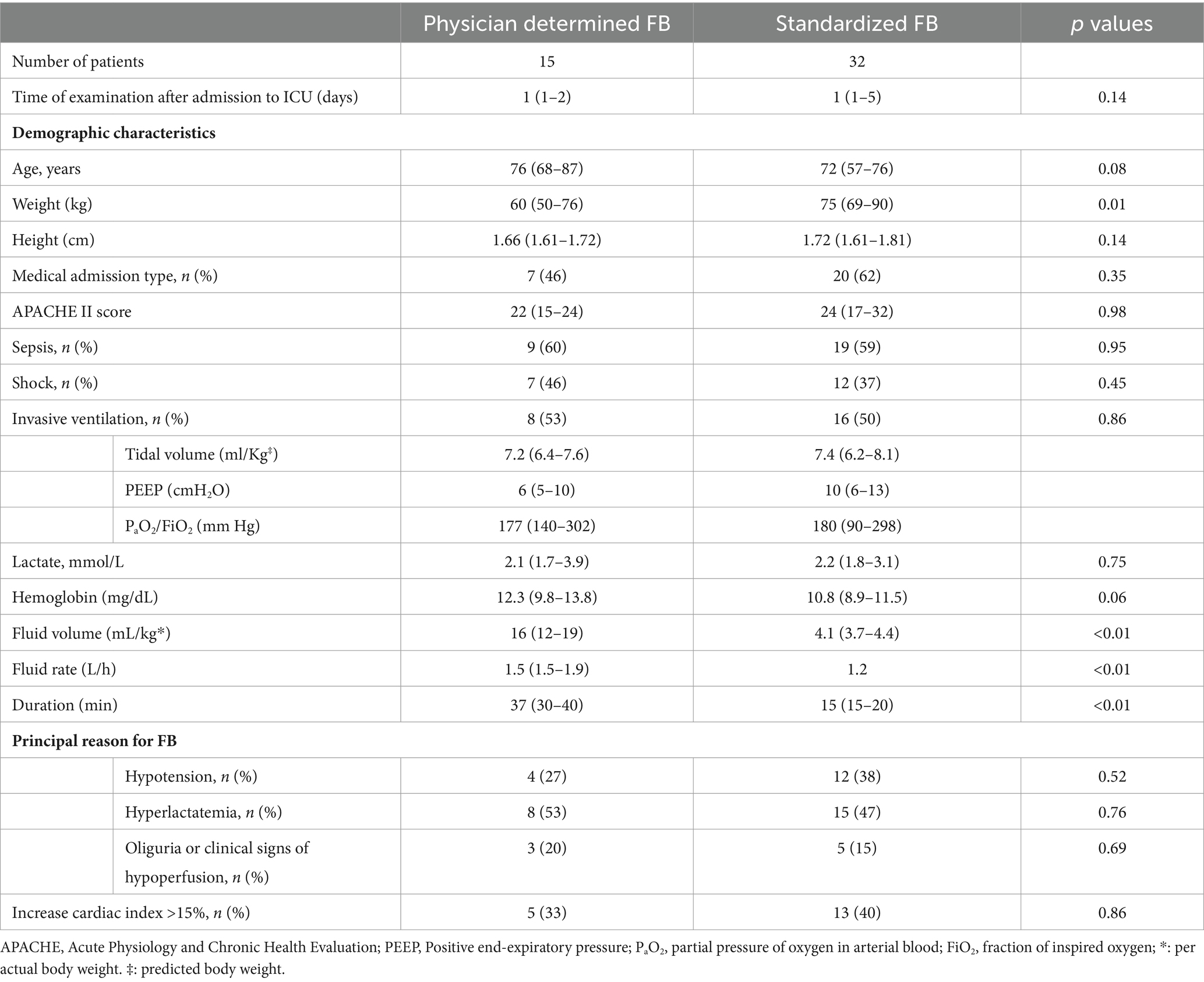
Of the 47 patients who met the entry criteria and were analyzed, 15 underwent treatment with FB wherein the volume and rate were determined by the attending physician, while 32 were treated using a standardized FB technique. The group treated with the physician-determined FB received more fluids, averaging 16 (12–19) mL/kg compared to 4.1 (3.7–4.4) mL/kg in the standardized group (p < 0.01). They also received fluids at a faster rate, with an average of 1.5 (1.5–1.9) L/h versus 1.2 (1.2–1.2) L/h (p = 0.01) in the standardized group. Tables 1, 2 reveals no significant baseline differences between the two groups regarding disease severity, metabolic characteristics, or hemodynamic characteristics. Most patients in both groups were diagnosed with sepsis at the time of FB and received noradrenaline support during FB administration.
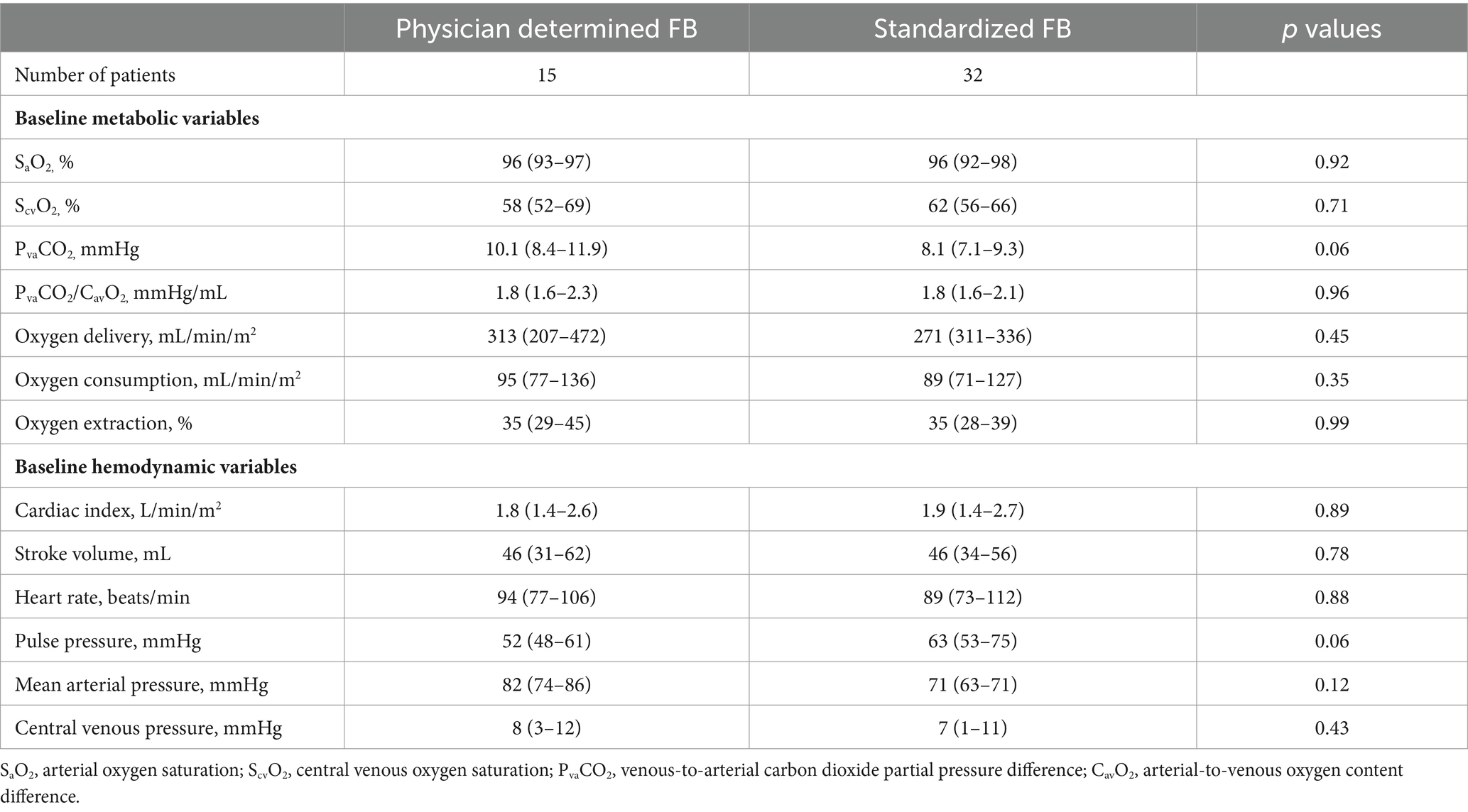
Table 2. Metabolic and hemodynamic variables before fluid bolus (FB) according to physician-determined or standardized (4 mL/kg, 1.2 L/h) FB technique.
Relation between fluid challenge technique and cardiac index changes
Patients in the standardized FB group (receiving 4 mL/kg) did not demonstrate a lower likelihood of experiencing a > 15% increase in CI compared to those treated with the physician-determined FB technique (odds ratio: 1.3, 95% CI: 0.37–5.18, p = 0.66) (Figure 1). The standardized technique resulted in a similar increase in CI to the physician-determined technique (8.4% [0.3–23.2%] vs. 8.8% [−0.1–19.9%], p = 0.76) despite a lower increase in CVP (2 [0–3] mmHg vs. 4 (2−6) mmHg, p = 0.01) (Table 3).
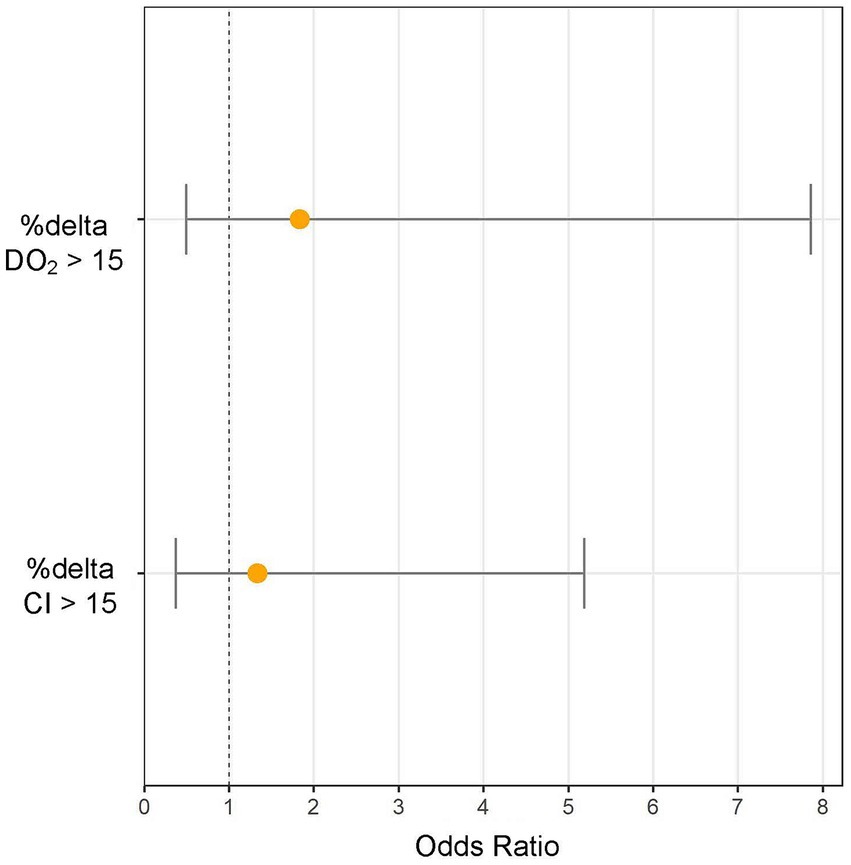
Figure 1. Increasing cardiac index (%Δ CI) and oxygen delivery (%Δ DO2) >15% odds ratios (and 95% CI) for standardized (4 mL/kg, 1.2 L/h) fluid bolus (FB) technique extracted from the multivariable logistic regression analysis with physician-determined FB as reference group.
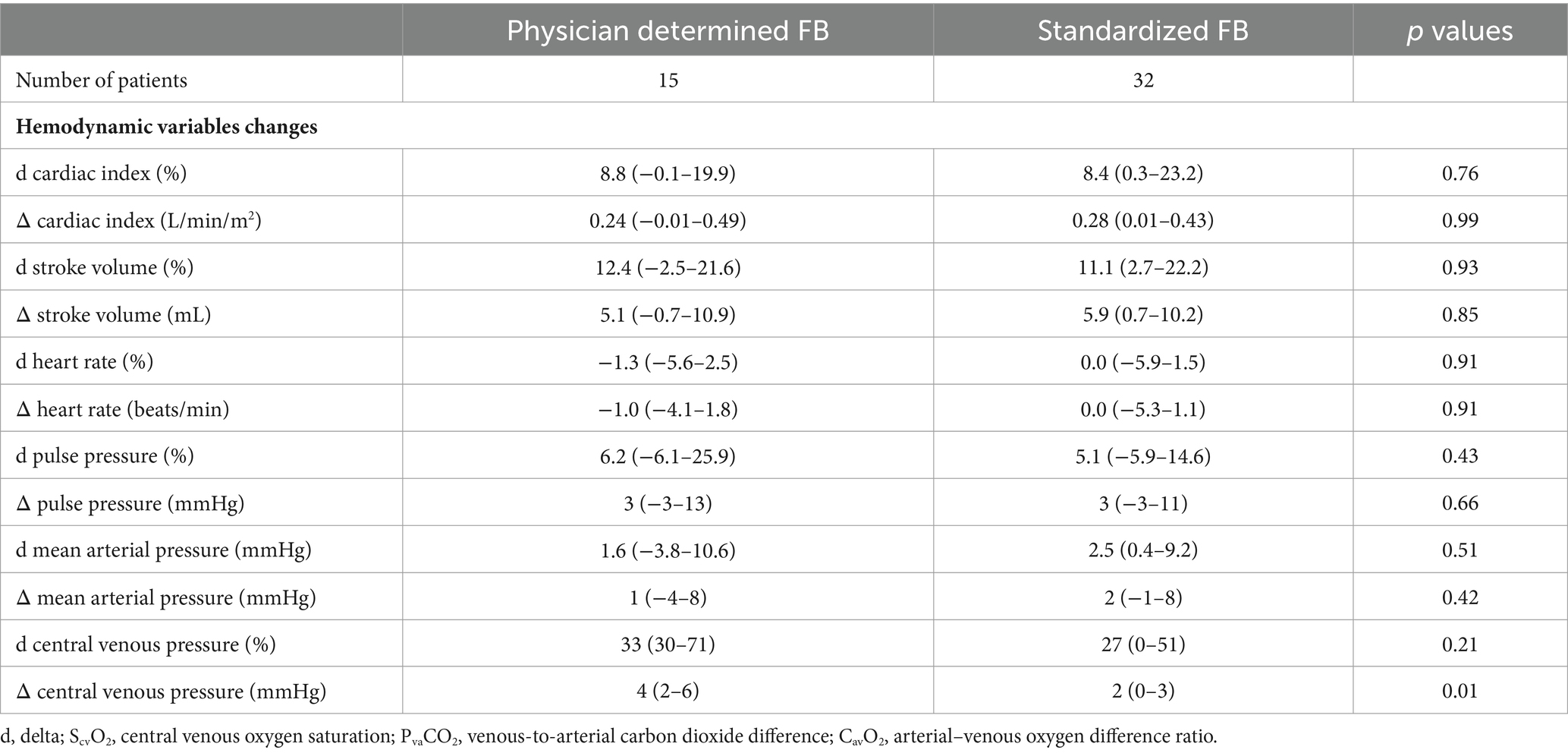
Table 3. Hemodynamic changes after fluid bolus (FB) according to physician-determined or standardized (4 mL/kg, 1.2 L/h) FB technique.
Relation between fluid challenge technique and oxygen delivery changes
Patients in the standardized FB group did not show a lower likelihood of a > 15% increase in DO2 compared to the physician-determined FB group (odds ratio: 1.83, 95% CI: 0.49–7.85, p = 0.38) (Figure 1). Although not statistically significant, the standardized FB technique resulted in a higher increase in DO2 compared to the physician-determined technique (7.2% [−3.8–19.3%] vs. -3.5% [−9.1–11.2%], p = 0.11) (Figure 2). Additionally, the standardized technique led to a smaller decrease in hemoglobin levels (−3.6% [−6.1−−1.8%] vs. -7.5% [−11.5−−3.6%], p = 0.01). A statistically significant decrease in oxygen saturation was observed with the physician-determined FB technique (−0.5% [−1.8–0.15%]) compared to an increase with the standardized FB technique (0.26% [−0.71–0.92%]) (p = 0.02) (Figure 2).
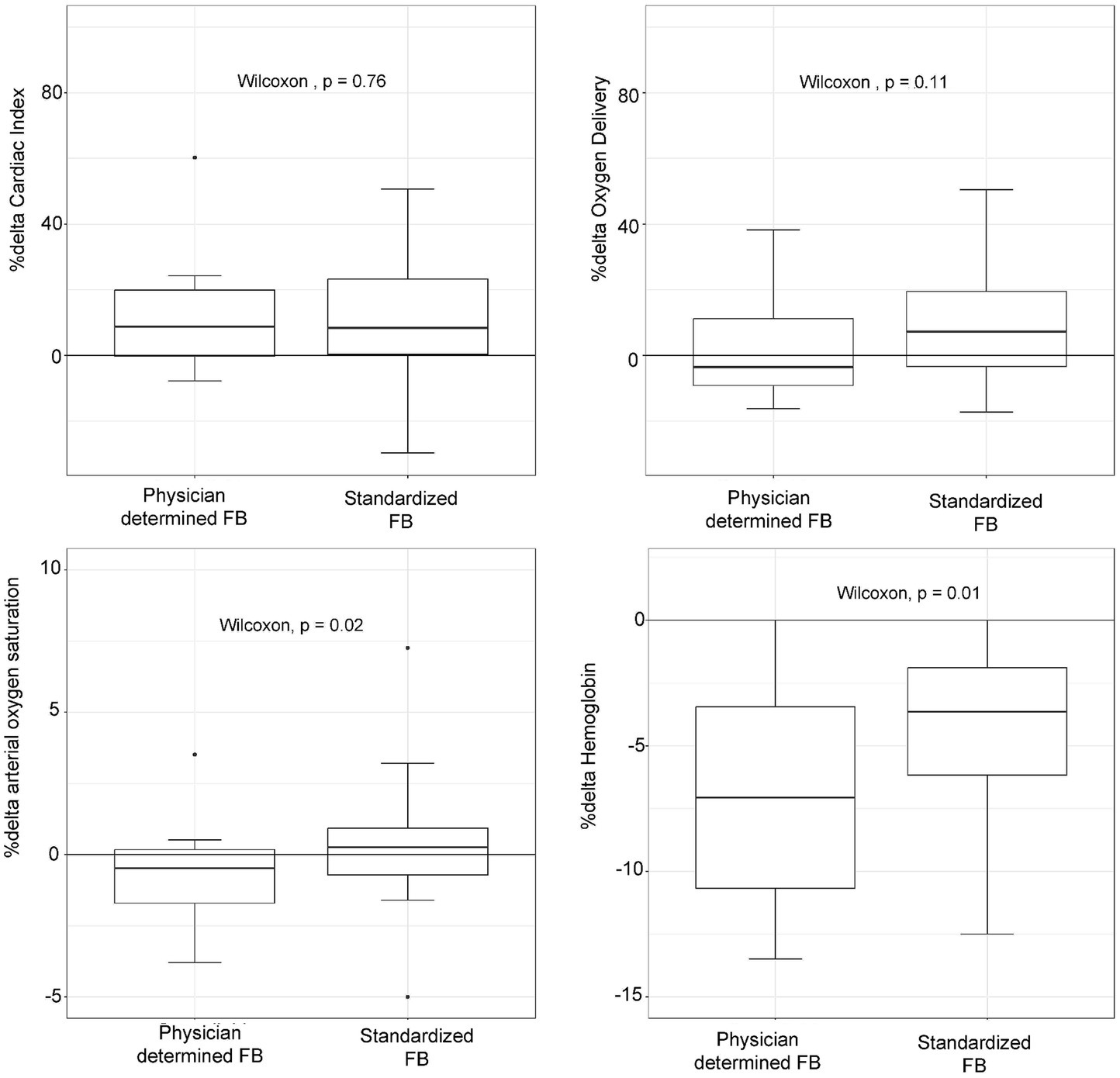
Figure 2. Oxygen delivery, cardiac index, hemoglobin concentration, and arterial oxygen saturation changes (%Δ) according to physician-determined or standardized (4 mL/kg, 1.2 L/h) fluid bolus (FB) technique.
Relation between fluid challenge technique and metabolic parameters changes
There were no statistically significant differences in baseline PvaCO2 values between patients treated with the standardized or physician-determined FB techniques (8.1 [7.1–9.3] mmHg vs. 10.1 [8.4–11.9] mmHg, p = 0.06) (Table 2). Both techniques resulted in comparable changes in PvaCO2 (−5.6% [−22–10%] for the standardized technique vs. -19.7% [−39–0.3%] for the physician-determined technique, p = 0.22) (Figure 3). Additionally, no significant variations in ScvO2, lactate, or oxygen consumption values were observed between the two groups (Figure 3).
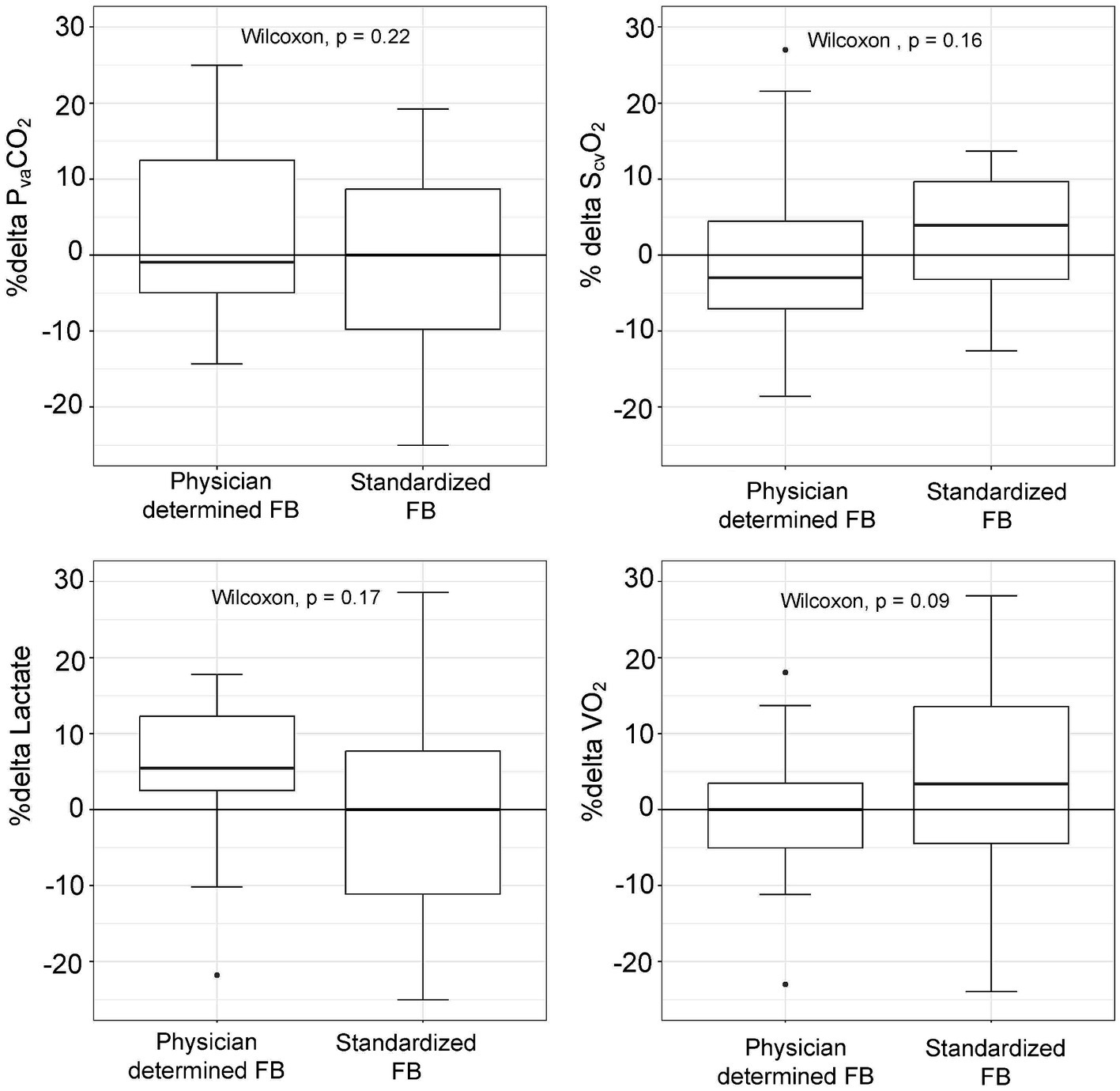
Figure 3. Changes in metabolic parameters (%delta) according to physician-determined or standardized (4 mL/kg, 1.2 L/h) fluid bolus (FB) technique.
Discussion
The study’s findings can be summed up as follows: (1) by employing a standardized FB technique, the volume of FB administered in relation to body weight was significantly reduced; (2) compared to those treated with higher volumes, critically ill patients who received crystalloids at a standardized dosage of 4 mL/kg not only exhibited a comparable likelihood of experiencing >15% increases in DO2 and CI, but also underwent metabolic effects comparable to those observed with a 16 mL/kg FB dosage; and (3) the standardized FB technique mitigated hemoglobin level reductions and reduced the risk of oxygen saturation decline.
The clinical significance of our study is highlighted by the existing variability in FB techniques in critical care practices (7). The type of fluids, volume, and rate are major components of the fluid challenge technique (1). In this study, we examined whether adhering to strict limits in these components could be effective under the pragmatic conditions of intensive care, demonstrating the hemodynamic and metabolic effectiveness of this approach. Notably, our findings indicate that patients who do not show improved hemodynamic or metabolic parameters after the initial standardized FB are unlikely to benefit from a repeated FB with a larger volume. This finding challenges the prevailing belief that higher fluid volumes are more efficacious, a concept our trial did not support (2). Our study demonstrates that a 4 mL/kg fluid bolus (FB) with a rate of 1.2 L/h of crystalloids can effectively assess fluid responsiveness and address hemodynamic and metabolic deficits, thus introducing a comprehensive and structured approach to FB in a field where standardization is lacking. While further research is required to determine if this standardized method outperforms physician-determined FB techniques in improving patient outcomes, our findings significantly address a critical gap in fluid resuscitation practices.
This study’s findings are consistent with previous research indicating that a FB of 4 mL/kg may have significant hemodynamic effects in post-cardiac surgery patients (4) and elective neurosurgical patients (19). We used the same amount of volume in non-selected critically ill patients. Almost half of those who received 4 mL/kg in our study experienced an increase in CI greater than 15%, comparable to the proportion of fluid responders in previous studies (20). The amount of fluid administered under a non-standardized technique resulted in a greater volume proportional to the patient’s weight but did not produce more pronounced hemodynamic effects. Therefore, the results of our study contribute to our comprehension of the optimal volume for FB in critically ill patients, suggesting that FB’s hemodynamic effects are no longer volume dependent for volumes greater than 4 mL/kg.
In the physician-determined FB technique, a higher fluid rate was used compared to the standardized FB, yet this did not result in a markedly stronger hemodynamic response. This finding contrasts with previous studies that associated fluid responsiveness with the rate of FB administration (3, 19). This disparity may stem from our study’s emphasis on the rate of fluid administration rather than its duration. In the physician-determined FB method, the duration surpassed 30 min, while in the standard approach, it was less than 20 min, despite a slower fluid rate. Moreover, the standardized technique employed a pump to ensure even fluid distribution throughout the FB process. Most patients were evaluated within 24 h of encountering a critical illness, a time often characterized by significant endothelial glycocalyx damage and increased vascular permeability (21). Given the short half-life of fluids in critically ill patients, approximately 15 min (22), the timing of assessing fluid responsiveness becomes pivotal. In the physician-determined FB group, the longer duration allowed for a substantial portion of the fluid to transition from the intravascular to the extravascular spaces. Additionally, the higher initial rate in this method may have exacerbated fluid leakage (23). Notably, some patients in the physician determined FB showed only minimal changes in hemoglobin levels after the FB, suggesting a modest expansion in intravascular volume. This likely attenuated the observable hemodynamic effects, explaining why a higher fluid rate did not lead to more pronounced results compared to the standardized FB technique. Therefore, this study sheds light on the hemodynamic response to FB at a commonly used in clinical practice rate of 1.2 L/h (7, 8), suggesting that higher rates may not significantly enhance this response.
In this study, we examined the effects of a standardized FB technique on DO2, alongside other metabolic parameters. While the primary objective of FB is to enhance CI and thereby increase DO2 (2), achieving this in practice can be challenging due to the unavoidable hemodilution effect of FB (24). Yet in clinical practice, it is essential to consider additional parameters like ScvO2 and blood lactate levels when assessing the adequacy of DO2 (25). Therefore, our evaluation included these metabolic markers in conjunction with DO2. Echoing findings from previous studies, we observed that reducing the volume of FB could mitigate the decrease in hemoglobin concentration (26). When comparing FB doses of 4 mL/kg and approximately 16 mL/kg, we noted a smaller decline in hemoglobin concentration at the lower dosage. This was correlated with a trend toward a higher increase in DO2 and ScvO2, as well as a more notable decrease in lactate levels. However, it is important to note that these differences did not achieve statistical significance. Therefore, our analysis reveals that while administering a 4 mL/kg volume of crystalloids can mitigate some hemodilution effects of FB, hemodilution remains considerable, leading to only subtle increases DO2 in several patients. This finding underscores the necessity of a multifaceted approach when evaluating the efficacy of FB in clinical settings, taking into account both hemodynamic and metabolic parameters.
In addition to hemodilution, significant adverse effects that may result from fluid administration regardless of the response to FB, such as deterioration of respiratory function, must be thoroughly investigated (5). CVP has been proposed as a safety parameter for patients receiving FB treatment (27). In our study, FB increased CVP in both groups. Nonetheless, FB with physician-determined volume and fluid rate caused a significant increase in CVP, reaching up to 6 mmHg in some patients. In contrast, patients receiving 4 mL/kg of FB exhibited modest increases in CVP, not exceeding 3 mmHg. Importantly, in this group of patients, oxygen saturation decreased less frequently than in patients who underwent the physician-determined FB technique. Therefore, the results of this investigation suggest that volume expansion with 4 mL/kg does not likely impair respiratory function by increasing cardiac filling pressure. Further evaluation is required to determine whether a 3-mmHg increase in CVP can be used as a safety limit in FB.
This study’s greatest asset is its comprehensive evaluation and comparison of FB techniques within a diverse group of critically ill patients, including a notable proportion with sepsis. Most of these patients were assessed early after admission. Hence, the study’s findings are particularly applicable to critically ill patients in the optimization and stabilization phase of their treatment, as well as during the de-escalation phase of fluid therapy (28). The uniformly elevated PvaCO2 levels across all patients in both cohorts signal a suboptimal pre-FB CI, a conclusion further reinforced by the high blood lactate levels observed in most of these patients. Consequently, FB emerges as a potentially effective intervention for patients in both cohorts. Furthermore, a consistent frequency of true fluid responders across both groups is anticipated, with minimal differences in the potential for hemodynamic and metabolic improvements. This uniformity is vital, especially considering our study’s primary goal of validating or refuting the equivalence of different FB techniques by minimizing the risk of Type II statistical error.
Our study has limitations that merit consideration. First, no formal sample size calculation was conducted, and the small sample size, albeit comparable to prior studies (4, 19), may affect the robustness of our findings. However, the observed benefits in CI and DO2 with the 4 mL/kg FB technique suggest that a larger sample is unlikely to contradict these results. As a secondary analysis of two prospective trials, potential biases from our retrospective approach and non-preplanned hypotheses, especially due to unmeasured confounders, need to be acknowledged. Our specific exclusion criteria, such as the absence of an acoustic window for cardiac ultrasound or the presence of atrial fibrillation, could limit the generalizability of our findings. The study’s comparability may be compromised by the historical control bias arising from different treatment periods (2015 vs. 2021–2023) and by the variability in treatment application, with the first cohort’s fluid bolus technique based on physician discretion and the second cohort following a standardized protocol, potentially confounding the outcomes. Another significant limitation is our omission of post-FB microcirculation alterations assessment. Additionally, the lack of systematic evaluation of septic patients within the first hour of sepsis diagnosis (29) may impact the findings’ applicability to early sepsis management. The study also did not investigate the shock state of patients or the duration of hemodynamic effects post-FB. The study inclusion criteria necessitated patient stability, as indicated by stable inotrope levels for 30 min prior to evaluations, which could further restrict the generalizability of our results in the early phase of rescue fluid therapy (28). Furthermore, our threshold of a 15% increase in CI for fluid responders may miss clinically significant but smaller CI increases due to the limitations of transthoracic echocardiography in detecting subtle changes, potentially underestimating the number of true fluid responders.
Conclusion
In a diverse cohort of critically ill patients, our study demonstrates that a standardized fluid bolus technique of 4 mL/kg at 1.2 L/h shows similar increases in cardiac index and oxygen delivery compared to techniques using larger volumes, with the additional benefit of reduced hemodilution and elevations in cardiac filling pressures. This balance of efficacy and safety suggests potential advantages in using this technique for fluid resuscitation in critical care scenarios.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by CHU-Brugmann ethical committee, Place Arthur Van Gehuchten 4 1020 Brussels (Laeken), Belgium. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RA: Data curation, Supervision, Validation, Writing – review & editing. TD: Conceptualization, Validation, Visualization, Writing – review & editing. DV: Supervision, Validation, Visualization, Writing – review & editing. SR: Supervision, Validation, Visualization, Writing – review & editing. MT: Supervision, Validation, Visualization, Writing – review & editing. CP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vincent, JL, and Weil, MH. Fluid challenge revisited. Crit Care Med. (2006) 34:1333–7. doi: 10.1097/01.CCM.0000214677.76535.A5
2. Vincent, JL, Cecconi, M, and De Backer, D. The fluid challenge. Crit Care. (2020) 24:703. doi: 10.1186/s13054-020-03443-y
3. Toscani, L, Aya, HD, Antonakaki, D, Bastoni, D, Watson, X, Arulkumaran, N, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. (2017) 21:207. doi: 10.1186/s13054-017-1796-9
4. Aya, HD, Rhodes, A, Chis Ster, I, Fletcher, N, Grounds, RM, and Cecconi, M. Hemodynamic effect of different doses of fluids for a fluid challenge: A quasi-randomized controlled study. Crit Care Med. (2017) 45:e161–8. doi: 10.1097/CCM.0000000000002067
5. Monnet, X, and Teboul, J-L. My patient has received fluid. How to assess its efficacy and side effects? Ann Intensive Care. (2018) 8:54. doi: 10.1186/s13613-018-0400-z
6. Fujimoto, N, Borlaug, BA, Lewis, GD, Hastings, JL, Shafer, KM, Bhella, PS, et al. Hemodynamic responses to rapid saline loading: The impact of age, sex, and heart failure. Circulation. (2013) 127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302
7. Cecconi, M, Hofer, C, Teboul, JL, Pettila, V, Wilkman, E, Molnar, Z, et al. Fluid challenges in intensive care: the FENICE study A global inception cohort study. Intensive Care Med. (2015) 41:1529–37. doi: 10.1007/s00134-015-3850-x
8. Zampieri, FG, Machado, FR, Biondi, RS, Freitas, FGR, Veiga, VC, and Figueiredo, RC. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the basics randomized clinical trial. JAMA. (2021) 326:830–8. doi: 10.1001/jama.2021.11444
9. Mallat, J. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. (2016) 5:47–56. doi: 10.5492/wjccm.v5.i1.47
10. Ltaief, Z, Schneider, AG, and Liaudet, L. Pathophysiology and clinical implications of the veno-arterial PCO2 gap. Crit Care. (2021) 25:318. doi: 10.1186/s13054-021-03671-w
11. Teboul, JL. How to integrate hemodynamic variables during resuscitation of septic shock? J Intens Med. (2023) 3:131–7. doi: 10.1016/j.jointm.2022.09.003
12. Bitar, ZI, Maadarani, OS, El-Shably, AM, Elshabasy, RD, and Zaalouk, TM. The Forgotten Hemodynamic (PCO2 Gap) in Severe Sepsis. Crit Care Res Pract. (2020) 2020:1–5. doi: 10.1155/2020/9281623
13. Pierrakos, C, de Bels, D, Nguyen, T, Velissaris, D, Attou, R, Devriendt, J, et al. Changes in central venous-to-arterial carbon dioxide tension induced by fluid bolus in critically ill patients. PLoS One. (2021) 16:e0257314. doi: 10.1371/journal.pone.0257314
14. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
15. Scheeren, TWL, Wicke, JN, and Teboul, JL. Understanding the carbon dioxide gaps. Curr Opin Crit Care. (2018) 24:181–9. doi: 10.1097/MCC.0000000000000493
16. Mekontso-Dessap, A, Castelain, V, Anguel, N, Bahloul, M, Schauvliege, F, Richard, C, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. (2002) 28:272–7. doi: 10.1007/s00134-002-1215-8
17. Jozwiak, M, Mercado, P, Teboul, JL, Benmalek, A, Gimenez, J, Dépret, F, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care. (2019) 23:116. doi: 10.1186/s13054-019-2413-x
18. Monnet, X, and Teboul, JL. Assessment of fluid responsiveness: Recent advances. Curr Opin Crit Care. (2018) 24:190–5. doi: 10.1097/MCC.0000000000000501
19. Messina, A, Palandri, C, De Rosa, S, Danzi, V, Bonaldi, E, Montagnini, C, et al. Pharmacodynamic analysis of a fluid challenge with 4 ml kg−1 over 10 or 20 min: a multicenter cross-over randomized clinical trial. J Clin Monit Comput. (2022) 36:1193–203. doi: 10.1007/s10877-021-00756-3
20. Messina, A, Calabrò, L, Pugliese, L, Lulja, A, Sopuch, A, Rosalba, D, et al. Fluid challenge in critically ill patients receiving haemodynamic monitoring: a systematic review and comparison of two decades. Crit Care. (2022) 26:186. doi: 10.1186/s13054-022-04056-3
21. Patterson, EK, Cepinskas, G, and Fraser, DD. Endothelial glycocalyx degradation in critical illness and injury. Front Med (Lausanne). (2022) 9:898592. doi: 10.3389/fmed.2022.898592
22. Hahn, RG, and Lyons, G. The half-life of infusion fluids. Eur J Anaesthesiol. (2016) 33:475–82. doi: 10.1097/EJA.0000000000000436
23. Hahn, RG, and Svensén, C. Plasma dilution and the rate of infusion of Ringer’s solution. Br J Anaesth. (1997) 79:64–7. doi: 10.1093/bja/79.1.64
24. Pierrakos, C, Nguyen, T, Velissaris, D, Gottignies, P, Devriendt, J, Honore, PM, et al. Acute oxygen delivery changes in relation to cardiac index changes after bolus fluid treatment in critically ill patients: Results of an observational study. J Clin Anesth. (2019) 57:9–10. doi: 10.1016/j.jclinane.2019.02.022
25. Bisarya, R, Shaath, D, Pirzad, A, Satterwhite, L, He, J, and Simpson, SQ. Serum lactate poorly predicts central venous oxygen saturation in critically ill patients: A retrospective cohort study. J Intensive Care. (2019) 7:47. doi: 10.1186/s40560-019-0401-5
26. Quispe-Cornejo, AA, Alves da Cunha, AL, Njimi, H, Mongkolpun, W, Valle-Martins, AL, Arébalo-López, M, et al. Effects of rapid fluid infusion on hemoglobin concentration: a systematic review and meta-analysis. Crit Care. (2022) 26:324. doi: 10.1186/s13054-022-04191-x
27. De Backer, D, and Vincent, JL. Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care. (2018) 22:43. doi: 10.1186/s13054-018-1959-3
28. Hoste, EA, Maitland, K, Brudney, CS, Mehta, R, Vincent, JL, Yates, D, et al. Four phases of intravenous fluid therapy: A conceptual model. Br J Anaesth. (2014) 113:740–7. doi: 10.1093/bja/aeu300
Keywords: fluid challenge, PCO2 gap, carbon dioxide, veins/metabolism, plasma substitutes, crystalloid solutions, hemodilution, hemodynamics
Citation: Attou R, Du T, Velissaris D, Redant S, Talpoș MT and Pierrakos C (2024) Evaluating the efficacy of a standardized 4 mL/kg fluid bolus technique in critically ill patients with elevated PvaCO2: secondary analysis of two prospective studies. Front. Med. 11:1348747. doi: 10.3389/fmed.2024.1348747
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Andrea Glotta, Istituto Cardiocentro Ticino, SwitzerlandFrancesco Zadek, University of Milano Bicocca, Italy
Copyright © 2024 Attou, Du, Velissaris, Redant, Talpoș and Pierrakos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charalampos Pierrakos, Y2hhcmFsYW1wb3NfcEBob3RtYWlsLmNvbQ==
 Rachid Attou1
Rachid Attou1 Thierry Du
Thierry Du Dimitrios Velissaris
Dimitrios Velissaris Sebastien Redant
Sebastien Redant Mircea T. Talpoș
Mircea T. Talpoș Charalampos Pierrakos
Charalampos Pierrakos